Exploring the Chemical Profiles and Biological Values of Two Spondias Species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
2.2. Chemicals and Reagents
2.3. Profile of Bioactive Compounds
2.4. Chromatographic Separation
2.5. Mass Chromatography Conditions
2.6. Determination of Antioxidant and Enzyme Inhibitory Effects
2.7. Statistical Analysis
3. Results and Discussion
3.1. Gallic Acid and Galloyl Derivatives
3.2. Ellagic Acid Derivatives and Ellagitannins
3.3. Hydroxybenzoic, Hydroxycinnamic, Acylquinic Acids and Derivatives
3.4. Flavonols
3.5. Flavanones, Flavanonols and Flavan 3-ols
3.6. Others
3.7. Enzyme Inhibitory Properties
3.8. Antioxidant Properties
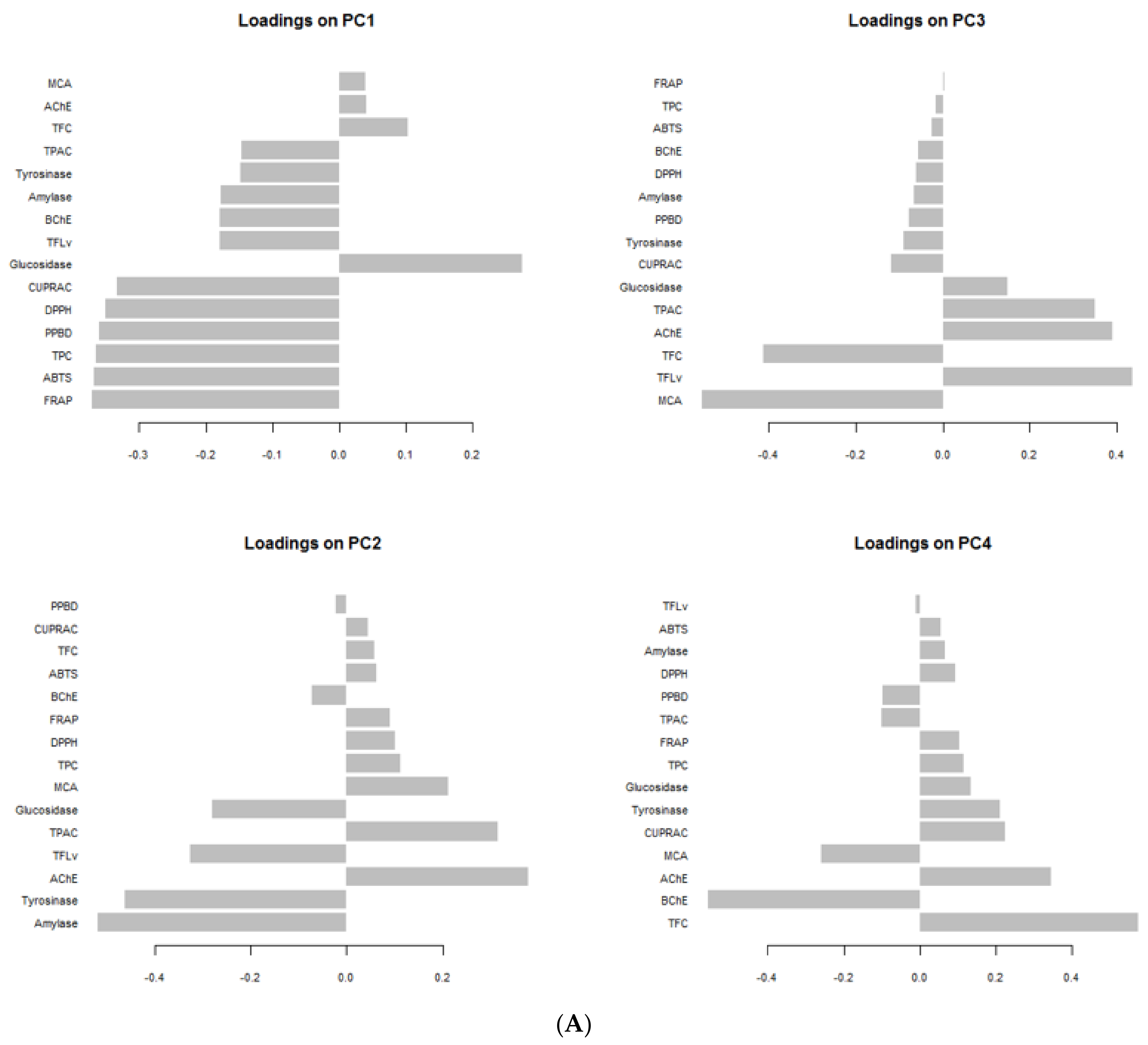
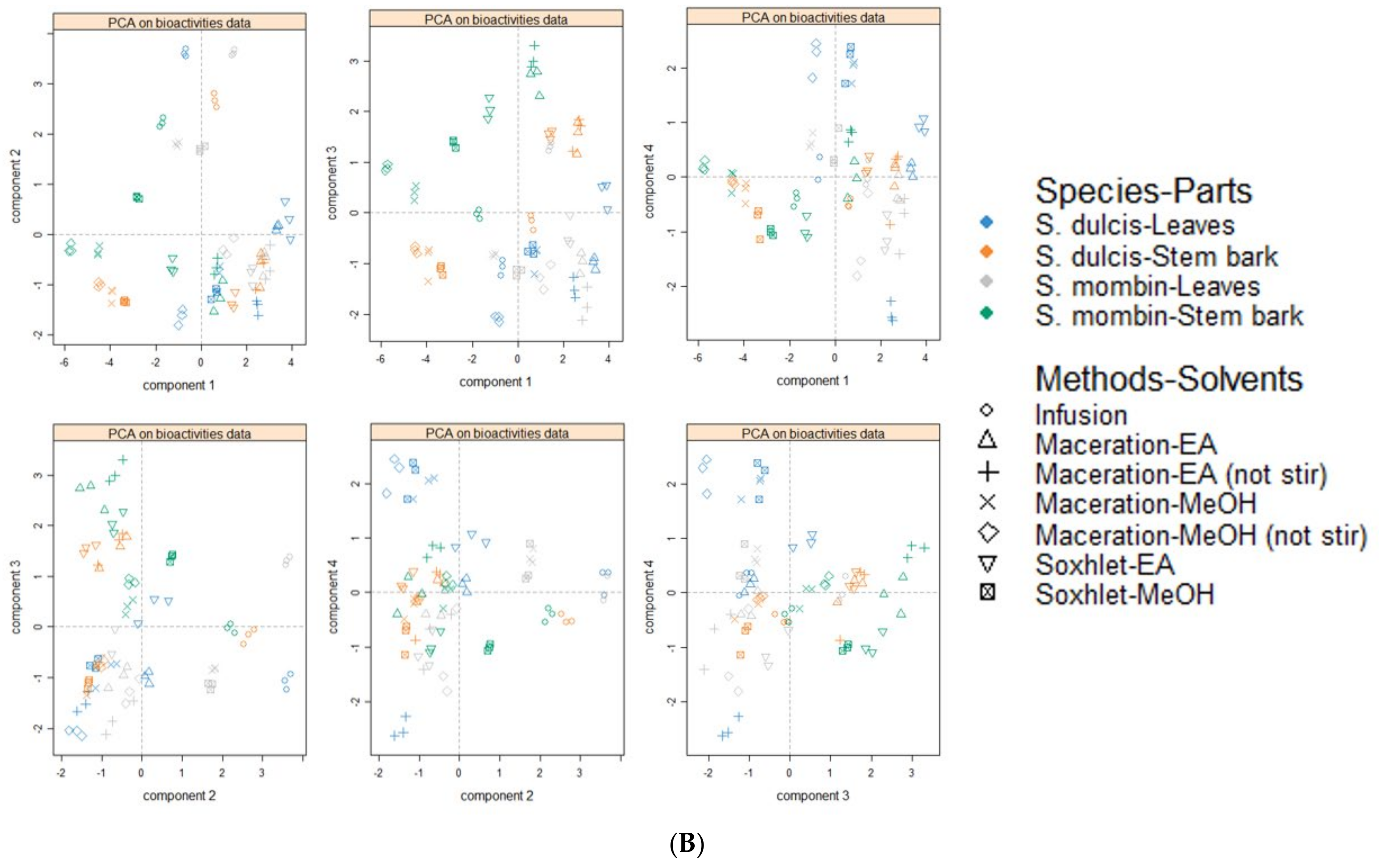
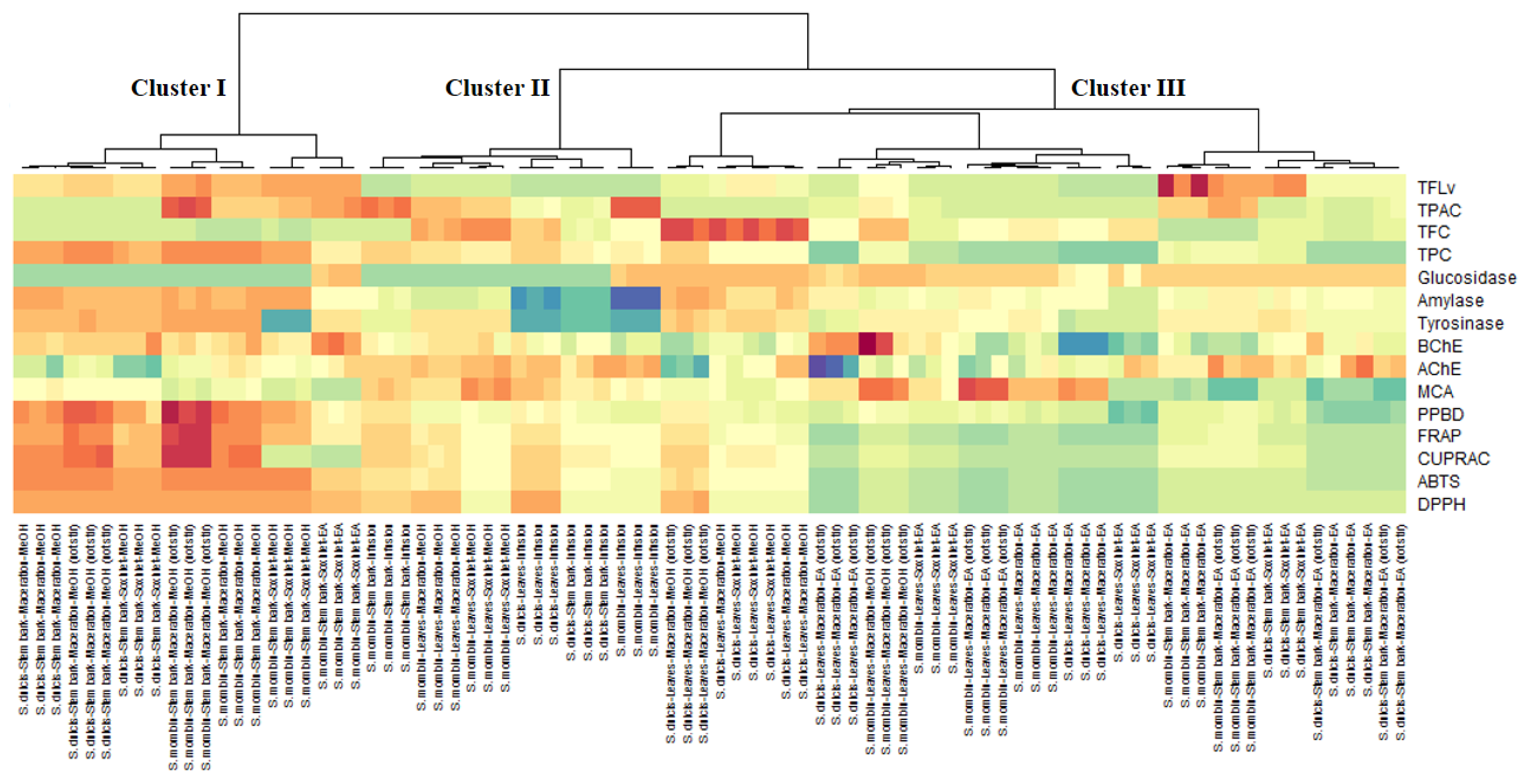
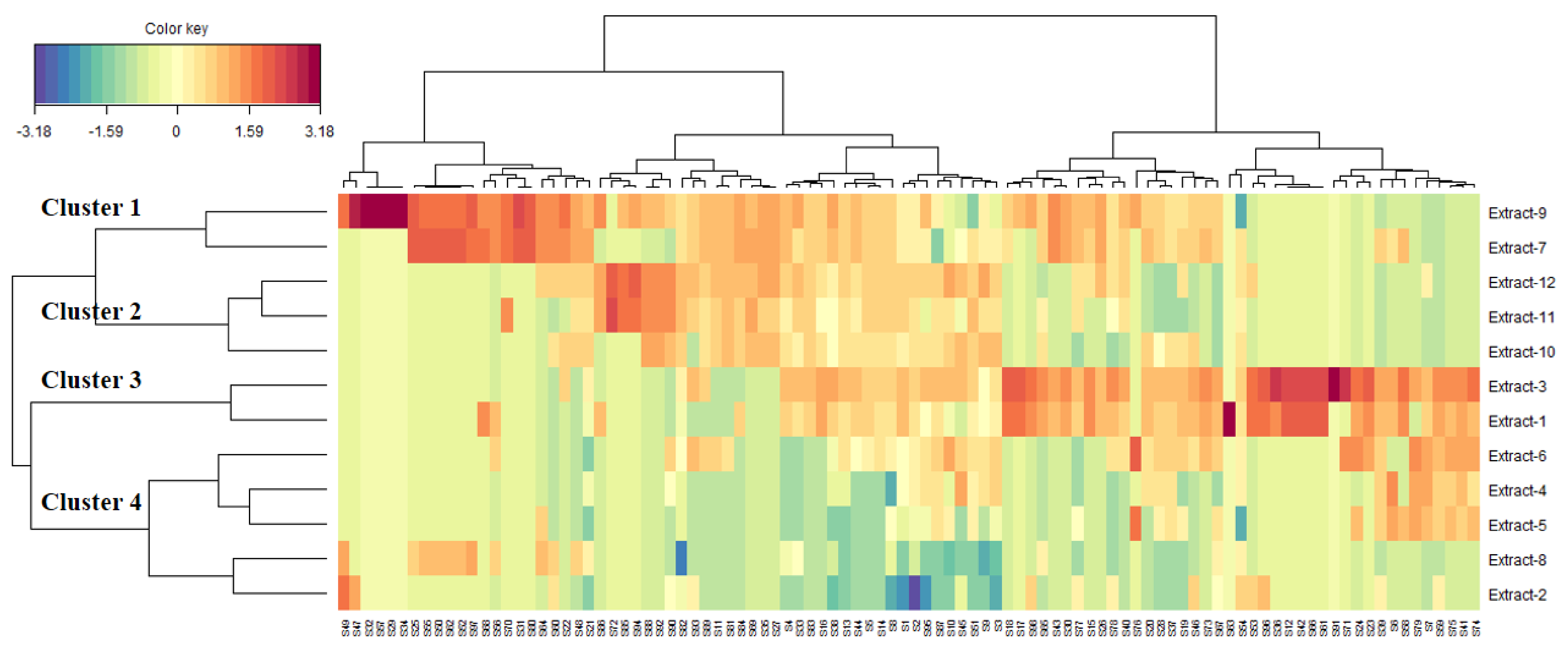
3.9. Data Mining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N.B. Comparative metabolic profiling of essential oils from Spondias pinnata (Linn. F.) Kurz and characterization of their antibacterial activities. Ind. Crops Prod. 2019, 137, 468–474. [Google Scholar] [CrossRef]
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N. Genus Spondias: A Phytochemical and Pharmacological Review. Evid.-Based Complementary Altern. Med. 2018, 2018, 5382904. [Google Scholar] [CrossRef] [Green Version]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef] [Green Version]
- Nwidu, L.L.; Elmorsy, E.; Oboma, Y.I.; Carter, W.G. Hepatoprotective and antioxidant activities of Spondias mombin leaf and stem extracts against carbon tetrachloride-induced hepatotoxicity. J. Taibah Univ. Med. Sci. 2018, 13, 262–271. [Google Scholar] [CrossRef]
- Fred-Jaiyesimi, A.; Kio, A.; Richard, W. α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem. 2009, 116, 285–288. [Google Scholar] [CrossRef]
- Ishola, I.O.; Ikuomola, B.O.; Adeyemi, O.O. Protective role of Spondias mombin leaf and Cola acuminata seed extracts against scopolamineinduced cognitive dysfunction. Alex. J. Med. 2018, 54, 27–39. [Google Scholar]
- Cabral, B.; Siqueira, E.; Bitencourt, M.A.; Lima, M.C.; Lima, A.K.; Ortmann, C.F.; Chaves, V.C.; Fernandes-Pedrosa, M.F.; Rocha, H.A.; Scortecci, K.C. Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev. Bras. Farmacogn. 2016, 26, 304–311. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Sampaio, T.I.; de Melo, N.C.; de Freitas Paiva, B.T.; da Silva Aleluia, G.A.; da Silva Neto, F.L.P.; da Silva, H.R.; Keita, H.; Cruz, R.A.S.; Sánchez-Ortiz, B.L.; Pineda-Peña, E.A.; et al. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio). J. Ethnopharmacol. 2018, 224, 563–578. [Google Scholar] [CrossRef]
- Ayoka, A.O.; Akomolafe, R.O.; Iwalewa, E.O.; Akanmu, M.A.; Ukponmwan, O.E. Sedative, antiepileptic and antipsychotic effects of Spondias mombin L. (Anacardiaceae) in mice and rats. J. Ethnopharmacol. 2006, 103, 166–175. [Google Scholar] [CrossRef]
- Accioly, M.P.; Bevilaqua, C.M.L.; Rondon, F.C.; de Morais, S.M.; Machado, L.K.; Almeida, C.A.; de Andrade Jr, H.F.; Cardoso, R.P. Leishmanicidal activity in vitro of Musa paradisiaca L. and Spondias mombin L. fractions. Vet. Parasitol. 2012, 187, 79–84. [Google Scholar] [CrossRef]
- Franquin, S.; Marcelin, O.; Aurore, G.; Reynes, M.; Brillouet, J.-M. Physicochemical characterisation of the mature-green Golden apple (Spondias cytherea Sonnerat). Fruits 2005, 60, 203–210. [Google Scholar] [CrossRef]
- Sarker, M.; Nimmi, I.; Kawsar, M.H. Preliminary screening of six popular fruits of Bangladesh for in vitro IgM production and proliferation of splenocytes. Bangladesh Pharm J. 2012, 15, 31–37. [Google Scholar]
- Islam, S.M.A.; Ahmed, K.T.; Manik, M.K.; Wahid, M.A.; Kamal, C.S.I. A comparative study of the antioxidant, antimicrobial, cytotoxic and thrombolytic potential of the fruits and leaves of Spondias dulcis. Asian Pac. J. Trop Biomed. 2013, 3, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Koubala, B.; Mbome, L.; Kansci, G.; Mbiapo, F.T.; Crepeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 2008, 106, 1202–1207. [Google Scholar] [CrossRef]
- Koubala, B.B.; Kansci, G.; Ralet, M.-C. Ambarella—Spondias cytherea. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: ondon, UK, 2018; pp. 15–22. [Google Scholar]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complementary Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Sayed, E.A.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K.; Ayoub, N.; Singab, A.-E.N.; El-Azizi, M. Chemical composition and bioactivity of Pleiogynium timorense (Anacardiaceae). Nat. Prod. Commun. 2010, 5, 1934578X1000500410. [Google Scholar] [CrossRef] [Green Version]
- Guedes, J.A.C.; Santiago, Y.G.; dos Reis Luz, L.; Silva, M.F.S.; Ramires, C.M.C.; de Lima, M.A.C.; de Oliveira, V.R.; do Ó Pessoa, C.; Canuto, K.M.; de Brito, E.S. Comparative analyses of metabolic fingerprint integrated with cytotoxic activity and in silico approaches of the leaves extract of Spondias mombin L. and Spondias tuberosa Arr. Cam. from Northeast, Brazil. Phytochem. Lett. 2020, 40, 26–36. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Correlation of oxidative transformations of hydrolyzable tannins and plant evolution. Phytochemistry 2000, 55, 513–529. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Kadir, K.A. Ellagitannin geraniin: A review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem. Rev. 2017, 16, 159–193. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, X.; Guo, M. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [Green Version]
- Corthout, J.; Pieters, L.; Claeys, M.; Berghe, D.V.; Vlietinck, A. Antiviral ellagitannins from Spondias mombin. Phytochemistry 1991, 30, 1129–1130. [Google Scholar] [CrossRef]
- Raffaella, P.; Acquavia, M.A.; Cataldi, T.R.; Alberto, O.; Donatella, C.; Bufo, S.A.; Laura, S.; Rosanna, C.; Guerrieri, A.; Giuliana, B. Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI (-)-MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem. 2020, 412, 3005–3015. [Google Scholar]
- de Rijke, E.; Out, P.; Niessen, W.M.; Ariese, F.; Gooijer, C.; Udo, A.T. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC–MS analyses of acylquinic acids in Geigeria alata (DC) Oliv. & Hiern. and their contribution to antioxidant and antimicrobial capacity. Phytochem. Anal. 2017, 28, 176–184. [Google Scholar]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.Å.; Smeds, A.I.; Sjöholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Gu, W.-Y.; Li, N.; Leung, E.L.-H.; Zhou, H.; Luo, G.-A.; Liu, L.; Wu, J.-L. Metabolites software-assisted flavonoid hunting in plants using ultra-high performance liquid chromatography-quadrupole-time of flight mass spectrometry. Molecules 2015, 20, 3955–3971. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.Y.; Snyder, P.J.; Wu, W.-C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Carmona, V.; Martín-Aragón, S.; Goldberg, J.; Schubert, D.; Bermejo-Bescós, P. Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutr. Neurosci. 2020, 23, 575–590. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.; Li, X.; Chen, L.; Taylor, K.D.; Rotter, J.I.; Rissman, R.A.; Aisen, P.S.; Apostolova, L.G. Deleterious Effect of Butyrylcholinesterase K-Variant in Donepezil Treatment of Mild Cognitive Impairment. J. Alzheimers Dis. 2017, 56, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesulam, M.-M. Butyrylcholinesterase in Alzheimer’s disease. In Alzheimer Disease; Springer, Birkhäuser: Boston, MA, USA, 1994; pp. 79–83. [Google Scholar]
- Elufioye, T.O.; Obuotor, E.M.; Agbedahunsi, J.M.; Adesanya, S.A. Anticholinesterase constituents from the leaves of Spondias mombin L. (Anacardiaceae). Biol. Targets Ther. 2017, 11, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asuquo, O.; Ekanem, T.; Udoh, P.; Mesembe, O. Antigonadotrophic Effect of Spondias Mombin Leaf Extract In Male Wistar Rats. J. Biol. Agric. Healthc. 2012, 2, 14–17. [Google Scholar]
- Bhuvanendran, S.; Hanapi, N.A.; Ahemad, N.; Othman, I.; Yusof, S.R.; Shaikh, M.F. Embelin, a Potent Molecule for Alzheimer’s Disease: A Proof of Concept From Blood-Brain Barrier Permeability, Acetylcholinesterase Inhibition and Molecular Docking Studies. Front. Neurosci. 2019, 13, 495. [Google Scholar] [CrossRef] [Green Version]
- Strachan, M.W.; Deary, I.J.; Ewing, F.M.; Frier, B.M. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care 1997, 20, 438–445. [Google Scholar] [CrossRef]
- Stewart, R.; Liolitsa, D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999, 16, 93–112. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crops Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Julfikar Hossain, S.; Tsujiyama, I.; Takasugi, M.; Biswas, R.; Aoshima, H. Total phenolic content, antioxidative, antiamylase, anti-glucosidase, and antihistamine release activities of Bangladeshi fruits. Food Sci. Technol. Res. 2008, 14, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Fadare, O.; Obuotor, E.M. Estimation of the kinetic parameters of the inhibition of tyrosinase by an extract of S. mombin (root bark) and the investigation of likely interactions of composite phytochemicals using molecular docking calculations. Am. J. Pharmacol. Sci. 2018, 6, 13–18. [Google Scholar]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Brandão, P.; Martins, M.A.; Silva, L.P.; Schreiner, T.B.; Fernandes, L.; Ferreira, O.; Pinho, S.P. Solubility and solid phase studies of isomeric phenolic acids in pure solvents. J. Mol. Liq. 2018, 272, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Zwick, W.R.; Velicer, W.F. Comparison of five rules for determining the number of components to retain. Psychol. Bull. 1986, 99, 432. [Google Scholar] [CrossRef]
- Sinan, K.I.; Ferrarese, I.; Aktumsek, A.; Peron, G.; Glamocilja, J.; Sokovic, M.; Nenadić, M.; Dall’Acqua, S.; Zengin, G. NMR and LC-MSn coupled with pharmacological network analysis for the assessment of phytochemical content and biopharmaceutical potential of Carapa procera extracts. J. Pharm. Biomed. Anal. 2021, 114184. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron Californicum. BMC Res. Notes 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Felhi, S.; Daoud, A.; Hajlaoui, H.; Mnafgui, K.; Gharsallah, N.; Kadri, A. Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis of Ecballium elaterium seeds and peels fruits. Food Sci. Technol. 2017, 37, 483–492. [Google Scholar] [CrossRef] [Green Version]

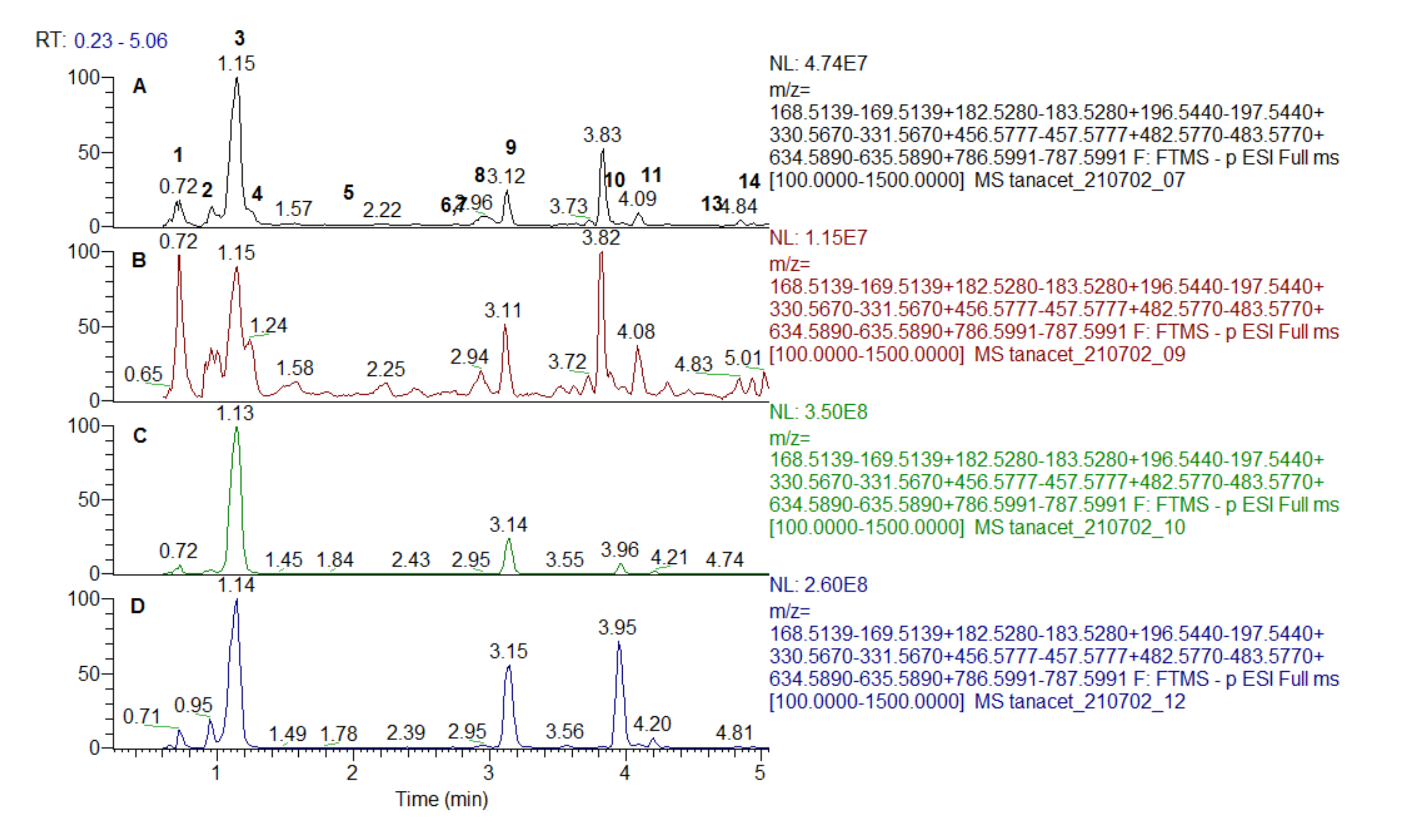

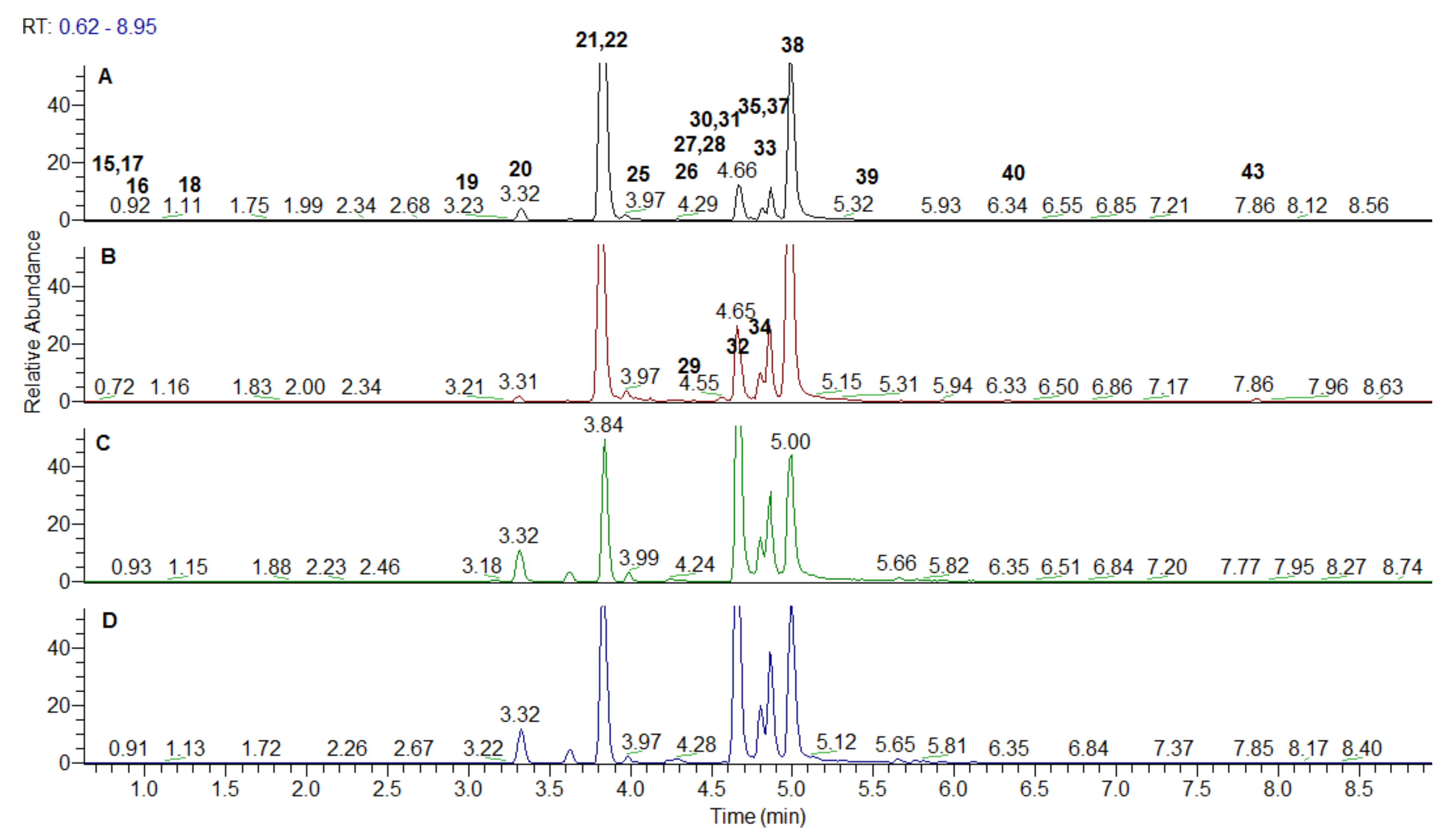
| No | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− | Distribution |
|---|---|---|---|---|
| Gallic acid and galloyl derivatives | ||||
| 1. | galloyl hexose | C13H16O10 | 331.0671 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 2. | galloyl hexose isomer | C13H16O10 | 331.0671 | 1,3,4,5,6,7,8,9,10,11,12 |
| 3. | gallic acid * | C7H6O5 | 169.0142 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 4. | gallic acid hexoside | C13H16O10 | 331.0671 | 1,3,7,8,9,10,11,12 |
| 5. | digalloyl hexoside | C20H20O14 | 483.0780 | 1,3,6,7,9,10,11,12 |
| 6. | digalloylquinic acid | C21H20O14 | 495.0776 | 1,3,4,5,6,7 |
| 7. | digalloylquinic acid isomer | C21H20O14 | 495.0790 | 1,3,4,5,6 |
| 8. | digalloyl hexose | C20H20O14 | 483.0780 | 1,3,5,6,7,8,9,10,11,12 |
| 9. | methylgallate | C8H8O5 | 183.0289 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 10. | epigallocatechin-O-gallate | C22H18O11 | 457.0776 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 11. | trigalloyl hexoside | C27H24O18 | 635.0890 | 6,7,8,9,10,11,12 |
| 12. | dimethylgallate | C9H10O5 | 197.0455 | 1,2,3 |
| 13. | trigalloyl hexoside isomer | C27H24O18 | 635.0890 | 1,3,4,6,7,9,10,11,12 |
| 14. | tetragalloyl hexoside | C34H28O22 | 787.0999 | 1,3,4,6,7,9,10,11,12 |
| Ellagic acid derivatives and ellagitannins | ||||
| 15. | HHDP-hexoside | C20H18O14 | 481.0624 | 1,3,7,9,12 |
| 16. | HHDP-hexoside isomer | C20H18O14 isomer | 481.0624 | 1,3,7,9,10,12 |
| 17. | galloyl-HHDP-hexoside | C27H22O18 | 633.0733 | 1,3,8,9 |
| 18. | galloyl-HHDP-hexoside isomer | C27H22O18 | 633.0733 | 1,3,7,9 |
| 19. | galloyl-HHDP-hexoside isomer | C27H22O18 | 633.0733 | 1,3,5,6,7,9,10,12 |
| 20. | brevifolin carboxylic acid | C13H8O8 | 291.0149 | 1,3,4,6,7,9,10 |
| 21. | galloyl-HHDP-hexoside isomer | C27H22O18 | 633.0733 | 1,3,4,6,7,8,9,10,11,12 |
| 22. | geraniin | C41H28O27 | 951.0745 | 3,7,10,12 |
| 23. | valoneic acid-dilactone | C21H10O13 | 469.0049 | 1,3,6 |
| 24. | digalloyl-HHDP-hexoside | C34H26O22 | 785.0843 | 1,3,5,6 |
| 25. | ellagic acid-hexoside | C20H16O13 | 463.0518 | 7,8,9 |
| 26. | brevifolin | C12H8O6 | 247.0245 | 1,3,7,9,12 |
| 27. | digalloyl-HHDP-hexoside isomer | C34H26O22 | 785.0843 | 7,9,10,11,12 |
| 28. | methylellagic acid-O-hexoside | C21H18O13 | 477.0675 | 1,3,4,5,6,7,9,10 |
| 29. | galloyl-geraniin | C48H32O31 | 1103.0855 | 9 |
| 30. | methyl brevifolin carboxylate | C14H10O8 | 305.0304 | 1,3,7,9 |
| 31. | galloyl-bisHHDP-hexoside | C41H30O27 | 953.0902 | 7,9 |
| 32. | ellagic acid- pentoside isomer | C19H14O12 | 433.0412 | 9 |
| 33. | ellagic acid deoxyhexoside | C20H16O12 | 447.0571 | 1,3,7,8,9,10,11,12 |
| 34. | ellagic acid- pentoside | C19H14O12 | 433.0412 | 9 |
| 35. | digalloyl-HHDP-hexoside isomer | C34H26O22 | 785.0843 | 7,9,10,11,12 |
| 36. | di HHDP-hexoside | C34H24O22 | 783.0686 | 1,3 |
| 37. | methylellagic acid O-hexoside | C21H18O13 | 477.0675 | 1,3,4,5,6,7,9,10 |
| 38. | ellagic acid * | C14H6O8 | 300.9991 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 39. | dimethylellagic acid O-hexoside | C22H20O13 | 491.0831 | 1,2,3,4,5,6,7 |
| 40. | methylellagic acid | C15H8O8 | 315.0146 | 1,3,6,7,9,12 |
| 41. | dimethylellagic acid O-hexoside | C22H20O13 | 491.0831 | 1,3,4,5,6 |
| 42. | trimethylellagic acid | C17H12O8 | 343.0459 | 1,3 |
| 43. | dimethylellagic acid | C16H10O8 | 329.0303 | 1,3,7,9 |
| Hydroxybenzoic, hydroxycinnamic, acylquinic acids and derivatives | ||||
| 44. | protocatechuic acid O-hexoside | C13H16O9 | 315.0723 | 1,3,6,7,9,10,11,12 |
| 45. | protocatechuic acid * | C7H6O4 | 153.0181 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 46. | syringic acid O-hexoside | C15H20O10 | 359.0956 | 1,2,3,6,7,9,10 |
| 47. | caffeic acid O-hexoside | C15H18O9 | 341.0883 | 9 |
| 48. | neochlorogenic acid * | C16H18O9 | 353.0878 | 7,8,9,10,11,12 |
| 49. | caffeic acid * | C9H8O4 | 179.0342 | 8,9 |
| 50. | chlorogenic acid * | C16H18O9 | 353.0877 | 7,8,9 |
| 51. | vanillic acid O-hexoside | C14H18O9 | 329.0888 | 1,3,4,5,6,7,10,11,12 |
| 52. | 4-caffeoylquinic aid | C16H18O9 | 353.0880 | 7,8 |
| 53. | syringic acid * | C9H10O5 | 197.0447 | 1,3,12 |
| 54. | salicylic acid | C7H6O3 | 137.0231 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| Flavonols | ||||
| 55. | quercetin 3-O-dihexoside | C27H30O17 | 625.1415 | 7,8,9 |
| 56. | quercetin 3-O-hexoside-O-pentoside | C26H28O16 | 595.1307 | 1,7,8,9 |
| 57. | kaempferol 3-O-dihexoside | C27H30O16 | 609.1436 | 9 |
| 58. | myricetin 3-O-pentoside | C20H18O12 | 449.0727 | 1,3,5,7 |
| 59. | myricitrin (myricetin 3-O-rhamnoside) * | C21H20O12 | 463.0882 | 1,3,4,5,6 |
| 60. | isoquercitrin (quercetin 3-O-glucoside) * | C21H20O12 | 463.0883 | 7,8,9,10,12 |
| 61. | myricetin 3-O-hexoside-7-O-deoxyhexoside | C27H30O17 | 625.1418 | 1,3 |
| 62. | kaempferol 3-O-pentosyl-hexoside | C26H28O15 | 579.1359 | 7,8,9 |
| 63. | quercetin 4′-O-hexuronide | C21H18O13 | 477.0680 | 1 |
| 64. | hyperoside (quercetin 3-O-galactoside) * | C21H20O12 | 463.0885 | 5,7,8,9,12 |
| 65. | quercetin-O-pentoside | C20H18O11 | 433.0776 | 1,3,7,8,9,10,11 |
| 66. | methylmyricetin-O-deoxyhexoside | C22H22O12 | 477.1038 | 1,3 |
| 67. | kaempferol 3-O-glucoside * | C21H20O11 | 447.0933 | 1,3,5,6,7,8,9 |
| 68. | quercitrin (quercetin 3-O-rhamnoside) * | C21H20O11 | 447.0933 | 1,6,7,9 |
| 69. | kaempferol 7-O-glucoside | C21H20O11 | 447.0941 | 7,9,10,11,12 |
| 70. | quercetin 4′-O-hexoside | C21H20O12 | 463.0892 | 7,9,11 |
| 71. | myricetin 7-O-deoxyhexoside | C21H20O12 | 463.0885 | 3,5,6 |
| 72. | isorhamnetin 3-O-glucoside * | C22H22O12 | 477.1042 | 11,12 |
| 73. | kaempferol-O-pentoside | C20H18O10 | 417.0828 | 1,3,6,7,9 |
| 74. | myricitrin-O-gallate | C28H24O16 | 615.0999 | 1,3,5,6 |
| 75. | kaempferol 3-O-deoxyhexoside | C21H20O10 | 431.0985 | 1,3,4,5,6 |
| 76. | rhamnetin 3-O-hexoside | C22H22O12 | 477.1035 | 5,6,9 |
| 77. | Quercetin * | C15H10O7 | 301.0347 | 1,3,5,7,9,11,12 |
| 78. | Myricetin * | C15H10O8 | 317.0301 | 1,3,6,7,9,11,12 |
| 79. | rhamnetin 3-O-deoxyhexoside | C22H22O11 | 461.1090 | 3,4,5,6 |
| 80. | Kaempferol * | C15H10O6 | 285.0407 | 7,9 |
| Flavanones, flavanonols and flavan-3-ols | ||||
| 81. | (epi)catechin O-hexoside | C21H24O11 | 451.1241 | 6,7,9,10,11,12 |
| 82. | (+)-catechin * | C15H14O6 | 289.0717 | 1,3,4,5,6,7,9,10,11,12 |
| 83. | taxifolin 3-O-hexoside | C21H22O12 | 465.1039 | 1,3,7,9,10,11,12 |
| 84. | taxifolin 7-O-hexoside | C21H22O12 | 465.1037 | 1,7,9,10,11,12 |
| 85. | (epi)catechin-gallate | C22H18O10 | 441.0828 | 9,11,12 |
| 86. | taxifolin 4′-O-hexoside | C21H22O12 | 465.1037 | 1,7,9,11,12 |
| 87. | naringenin 7-O-glucoside (prunin) * | C21H22O10 | 433.1140 | 1,3,4,5,6,9,10,11,12 |
| 88. | (epi)catechin-gallate isomer I | C22H18O10 | 441.0826 | 9,10,11,12 |
| 89. | eriodictiol 7-O-hexoside | C21H22O11 | 449.1090 | 3,6,7,9,10,11,12 |
| 90. | (epi)catechin-gallate isomer II | C22H18O10 | 441.0826 | 1,4,6,9,10,11,12 |
| 91. | naringenin 8-C-hexoside | C21H22O10 | 433.1144 | 3 |
| 92. | pinocembrin-O-hexoside | C21H22O9 | 417.1195 | 9,10,11,12 |
| 93. | naringenin | C15H12O5 | 271.0614 | 3,5,6,7,9,11,12 |
| 94. | pinocembrin | C15H12O4 | 255.0664 | 9,11,12 |
| Others | ||||
| 95. | sucrose | C12H22O11 | 341.1088 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 96. | tuberonic acid-hexoside | C18H28O9 | 387.1657 | 1,3 |
| 97. | cinchonain I | C24H20O9 | 451.1037 | 7,8,9 |
| 98. | pinoresinol (Eklund et al., 2008) | C20H22O6 | 357.1345 | 1,3,9 |
| Species | Parts | Methods-Solvents | AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) |
|---|---|---|---|---|---|---|---|
| Spondias dulcis | Leaves | Infusion | 10.10 ± 0.11 ab | 3.83 ± 0.48 cdefg | na | 0.25 ± 0.04 n | na |
| Maceration-EA | 8.60 ± 0.23 bcdef | na | 82.27 ± 8.84 n | 0.71 ± 0.01 efghi | 11.07 ± 0.21 d | ||
| Maceration-MeOH | 9.79 ± 0.79 abcd | 3.44 ± 0.25 cdefg | 165.44 ± 2.39 d | 0.88 ± 0.03 c | 17.37 ± 0.11 a | ||
| Maceration-EA (not stir) | 5.77 ± 0.66 h | 6.21 ± 0.18 ab | 94.17 ± 5.97 m | 0.76 ± 0.01 de | 14.73 ± 0.74 bc | ||
| Maceration-MeOH (not stir) | 6.89 ± 0.21 gh | 1.98 ± 0.44 ghi | 184.49 ± 2.51 c | 0.97 ± 0.03 a | 17.70 ± 0.04 a | ||
| Soxhlet-EA | 9.51 ± 0.99 abcd | 1.21 ± 0.44 hi | 79.88 ± 2.02 n | 0.54 ± 0.01 l | 14.27 ± 2.96 c | ||
| Soxhlet-MeOH | 8.82 ± 0.25 abcde | 2.55 ± 0.72 efgh | 178.97 ± 1.28 c | 0.80 ± 0.01 d | 17.61 ± 0.03 a | ||
| Stem barks | Infusion | 9.95 ± 0.53 abcd | 3.36 ± 0.68 cdefgh | 15.72 ± 1.90 o | 0.32 ± 0.01 m | na | |
| Maceration-EA | 10.33 ± 1.09 a | 3.62 ± 0.39 cdefg | 119.85 ± 1.47 k | 0.70 ± 0.01 efghi | 16.88 ± 0.02 a | ||
| Maceration-MeOH | 7.81 ± 0.62 efg | 4.83 ± 0.14 abcd | 197.72 ± 1.43 b | 0.99 ± 0.01 a | na | ||
| Maceration-EA (not stir) | 9.58 ± 0.77 abcd | 3.68 ± 1.49 cdefg | 116.15 ± 3.31 k | 0.67 ± 0.01 hij | 16.94 ± 0.11 a | ||
| Maceration-MeOH (not stir) | 8.39 ± 0.19 cdefg | 5.09 ± 0.08 abc | 201.48 ± 1.17 ab | 0.94 ± 0.01 abc | na | ||
| Soxhlet-EA | 9.98 ± 0.23 abc | 3.55 ± 0.37 cdefg | 155.95 ± 3.27 efg | 0.71 ± 0.04 efgh | 16.91 ± 0.03 a | ||
| Soxhlet-MeOH | 7.00 ± 0.14 fgh | 5.39 ± 0.72 abc | 198.93 ± 0.85 ab | 0.90 ± 0.01 bc | na | ||
| Spondias mombin | Leaves | Infusion | 10.45 ± 0.16 a | 2.54 ± 0.56 efgh | na | 0.13 ± 0.01 o | 17.12 ± 0.13 a |
| Maceration-EA | 8.66 ± 0.77 bcde | 2.88 ± 0.52 defgh | 140.14 ± 2.50 i | 0.69 ± 0.04 fghi | 16.20 ± 0.13 ab | ||
| Maceration-MeOH | 10.01 ± 0.13 abc | 4.48 ± 0.21 bcde | 159.58 ± 1.37 def | 0.55 ± 0.01 l | na | ||
| Maceration-EA (not stir) | 7.77 ± 0.97 efg | 2.17 ± 0.87 fgh | 129.52 ± 1.19 j | 0.73 ± 0.02 efg | 16.23 ± 0.50 ab | ||
| Maceration-MeOH (not stir) | 8.59 ± 0.45 bcdef | 6.89 ± 1.95 a | 153.70 ± 0.62 fgh | 0.62 ± 0.01 jk | 17.41 ± 0.19 a | ||
| Soxhlet-EA | 8.33 ± 0.65 defg | 4.15 ± 0.44 bcdef | 147.24 ± 1.93 hi | 0.68 ± 0.02 fghij | 16.79 ± 0.40 a | ||
| Soxhlet-MeOH | 10.37 ± 0.07 a | 4.32 ± 0.94 bcde | 156.41 ± 1.05 efg | 0.57 ± 0.01 kl | na | ||
| Stem barks | Infusion | 9.93 ± 0.04 abcd | 3.71 ± 0.34 cdefg | 104.08 ± 1.11 l | 0.65 ± 0.04 ij | na | |
| Maceration-EA | 9.27 ± 0.03 abcde | 2.89 ± 1.03 defgh | 150.72 ± 1.82 gh | 0.68 ± 0.03 ghij | 16.96 ± 0.04 a | ||
| Maceration-MeOH | 9.34 ± 0.21 abcde | 5.26 ± 0.57 abc | 203.72 ± 1.02 ab | 0.94 ± 0.03 abc | na | ||
| Maceration-EA (not stir) | 10.31 ± 0.47 a | 1.97 ± 0.19 ghi | 146.82 ± 0.19 hi | 0.74 ± 0.01 def | 16.82 ± 0.03 a | ||
| Maceration-MeOH (not stir) | 8.59 ± 0.12 bcdef | 4.51 ± 0.30 bcde | 207.00 ± 0.73 a | 0.95 ± 0.01 ab | na | ||
| Soxhlet-EA | 9.39 ± 0.39 abcde | 6.28 ± 0.33 ab | 163.23 ± 2.54 de | 0.72 ± 0.01 efgh | 17.16 ± 0.03 a | ||
| Soxhlet-MeOH | 9.02 ± 0.14 abcde | 4.36 ± 0.09 bcde | na | 0.96 ± 0.01 ab | na |
| Species | Parts | Methods-Solvents | TPC (mg GAE/g) | TFC (mg RE/g) | TPAC (mg CAE/g) | TFlv (mg CE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|---|---|
| Spondias dulcis | Leaves | Infusion | 179.89 ± 0.14 ef | 25.30 ± 0.59 d | 6.91 ± 0.74 h | 0.46 ± 0.01 k | 4.89 ± 0.14 efg |
| Maceration-EA | 29.27 ± 0.91 qr | 13.80 ± 0.49 g | nd | 1.82 ± 0.06 jk | 3.33 ± 0.14 k | ||
| Maceration-MeOH | 134.39 ± 1.96 j | 43.41 ± 1.08 a | nd | 7.35 ± 0.04 gh | 3.94 ± 0.12 hijk | ||
| Maceration-EA (not stir) | 32.60 ± 0.32 pq | 13.21 ± 0.59 g | 2.63 ± 0.22 ij | 2.33 ± 0.02 jk | 3.49 ± 0.14 ijk | ||
| Maceration-MeOH (not stir) | 182.53 ± 1.15 e | 43.11 ± 3.28 a | nd | 4.85 ± 0.05 hij | 4.98 ± 0.02 ef | ||
| Soxhlet-EA | 25.85 ± 0.97 r | 21.26 ± 0.26 e | nd | 1.22 ± 0.01 jk | 1.75 ± 0.12 l | ||
| Soxhlet-MeOH | 136.96 ± 1.86 ij | 40.71 ± 3.63 a | nd | 9.48 ± 0.01 fg | 3.87 ± 0.25 hijk | ||
| Stem barks | Infusion | 143.60 ± 0.90 h | 8.53 ± 0.43 h | 4.28 ± 0.52 i | 1.32 ± 0.01 jk | 4.27 ± 0.09 fgh | |
| Maceration-EA | 47.28 ± 1.02 o | 4.42 ± 0.22 ijk | nd | 7.48 ± 0.05 gh | 2.16 ± 0.08 l | ||
| Maceration-MeOH | 230.08 ± 3.14 b | 2.30 ± 0.20 kl | nd | 12.52 ± 0.17 def | 6.82 ± 0.28 cd | ||
| Maceration-EA (not stir) | 44.19 ± 0.93 o | 5.85 ± 0.08 hi | 3.77 ± 0.81 ij | 6.24 ± 0.13 ghi | 2.31 ± 0.11 l | ||
| Maceration-MeOH (not stir) | 240.24 ± 1.08 a | 2.40 ± 0.09 kl | nd | 14.27 ± 0.07 cde | 7.77 ± 0.18 b | ||
| Soxhlet-EA | 92.69 ± 0.99 m | 6.47 ± 0.23 hi | nd | 18.95 ± 0.47 b | 3.43 ± 0.27 jk | ||
| Soxhlet-MeOH | 216.16 ± 1.00 c | 2.47 ± 0.07 jkl | nd | 11.66 ± 0.02 ef | 6.24 ± 0.70 d | ||
| Spondias mombin | Leaves | Infusion | 143.12 ± 1.00 hi | 12.14 ± 0 11 g | 27.16 ± 0.64 a | 0.60 ± 0.01 k | 3.65 ± 0.14 hijk |
| Maceration-EA | 46.37 ± 0.41 o | 17.06 ± 0.33 f | nd | 2.81 ± 0.02 ijk | 3.52 ± 0.29 hijk | ||
| Maceration-MeOH | 174.93 ± 1.00 f | 29.02 ± 0.69 c | 16.87 ± 0.51 d | 2.09 ± 0.02 jk | 4.18 ± 0.08 ghij | ||
| Maceration-EA (not stir) | 36.97 ± 0.98 p | 13.51 ± 0.34 g | nd | 2.23 ± 0.03 jk | 3.85 ± 0.18 hijk | ||
| Maceration-MeOH (not stir) | 112.14 ± 0.24 l | 27.51 ± 0.12 cd | 9.64 ± 0.56 g | 8.78 ± 0.08 fg | 4.26 ± 0.21 fghi | ||
| Soxhlet-EA | 59.54 ± 0.24 n | 5.58 ± 0.37 hij | 2.43 ± 0.77 j | 3.04 ± 0.01 ijk | 3.76 ± 0.18 hijk | ||
| Soxhlet-MeOH | 151.20 ± 1.55 g | 33.16 ± 0.15 b | 14.76 ± 0.07 ef | 3.31 ± 0.03 ijk | 4.29 ± 0.13 fgh | ||
| Stem barks | Infusion | 189.85 ± 1.25 d | 3.39 ± 0.09 ijkl | 23.25 ± 1.18 b | 0.85 ± 0.02 k | 5.34 ± 0.09 e | |
| Maceration-EA | 107.43 ± 1.35 l | 1.21 ± 0.14 l | 14.50 ± 0.91 f | 25.83 ± 6.22 a | 3.71 ± 0.07 hijk | ||
| Maceration-MeOH | 245.50 ± 3.20 a | 1.41 ± 0.11 kl | 15.22 ± 0.23 def | 15.48 ± 0.29 bcd | 7.20 ± 0.30 bc | ||
| Maceration-EA (not stir) | 119.57 ± 4.83 k | 1.26 ± 0.04 kl | 19.32 ± 1.40 c | 18.50 ± 0.39 b | 4.05 ± 0.22 hijk | ||
| Maceration-MeOH (not stir) | 244.28 ± 5.42 a | 1.77 ± 0.21 kl | 27.54 ± 0.73 a | 18.13 ± 0.43 b | 8.76 ± 0.49 a | ||
| Soxhlet-EA | 156.46 ± 1.60 g | 1.94 ± 0.04 kl | 20.12 ± 0.93 c | 18.03 ± 0.41 bc | 4.89 ± 0.18 efg | ||
| Soxhlet-MeOH | 228.18 ± 2.31 b | 1.55 ± 0.07 kl | 16.44 ± 0.29 de | 17.68 ± 0.05 bc | 6.50 ± 0.34 cd |
| Species | Parts | Methods-Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal Chelating (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| Spondias dulcis | Leaves | Infusion | 619.35 ± 1.10 b | 1115.63 ± 3.70 f | 1109.52 ± 7.96 f | 687.23 ± 5.19 f | 30.97 ± 2.15 def |
| Maceration-EA | 17.82 ± 2.41 jk | 29.11 ± 6.59 p | 104.60 ± 1.36 q | 37.64 ± 0.64 o | 34.78 ± 1.64 cd | ||
| Maceration-MeOH | 259.85 ± 0.14 e | 658.05 ± 0.53 j | 733.72 ± 22.82 j | 405.48 ± 4.73 j | 19.78 ± 0.31 lm | ||
| Maceration-EA (not stir) | 16.23 ± 1.92 k | 21.21 ± 0.17 p | 122.46 ± 2.02 q | 45.69 ± 1.21 no | 25.67 ± 0.67 ghi | ||
| Maceration-MeOH (not stir) | 609.71 ± 1.58 b | 1076.92 ± 6.96 g | 1107.96 ± 16.31 f | 632.18 ± 5.99 g | 17.58 ± 0.97 mn | ||
| Soxhlet-EA | 28.51 ± 2.58 j | 15.98 ± 4.21 p | 93.71 ± 3.86 q | 47.77 ± 1.24 no | 10.02 ± 0.37 pq | ||
| Soxhlet-MeOH | 261.69 ± 0.25 e | 662.37 ± 0.81 j | 884.09 ± 12.38 h | 502.83 ± 5.24 i | 14.64 ± 1.08 no | ||
| Stem barks | Infusion | 252.51 ± 0.37 e | 660.88 ± 0.86 j | 810.96 ± 7.10 i | 494.47 ± 7.75 i | 24.68 ± 0.83 hijk | |
| Maceration-EA | 117.97 ± 1.11 gh | 180.04 ± 1.64 m | 204.32 ± 4.55 o | 97.41 ± 3.58 mn | 5.77 ± 0.56 r | ||
| Maceration-MeOH | 657.31 ± 0.45 a | 1601.11 ± 10.38 b | 1567.07 ± 27.85 d | 954.81 ± 28.93 c | 21.45 ± 2.88 jklm | ||
| Maceration-EA (not stir) | 107.54 ± 0.81 h | 156.72 ± 2.25 mn | 186.75 ± 2.23 o | 96.84 ± 1.94 mn | na | ||
| Maceration-MeOH (not stir) | 656.25 ± 0.54 a | 1657.40 ± 1.72 a | 1787.56 ± 13.10 b | 1065.65 ± 23.89 b | 20.80 ± 0.67 klm | ||
| Soxhlet-EA | 131.03 ± 0.13 f | 330.61 ± 0.12 l | 409.34 ± 13.29 m | 218.78 ± 13.82 l | 11.89 ± 0.56 opq | ||
| Soxhlet-MeOH | 657.27 ± 0.59 a | 1479.83 ± 7.59 d | 1309.52 ± 12.49 e | 814.43 ± 27.99 d | 20.41 ± 1.40 lm | ||
| Spondias mombin | Leaves | Infusion | 254.63 ± 2.05 e | 628.26 ± 4.35 k | 664.65 ± 4.24 k | 490.70 ± 2.10 i | 21.86 ± 0.63 ijkl |
| Maceration-EA | 59.66 ± 2.18 i | 89.25 ± 4.67 o | 178.94 ± 3.24 op | 71.95 ± 2.74 mno | 31.71 ± 0.94 de | ||
| Maceration-MeOH | 570.90 ± 14.85 c | 944.48 ± 12.25 i | 955.54 ± 29.11 g | 613.45 ± 24.53 gh | 27.27 ± 0.40 fgh | ||
| Maceration-EA (not stir) | 24.61 ± 1.25 jk | 21.50 ± 2.48 p | 137.66 ± 6.38 pq | 52.94 ± 0.56 no | 45.19 ± 0.93 a | ||
| Maceration-MeOH (not stir) | 125.91 ± 0.06 fg | 329.50 ± 0.28 l | 443.61 ± 33.81 m | 225.48 ± 29.90 l | 40.40 ± 1.59 b | ||
| Soxhlet-EA | 65.04 ± 1.99 i | 133.21 ± 7.15 n | 261.95 ± 3.73 n | 112.77 ± 1.64 m | 25.09 ± 2.85 hij | ||
| Soxhlet-MeOH | 258.87 ± 0.20 e | 658.11 ± 0.21 j | 776.86 ± 12.30 ij | 466.31 ± 4.46 i | 36.54 ± 1.01 bc | ||
| Stem barks | Infusion | 528.64 ± 2.15 d | 1009.25 ± 9.40 h | 1118.43 ± 6.39 f | 749.16 ± 3.86 e | 29.51 ± 1.14 efg | |
| Maceration-EA | 130.13 ± 0.22 f | 331.05 ± 0.27 l | 527.95 ± 3.27 l | 299.55 ± 2.14 k | 8.46 ± 1.68 qr | ||
| Maceration-MeOH | 657.82 ± 0.32 a | 1584.71 ± 24.46 bc | 1672.05 ± 20.01 c | 1030.53 ± 22.80 b | 18.49 ± 1.56 lmn | ||
| Maceration-EA (not stir) | 131.46 ± 0.40 f | 331.55 ± 0.14 l | 627.77 ± 23.13 k | 375.28 ± 10.25 j | na | ||
| Maceration-MeOH (not stir) | 660.19 ± 1.18 a | 1659.38 ± 1.31 a | 2123.67 ± 28.84 a | 1379.24 ± 35.00 a | 12.80 ± 1.14 op | ||
| Soxhlet-EA | 560.05 ± 10.56 c | 1219.18 ± 6.47 e | 180.16 ± 5.12 op | 572.17 ± 37.58 h | 9.26 ± 0.61 pqr | ||
| Soxhlet-MeOH | 656.01 ± 0.63 a | 1566.78 ± 22.64 c | 285.80 ± 4.55 n | 845.72 ± 16.88 d | 13.05 ± 1.04 op |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Picot-Allain, M.C.N.; Dall’Acqua, S.; Behl, T.; Goh, B.H.; Ying, P.T.S.; Mahomoodally, M.F. Exploring the Chemical Profiles and Biological Values of Two Spondias Species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products. Antioxidants 2021, 10, 1771. https://doi.org/10.3390/antiox10111771
Sinan KI, Zengin G, Zheleva-Dimitrova D, Gevrenova R, Picot-Allain MCN, Dall’Acqua S, Behl T, Goh BH, Ying PTS, Mahomoodally MF. Exploring the Chemical Profiles and Biological Values of Two Spondias Species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products. Antioxidants. 2021; 10(11):1771. https://doi.org/10.3390/antiox10111771
Chicago/Turabian StyleSinan, Kouadio Ibrahime, Gokhan Zengin, Dimitrina Zheleva-Dimitrova, Reneta Gevrenova, Marie Carene Nancy Picot-Allain, Stefano Dall’Acqua, Tapan Behl, Bey Hing Goh, Patrick Tang Siah Ying, and Mohamad Fawzi Mahomoodally. 2021. "Exploring the Chemical Profiles and Biological Values of Two Spondias Species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products" Antioxidants 10, no. 11: 1771. https://doi.org/10.3390/antiox10111771
APA StyleSinan, K. I., Zengin, G., Zheleva-Dimitrova, D., Gevrenova, R., Picot-Allain, M. C. N., Dall’Acqua, S., Behl, T., Goh, B. H., Ying, P. T. S., & Mahomoodally, M. F. (2021). Exploring the Chemical Profiles and Biological Values of Two Spondias Species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products. Antioxidants, 10(11), 1771. https://doi.org/10.3390/antiox10111771












