Abstract
Both exposure to higher levels of polluted air and physical inactivity are crucial risk factors for the development and progression of major noncommunicable diseases and, in particular, of cardiovascular disease. In this context, the World Health Organization estimated 4.2 and 3.2 million global deaths per year in response to ambient air pollution and insufficient physical activity, respectively. While regular physical activity is well known to improve general health, it may also increase the uptake and deposit of air pollutants in the lungs/airways and circulation, due to increased breathing frequency and minute ventilation, thus increasing the risk of cardiovascular disease. Thus, determining the tradeoff between the health benefits of physical activity and the potential harmful effects of increased exposure to air pollution during physical activity has important public health consequences. In the present comprehensive review, we analyzed evidence from human and animal studies on the combined effects of physical activity and air pollution on cardiovascular and other health outcomes. We further report on pathophysiological mechanisms underlying air pollution exposure, as well as the protective effects of physical activity with a focus on oxidative stress and inflammation. Lastly, we provide mitigation strategies and practical recommendations for physical activity in areas with polluted air.
1. Introduction
Physical inactivity is among the leading risk factors for major noncommunicable diseases, including cardiometabolic disease and cancer. The beneficial health effects of physical activity are well known, with further positive impacts on mental health, quality of life, cognitive function, and healthy weight [1]. On a global scale, more than every fourth adult is insufficiently physically active, which means that they do not achieve the minimum recommended level of 150 min of moderate intensity or 75 min of vigorous intensity physical activity per week, as indicated by recent data from Guthold et al. [2]. Environmental factors, such as air pollution, heavy metals, pesticides, and traffic noise, are increasingly recognized as endocrine disruptors that contribute to cardiometabolic diseases such as metabolic syndrome and type 2 diabetes [3,4,5,6]. Likewise, the World Health Organization (WHO) concludes that air pollution is a major contributor to the global burden of disease, with 9 out of 10 people worldwide breathing polluted air, exceeding the WHO guideline values for ambient air quality [7]. Both air pollution and physical inactivity were demonstrated to cause excess deaths from noncommunicable diseases, including cardiovascular disease, respiratory disease (mainly induced by air pollution), type 2 diabetes, and certain types of cancers [8]. Especially, the risk for diabetes is largely increased in areas with polluted air [9,10], with a central role for low grade inflammation and altered lipid metabolism by air pollution constituents [11]. For comparison, WHO estimated that ambient air pollution was associated with 4.2 million deaths in 2016 [12], while insufficient physical activity accounted for 3.2 million deaths for the same year [13]. Of note, we recently used a novel hazard ratio function, the estimate of the Global Exposure-Mortality Model (GEMM), to reveal 8.79 million global premature deaths in 2019, as well as 790,000 excess deaths per year in Europe, attributable to air pollution, thus greatly exceeding the calculations from WHO [14]. The interplay between air pollution and physical activity and the joint effects on health outcomes constitute an emerging area of investigation with important public health implications. For instance, higher air pollution may interfere with physical activity behavior, as it could create a barrier for doing physical activity that is, importantly, not restricted to outdoor environments as outdoor and indoor environments are fundamentally connected [8]. These circumstances lead to a key question—should physical activity be encouraged in areas with polluted air? While regular physical activity is in general regarded to improve somatic and mental health, it may also influence the uptake and deposit of air pollutants in the lungs and airways due to increased breathing frequency and minute ventilation, thus increasing the risk of several noncommunicable diseases, mainly from cardiorespiratory origin [15]. Hence, determining the tradeoff between the health benefits of physical activity and the potential harmful effects of increased exposure to air pollution during physical activity is an ongoing target. In the present review, we provide an updated overview of the combined effects of physical activity and air pollution on (cardiovascular) health outcomes on the basis of human and animal data. We also describe the pathomechanisms of air pollution, as well as the protective effects of physical activity, with focus on oxidative stress and inflammation. To end, we will report on mitigation strategies and practical recommendations for physical activity in air polluted areas.
2. Pathomechanisms of Air Pollution with Focus on Oxidative Stress and Inflammation
Oxidative stress and inflammation represent the common denominator of the adverse cardiorespiratory health effects of air pollution. Recent evidence from human and animal studies suggests that exposure to multiple air pollutants may have the potential to increase systemic oxidative stress and inflammation, both relevant to mediating disease risk (reviewed in [16,17]). However, understanding the complete picture of underlying pathomechanisms is still ongoing, and complex interactions with other risk and lifestyle factors, such as physical activity, are very likely. As shown in Table 1, the short and long-term impacts of air pollution constituents on various markers of oxidative stress and inflammation are supported by a number of recent clinical studies. Exemplarily, Liu et al. evidenced a positive association between polycyclic aromatic hydrocarbons (PAHs) and levels of malondialdehyde in a panel study of 40 chronic obstructive pulmonary disease patients and 75 healthy controls, which was even more pronounced in subjects with impaired lung function [18]. Nassan et al. performed a mass spectrometry based metabolomic profiling of plasma samples among 456 men, to show that acute and chronic exposures to fine particulate matter (PM2.5) were related to metabolic pathways involved in inflammation, oxidative stress, immunity, and nucleic acid damage and repair [19]. These observations can be mechanistically explained, at least in part, by air pollution induced mitochondrial damage and dysfunction, as reviewed in [20]. Abohashem et al. demonstrated that higher PM2.5 exposure was associated with an increase in major adverse cardiovascular events, and that this was mediated by an increase in leucopoietic activity and arterial inflammation [21]. These results are not restricted to adult populations, as indicated by a recent study from Mann et al. that indicates that acute increases in traffic related air pollutants were associated with 8-isoprostane levels in 299 children (aged 6–8) [22]. Likewise, adolescents (n = 100) were demonstrated to be a susceptible group, by showing that exposure to multiple air pollutants was related to markers of oxidative stress, acute inflammation, altered hemostasis, endothelial dysfunction, monocyte enrichment, and high blood pressure [23]. In a larger cohort of 3996 subjects, Li et al. found acute increases in PM2.5 and sulfate to be associated with increased C-reactive protein levels, which was also true for nitrogen dioxide (NO2) in the case of interleukin (IL)-6, and for black carbon (BC), sulfate, and ozone (O3) in the case of tumor necrosis factor receptor 2 [24]. Conversely, BC, sulfate, and NOx were negatively associated with fibrinogen, and sulfate was negatively associated with tumor necrosis factor (TNF)-α levels.
Cell culture exposures suffer from often unrealistic interaction between certain cell types and air pollution constituents, and controlled human exposure studies are hard to perform due to ethical concerns. This makes the use of animal models an indispensable tool in probing the mechanisms of air pollution induced pathology. For a long time, O3 was the main focus of air pollution research, with its effects on human health being well characterized [25] and only recently has PM2.5 taken its place. Other gaseous constituents, such as NO2 and sulfur dioxide (SO2), also play a prominent role in the air pollution induced pathology, but receive less attention [26,27].
O3 is still an important contributor to air pollution and there is a large body of literature dedicated to its effects on health. One of the main ways in which O3 influences the onset and progression of cardiovascular disease is through oxidative stress and inflammation. A study in mice found that O3 exposure modulated vascular tone regulation and increased oxidant stress and mitochondrial DNA damage [28]. Authors have also observed a larger increase in atherosclerotic plaques in ApoE−/− mice exposed to O3, pointing to a proinflammatory phenotype. Endothelial dysfunction due to O3 exposure was also observed to be CD36 dependent, as a study showed that vasorelaxation by acetylcholine was not impaired in CD36−/− mice [29]. The same study demonstrated an increase in the number of lung macrophages and neutrophils after O3 exposure in wild type mice. In diabetes prone mice, O3 exposure not only impaired insulin response but also caused systemic inflammation that was observed through the elevation of interferon (IFN)-γ, TNF-α, and IL-12 in visceral adipose tissue [30]. Expression of oxidative stress related genes, such as Cox4 and Nrf2, was also increased. Interestingly, a study on a successive O3 and carbon black particles exposure revealed that heart rate and heart rate variability were both changed in mice exposed to O3 and then carbon black particles, but not in mice exposed to clean air and then carbon black alone, indicating that O3 is a major contributor to air pollution induced cardiovascular disease [31].
PM, especially PM2.5, is now known to influence the onset and progression of cardiovascular disease, mainly through oxidative stress and inflammation pathways [32]. Exposure of mice to PM2.5 was shown to increase the number of circulating monocytes and their infiltration into the vasculature [33]. The same study found that inflammation markers such as TNF-α and monocyte chemoattractant protein 1 (MCP-1) were upregulated in the lung tissue, together with markers of lipid peroxidation, and that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) was activated in the aorta. More evidence that NADPH oxidase is a major contributor to oxidative stress was demonstrated in a study where superoxide production was increased in rat aortic rings after exposure to PM2.5, but the effect was abolished after inhibition of NADPH oxidase [34]. Endothelial function, a potent early prognostic functional parameter for later cardiovascular events [35,36,37], is partly dependent on the ability of eNOS to produce nitric oxide (NO), and the presence of superoxide radical is detrimental to the NO signaling, because NO and O2− react to produce peroxynitrite (ONOO−) [38,39]. It was shown that 3-nitrotyrosine, a marker of protein nitration by ONOO−, was elevated in the thoracic aorta of ApoE−/− mice exposed to concentrated ambient PM [40]. PM can also impair NO signaling through the uncoupling of eNOS, as was shown in a study where rats were exposed to diesel PM [41]. Oxidative stress in the aorta as a result of PM exposure was normalized after treatment with BH4, an eNOS cofactor. A decrease in eNOS, accompanied by oxidative stress and an increase in Mn-SOD, was also observed in the pulmonary artery of PM2.5 exposed rats [42]. Inflammation in the mesenteric arteries, as defined by increased expression of TNF-α, IL-6, MCP-1, E-selectin, and vascular cell adhesion molecule 1 (VCAM-1), was observed after the concentrated ambient PM exposure of ApoE−/− mice [43]. Progression of atherosclerosis through the accumulation of oxidized lipids and the infiltration of immune cells was associated with PM2.5 exposure in ApoE−/− mice [44]. Accumulation of oxidized lipids such as 7-ketocholesterol was suggested to be CD36 mediated. In most of the above mentioned studies, endothelial function, as measured by the endothelium dependent relaxation of isolated aortic rings, was impaired. Interestingly, a study on rats showed significant impairment of endothelial function after the instillation of ambient PM, but not after the instillation of carbon black or TiO2 particles, suggesting that the complex composition of ambient PM is responsible for the observed cardiovascular effects [45]. An overview of the mechanisms by which air pollution confers negative cardiovascular effects is presented in Figure 1.
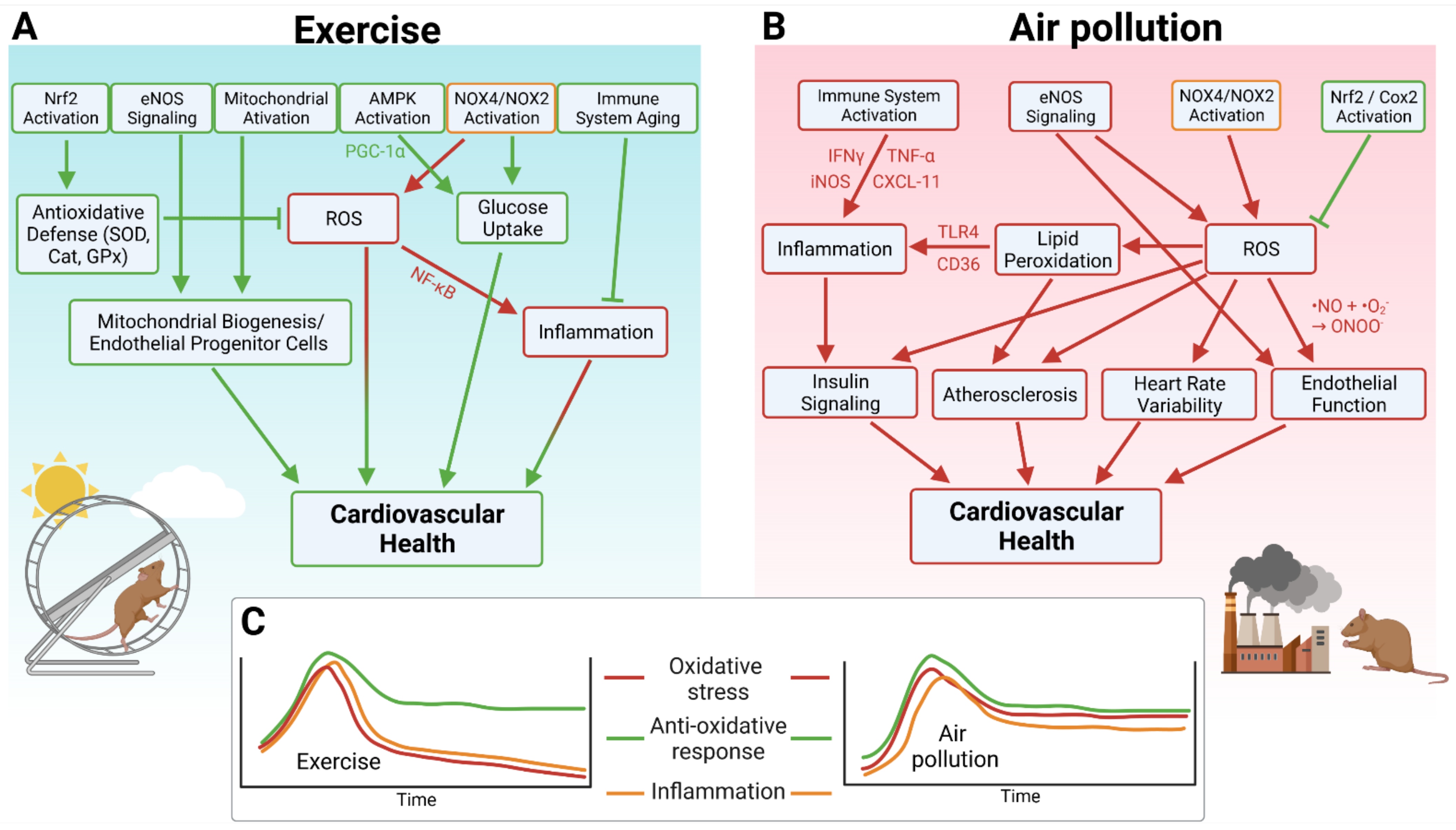
Figure 1.
(A) Major pathways by which exercise improves cardiovascular health as inferred from animal studies. (B) Major pathways by which air pollution negatively influences cardiovascular health. (C) Chronic versus acute exposure to exercise or air pollution. Oxidative stress and inflammation are acutely elevated during both exercise and air pollution exposure, but decrease after exercise and remain high after air pollution. Antioxidative defense also increases acutely following either exercise or air pollution exposure, but remains high in both scenarios, resulting in a long-term benefit of exercise. Created with BioRender.com, accessed on 4 November 2021.

Table 1.
Human studies on the association of inflammation and/or oxidative stress with air pollution.
Table 1.
Human studies on the association of inflammation and/or oxidative stress with air pollution.
| First Author/Year | Population/Cohort | Air Pollutants | Major Outcomes | Ref. |
|---|---|---|---|---|
| Liu, 2021 | 40 chronic obstructive pulmonary disease patients and 75 controls | PAHs | A one fold increase in hydroxylated PAHs was associated with a 4.1–15.1% elevation of malondialdehyde, which was stronger in subjects with impaired lung function. | [18] |
| Abohashem, 2021 | 503 subjects without cardiovascular disease | PM2.5 | Higher PM2.5 was associated with increased risk for major adverse cardiovascular events, mediated by an increase in leucopoietic activity and arterial inflammation. | [21] |
| Ni, 2021 | 740 subjects | PM2.5 | Acute increases in PM2.5 were associated with increased soluble lectin like oxidized LDL receptor-1, but not with nitrite. | [46] |
| Nassan, 2021 | 456 men | PM2.5 species | Acute increases in PM2.5 species were associated with metabolic pathways involved in inflammation, oxidative stress, immunity, and nucleic acid damage and repair. | [19] |
| Mann, 2021 | 299 children | Traffic related air pollutants (sum of PAH456, NO2, elemental carbon, PM2.5) | Acute increases in traffic related air pollutants were associated with 8-isoprostane. | [22] |
| Prunicki, 2020 | 100 subjects | PM2.5, NO, NO2, CO, PAHs | Air pollutants were associated with oxidative stress, acute inflammation, altered hemostasis, endothelial dysfunction, monocyte enrichment, and diastolic blood pressure. | [23] |
| Riggs, 2020 | 100 subjects | PM2.5 | A 10 μg/m3 increase in PM2.5 was associated with a 12.4% decrease in reactive hyperemia index (95% CI −21.0–−2.7). Increased PM2.5 was associated with elevated F-2 isoprostane metabolite, angiopoietin 1, vascular endothelial growth factor, placental growth factor, intracellular adhesion molecule-1, and matrix metalloproteinase-9 as well as reduced vascular adhesion molecule-1. | [47] |
| Li, 2019 | 73 subjects | PM2.5, BC, NO2, CO | Increases in air pollutants were associated with reductions in circulating high density lipoprotein cholesterol and apolipoprotein A-I, as well as elevations in HDL oxidation index, oxidized LDL, malondialdehyde, and C-reactive protein. | [48] |
| Lin, 2019 | 26 subjects | PAHs | Increases in 5-, 12-, and 15-hydroxyeicosatetraenoic acid, as well as 9- and 13-hydroxyoctadecadienoic acid, were observed. Decreases in paraoxonase and arylesterase, as well increases in C-reactive protein and fibrinogen, were observed. | [49] |
| Balmes, 2019 | 87 subjects | O3 | Acute O3 exposure did not alter C-reactive protein, monocyte–platelet conjugates, and microparticle associated tissue factor activity, whereas increases in endothelin-1 and decreases in nitrotyrosine were observed. | [50] |
| Han, 2019 | 60 subjects with prediabetes and 60 healthy subjects | PM2.5 | Acute exposure to PM2.5 resulted in increased exhaled NO, white blood cells, neutrophils, interleukin-1α, and glycated hemoglobin. Compared to healthy subjects, prediabetic subjects displayed pronounced PM2.5 associated systemic inflammation, elevated systolic and diastolic blood pressure, impaired endothelial function, and elevated fasting glucose. | [51] |
| Xia, 2019 | 215 pregnant women | PM2.5 | Acute increases in PM2.5 and lead constituent were associated with endothelial dysfunction (increased endothelin-1, E-selectin, and intracellular adhesion molecule-1) and inflammation (increased interleukin-1β, interleukin-6, tumor necrosis factor-α). Endothelial dysfunction and elevated inflammation were partially mediated by the effect of PM2.5 and lead constituent on blood pressure. | [52] |
| Li, 2019 | 3820 subjects | PM2.5, BC, O3, sulfate, NOX | Negative associations of acute PM2.5 and BC with P-selectin, of O3 with monocyte chemoattractant protein 1, and of sulfate and NOx with osteoprotegerin were found. | [53] |
| Li, 2017 | 3996 subjects | PM2.5, sulfate, NOx, BC, O3 | Acute increases in PM2.5 and sulfate were associated with increased C-reactive protein, which was also true for NOx in case of interleukin-6 and for BC, sulfate, and O3 in case of tumor necrosis factor receptor 2. Conversely, BC, sulfate, and NOx were negatively associated with fibrinogen, and sulfate was negatively associated with tumor necrosis factor α. | [24] |
| Mirowsky, 2017 | 13 subjects with coronary artery disease | O3 | Per acute IQR increase in O3, changes in tissue plasminogen factor (6.6%, 95% CI 0.4–13.2), plasminogen activator inhibitor-1 (40.5%, 95% CI 8.7–81.6), neutrophils (8.7%, 95% CI 1.5–16.4), monocytes (10.2%, 95% CI 1.0–20.1), interleukin-6 (15.9%, 95% CI 3.6–29.6), large artery elasticity index (−19.5%, 95% CI −34.0–−1.7), and the baseline diameter of the brachial artery (−2.5%, 95% CI −5.0–0.1) were observed. | [54] |
| Pope 3rd, 2016 | 24 subjects | PM2.5 | Episodic increases in PM2.5 were associated with increased endothelial cell apoptosis, an anti-angiogenic plasma profile, and elevated circulating monocytes, and T, but not B, lymphocytes. | [55] |
| Wu, 2016 | 89 subjects | PM2.5, NO2 | Acute increases in PM2.5 were associated with brachial–ankle pulse wave velocity, whereas no association was found for NO2. NO2 was associated with increased C-reactive protein. | [56] |
PAHs: polycyclic aromatic hydrocarbons, PM(diameter size): particulate matter, NO2: nitrogen dioxide, NO: nitric oxide, CO: carbon monoxide, BC: black carbon, O3: ozone, NOx: nitrogen oxides, IQR: interquartile range, CI: confidence interval.
3. Key Mechanisms of Antioxidant and Anti-Inflammatory Effects of Physical Activity
While there is a general consensus that regular physical activity can improve cardiorespiratory fitness and health, and contribute to the prevention of disease, it is important to note that different types and contexts of physical activity may result in differential health effects. These relationships may strongly depend on sociodemographic characteristics (e.g., age, sex, and socioeconomic status), settings (e.g., leisure time physical activity, commuting, and sports), types (e.g., anaerobic/strength or aerobic/endurance activities), extent of physical activity (intensity, frequency, and duration), and overall fitness level and health status [57]. Several studies have investigated these differential health effects induced by different modes of physical activity. In the German Gutenberg Health study (n = 12,650), Arnold et al. recently demonstrated arterial stiffness (measured by stiffness index), a well established marker of cardiovascular disease risk that is fundamentally influenced by oxidative stress and inflammatory processes, to be favorably associated with sports related endurance activities, but also with active commuting [58]. Conversely, physical activities associated with intense work, such as heavy occupational activity, were shown to be associated with higher arterial stiffness. Of special importance is that the combination of both engaging in endurance training and having lower arterial stiffness (below median) resulted in decreased risk of all-cause mortality over a follow up period of eight years. In line with this, a prospective study of 723 subjects showed that sports and habitual activities possessed beneficial effects on physical fitness (13 motor performance tests) and health status (physician diagnosis concerning orthopedics, neurology, and cardiovascular system) [57]. In contrast, comparable amounts of work related activities did not substantially influence physical fitness or health status. On a mechanistic level, leisure time physical activities seem also to be accompanied by improved antioxidant defense and immune response [59]. In support of this, the beneficial impact of daily walking and gait on oxidative stress levels and inflammation was shown in patients with peripheral artery disease [60,61]. In further studies, leisure time physical activities were found to be inversely related to levels of inflammation, such as C-reactive protein, plasma fibrinogen, white blood cell count, and adhesion molecules [62,63,64,65]. In a more recent randomized controlled trial, the acute oxidative stress response following different types of activities, i.e., anaerobic, aerobic, and combined, were compared in healthy untrained young males [66]. The results revealed that aerobic, anaerobic, as well as combined activities may have the potential to acutely increase oxidative stress (in the sense of beneficial eustress [67,68]) and antioxidant responses, with a differential pattern of results related to the intensity and the duration of the physical activity.
Understanding of the relationship between exercise, oxidative stress, and antioxidant capacity has been cultivated over decades of study. Aerobic cellular metabolism produces free radicals as a byproduct of ATP synthesis that are, in normal conditions, detoxified by robust physiological defenses before serious damage to proteins, lipids, and DNA occurs [69]. Detoxification by antioxidant systems (e.g., the Nrf2 controlled gene pathway, the glutathione system, and nonenzymatic antioxidants) is essential to the life of the organism [69,70]. Despite the obvious benefits in cardiorespiratory and metabolic health, muscular exercise has long been known to cause the formation of reactive oxygen species (ROS), which initially seems somewhat incongruent to the well documented benefits to health. In 1978, markers of lipid peroxidation in the form of expired pentane were detected following 60 min of exercise [71] and a year later in rats [72]. Other studies later determined that vitamin E appeared to be important in protecting cellular membranes from the damage incurred by the greater oxidative load caused by exercise [73,74].
While the findings of higher ROS burden in exercise are consistent, there are varying reports as to the source of these oxidants. Mitochondria have been quite intuitively indicated as the primary source of ROS during exercise, but others report that mitochondria may not be the primary source of ROS, and other enzymatic sources may be critical [75]. NADPH oxidases, a group of enzymes whose endogenous role is the production of superoxide, have also been reported to be active in exercise induced ROS production. NOX-4, an isozyme present in the myocardium and also in skeletal muscle fibers, was demonstrated to increase in expression in the mouse myocardium and also to trigger Nrf2 in response to exercise. Mice deficient in cardiomyocyte NOX-4 were reported to have decreased exercise performance [76]. NOX-2 is also expressed in skeletal muscle fibers, and loss of function studies indicate that it is likely the primary source of cytosolic ROS during exercise and also plays a role in exercise stimulated glucose metabolism [77]. Interestingly, another study indicated a somewhat tissue dependent role for both NOX-2 and NOX-4 in exercise, alongside the stimulation of antioxidant defenses [78]. Another ROS-producing enzyme found in the plasma membranes of skeletal muscle is xanthine oxidase, which has also been implicated as a source of ROS following exercise, as indicated by protection following inhibition by allopurinol [79]. Importantly, ROS generation and detoxification capacity appear to be highly tissue and stimulus dependent [80]. The contribution of multiple ROS sources to the “eustress” mechanism by physical exercise can be best explained by the crosstalk concept of different ROS sources via redox switches in the involved enzymatic pathways [81,82,83,84], in the case of exercise most likely initial formation of mitochondrial ROS leading to the cross activation of NADPH oxidases and xanthine oxidase to orchestrate the concert of harmful and beneficial redox signaling pathways [85,86].
In line with these findings of greater free radical formation, exercise appears to cause the upregulation of endogenous antioxidant enzymes, namely, mitochondrial superoxide dismutase (SOD) and catalase were reported to be increased by 37% in the muscles of rats following a running program [87]. Mice placed on a long term swim program (21 weeks, 1 h/d 5 d/w) also expressed higher levels of catalase, SOD, and glutathione peroxidase in the plasma, heart, and liver [88]. Importantly, these two studies (amongst many others, reviewed in [89]) demonstrate that the physiological response to exercise induced oxidant load is plastic and occurs acutely in response to ongoing exercise, but is also maintained at higher levels over the course of training, leading to an increase in basal nonexertion levels. Many other studies support these findings, where SOD1, SOD2, and glutathione peroxidase are increased in regularly trained muscles [90,91,92,93]. In addition, the activation of the AMP activated protein kinase (AMPK) is a feature of exercise beneficial health effects, with downstream activation of protective pathways based on PGC-1α, improved angiogenesis and mitochondrial biogenesis, thrombus stabilization, anti-inflammatory and antioxidant effects, prevention of smooth muscle cell proliferation, and endothelial cell apoptosis [94]. AMPK is required for exercise protective effects on endothelial function and a higher number of endothelial progenitor cells, as well as against vascular senescence [95].
Although it was initially believed that moderate to high intensity exercise leads to a suppression of the immune system, finer analysis and more current data suggest that exercise leads to a redistribution of immune cells into tissues, resulting in heightened immune surveillance and possibly delaying the aging of the immune system (reviewed in [96]). Leukocytes migrate into active muscle and there is a transient increase in the levels of cytokines produced by immune cells. The identity of these upregulated cytokines is not consistent across many studies, however, C-reactive protein, IL-6, and IL-10 appear to be amongst these. Notably, oxidative stress and inflammation are intimately tied. NF-κB is an important transcription factor involved in cytokine production and regulating the immune response to physiological stressors that is also redox sensitive [97], particularly to the redox status of glutathione-s-transferase [98]. As previously discussed, exercise causes an increase in ROS and a subsequent increase in antioxidant capacity, possibly linking these two mechanisms. An overview of the mechanisms by which exercise promotes cardiovascular health is shown in Figure 2. Taking all of these beneficial effects of exercise into account, it is not surprising that exercise was proposed as a powerful nonpharmacological intervention against several environmental and behavioral risk factors, such as noise exposure, smoking, and mental stress [99] but also against common complications during the aging process [100]. Besides exercise, intermittent fasting was also discussed as an efficient nonpharmacological intervention against disease development and progression, which may be also of interest for the present overview since intermittent fasting shares many features with physical exercise with respect to the underlying protective mechanisms [101]. It remains to be fully established whether the beneficial effects of exercise always dominate and prevent the harmful health effects of air pollution.
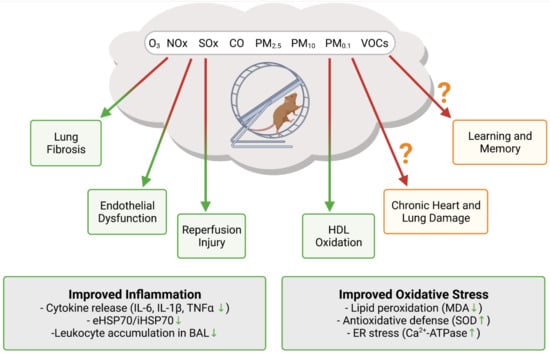
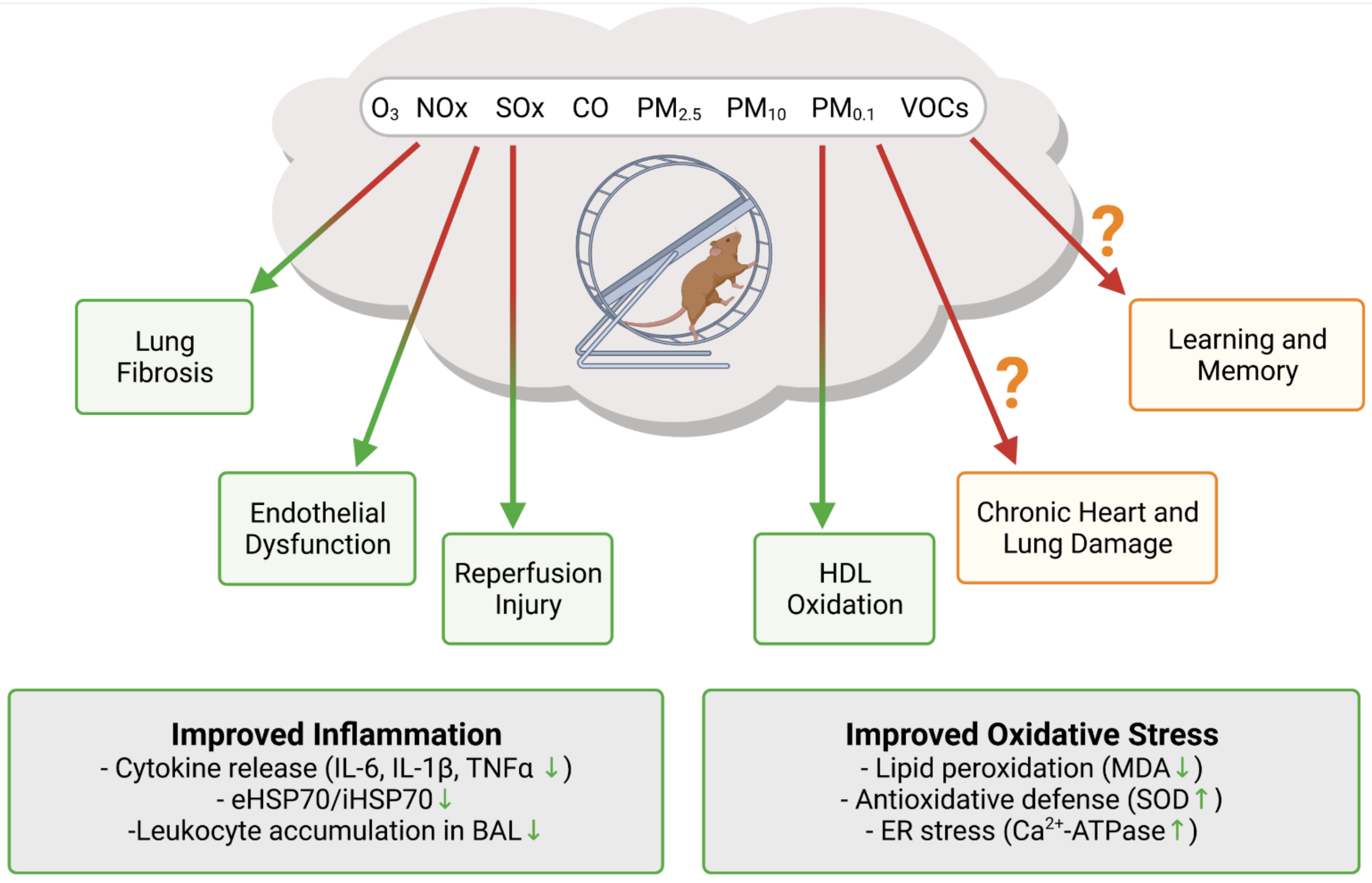
Figure 2.
Insight into the synergistic effects of exercise and air pollution exposure from animal studies. Exercise was shown to offset air pollution mediated increase in oxidative stress and inflammation. It is still not clear if exercise in polluted air can ameliorate the detrimental effects of air pollution in the long term. Created with BioRender.com, accessed on 4 November 2021.
4. Recent Epidemiological Evidence on the Association between Physical Activity, Long Term Exposure to Air Pollution, and (Cardiovascular) Health
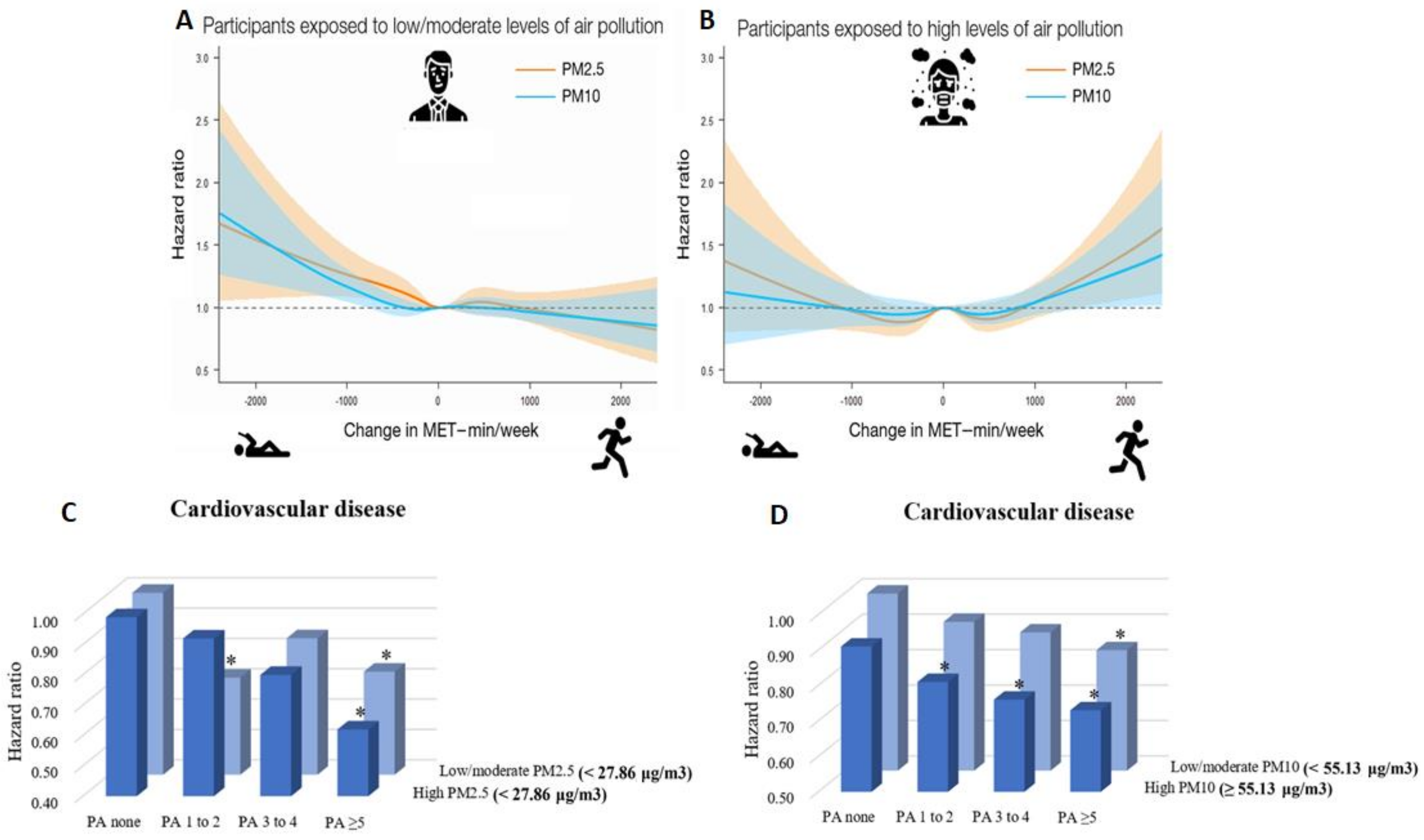
In a recent nationwide cohort study in South Korea including 1,469,972 young adults (aged 20–39 years), Kim et al. examined the tradeoff between the cardiovascular health benefits of physical activity and the potential harmful effects of increased exposure to air pollution during outdoor physical activity [102,103]. The authors demonstrated an increased risk of cardiovascular disease (including coronary heart disease and stroke) in subjects who decreased their physical activity from ≥1000 min of metabolic equivalent tasks per week (MET-min/week) to 1–499 MET-min/week (hazard ratio (HR) 1.22, 95% confidence interval (CI) 1.00–1.48 for PM10) and to 0 MET-min/week (physically inactive, HR 1.38, 95% CI 1.07–1.78 for PM10) compared to subjects who continuously engaged in ≥1000 MET-min/week in the setting of exposure to low to moderate levels of PM2.5 or PM10. Importantly, increases in physical activity above 1000 MET-min/week was associated with increased risk of cardiovascular disease among subjects exposed to high levels of PM2.5 or PM10, indicating that large increases in physical activity may adversely affect cardiovascular health in areas where high air pollution is present (Figure 3). In a further recent study from Kim et al., the combined effects of physical activity and disease were analyzed using data from 189,771 South Korean adults (aged ≥ 40 years) [104]. The study revealed that higher physical activity, i.e., moderate to vigorous physical activity ≥5 times/week, was associated with lower risk of cardiovascular disease (HR 0.73, 95% CI 0.62–0.87), coronary heart disease (HR 0.76, 95% CI 0.59–0.98), and stroke (HR 0.70, 95% CI 0.56–0.88) among subjects exposed to high levels of PM10. This was also the case for subjects exposed to low levels of PM10. Consistently, subjects engaging in moderate to vigorous physical activity ≥5 times/week experienced decreased risk of cardiovascular disease within groups of both high and low PM2.5 exposure (except for coronary heart disease) (Figure 3).
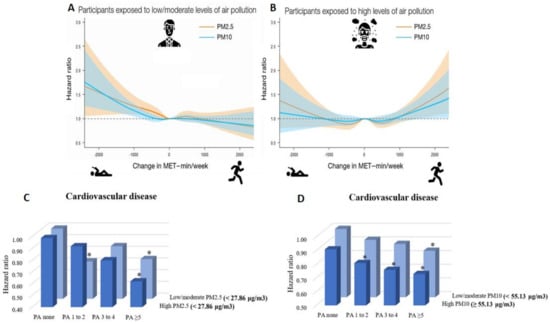
Figure 3.
(A) Combined effects of low/moderate levels of PM2.5/10 and changes in physical activity on subsequent cardiovascular disease risk in young adults, suggesting large increases in physical activity to positively affect cardiovascular health in low/moderate air polluted areas. (B) Combined effects of high levels of PM2.5/10 and changes in physical activity on subsequent cardiovascular disease risk in young adults, suggesting large increases in physical activity to negatively affect cardiovascular health in highly air polluted areas. (A,B) reused from [102] with permission; Copyright © 2021, Oxford University Press. (C) Moderate to vigorous physical activity (PA) frequency was significantly (* p < 0.05, subjects with no moderate to vigorous physical activity and low/moderate PM2.5 exposure are the reference group) associated with decreased risk of cardiovascular disease in subjects exposed to low/moderate as well as high levels of PM2.5. (D) Moderate to vigorous physical activity (PA) frequency was significantly (* p < 0.05, subjects with no moderate to vigorous PA and low/moderate PM10 exposure are the reference group) associated with decreased risk of cardiovascular disease in subjects exposed to low/moderate as well as high levels of PM10. (C,D) reused from [104] with permission; © 2021 The Authors; published on behalf of the American Heart Association, Inc., by Wiley; open access article under the terms of the http://creativecommons.org/licenses/by-nc-nd/4.0/, accessed on 4 November 2021, License.
Raza et al. aimed to analyze a potential interaction effect between physical activity and exposure to air pollution on the incident risk of ischemic heart disease and stroke in 2221 Swedish adults [105]. An increased risk of ischemic heart disease (HR 1.13, 95% CI 0.87–1.45) and stroke (HR 1.21, 95% CI 0.81–1.80) in response to PM2.5 exposure above the median (5.48 µg/m3) was observed. However, these increased risks were only present in subjects that exercised at most once a week and no interaction effect between physical activity and PM2.5 exposure was found, suggesting that physical activity may reduce PM2.5 induced risk of incident cardiovascular events. A further study by Raza et al., including 34,748 Swedish adults, confirmed these results by showing that being physically active at least twice a week was associated with decreased risk of incident ischemic heart disease among subjects exposed to high levels of PM2.5 (HR 0.60, 95% CI 0.44–0.82) and PM10 (HR 0.55, 95% CI 0.4–0.76) [106]. Conversely, this effect was weaker/not present among subjects exposed to low levels of PM2.5 (HR 0.94, 95% CI 0.72–1.22) and PM10 (HR 0.99, 95% CI 0.76–1.29). An increased PM associated risk was only observed among those who exercised less (i.e., not performing any active commuting or at most once a week). In a rural Chinese adult population including 31,162 subjects (aged 35–74 years), Tu et al. examined the interaction effect of multiple air pollutants and physical activity on 10-year atherosclerotic cardiovascular disease risk [107]. The authors observed that exposure to PM1, PM2.5, PM10, and NO2 was associated with 4.4% (odds ratio (OR), 1.044, 95% CI 1.034–1.056), 9.1% (OR 1.091, 95% CI 1.079–1.104), 4.6% (OR 1.046, 95% CI 1.040–1.051), and 6.4% (OR 1.064, 95% CI 1.055–1.072) higher odds of high 10-year atherosclerotic cardiovascular disease risk per 1 µg/m3 increase, respectively. Furthermore, increases in physical activity per one unit in MET-hour/day were associated with a 1.8% decrease (OR 0.982, 95% CI 0.980–0.985) in high 10-year atherosclerotic cardiovascular disease risk. Of special importance is that an inverse interaction effect of physical activity, PM1, PM2.5, PM10, and NO2 on high 10-year atherosclerotic cardiovascular disease risk was observed, implicating that physical activity attenuated the air pollution induced cardiovascular health risks.
Sun et al. analyzed the interaction effect of PM2.5 exposure and physical activity on cardiovascular and respiratory mortality among 66,820 older (aged ≥65 years) subjects from Hong Kong [108]. An inverse association between physical activity and risk of cardiovascular and respiratory mortality, as well as a positive association between PM2.5 and death risks, were found. Interestingly, engaging in traditional Chinese exercise (e.g., Tai Chi) and aerobic exercise (e.g., cycling) was associated with pronounced health benefits. There was minor evidence of an interaction between physical activity and PM2.5 exposure, suggesting that the beneficial health effects of physical activity outweigh the adverse effects of exposure to PM2.5. The interaction between PM2.5 exposure and physical activity, and its association with incident cardiovascular disease risk and overall mortality, was also the subject of a U.S. study of 104,990 female subjects [109]. As expected, PM2.5 exposure was associated with increases in cardiovascular disease risk (including myocardial infarction and stroke) and overall mortality, while increased overall physical activity was related to a decreased risk. No interaction effect between PM2.5 exposure and physical activity (overall, walking, vigorous activity) on cardiovascular disease risk and excess mortality was observed, implicating that higher physical activity reduced disease risk and overall mortality at all levels of PM2.5 exposure.
Beside the cardiorespiratory effects induced by the interplay between air pollution and physical activity, the related metabolic consequences are also an active area of investigation. In a rural Chinese adult population of 39,089 subjects, Hou et al. demonstrated that the prevalence of metabolic syndrome was increased in response to PM1 (OR 1.251, 95% CI 1.199–1.306), PM2.5 (OR 1.424, 95% CI 1.360–1.491), PM10 (OR 1.228, 95% CI 1.203–1.254), and NO2 exposure (OR 1.408, 95% CI 1.363–1.455 per 5 µg/m3), and decreased in cases of higher physical activity (OR 0.814, 95% CI 0.796–0.833 per 10 MET-hour/day) [3]. Importantly, when air pollution levels increased, the protective effect of physical activity on metabolic syndrome was diminished. In good agreement is a large study of 1,259,871 older adults (aged ≥58 years) from South Korea, which showed moderate to vigorous physical activity ≥5 times/week to be associated with lower risk of diabetes within both groups of subjects exposed to high and low/moderate levels of PM10 (HR 0.91, 95% CI 0.89–0.93 for low/moderate PM10; HR 0.97, 95% CI 0.94–0.99 for high PM10) and PM2.5 (HR 0.88, 95% CI 0.85–0.90 for low/moderate PM2.5; HR 0.95, 95% CI 0.91–0.99 for high PM10) [110]. Among subjects who were exposed to high levels of PM, the protective effect of physical activity tended to be slightly attenuated. In 156,314 nondiabetic adults (aged ≥18 years) from Taiwan, moderate and inactive/low physical activity (vs. high) were shown to increase risk of type 2 diabetes (HR 1.31, 95% CI 1.22–1.41 and HR 1.56, 95% CI 1.46–1.68, respectively) [111]. The same pattern was observed for subjects exposed to moderate/high levels of PM2.5. Analyzing the joint effects revealed that subjects engaging in high physical activity and exposed to low levels of PM2.5 had a 64% lower risk of type 2 diabetes than those with inactive/low physical activity and high PM2.5 exposure. Based on the present data, it is not entirely clear whether physical activity in highly polluted air is (less) beneficial or even harmful, as large scale studies exist reporting opposite effects (Figure 3 and Figure 4).
In contrast, practically no evidence is available concerning the joint effects of physical activity and air pollution on mental health. Besides the proposed biological effects centered on oxidative stress and inflammatory processes (that are also involved in the pathogenesis of mental disorders [16,17]), high levels of air pollution may also affect mental health through interference with health related behaviors such as less outdoor physical activity with face to face social contact, which represents an effective way to cope with mental health related stressors [8]. Interestingly, a recent study found lower PM10 exposure to be associated with life satisfaction, more self-esteem, and higher stress resilience in 3020 German adults [112].
5. Evidence on the (Cardiovascular) Health Effects of Physical Activity and Air Pollution from Animal Studies
Translational research into the potentially offsetting effects of air pollution on the benefits of exercise is very scarce. An overview of the available data on exercise in polluted air from animal studies is shown in Figure 2. The majority of research investigating the combined effects focuses on the pulmonary system, as the lung is usually the first organ to interact with PM after inhalation. Lung fibrosis is known to be both caused and worsened by ambient PM [113,114]. A study on bleomycin induced lung fibrosis demonstrated that aerobic exercise reduced the severity of inflammation and fibrosis in this model [115]. Most notably, inflammation markers IL-6 and IL-1β were reduced in the bronchoalveolar lavage (BAL), together with collagen fiber deposition and Akt phosphorylation in the lung tissue. Pulmonary inflammation in response to the PM2.5 and PM10 exposure of mice, as measured by the accumulation of neutrophils and macrophages in the BAL, was significantly reduced after treadmill training [116]. Importantly, these effects also appear to be systemic: inflammation markers in the plasma, IL-1β, TNF-α, and CXCL1/KC were also reduced in the treadmill exercise group. A study on thoroughbred racehorses showed that accumulation of tracheal mucus was associated with the breathing PM10 concentration, adding evidence that the pulmonary system suffers from air pollution [117]. The anti-inflammatory effects of exercise were also demonstrated in mice exposed to diesel PM [118] and hamsters exposed to O3, as neutrophil levels were increased after O3 exposure, but unchanged after exercise [119]. Not all research reports point to a beneficial effect of exercise on the air pollution induced lung injury. The study on hamsters also demonstrated increased levels of F2-isoprostanes, a marker of lipid peroxidation, after exercise in O3-rich environment [119]. A study on bronchoalveolar mucosal permeability to bovine serum albumin and diethylenetriamine pentaacetate in rats exposed to O3 and NO2 with and without exercise, showed that airway permeability was greater in the exercise group, however, without providing data on the enzymatic antioxidant capacity [120]. In addition, airway permeability remained high 48 h after the O3 treatment in the exercise group but not in the inactive group, where it returned to the level of the fresh air control. Another study on diesel PM exposed mice also demonstrated that airway permeability was more pronounced in the exercise group in comparison to the inactive group [121]. The authors of this study concluded that exercise does not exert an anti-inflammatory effect in the lung, but, rather, provides physiological adaption of the airways. These studies, while not directly addressing the cardiovascular consequences or benefits of exercising in polluted air, indicate that, under these circumstances, increases in ventilation rate may accelerate systemic inflammation, a known cardiovascular risk factor [122,123].
Recently, more research on exercising in polluted air focused on the cardiovascular system, as cardiovascular health is known to greatly improve with exercise. A study on exercising and nonexercising rats exposed to carbon monoxide (CO), an important factor of urban air pollution, showed that exercise reduces heart vulnerability to ischemic reperfusion injury [124]. Authors observed that cardiomyocyte antioxidant status and Ca2+ handling were disturbed in the CO exposed sedentary rats, but not in the exercising rats. Mice exposed to low levels of PM2.5 and submitted to either moderate or high intensity training showed a marked anti-inflammatory profile [125]. The ratio of intracellular to extracellular levels of heat shock protein HSP70, a marker of inflammation, was decreased in both exercise groups, but significantly only in the high intensity training group. On the other hand, malondialdehyde, a marker of lipid peroxidation, was increased in the moderate intensity training group, but not in the sedentary or high intensity training group. The same group of authors performed a study on obese mice and found that PM2.5 exposure changed the effects of exercise from anti-inflammatory to proinflammatory [126]. In addition, the inability of obese mice to finish an exercise session may have been the reason for the observed change in inflammation status. In rats exposed to residual oil fly ash (PM), no changes were observed in the expression of antioxidant enzymes SOD and catalase in the heart and lung tissue, either with or without treadmill exercise [127]. Worsening of the antioxidant enzyme expression and subsequent improvement after exercise were only observed in the gastrocnemius muscle. A recent study on rats demonstrated that exercise prevented the impairment of endothelium dependent vasorelaxation and a reduction in NO bioavailability from PM2.5 exposure [128]. The authors also observed that exercise increased the HDL cholesterol levels and reduced the oxidation of HDL cholesterol. In a 6-week PM2.5 exposure study, exercising rats were found to have lower levels of inflammatory markers in both lung and heart, and improved heart rate variability, in comparison to sedentary rats [129]. Even though exercise improved the inflammation status of heart and lung, authors concluded that it was not enough to prevent chronic lung and heart damage.
Other effects of exercising in polluted air were observed as well. The impairment of spatial learning and memory by air pollution was not rescued by exercise, pointing to a permanent negative effect on the hippocampus [130]. Cigarette smoking and secondhand smoke can be considered as a kind of air pollution as many of the toxic components, including PM and PAHs, are shared. Quite a few studies have demonstrated the benefits of exercise in reducing cigarette smoke induced cardiopulmonary damage [131,132,133,134].
6. Mitigation Strategies and Practical Recommendations
The individual person can only partially protect his/her health by personal protection measures, as briefly described below and reviewed previously [135]. As suggested by the report of The Lancet Commission on Pollution and Public Health, it is more important that governments and health decision makers improve the protection of the general population, e.g., by lowering legal thresholds for air pollutants [136], which is also strongly recommended by planetary health experts [137]. The problem is that national legislation does not uniformly implement the air quality limits (e.g., average exposure to maximally 5 µg/m3 of PM2.5), as recommended by the WHO [138] and atmospheric chemistry experts [139]. Whereas the legal thresholds for annual mean PM2.5 are currently 12 µg/m3 in the USA, 10 µg/m3 in Canada, and even 8 µg/m3 in Australia, the EU still recommends an average maximum exposure of 25 µg/m3 [32], which is clearly too high, as demonstrated by significant health effects at lower concentrations [140,141]. Three prominent examples have demonstrated the dramatic health improvement and also lowering of health costs by strict adherence to higher air quality standards: (1) the pollution control measures before the 2008 Beijing Olympic Games leading to a lower output of traffic and industrial exhaust pollutants, with the dramatic improvement of air quality and beneficial health effects [142] that, however, immediately returned to the same levels as before the Olympic Games when pollution control measures were stopped. (2) A decrease in diesel emissions by new restrictive laws in Tokyo, leading to a 44% decrease in PM2.5 from traffic over the period 2003–2012 and a decrease in cardiovascular mortality by 11% (mainly due to a 10% decrease in ischemic heart disease mortality) [143]. (3) The reduction of air pollution during the COVID-19 pandemic caused by the shutdown of major factories and low transport volume was estimated to be associated with a significant decrease of up to 13,600 premature deaths in Europe [144]. Besides the legal thresholds, healthy city design (urban planning) with a lot of green spaces, placing main roads and airports to the outskirts with low population density, short distances between residences, working places, schools, shops and places of social life, and efficient ecological public transportation, contribute largely to better air quality and the improvement of (cardiovascular) health [145,146,147,148]. Providing attractive and accessible urban environments may encourage people to spend more time outdoors and facilitate physical activity. Herein, the quality of the urban green space is an important factor facilitating physical activity in older and the most susceptible populations. Numerous studies have demonstrated that increased physical activity is associated with access to, and use of, green space among senior citizen, working adults, and children. The availability of green space has also been associated with reduced prevalence of obesity and type 2 diabetes, reduced cardiovascular morbidity and mortality, improved mental health and cognitive function, improved pregnancy outcomes, and overall reduced all-cause mortality and increased life span [149,150,151,152]. In addition, the presence of trees in urban green spaces has been related with improvements in air quality, due to trees’ capacity of removing pollutants from the atmosphere [153]. This reduction can occur directly by deposition on the tree surface and/or by stomatal uptake of gases [154]. Due to the shading effect trees have on surfaces and/or the cooling effect of the water they transpire, they can also mitigate extreme air temperatures by changing microclimatic conditions on their surroundings. Thus, increasing urban green space may result in a win–win situation, related to increases in physical activity and improvements of air quality. However, a sustainable and striking improvement in air quality, e.g., by significantly lowering PM concentrations, can probably only be achieved if we quit fossil fuel-based energy sources [14,155,156]. Moreover, recent studies indicate biodiversity to be a cornerstone of human (mental) health and wellbeing. Importantly, pathways linking biodiversity to beneficial human health effects include less environmental exposures, such as air and noise pollution, as well as increased building capacities, such as green space, to promote physical activity [157,158,159].
During the transition process of lowering air pollution concentrations by (inter)national policies, personal protection measures are the only possibility for the individual person to decrease air pollutant exposure and, thereby, the associated health risks [160,161]. Several clinical field studies demonstrated that prevention of PM2.5 exposure by N95 filtration masks prevents the increase in systolic blood pressure induced by particle exposure, and has beneficial effects on heart rate variability [161]. Other clinical trials support protective effects against air pollution by home air filtration systems by preventing endothelial dysfunction, hypertension, and pro-atherothrombotic changes induced by air pollutants [160,161]. Whereas wearing face masks during outdoor exercise seems an inacceptable mitigation strategy, clean air systems may efficiently decrease exposure levels to air pollutants (mostly PM of different categories) and thereby make home based physical exercise healthier. Whereas cloth or surgical face masks seem to have no direct negative effects on physical exercise performance and health outcome [162], N95 masks slightly but significantly increased heart rate [163] and FFP2 masks caused a significant but modest impairment of spirometry and cardiorespiratory parameters at rest and peak exercise that was mostly based on decreased ventilation by higher airflow resistance [164]. Taken together, wearing a face mask during exercise is highly annoying but seems to be more or less safe, although some experts warrant caution due to development of hypercapnic hypoxia with negative health effects, which may become more significant with N95 face masks [165]. Therefore, avoidance of running in highly polluted areas (main roads, industrial parks) but, rather, exercising in green space areas or even rural places, represents another mitigation strategy for the individual to prevent high exposure levels of air pollutants, also because face masks will only filter the solid air pollutants, such as PM10 and PM2.5, whereas gaseous or ultrafine particles are not retained by most masks. However, in a comparative risk assessment study, Tainio et al. [166] concluded that active travel (cycling and walking) should be promoted, since the associated benefits outweigh the health risks (all-cause mortality) from air pollution in the vast majority of settings, which was recently also confirmed by Giallouros et al. [167]. In addition to a healthy environment, lifestyle changes are also paramount in preventing cardiovascular disease. Smoking cessation should be an essential strategy to help prevent the onset and progression of cardiovascular disease, as smoking is not only a form of PM air pollution [168], but smokers are generally less likely to engage in physical activity [169]. Exercise has also been shown to help with smoking cessation, providing an additional beneficial effect for cardiovascular health [170].
Finally, pharmacological interventions may help to lower the negative health impact of air pollution and represent another mitigation measure at the individual level [135], especially for those who have to run in highly polluted air. Dietary fish oil administration partially prevented the PM2.5 dependent induction of inflammation, coagulation, endothelial function, oxidative stress, and neuroendocrine stress response [171]. In addition, dietary AMPK or Nrf2 activation, as conferred by many vegetables, may help to prevent the detrimental health effects of PM2.5 exposure, as shown in animal studies [172]. However, the ultimate proof of an efficient nutraceutical protection from air pollution mediated adverse health effects needs further clinical investigations. Since air pollution is increasingly recognized as a cardiovascular risk factor [173,174], the optimal medication of patients with traditional cardiovascular risk factors (e.g., hypertension, diabetes) is also a main option in the primary and secondary prevention of the onset and progression of atherosclerotic disease by air pollutants such as PM2.5 [135]. However, recognition of air pollution by the national guidelines for the therapy and prevention of cardiovascular disease would be of great importance, to also put pressure on decision makers to lower the legal thresholds.
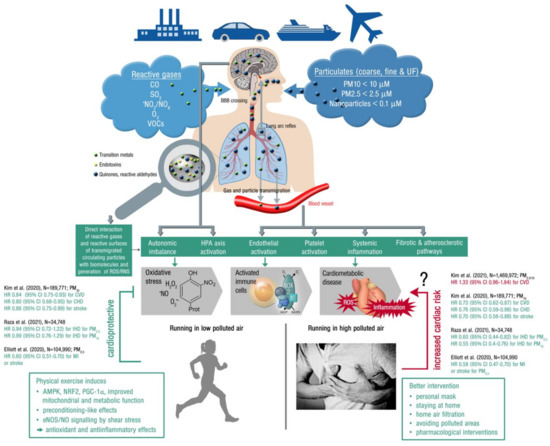
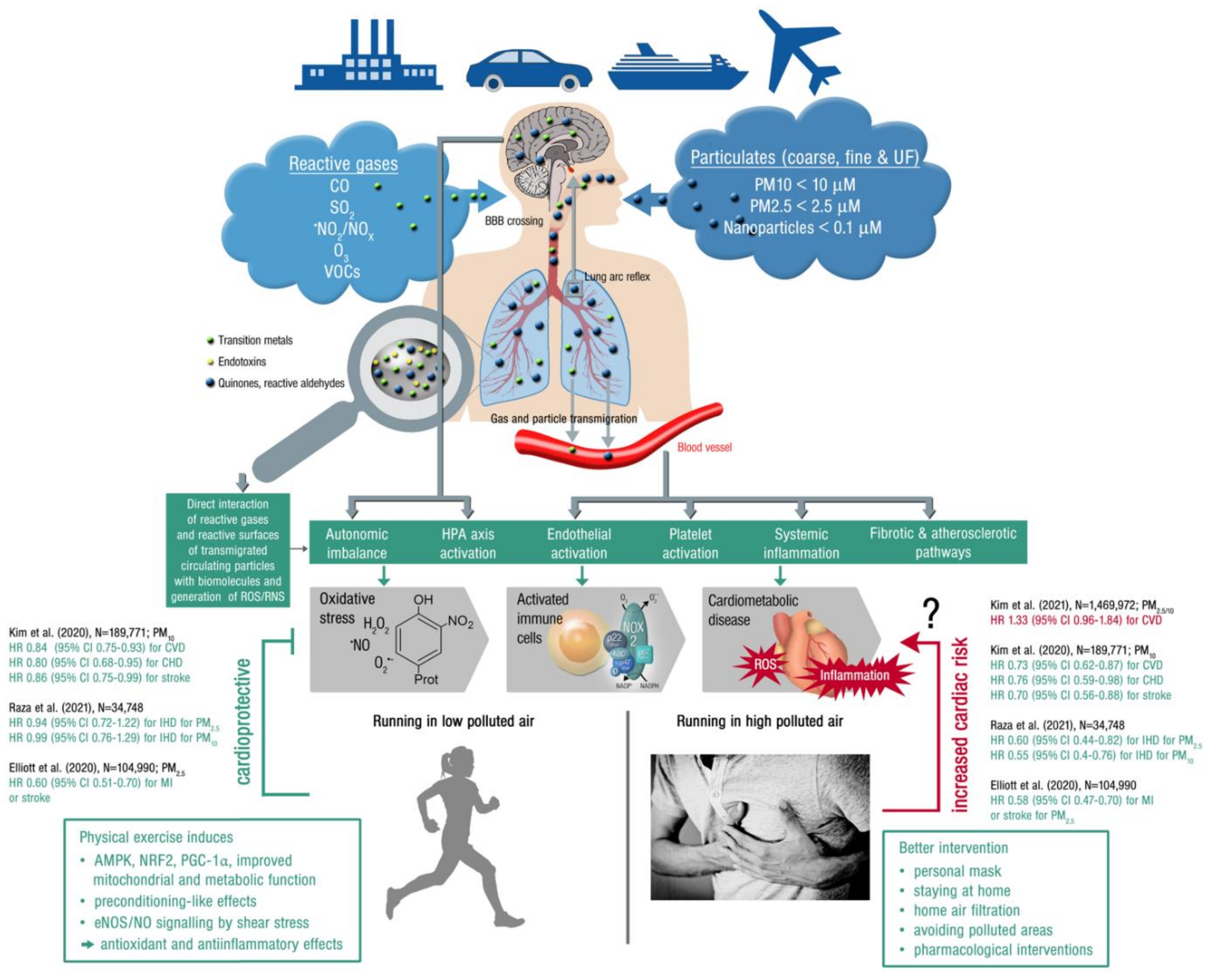
Figure 4.
Upper part: Summary of pathophysiological mechanisms by which air pollutants cause increased oxidative stress and inflammation, thereby contributing to cardiometabolic disease. Uptake and cardiorespiratory health effects triggered by air pollution constituents. Middle part: Key events that contribute to increased cardiometabolic risk by air pollution constituents. Ambient PM is often loaded with environmental toxins stemming from particle “aging” by UV induced photoreactions or modifications upon interaction with reactive gases in the atmosphere [175]. In addition, loading of the particles with environmental endotoxins and transition metals enhances their direct biochemical reactivity. Summarized from [32] (upper part; Copyright © 2021, Oxford University Press) and [176] (middle part; © 2021 International Union of Biochemistry and Molecular Biology) with permission. BBB, blood–brain barrier; UF, ultrafine; ROS/RNS, reactive oxygen and nitrogen species; HPA, hypothalamic–pituitary–adrenal. Lower part: Physical activity (running) in areas with low air pollution is cardioprotective (by the well known beneficial effects of exercise, see text box on the left), whereas extensive exercise in areas with high air pollution may increase the cardiovascular risk, representing the main message of a recent high quality study by Kim et al. [102]. Therefore, other mitigation strategies may be required in highly polluted areas, such as personal masks or pharmacological interventions (see text box on the left). However, the majority of clinical studies indicates that physical activity is always cardioprotective irrespective of high or low levels of pollution, as shown by the inserted hazard ratios on the left and right part of the figure (green HR = beneficial effect of exercise in polluted air; red HR = detrimental effect of exercise in highly polluted air; CVD, cardiovascular disease; CHD, coronary heart disease; IHD, ischemic heart disease; MI, myocardial infarction). Open source “Heart attack” image (right bottom) taken from https://pixabay.com/de/photos/mann-herzenskummer-brustschmerzen-1846050/, accessed on 4 November 2021.
Figure 4.
Upper part: Summary of pathophysiological mechanisms by which air pollutants cause increased oxidative stress and inflammation, thereby contributing to cardiometabolic disease. Uptake and cardiorespiratory health effects triggered by air pollution constituents. Middle part: Key events that contribute to increased cardiometabolic risk by air pollution constituents. Ambient PM is often loaded with environmental toxins stemming from particle “aging” by UV induced photoreactions or modifications upon interaction with reactive gases in the atmosphere [175]. In addition, loading of the particles with environmental endotoxins and transition metals enhances their direct biochemical reactivity. Summarized from [32] (upper part; Copyright © 2021, Oxford University Press) and [176] (middle part; © 2021 International Union of Biochemistry and Molecular Biology) with permission. BBB, blood–brain barrier; UF, ultrafine; ROS/RNS, reactive oxygen and nitrogen species; HPA, hypothalamic–pituitary–adrenal. Lower part: Physical activity (running) in areas with low air pollution is cardioprotective (by the well known beneficial effects of exercise, see text box on the left), whereas extensive exercise in areas with high air pollution may increase the cardiovascular risk, representing the main message of a recent high quality study by Kim et al. [102]. Therefore, other mitigation strategies may be required in highly polluted areas, such as personal masks or pharmacological interventions (see text box on the left). However, the majority of clinical studies indicates that physical activity is always cardioprotective irrespective of high or low levels of pollution, as shown by the inserted hazard ratios on the left and right part of the figure (green HR = beneficial effect of exercise in polluted air; red HR = detrimental effect of exercise in highly polluted air; CVD, cardiovascular disease; CHD, coronary heart disease; IHD, ischemic heart disease; MI, myocardial infarction). Open source “Heart attack” image (right bottom) taken from https://pixabay.com/de/photos/mann-herzenskummer-brustschmerzen-1846050/, accessed on 4 November 2021.
7. Gaps in Current Knowledge and Conclusions
The decision to take part in (outdoor) physical activities or not clearly depends on the assessment of external environmental exposures, especially in cities, where novel technologies may indeed bring great advancements. It is important that, nowadays, cheap sensors are available to quantify environmental exposures including noise, temperature, and air pollution. Even within a city, variations in exposure to heat, air pollution, noise, and green space can be measured. Novel technologies have to be distributed, such as smartphones and GPS assessments, in order to improve the personal assessment of exposure within the city and to catch variations of exposure to various environmental stressors [177]. Smartphone apps may be very helpful in providing exposure information, even allowing an estimation of the inhaled dose of polluted air along with the health side effects. These apps may also be helpful in determining the ideal, less polluted exercise routes [177].
Nevertheless, further research is urgently needed to determine the exact thresholds of when environmental exposures may be harmful for human health, although the majority of published data supports a beneficial effect of exercise even at higher air pollution levels (Figure 4). We still do not know enough concerning the adverse health effects of exercise in polluted air with respect to people with preexisting cardiovascular disease, lung disease, children, and the elderly, in particular. A further challenge seems to be the combined assessment of the health side effects of air pollution and noise, since these environmental stressors show a large overlap of their sources and share many pathomechanistic features. We also have to take into account interventions in the built environment and the impact of potential interventions concerning compactness of the city, transport systems, heat islands, and green space on air pollution and physical activity [8,145,178]. Ultimately, stricter legislative air quality guidelines and air pollution thresholds must be implemented in Europe, as the most recent WHO global air quality guidelines recommend an annual exposure limit for PM2.5 of 5 µg/m3 [138] that is almost met by countries such as Australia, whereas the European legislation got stuck at a threshold of 25 µg/m3.
Author Contributions
All authors contributed to writing of the different sections of the review article, helped drafting the figures, checked and critically revised the final version of the manuscript. O.H., M.K., A.D. and T.M. wrote most parts of the article. All authors have read and agreed to the published version of the manuscript.
Funding
A.D. and T.M. were supported by vascular biology research grants from the Boehringer Ingelheim Foundation for the collaborative research group “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutics” and through continuous research support from the Foundation Heart of Mainz (O.H., A.D. and T.M.). T.M. is PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany. M.K. holds and K.F. held a TransMed PhD stipend funded by the Boehringer Ingelheim Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMPK | AMP-activated protein kinase |
| ApoE−/− | Apolipoprotein E knockout |
| BC | Black carbon |
| BAL | Bronchoalveolar lavage |
| Ca2+ | Tetrahydrobiopterin |
| Cat | Catalase |
| CD36 | Cluster of differentiation 36 |
| CHD | Coronary heart disease |
| CI | Confidence interval |
| CO | Carbon monoxide |
| Cox2/Cox4 | Cytochrome C oxidase 2/4 |
| CVD | Cardiovascular disease |
| CXCL1/KC | Chemokine (C-X-C motif) ligand 1 |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| GEMM | Global Exposure-Mortality Model |
| GPx | Glutathione peroxidase |
| HDL | High-density lipoprotein |
| eHSP70/iHSP70 | Extracellular/intracellular heat shock protein 70 |
| HR | Hazard ratio |
| IFNγ | Interferon gamma |
| iNOS | Inducible nitric oxide synthase |
| IHD | Ischemic heart disease |
| IL-6/IL-1β | Interleukin 6/1β |
| IQR | Interquartile range |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MET-min/week | Minutes of metabolic equivalent tasks per week |
| MI | Myocardial infarction |
| Mn-SOD | Manganese superoxide dismutase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | NADPH oxidase |
| NO | Nitric oxide/nitrogen monoxide |
| NO2 | Nitrogen dioxide |
| NOx | Nitrogen oxides |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| O2− | Superoxide anion |
| O3 | Ozone |
| ONOO− | Peroxynitrite |
| OR | Odds ratio |
| PA | Physical activity |
| PAHs | Polycyclic aromatic hydrocarbons |
| PGC-1α | Peroxisome proliferator activated receptor gamma coactivator 1-alpha |
| PM(diameter size) | Particulate matter |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TiO2 | Titanium dioxide |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VOCs | Volatile organic compounds |
| WHO | World Health Organization |
References
- Hallal, P.C.; Victora, C.G.; Azevedo, M.R.; Wells, J.C. Adolescent physical activity and health: A systematic review. Sports Med. 2006, 36, 1019–1030. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Liu, X.; Tu, R.; Dong, X.; Zhai, Z.; Mao, Z.; Huo, W.; Chen, G.; Xiang, H.; Guo, Y.; et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ. Int. 2020, 136, 105459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Paul, K.; Arah, O.A.; Mayeda, E.R.; Wu, J.; Lee, E.; Shih, I.F.; Su, J.; Jerrett, M.; Haan, M.; et al. Air pollution, noise exposure, and metabolic syndrome—A cohort study in elderly Mexican-Americans in Sacramento area. Environ. Int. 2020, 134, 105269. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chan, T.C.; Huang, Y.J.; Pan, W.C. The Association between Noise Exposure and Metabolic Syndrome: A Longitudinal Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 4236. [Google Scholar] [CrossRef]
- World Health Organization. 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries Are Taking Action. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-peopleworldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 6 August 2021).
- Tainio, M.; Jovanovic Andersen, Z.; Nieuwenhuijsen, M.J.; Hu, L.; de Nazelle, A.; An, R.; Garcia, L.M.T.; Goenka, S.; Zapata-Diomedi, B.; Bull, F.; et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ. Int. 2021, 147, 105954. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Sbihi, H.; Tamburic, L.; Brauer, M.; Frank, L.D.; Davies, H.W. Association of Long-Term Exposure to Transportation Noise and Traffic-Related Air Pollution with the Incidence of Diabetes: A Prospective Cohort Study. Environ. Health Perspect. 2017, 125, 087025. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ying, Z.; Harkema, J.; Sun, Q.; Rajagopalan, S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2013, 41, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, S.; Brook, R.D. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 2012, 61, 3037–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 6 August 2021).
- World Health Organization. Physical Activity. Available online: https://www.who.int/westernpacific/health-topics/physical-activity (accessed on 6 August 2021).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Personal Interventions and Risk Communication on air Pollution. Available online: https://www.who.int/publications/i/item/9789240000278 (accessed on 6 August 2021).
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Frenis, K.; Kuntic, M.; Daiber, A.; Munzel, T. Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int. J. Mol. Sci. 2021, 22, 2419. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Qiu, X.; Zhang, H.; Lu, X.; Li, H.; Chen, W.; Zhang, L.; Que, C.; Zhu, T. Association between exposure to polycyclic aromatic hydrocarbons and lipid peroxidation in patients with chronic obstructive pulmonary disease. Sci. Total Environ. 2021, 780, 146660. [Google Scholar] [CrossRef]
- Nassan, F.L.; Wang, C.; Kelly, R.S.; Lasky-Su, J.A.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J.D. Ambient PM2.5 species and ultrafine particle exposure and their differential metabolomic signatures. Environ. Int. 2021, 151, 106447. [Google Scholar] [CrossRef]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Munzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress—Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef] [PubMed]
- Abohashem, S.; Osborne, M.T.; Dar, T.; Naddaf, N.; Abbasi, T.; Ghoneem, A.; Radfar, A.; Patrich, T.; Oberfeld, B.; Tung, B.; et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur. Heart J. 2021, 42, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Lutzker, L.; Holm, S.M.; Margolis, H.G.; Neophytou, A.M.; Eisen, E.A.; Costello, S.; Tyner, T.; Holland, N.; Tindula, G.; et al. Traffic-related air pollution is associated with glucose dysregulation, blood pressure, and oxidative stress in children. Environ. Res. 2021, 195, 110870. [Google Scholar] [CrossRef] [PubMed]
- Prunicki, M.; Cauwenberghs, N.; Ataam, J.A.; Movassagh, H.; Kim, J.B.; Kuznetsova, T.; Wu, J.C.; Maecker, H.; Haddad, F.; Nadeau, K. Immune biomarkers link air pollution exposure to blood pressure in adolescents. Environ. Health 2020, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1793–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prows, D.R.; Shertzer, H.G.; Daly, M.J.; Sidman, C.L.; Leikauf, G.D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997, 17, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Zirlik, A.; Wolf, D. Pathogenic Role of Air Pollution Particulate Matter in Cardiometabolic Disease: Evidence from Mice and Humans. Antioxid. Redox Signal. 2020, 33, 263–279. [Google Scholar] [CrossRef]
- Sunyer, J.; Ballester, F.; Tertre, A.L.; Atkinson, R.; Ayres, J.G.; Forastiere, F.; Forsberg, B.; Vonk, J.M.; Bisanti, L.; Tenias, J.M.; et al. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study). Eur. Heart J. 2003, 24, 752–760. [Google Scholar] [CrossRef]
- Chuang, G.C.; Yang, Z.; Westbrook, D.G.; Pompilius, M.; Ballinger, C.A.; White, C.R.; Krzywanski, D.M.; Postlethwait, E.M.; Ballinger, S.W. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L209–L216. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Colombo, E.S.; Lucas, S.N.; Hall, P.R.; Febbraio, M.; Paffett, M.L.; Campen, M.J. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol. Sci. 2013, 134, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Allen, K.; Rao, X.; Ying, Z.; Braunstein, Z.; Kankanala, S.R.; Xia, C.; Wang, X.; Bramble, L.A.; Wagner, J.G.; et al. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal. Toxicol. 2016, 28, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Hamade, A.K.; Rabold, R.; Tankersley, C.G. Adverse cardiovascular effects with acute particulate matter and ozone exposures: Interstrain variation in mice. Environ. Health Perspect. 2008, 116, 1033–1039. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef] [Green Version]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Arora, R.C.; Hiebert, B.M.; Lerner, B.; Szwajcer, A.; McDonald, K.; Rigatto, C.; Komenda, P.; Sood, M.M.; Tangri, N. Non-invasive endothelial function testing and the risk of adverse outcomes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 736–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Crow, J.P.; Beckman, J.S. Reaction between Nitric Oxide, Superoxide, and Peroxynitrite: Footprints of Peroxynitrite in Vivo. Adv. Pharmacol. 1995, 35, 17–43. [Google Scholar]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherng, T.W.; Paffett, M.L.; Jackson-Weaver, O.; Campen, M.J.; Walker, B.R.; Kanagy, N.L. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 2011, 119, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Davel, A.P.; Lemos, M.; Pastro, L.M.; Pedro, S.C.; de Andre, P.A.; Hebeda, C.; Farsky, S.H.; Saldiva, P.H.; Rossoni, L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012, 295, 39–46. [Google Scholar] [CrossRef]
- Ying, Z.; Xu, X.; Chen, M.; Liu, D.; Zhong, M.; Chen, L.C.; Sun, Q.; Rajagopalan, S. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol. Sci. 2013, 135, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Zhong, J.; Maiseyeu, A.; Gopalakrishnan, B.; Villamena, F.A.; Chen, L.C.; Harkema, J.R.; Sun, Q.; Rajagopalan, S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014, 115, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Courtois, A.; Andujar, P.; Ladeiro, Y.; Baudrimont, I.; Delannoy, E.; Leblais, V.; Begueret, H.; Galland, M.A.; Brochard, P.; Marano, F.; et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: Comparison of urban particulate matter and manufactured nanoparticles. Environ. Health Perspect. 2008, 116, 1294–1299. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Tracy, R.P.; Cornell, E.; Kaufman, J.D.; Szpiro, A.A.; Campen, M.J.; Vedal, S. Short-term exposure to air pollution and biomarkers of cardiovascular effect: A repeated measures study. Environ. Pollut. 2021, 279, 116893. [Google Scholar] [CrossRef]
- Riggs, D.W.; Zafar, N.; Krishnasamy, S.; Yeager, R.; Rai, S.N.; Bhatnagar, A.; O’Toole, T.E. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ. Res. 2020, 180, 108890. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Ramanathan, G.; Zhu, Y.; Yin, F.; Rea, N.D.; Lu, X.; Tseng, C.H.; Faull, K.F.; Yoon, A.J.; Jerrett, M.; et al. Pro-Oxidative and Proinflammatory Effects After Traveling from Los Angeles to Beijing: A Biomarker-Based Natural Experiment. Circulation 2019, 140, 1995–2004. [Google Scholar] [CrossRef]
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in oldEr Subjects (MOSES). PLoS ONE 2019, 14, e0222601. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Li, W.; Chen, X.; Xue, T.; Chen, W.; Fan, Y.; Qiu, X.; Zhu, T. Susceptibility of prediabetes to the health effect of air pollution: A community-based panel study with a nested case-control design. Environ. Health 2019, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Zhou, Y.; Zhu, Q.; Zhao, Y.; Wang, Y.; Ge, W.; Yang, Q.; Zhao, Y.; Wang, P.; Si, J.; et al. Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ. Pollut. 2019, 250, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-term exposure to ambient air pollution and circulating biomarkers of endothelial cell activation: The Framingham Heart Study. Environ. Res. 2019, 171, 36–43. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R.; et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.F.; Shen, F.H.; Li, Y.R.; Tsao, T.M.; Tsai, M.J.; Chen, C.C.; Hwang, J.S.; Hsu, S.H.; Chao, H.; Chuang, K.J.; et al. Association of short-term exposure to fine particulate matter and nitrogen dioxide with acute cardiovascular effects. Sci. Total Environ. 2016, 569–570, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.C.E.; Tittlbach, S.; Bos, K.; Woll, A. Different Types of Physical Activity and Fitness and Health in Adults: An 18-Year Longitudinal Study. Biomed. Res. Int. 2017, 2017, 1785217. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Deiseroth, A.; Hahad, O.; Diestelmeier, S.; Schulz, A.; Daubenbuchel, A.; Gori, T.; Binder, H.; Pfeiffer, N.; Prochaska, J.; et al. Domains of Physical Activity in Relation to Stiffness Index in the General Population. J. Am. Heart Assoc. 2021, 10, e020930. [Google Scholar] [CrossRef] [PubMed]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age 2016, 38, 64. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Montgomery, P.S.; Zhao, Y.D.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A.; Sonntag, W.E. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2017, 65, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002, 13, 561–568. [Google Scholar] [CrossRef]
- Mora, S.; Lee, I.M.; Buring, J.E.; Ridker, P.M. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006, 295, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Ye, X.; Wang, J.; Qi, Q.; Franco, O.H.; Rennie, K.L.; Pan, A.; Li, H.; Liu, Y.; Hu, F.B.; et al. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older chinese people. Circulation 2009, 119, 2969–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Borjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—A systematic review. Atherosclerosis 2014, 235, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glenn, J.M.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; et al. Effects of Aerobic-, Anaerobic- and Combined-Based Exercises on Plasma Oxidative Stress Biomarkers in Healthy Untrained Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 2601. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44. [Google Scholar] [CrossRef]
- Dillard, C.J.; Litov, R.E.; Savin, W.M.; Dumelin, E.E.; Tappel, A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.S.; Brady, L.J.; Ullrey, D.E. Selenium, vitamin E and the response to swimming stress in the rat. J. Nutr. 1979, 109, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H. Vitamin E and skeletal muscle. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 224–239. [Google Scholar] [CrossRef]
- Quintanilha, A.T.; Packer, L. Vitamin E, physical exercise and tissue oxidative damage. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 56–69. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, M.; Hafstad, A.D.; Nabeebaccus, A.A.; Catibog, N.; Logan, A.; Smyrnias, I.; Hansen, S.S.; Lanner, J.; Schroder, K.; Murphy, M.P.; et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife 2018, 7, e41044. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rego Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardo, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kroller-Schon, S.; Steven, S.; Schulz, E.; Munzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Schulz, E.; Wenzel, P.; Munzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324. [Google Scholar] [CrossRef]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Cartier, L.J.; Chen, M.; Holloszy, J.O. Superoxide dismutase and catalase in skeletal muscle: Adaptive response to exercise. J. Gerontol. 1985, 40, 281–286. [Google Scholar] [CrossRef]
- Kanter, M.M.; Hamlin, R.L.; Unverferth, D.V.; Davis, H.W.; Merola, A.J. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. 1985, 59, 1298–1303. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Fu, R.; Mitchell, E.W. Glutathione and antioxidant enzymes in skeletal muscle: Effects of fiber type and exercise intensity. J. Appl. Physiol. 1992, 73, 1854–1859. [Google Scholar] [CrossRef]
- Ji, L.L.; Stratman, F.W.; Lardy, H.A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch. Biochem. Biophys. 1988, 263, 150–160. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990, 68, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Kvandova, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Munzel, T.; Kroller-Schon, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Kroller-Schon, S.; Jansen, T.; Hauptmann, F.; Schuler, A.; Heeren, T.; Hausding, M.; Oelze, M.; Viollet, B.; Keaney, J.F., Jr.; Wenzel, P.; et al. alpha1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1632–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Kossmann, S.; Munzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef]
- Sen, C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol. Cell Biochem. 1999, 196, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Li, H.; Laher, I. Oxidative Stress: A Unifying Mechanism for Cell Damage Induced by Noise, (Water-Pipe) Smoking, and Emotional Stress-Therapeutic Strategies Targeting Redox Imbalance. Antioxid. Redox Signal. 2018, 28, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diab. Rep. 2017, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Cho, Y.; Oh, Y.H.; Lee, G.; Son, J.S.; Kim, K.H.; et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur. Heart J. 2021, 42, 2487–2497. [Google Scholar] [CrossRef]
- Munzel, T.; Hahad, O.; Daiber, A. Running in polluted air is a two-edged sword—Physical exercise in low air pollution areas is cardioprotective but detrimental for the heart in high air pollution areas. Eur. Heart J. 2021, 42, 2498–2500. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, S.; Keum, N.; Park, S.M. Combined Effects of Physical Activity and Air Pollution on Cardiovascular Disease: A Population-Based Study. J. Am. Heart Assoc. 2020, 9, e013611. [Google Scholar] [CrossRef]
- Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? Int. J. Environ. Res. Public Health 2021, 18, 2631. [Google Scholar] [CrossRef]
- Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Air pollution, physical activity and ischaemic heart disease: A prospective cohort study of interaction effects. BMJ Open 2021, 11, e040912. [Google Scholar] [CrossRef]
- Tu, R.; Hou, J.; Liu, X.; Li, R.; Dong, X.; Pan, M.; Mao, Z.; Huo, W.; Chen, G.; Guo, Y.; et al. Physical activity attenuated association of air pollution with estimated 10-year atherosclerotic cardiovascular disease risk in a large rural Chinese adult population: A cross-sectional study. Environ. Int. 2020, 140, 105819. [Google Scholar] [CrossRef]
- Sun, S.; Cao, W.; Qiu, H.; Ran, J.; Lin, H.; Shen, C.; Siu-Yin Lee, R.; Tian, L. Benefits of physical activity not affected by air pollution: A prospective cohort study. Int. J. Epidemiol. 2020, 49, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.G.; Laden, F.; James, P.; Rimm, E.B.; Rexrode, K.M.; Hart, J.E. Interaction between Long-Term Exposure to Fine Particulate Matter and Physical Activity, and Risk of Cardiovascular Disease and Overall Mortality in U.S. Women. Environ. Health Perspect. 2020, 128, 127012. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Choi, D.; Choi, S.; Kim, K.; Lee, G.; Son, J.S.; Kim, K.H.; Park, S.M. Association of combined effects of physical activity and air pollution with diabetes in older adults. Environ. Int. 2020, 145, 106161. [Google Scholar] [CrossRef]
- Guo, C.; Yang, H.T.; Chang, L.Y.; Bo, Y.; Lin, C.; Zeng, Y.; Tam, T.; Lau, A.K.H.; Hoek, G.; Lao, X.Q. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: A longitudinal cohort study. Diabetologia 2021, 64, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Petrowski, K.; Buhrer, S.; Strauss, B.; Decker, O.; Brahler, E. Examining air pollution (PM10), mental health and well-being in a representative German sample. Sci. Rep. 2021, 11, 18436. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Bijnens, E.; Nemery, B.; Nawrot, T.S.; Dupont, L.J. Impact of traffic related air pollution indicators on non-cystic fibrosis bronchiectasis mortality: A cohort analysis. Respir. Res. 2014, 15, 108. [Google Scholar] [CrossRef] [Green Version]
- Majewski, S.; Piotrowski, W.J. Air Pollution-An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2020, 10, 77. [Google Scholar] [CrossRef]
- Pereira, P.R.; Oliveira-Junior, M.C.; Mackenzie, B.; Chiovatto, J.E.; Matos, Y.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Brugemman, T.R.; Delle, H.; Idzko, M.; et al. Exercise Reduces Lung Fibrosis Involving Serotonin/Akt Signaling. Med. Sci. Sports Exerc. 2016, 48, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Silva-Renno, A.; Baldivia, G.C.; Oliveira-Junior, M.C.; Brandao-Rangel, M.A.R.; El-Mafarjeh, E.; Dolhnikoff, M.; Mauad, T.; Britto, J.M.; Saldiva, P.H.N.; Oliveira, L.V.F.; et al. Exercise Performed Concomitantly with Particulate Matter Exposure Inhibits Lung Injury. Int. J. Sports Med. 2018, 39, 133–140. [Google Scholar] [CrossRef]
- Millerick-May, M.L.; Karmaus, W.; Derksen, F.J.; Berthold, B.; Robinson, N.E. Airborne particulates (PM10) and tracheal mucus: A case-control study at an American Thoroughbred racetrack. Equine Vet. J. 2015, 47, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Toledo, A.C.; Silva, L.B.; Almeida, F.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Santos, A.B.; Rivero, D.H.; Hizume, D.C.; Lopes, F.D.; et al. Anti-inflammatory effects of aerobic exercise in mice exposed to air pollution. Med. Sci. Sports Exerc. 2012, 44, 1227–1234. [Google Scholar] [CrossRef]
- Long, N.C.; Suh, J.; Morrow, J.D.; Schiestl, R.H.; Murthy, G.G.; Brain, J.D.; Frei, B. Ozone causes lipid peroxidation but little antioxidant depletion in exercising and nonexercising hamsters. J. Appl. Physiol. 2001, 91, 1694–1700. [Google Scholar] [CrossRef]
- Bhalla, D.K.; Mannix, R.C.; Lavan, S.M.; Phalen, R.F.; Kleinman, M.T.; Crocker, T.T. Tracheal and bronchoalveolar permeability changes in rats inhaling oxidant atmospheres during rest or exercise. J. Toxicol. Environ. Health 1987, 22, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Decaesteker, T.; Vanhoffelen, E.; Trekels, K.; Jonckheere, A.C.; Cremer, J.; Vanstapel, A.; Dilissen, E.; Bullens, D.; Dupont, L.J.; Vanoirbeek, J.A. Differential effects of intense exercise and pollution on the airways in a murine model. Part. Fibre Toxicol. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II2-10. [Google Scholar] [CrossRef] [Green Version]
- Farah, C.; Meyer, G.; Andre, L.; Boissiere, J.; Gayrard, S.; Cazorla, O.; Richard, S.; Boucher, F.; Tanguy, S.; Obert, P.; et al. Moderate exercise prevents impaired Ca2+ handling in heart of CO-exposed rat: Implication for sensitivity to ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H2076–H2081. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.S.; Dos Santos, A.B.; Beber, L.C.C.; Basso, R.D.B.; Sulzbacher, L.M.; Goettems-Fiorin, P.B.; Frizzo, M.N.; Rhoden, C.R.; Ludwig, M.S.; Heck, T.G. Exercise Training under Exposure to Low Levels of Fine Particulate Matter: Effects on Heart Oxidative Stress and Extra-to-Intracellular HSP70 Ratio. Oxid. Med. Cell Longev. 2017, 2017, 9067875. [Google Scholar] [CrossRef]
- Kostrycki, I.M.; Wildner, G.; Donato, Y.H.; Dos Santos, A.B.; Beber, L.C.C.; Frizzo, M.N.; Ludwig, M.S.; Keane, K.N.; Cruzat, V.; Rhoden, C.R.; et al. Effects of High-Fat Diet on eHSP72 and Extra-to-Intracellular HSP70 Levels in Mice Submitted to Exercise under Exposure to Fine Particulate Matter. J. Diabetes Res. 2019, 2019, 4858740. [Google Scholar] [CrossRef]
- Marmett, B.; Nunes, R.B.; de Souza, K.S.; Lago, P.D.; Rhoden, C.R. Aerobic training reduces oxidative stress in skeletal muscle of rats exposed to air pollution and supplemented with chromium picolinate. Redox Rep. 2018, 23, 146–152. [Google Scholar] [CrossRef]
- Feng, B.; Qi, R.; Gao, J.; Wang, T.; Xu, H.; Zhao, Q.; Wu, R.; Song, X.; Guo, J.; Zheng, L.; et al. Exercise training prevented endothelium dysfunction from particulate matter instillation in Wistar rats. Sci. Total Environ. 2019, 694, 133674. [Google Scholar] [CrossRef]
- Olivo, C.R.; Castro, T.B.P.; Riane, A.; Regonha, T.; Rivero, D.; Vieira, R.P.; Saraiva-Romanholo, B.M.; Lopes, F.; Tiberio, I.; Martins, M.A.; et al. The effects of exercise training on the lungs and cardiovascular function of animals exposed to diesel exhaust particles and gases. Environ. Res. 2021, 203, 111768. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Kiray, M.; Sisman, A.R.; Baykara, B.; Aksu, I.; Dayi, A.; Gencoglu, C.; Evren, M.; Buyuk, E.; Cetin, F.; et al. Effects of exercise and poor indoor air quality on learning, memory and blood IGF-1 in adolescent mice. Biotech. Histochem. 2014, 89, 126–135. [Google Scholar] [CrossRef]
- Toledo, A.C.; Magalhaes, R.M.; Hizume, D.C.; Vieira, R.P.; Biselli, P.J.; Moriya, H.T.; Mauad, T.; Lopes, F.D.; Martins, M.A. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 2012, 39, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Nesi, R.T.; de Souza, P.S.; Dos Santos, G.P.; Thirupathi, A.; Menegali, B.T.; Silveira, P.C.; da Silva, L.A.; Valenca, S.S.; Pinho, R.A. Physical exercise is effective in preventing cigarette smoke-induced pulmonary oxidative response in mice. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.B.; Liao, Y.W.; Su, K.H.; Chang, T.M.; Shyue, S.K.; Kou, Y.R.; Lee, T.S. Prior exercise training alleviates the lung inflammation induced by subsequent exposure to environmental cigarette smoke. Acta Physiol. 2012, 205, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.R.; Nai, G.A.; Laurindo, C.P.; Gregorio, K.C.R.; Olean-Oliveira, T.; Teixeira, M.F.S.; Seraphim, P.M. Resistance training prevents right ventricle hypertrophy in rats exposed to secondhand cigarette smoke. PLoS ONE 2020, 15, e0236988. [Google Scholar] [CrossRef]
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.D.; Munzel, T.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis: Insights Into Dose, Time, and Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 628–637. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 27 September 2021).
- Lelieveld, J.; Poschl, U. Chemists can help to solve the air-pollution health crisis. Nature 2017, 551, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Daiber, A. The air pollution constituent particulate matter (PM2.5) destabilizes coronary artery plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, S.P.; Park, J.B.; Lee, H.; Kang, S.H.; Lee, S.E.; Kim, J.B.; Choi, S.Y.; Kim, Y.J.; Chang, H.J. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Rich, D.Q.; Kipen, H.M.; Huang, W.; Wang, G.; Wang, Y.; Zhu, P.; Ohman-Strickland, P.; Hu, M.; Philipp, C.; Diehl, S.R.; et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 2012, 307, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S.; Doi, H. Fine-particulate Air Pollution from Diesel Emission Control and Mortality Rates in Tokyo: A Quasi-experimental Study. Epidemiology 2016, 27, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Giani, P.; Castruccio, S.; Anav, A.; Howard, D.; Hu, W.; Crippa, P. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: A modelling study. Lancet Planet. Health 2020, 4, e474–e482. [Google Scholar] [CrossRef]
- Munzel, T.; Sorensen, M.; Lelieveld, J.; Hahad, O.; Al-Kindi, S.; Nieuwenhuijsen, M.; Giles-Corti, B.; Daiber, A.; Rajagopalan, S. Heart healthy cities: Genetics loads the gun but the environment pulls the trigger. Eur. Heart J. 2021, 42, 2422–2438. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J. Influence of urban and transport planning and the city environment on cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; Gascon, M.; Perez-Leon, D.; Mudu, P. Green spaces and mortality: A systematic review and meta-analysis of cohort studies. Lancet Planet. Health 2019, 3, e469–e477. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuijsen, M.J. New urban models for more sustainable, liveable and healthier cities post covid19; reducing air pollution, noise and heat island effects and increasing green space and physical activity. Environ. Int. 2021, 157, 106850. [Google Scholar] [CrossRef] [PubMed]
- Sallis, J.F.; Cerin, E.; Conway, T.L.; Adams, M.A.; Frank, L.D.; Pratt, M.; Salvo, D.; Schipperijn, J.; Smith, G.; Cain, K.L.; et al. Physical activity in relation to urban environments in 14 cities worldwide: A cross-sectional study. Lancet 2016, 387, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.; Maheswaran, R. The health benefits of urban green spaces: A review of the evidence. J. Public Health 2011, 33, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Cerin, E.; Owen, N.; Oyeyemi, A.L.; Conway, T.L.; Van Dyck, D.; Schipperijn, J.; Macfarlane, D.J.; Salvo, D.; Reis, R.S.; et al. Perceived neighbourhood environmental attributes associated with adults recreational walking: IPEN Adult study in 12 countries. Health Place 2014, 28, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinemets, U.; Fares, S.; Harley, P.; Jardine, K.J. Bidirectional exchange of biogenic volatiles with vegetation: Emission sources, reactions, breakdown and deposition. Plant Cell Environ. 2014, 37, 1790–1809. [Google Scholar] [CrossRef] [PubMed]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marselle, M.R.; Hartig, T.; Cox, D.T.C.; de Bell, S.; Knapp, S.; Lindley, S.; Triguero-Mas, M.; Bohning-Gaese, K.; Braubach, M.; Cook, P.A.; et al. Pathways linking biodiversity to human health: A conceptual framework. Environ. Int. 2021, 150, 106420. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.T.C.; Shanahan, D.F.; Hudson, H.L.; Plummer, K.E.; Siriwardena, G.M.; Fuller, R.A.; Anderson, K.; Hancock, S.; Gaston, K.J. Doses of Neighborhood Nature: The Benefits for Mental Health of Living with Nature. BioScience 2017, 67, 147–155. [Google Scholar] [CrossRef]
- Methorst, J.; Rehdanz, K.; Mueller, T.; Hansjürgens, B.; Bonn, A.; Böhning-Gaese, K. The importance of species diversity for human well-being in Europe. Ecol. Econ. 2021, 181, 106917. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- Shaw, K.; Butcher, S.; Ko, J.; Zello, G.A.; Chilibeck, P.D. Wearing of Cloth or Disposable Surgical Face Masks has no Effect on Vigorous Exercise Performance in Healthy Individuals. Int. J. Environ. Res. Public Health 2020, 17, 8110. [Google Scholar] [CrossRef]
- Ahmadian, M.; Ghasemi, M.; Nasrollahi Borujeni, N.; Afshan, S.; Fallah, M.; Ayaseh, H.; Pahlavan, M.; Nabavi Chashmi, S.M.; Haeri, T.; Imani, F.; et al. Does wearing a mask while exercising amid COVID-19 pandemic affect hemodynamic and hematologic function among healthy individuals? Implications of mask modality, sex, and exercise intensity. Phys. Sportsmed. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Salvioni, E.; De Martino, F.; Mattavelli, I.; Gugliandolo, P.; Vignati, C.; Farina, S.; Palermo, P.; Campodonico, J.; Maragna, R.; et al. “You can leave your mask on”: Effects on cardiopulmonary parameters of different airway protection masks at rest and during maximal exercise. Eur. Respir. J. 2021, 58, 2004473. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Fernandes, S. “Exercise with facemask; Are we handling a devil’s sword?”—A physiological hypothesis. Med. Hypotheses 2020, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Tainio, M.; de Nazelle, A.J.; Gotschi, T.; Kahlmeier, S.; Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; de Sa, T.H.; Kelly, P.; Woodcock, J. Can air pollution negate the health benefits of cycling and walking? Prev. Med. 2016, 87, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Giallouros, G.; Kouis, P.; Papatheodorou, S.I.; Woodcock, J.; Tainio, M. The long-term impact of restricting cycling and walking during high air pollution days on all-cause mortality: Health impact Assessment study. Environ. Int. 2020, 140, 105679. [Google Scholar] [CrossRef]
- Hahad, O.; Daiber, A.; Michal, M.; Kuntic, M.; Lieb, K.; Beutel, M.; Munzel, T. Smoking and Neuropsychiatric Disease-Associations and Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 7272. [Google Scholar] [CrossRef] [PubMed]
- Olchowski, A.E.; Graham, J.W.; Beverly, E.A.; Dupkanick, C.W. Cigarette Smoking, Physical Activity, and the Health Status of College Students. J. Appl. Soc. Psychol. 2009, 39, 683–706. [Google Scholar] [CrossRef]
- Ussher, M.H.; Taylor, A.H.; Faulkner, G.E. Exercise interventions for smoking cessation. Cochrane Database Syst. Rev. 2014, CD002295. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, R.; Jiang, Y.; Xia, Y.; Niu, Y.; Wang, C.; Liu, C.; Chen, C.; Ge, Y.; Wang, W.; et al. Cardiovascular Benefits of Fish-Oil Supplementation Against Fine Particulate Air Pollution in China. J. Am. Coll. Cardiol. 2019, 73, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, X.; Tian, G.; Shi, X.; Huang, W.; Wu, Y.; Sun, L.; Peng, C.; Liu, S.; Huang, Y.; et al. AMPKalpha2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radic. Biol. Med. 2018, 121, 202–214. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, S.; Brauer, M.; Bhatnagar, A.; Bhatt, D.L.; Brook, J.R.; Huang, W.; Munzel, T.; Newby, D.; Siegel, J.; Brook, R.D.; et al. Personal-Level Protective Actions Against Particulate Matter Air Pollution Exposure: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e411–e431. [Google Scholar] [CrossRef] [PubMed]
- Poschl, U.; Shiraiwa, M. Multiphase chemistry at the atmosphere-biosphere interface influencing climate and public health in the anthropocene. Chem. Rev. 2015, 115, 4440–4475. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kroller-Schon, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Vujacic-Mirski, K.; Kuntic, M.; Bayo Jimenez, M.T.; Helmstadter, J.; Steven, S.; et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction—Signatures of the internal exposome. Biofactors 2019, 45, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M.J. Urban and transport planning, environmental exposures and health-new concepts, methods and tools to improve health in cities. Environ. Health 2016, 15 (Suppl. S1), 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzel, T.; Hahad, O.; Sorensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental risk factors and cardiovascular diseases: A comprehensive review. Cardiovasc. Res. 2021, cvab316. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).