LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Extraction of Phenolic Compounds
2.3. Antioxidant Activities
2.4. LC-MS/MS Characterization of Phenolic Compounds
2.5. HPLC-MS Semi-Quantification of Phenolic Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols Estimation of Herbs
3.2. Antioxidant Activities of Herbs
3.3. LC-MS/MS Screening and Identification of Polyphenolic Compounds
3.3.1. Phenolic Acids
3.3.2. Flavonoids
3.3.3. Other polyphenols
3.3.4. Lignans and Stilbenes
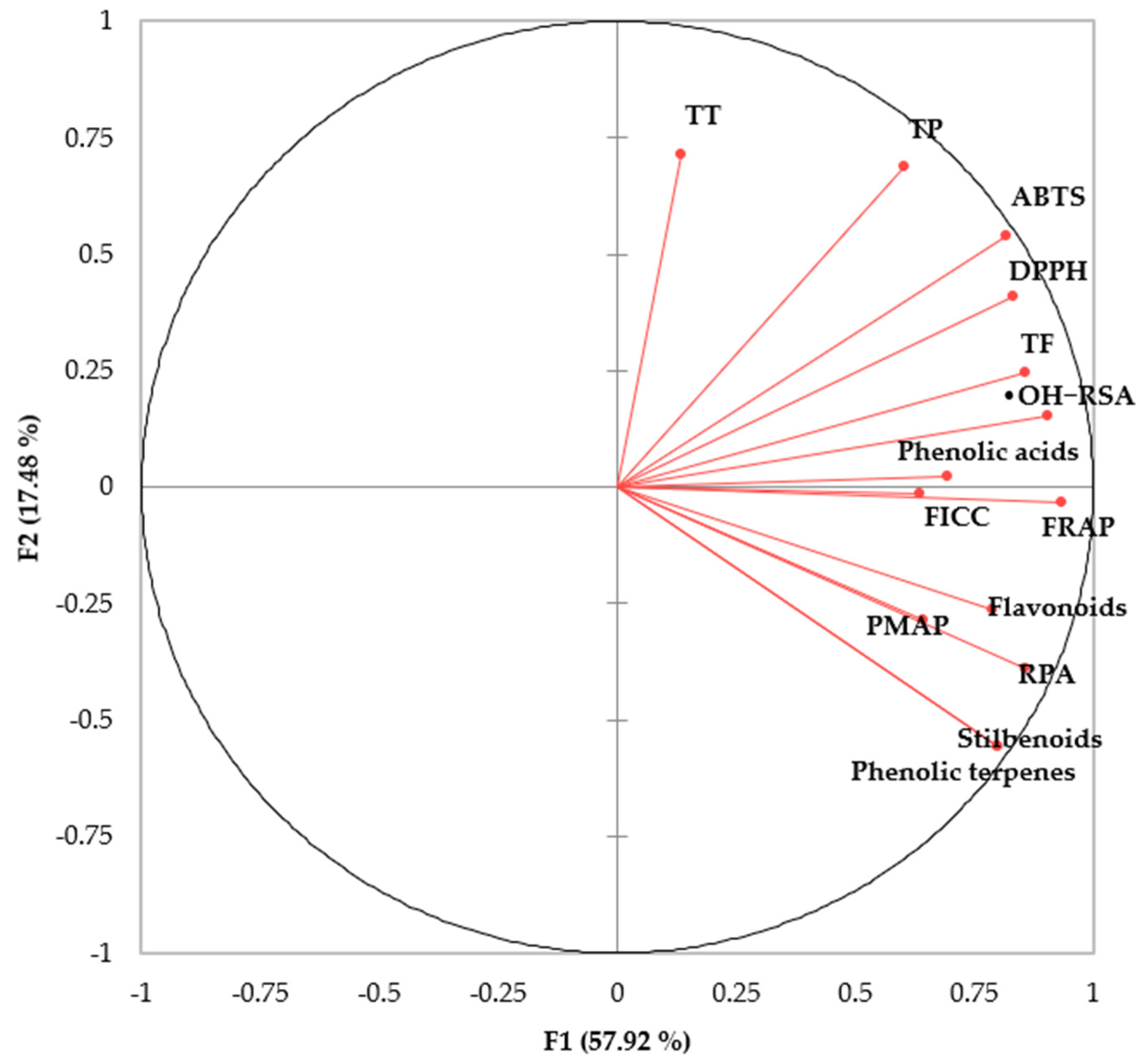
3.4. HPLC-MS Semi-Quantification of Phenolics from Herbs
3.5. Pearson’s Correlation among Polyphenolics and Their Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through lc-esi-qtof-ms(2) and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef]
- Wolf, C.P.J.G.; Rachow, T.; Ernst, T.; Hochhaus, A.; Zomorodbakhsch, B.; Foller, S.; Rengsberger, M.; Hartmann, M.; Hübner, J. Interactions in cancer treatment considering cancer therapy, concomitant medications, food, herbal medicine and other supplements. J. Cancer Res. Clin. Oncol. 2021, 1–13. [Google Scholar]
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices? A mini review. Acta. Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M. Pharmacological properties and traditional therapeutic uses of important Indian spices: A review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Razzaq, P.A.; Iftikhar, M.; Faiz, A.; Aman, F.; Ijaz, A.; Iqbal, S.; Khalid, A.; Sarwar, S. A comprehensive review on antidiabetic properties of turmeric. Life Sci. J. 2020, 17, 26–39. [Google Scholar]
- Hussain, S.A.; Panjagari, N.R.; Singh, R.R.B.; Patil, G.R. Potential herbs and herbal nutraceuticals: Food applications and their interactions with food components. Crit. Rev. Food Sci. Nutr. 2015, 55, 94–122. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants–a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A Natural Feed Additive for Poultry Health and Production-A Review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R.J.F. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Ponnampalam, E.N.; Suleria, H.A.R.; Cottrell, J.J.; Dunshea, F.R. Lc-esi/qtof-ms Profiling of Chicory and Lucerne Polyphenols and Their Antioxidant Activities. Antioxidants 2021, 10, 932. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Słowianek, M.; Leszczyńska, J. Antioxidant properties of selected culinary spices. Herba Pol. 2016, 62, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-Y.; Lin, Y.-C.; Hsieh, C.-L. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007, 104, 1418–1424. [Google Scholar] [CrossRef]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020, 44, e14497. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chin. J. Nat. Med. 2013, 11, 149–157. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and Characterization of Phenolic Compounds from Australian Grown Bananas and Their Antioxidant Capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of phenolic compounds from Pyracantha coccinea M. Roem. and their antioxidant capacity. Cellu. Molec. Biol. 2021, 67, 201–211. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumara, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by hplc-esi-qtof-ms/ms. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Lescano, C.H.; Freitas de Lima, F.; Caires, A.R.L.; de Oliveira, I.P. Chapter 25–Polyphenols Present in Campomanesia Genus: Pharmacological and Nutraceutical Approach. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 407–420. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by lc-esi-ms/ms. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, Y.; Gao, X.; Chang, Y.; Wang, M.; Zhang, P. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Dan-Lou tablet by ultra high performance liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013, 80, 50–62. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep. 2002, 7, 41–46. [Google Scholar]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by hplc-esi-qtof-ms/ms. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.-C.; Wang, H.; Fu, Z.-F.; Wen, Q.-H.; Chang, H.-X.; Huang, X.-Q. Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by hplc-qtof-ms2. Food Res. Int. 2015, 70, 101–109. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Ammar, S.; del Mar Contreras, M.; Belguith-Hadrich, O.; Bouaziz, M.; Segura-Carretero, A. New insights into the qualitative phenolic profile of Ficus carica L. fruits and leaves from Tunisia using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry and their antioxidant activity. RSC Adv. 2015, 5, 20035–20050. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Geng, C.-A.; Chen, H.; Chen, X.-L.; Zhang, X.-M.; Lei, L.-G.; Chen, J.-J. Rapid characterization of chemical constituents in Saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Boue, S.M.; Shih, B.Y.; Burow, M.E.; Eggleston, G.; Lingle, S.; Pan, Y.-B.; Daigle, K.; Bhatnagar, D. Postharvest accumulation of resveratrol and piceatannol in sugarcane with enhanced antioxidant activity. J. Agric. Food Chem. 2013, 61, 8412–8419. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res./Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef]
- Achour, M.; Bravo, L.; Sarriá, B.; Fredj, M.B.; Nouira, M.; Mtiraoui, A.; Saguem, S.; Mateos, R. Bioavailability and nutrikinetics of rosemary tea phenolic compounds in humans. Food Res. Int. 2021, 139, 109815. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of uhplc-esi-qtof-ms in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Planta Med. Int. Open 2020, 7, 133–144. [Google Scholar]
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Li, G.Q. Variability of the polyphenolic content and antioxidant capacity of methanolic extracts of pomegranate peel. Nat. Prod. Commu. 2013, 8, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef]
| Herbs | TP (mg GAE/g) | TF (mg QE/g) | TT (mg CE/g) |
|---|---|---|---|
| Oregano | 140.59 ± 9.52 a | 5.15 ± 0.12 bc | 5.23 ± 0.50 c |
| Mint | 103.28 ± 8.08 b | 7.05 ± 0.43 ab | 8.31 ± 1.58 a |
| Thyme | 43.16 ± 1.54 d | 4.30 ± 0.26 c | 4.93 ± 0.26 d |
| Basil | 39.91 ± 1.39 de | 3.62 ± 0.16 d | 6.16 ± 0.31 b |
| Rosemary | 58.66 ± 1.68 c | 8.19 ± 0.74 a | 4.69 ± 0.23 d |
| Bay | 33.93 ± 2.07 e | 1.84 ± 0.38 e | 6.09 ± 0.14 b |
| Sage | 47.62 ± 2.03 d | 6.05 ± 0.76 b | 4.41 ± 0.43 d |
| Dill | 16.41 ± 0.61 f | 3.70 ± 0.23 d | 5.28 ± 0.65 c |
| Parsley | 12.43 ± 3.20 g | 1.51 ± 0.13 e | 5.14 ± 0.68 c |
| Fenugreek | 7.58 ± 0.35 h | 1.57 ± 0.16 e | 3.46 ± 0.20 e |
| Herbs | DPPH (mg AAE/g) | FRAP (mg AAE/g) | ABTS (mg AAE/g) | RPA (mg AAE/g) | FICC (mg EDTA/g) | •OH−RSA (mg AAE/g) | PMAP (mg AAE/g) |
|---|---|---|---|---|---|---|---|
| Oregano | 23.24 ± 1.23 ab | 10.72 ± 1.44 b | 111.12 ± 2.81 a | 16.98 ± 1.34 b | 0.73 ± 0.08 c | 17.72 ± 0.35 b | 10.06 ± 0.21 c |
| Mint | 21.65 ± 0.36 ab | 6.91 ± 0.77 c | 106.99 ± 2.90 ab | 8.61 ± 3.16 d | 1.22 ± 0.04 b | 16.22 ± 0.16 b | 12.82 ± 0.28 c |
| Thyme | 18.71 ± 0.52 b | 3.45 ± 1.67 d | 69.27 ± 0.56 d | 10.05 ± 0.88 cd | 1.35 ± 0.08 b | 14.74 ± 0.23 b | 7.93 ± 0.14 d |
| Basil | 18.64 ± 0.38 b | 7.56 ± 5.15 c | 65.73 ± 2.38 d | 8.00 ± 1.25 d | 1.13 ± 0.03 b | 17.49 ± 0.41 b | 10.51 ± 0.36 c |
| Rosemary | 25.09 ± 0.67 a | 17.21 ± 0.54 a | 98.91 ± 3.40 b | 37.20 ± 3.85 a | 1.68 ± 0.04 a | 26.09 ± 1.73 a | 21.93 ± 2.82 a |
| Bay | 18.56 ± 0.65 b | 1.74 ± 1.01 e | 58.85 ± 1.19 d | 18.78 ± 4.73 b | 0.16 ±0.04 d | 9.67 ± 0.69 c | 18.53 ± 0.16 ab |
| Sage | 21.43 ± 0.51 ab | 3.98 ± 0.56 d | 73.78 ± 1.49 cd | 9.52 ± 3.42 cd | 1.08 ± 0.04 b | 17.69 ± 0.13 b | 17.64 ± 0.08 ab |
| Dill | 7.26 ± 0.31 c | 3.19 ± 1.85 d | 13.02 ± 1.15 e | 7.35 ± 2.67 d | 0.56 ± 0.05 c | 13.09 ± 0.50 bc | 10.35 ± 0.33 c |
| Parsley | 4.02 ± 0.14 d | 1.85 ± 1.18 e | 7.16 ± 0.34 f | 9.48 ± 1.19 cd | 0.93 ± 0.06 bc | 7.22 ± 0.35 cd | 10.98 ± 0.33 c |
| Fenugreek | 4.34 ± 1.99 d | 1.48 ± 1.21 e | 3.31 ± 0.14 g | 4.22 ± 0.13 e | 0.58 ± 0.09 c | 3.19 ± 0.29 e | 7.61 ± 0.21 d |
| No | Proposed Compounds | Molecular Formula | RT (min) | Ionization ESI (+/−) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS Product Ions | Herbs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||||
| Hydroxybenzoic acids | |||||||||||
| 1 | Gallic acid | C7H6O5 | 10.544 | [M − H]− | 170.0215 | 169.0142 | 169.0144 | 0.3 | 125 | * Bl, M | |
| 2 | 3-O-Methylgallic acid | C8H8O5 | 10.632 | [M − H]− | 184.0372 | 183.0299 | 183.0294 | −2.7 | 168, 140, 124 | T | |
| 3 | Gallic acid 4-O-glucoside | C13H16O10 | 10.713 | [M − H]− | 332.0743 | 331.0670 | 331.0662 | −2.4 | 169, 125 | * T, By | |
| 4 | 3,4-O-Dimethylgallic acid | C9H10O5 | 12.632 | ** [M + H]+ | 198.0528 | 199.0601 | 199.0595 | 3.0 | 153, 139, 125, 111 | * Bl, M, R, S, T | |
| 5 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 12.620 | [M − H]− | 316.0794 | 315.0721 | 315.0716 | −1.6 | 153 | * R, T, M, Bl | |
| 6 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 14.097 | [M − H]− | 154.0266 | 153.0193 | 153.0186 | −4.6 | 109 | * R, M, T, Bl | |
| 7 | 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 16.006 | [M − H]− | 300.0845 | 299.0772 | 299.0756 | −0.7 | 255, 137 | * Bl, T, S | |
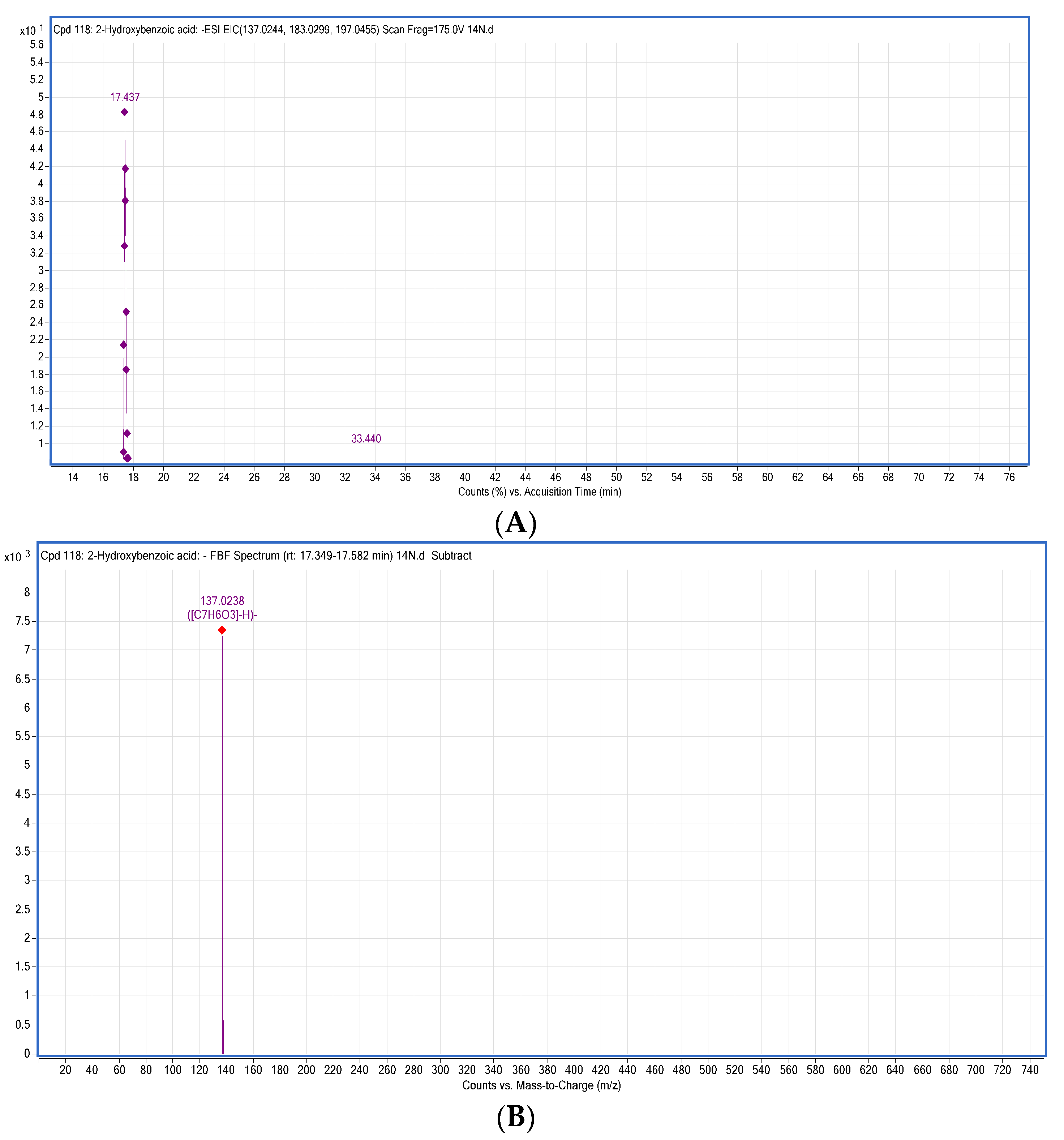
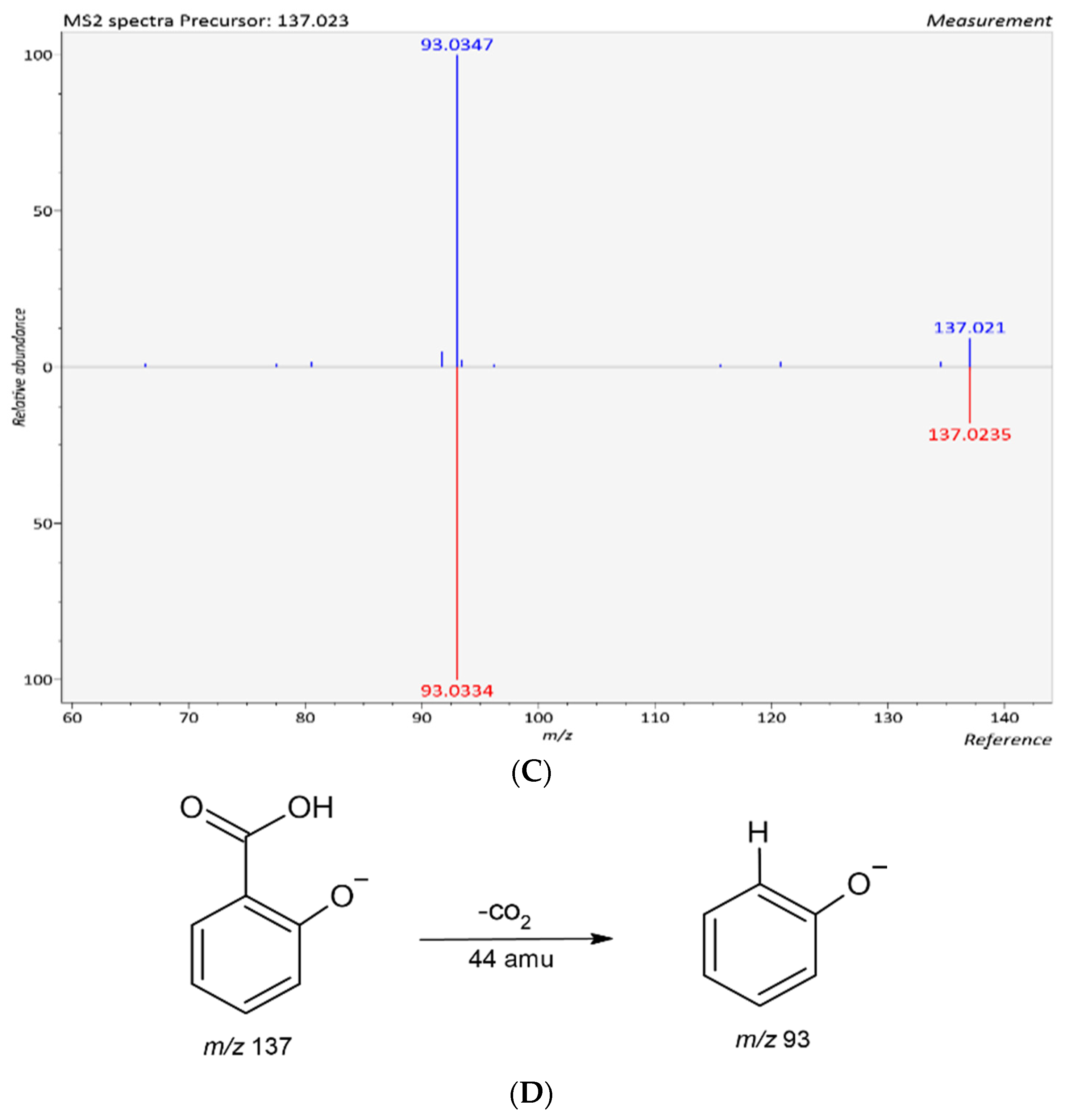
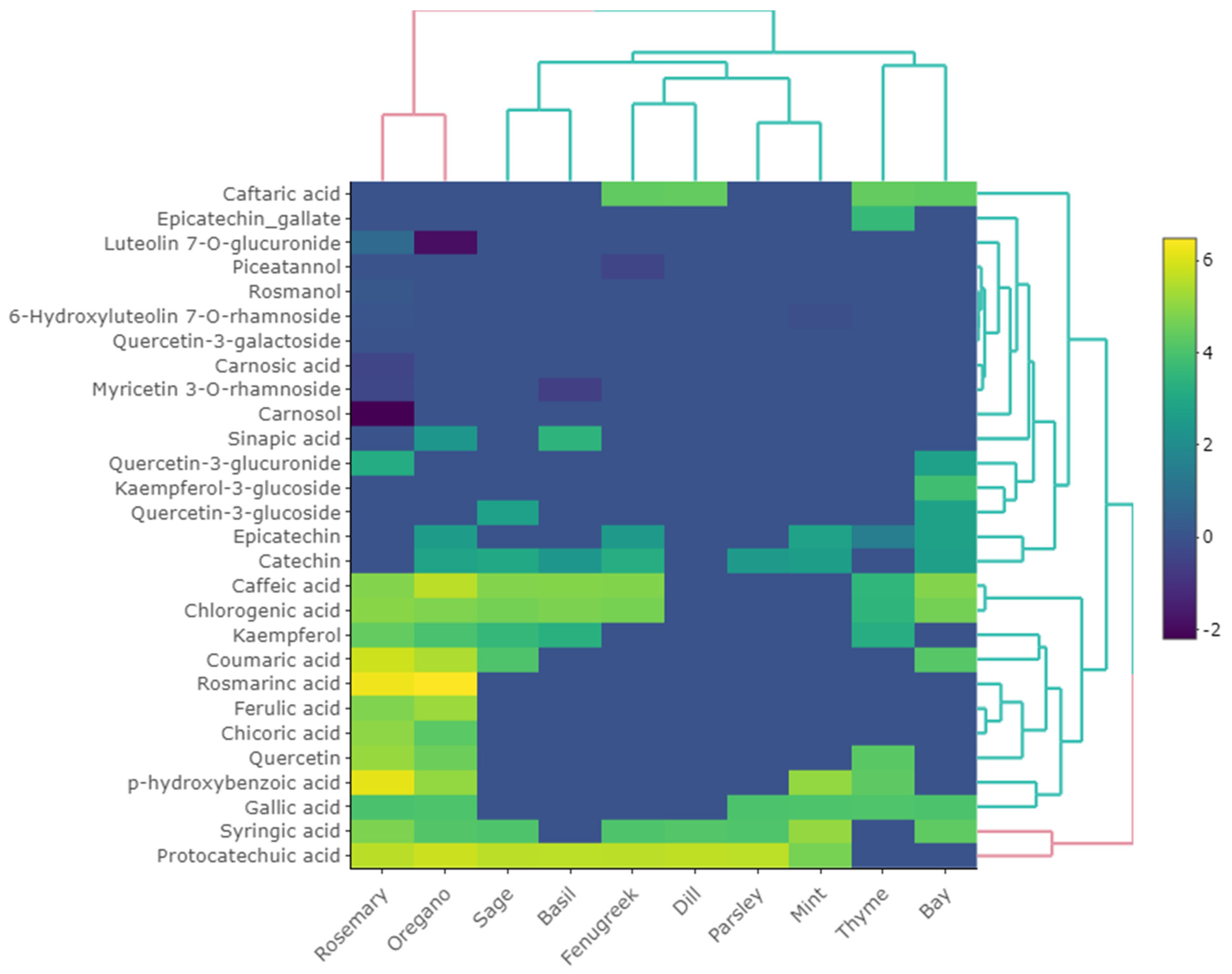
| 8 | 2-Hydroxybenzoic acid | C7H6O3 | 17.155 | [M − H]− | 138.0317 | 137.0244 | 137.0238 | −0.6 | 93 | * T, R, M, S, By, Bl, O | |
| Hydroxycinnamic acids | |||||||||||
| 9 | Caffeoyl glucose | C15H18O9 | 14.513 | [M − H]− | 342.0951 | 341.0878 | 341.0877 | −0.3 | 179, 161 | * T, R, Bl | |
| 10 | Caffeoyl tartaric acid | C13H12O9 | 15.864 | [M − H]− | 312.0481 | 311.0408 | 311.0401 | −2.3 | 161 | * M, Bl | |
| 11 | 3-p-Coumaroylquinic acid | C16H18O8 | 16.456 | [M − H]− | 338.1002 | 337.0929 | 337.0929 | 0.0 | 265, 173, 162 | * M, R, S | |
| 12 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 16.984 | [M − H]− | 326.1002 | 325.0929 | 325.0917 | −3.7 | 163 | * T, Bl | |
| 13 | 3-Caffeoylquinic acid | C16H18O9 | 17.680 | [M − H]− | 354.0951 | 353.0878 | 353.0870 | −2.3 | 253, 190, 144 | * T, M, R, S, Bl | |
| 14 | 3-Feruloylquinic acid | C17H20O9 | 17.858 | [M − H]− | 368.1107 | 367.1034 | 367.1032 | −0.5 | 298, 288, 192, 191 | M | |
| 15 | Sinapic acid | C11H12O5 | 18.318 | [M + H]+ | 224.0685 | 225.0758 | 225.0760 | 0.9 | 193, 179, 149 134, | Bl, O | |
| 16 | m-Coumaric acid | C9H8O3 | 20.039 | ** [M − H]− | 164.0473 | 163.0400 | 163.0405 | 3.1 | 119 | * Bl, R, O, S, By | |
| 17 | Ferulic acid 4-O-glucoside | C16H20O9 | 20.816 | [M − H]− | 356.1107 | 355.1034 | 355.1028 | −1.7 | 193, 178, 149, 134 | * R, S | |
| 18 | Feruloyl tartaric acid | C14H14O9 | 21.620 | [M − H]− | 326.0638 | 325.0565 | 325.0542 | −4.6 | 193, 149 | * S, M, Bl | |
| 19 | Caffeic acid | C9H8O4 | 21.084 | [M − H]− | 180.0423 | 179.0350 | 179.0345 | −1.7 | 161, 135 | * Bl, R, M, O, S, T | |
| 20 | Ferulic acid | C10H10O4 | 21.595 | [M − H]− | 194.0579 | 193.0506 | 193.0501 | −2.6 | 178, 149, 134 | * S, Bl | |
| 21 | p-Coumaroyl tartaric acid | C13H12O8 | 20.039 | ** [M − H]− | 296.0532 | 295.0459 | 295.0446 | −1.7 | 115 | * Bl, S | |
| 22 | Chicoric acid | C22H18O12 | 30.115 | ** [M − H]− | 474.0798 | 473.0725 | 473.0736 | 2.3 | 293, 311 | Bl | |
| 23 | Rosmarinic acid | C18H16O8 | 33.487 | [M − H]− | 360.0845 | 359.0772 | 359.0754 | −3.2 | 179, 161, 135 | * R, T, M, S, O, Bl, By | |
| 24 | Cinnamoyl glucose | C15H18O7 | 42.212 | [M − H]− | 310.1053 | 309.0980 | 309.0995 | 4.9 | 147, 131, 103 | * T, M, O, R, S | |
| Hydroxyphenyl acetic acids | |||||||||||
| 25 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 12.532 | [M − H]− | 168.0423 | 167.0350 | 167.0349 | −0.6 | 149, 123 | * R, M, T, Bl | |
| Hydroxyphenylpentanoic acids | |||||||||||
| 26 | 5-(3′,4′,-dihydroxyphenyl)-γ-valerolactone | C11H12O4 | 41.689 | [M − H]− | 208.0736 | 207.0663 | 207.0653 | −4.8 | 163, 119 | S | |
| Hydroxyphenylpropanoic acids | |||||||||||
| 27 | 3-Hydroxy-3-(3-hydroxyphenyl)propionic acid | C9H10O4 | 14.984 | [M − H]− | 182.0579 | 181.0506 | 181.0512 | 3.3 | 163, 135, 119 | * T, S | |
| 28 | Dihydroferulic acid 4-O-glucuronide | C16H20O10 | 22.759 | [M − H]− | 372.1056 | 371.0983 | 371.1014 | 3.4 | 195 | * T, R, S | |
| Flavonoids | |||||||||||
| Flavanols | |||||||||||
| 29 | (+)-Gallocatechin | C15H14O7 | 16.244 | [M − H]− | 306.0740 | 305.0667 | 305.0639 | −4.3 | 261, 219 | * By, S | |
| 30 | (+)-Catechin | C15H14O6 | 21.158 | [M − H]− | 290.0790 | 289.0710 | 289.0701 | −0.3 | 245, 205, 179 | By | |
| 31 | 3′-O-Methyl-(−)-epicatechin-7-O-glucuronide | C22H24O12 | 25.668 | [M − H]− | 480.1268 | 479.1195 | 479.1184 | −2.3 | 149, 121 | R | |
| 32 | 4′-O-Methyl-(−)-epigallocatechin-7-O-glucuronide | C22H24O13 | 28.364 | [M − H]− | 496.1217 | 495.1144 | 495.1155 | 2.2 | 451, 313 | T | |
| 33 | Chrysoeriol 7-O-glucoside | C22H22O11 | 40.831 | [M − H]− | 462.1162 | 461.1089 | 461.1075 | −3.0 | 299, 285 | * R, S, By | |
| Flavones | |||||||||||
| 34 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 20.343 | [M − H]− | 594.1585 | 593.1512 | 593.1515 | 0.5 | 503, 473 | * R, M, By | |
| 35 | Apigenin 6-C-glucoside | C21H20O10 | 27.103 | [M − H]− | 432.1056 | 431.0983 | 431.0979 | −0.9 | 413, 341, 311 | * T, R, S, By | |
| 36 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 28.258 | ** [M−H]− | 448.1006 | 447.0933 | 447.0926 | −1.6 | 301 | * R, M, S, T, D, By, Bl, F | |
| 37 | Luteolin 7-O-glucuronide | C21H18O12 | 28.447 | [M − H]− | 462.0798 | 461.0725 | 461.0716 | −2.4 | 285, 216 | * M, T, R, S, O | |
| 38 | Rhoifolin | C27H30O14 | 30.045 | [M − H]− | 578.1636 | 577.1563 | 577.1525 | −4.3 | 413, 269 | * R, M, S, D, F | |
| 39 | Apigenin 7-O-glucuronide | C21H18O11 | 32.538 | [M − H]− | 446.0849 | 445.0776 | 445.0765 | −3.5 | 269, 175 | * T, R, S | |
| Flavanones | |||||||||||
| 40 | Neoeriocitrin | C27H32O15 | 25.227 | [M − H]− | 596.1741 | 595.1668 | 595.1669 | 0.2 | 431, 287 | * R, M, S | |
| 41 | Narirutin | C27H32O14 | 28.232 | [M − H]− | 580.1792 | 579.1719 | 579.1708 | −1.9 | 271 | M | |
| 42 | Hesperetin 3′-O-glucuronide | C22H22O12 | 29.202 | [M−H]− | 478.1111 | 477.1038 | 477.1022 | −3.4 | 301, 175, 113, 85 | * R, S | |
| 43 | Hesperidin | C28H34O15 | 30.888 | [M − H]− | 610.1898 | 609.1825 | 609.1803 | −3.6 | 301 | R | |
| 44 | Naringenin 7-O-glucoside | C21H22O10 | 31.079 | [M − H]− | 434.1213 | 433.1140 | 433.1136 | −0.9 | 373, 343, 303 | T | |
| Flavonols | |||||||||||
| 45 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 24.228 | [M − H]− | 610.1534 | 609.1461 | 609.1464 | 0.5 | 447, 285 | * R, M, S, T, By | |
| 46 | Myricetin 3-O-rhamnoside | C21H20O12 | 28.520 | [M − H]− | 464.0955 | 463.0882 | 463.0887 | 0.7 | 317 | * Bl, R, S, T, By | |
| 47 | Quercetin 3′-O-glucuronide | C21H18O13 | 25.113 | [M − H]− | 478.0747 | 477.0674 | 477.0676 | 0.4 | 301 | * R, S | |
| 48 | Quercetin 3-O-(6″-malonyl-glucoside) | C24H22O15 | 28.196 | [M − H]− | 550.0959 | 549.0886 | 549.0886 | 0.0 | 445, 300, 160 | * T, Bl | |
| 49 | Isorhamnetin 3-O-glucuronide | C22H20O13 | 29.864 | [M − H]− | 492.0904 | 491.0831 | 491.0822 | −1.8 | 315, 300, 272, 255 | * R, S | |
| 50 | Quercetin 3-O-arabinoside | C20H18O11 | 30.940 | [M − H]− | 434.0849 | 433.0776 | 433.0756 | −4.6 | 301 | By | |
| 51 | Isorhamnetin | C16H12O7 | 36.775 | [M − H]− | 316.0583 | 315.0510 | 315.0498 | −3.8 | 300, 271 | * M, S | |
| 52 | 3,7-Dimethylquercetin | C17H14O7 | 45.315 | ** [M − H]− | 330.0740 | 329.0667 | 329.0660 | −1.1 | 314, 299, 271 | * M, O, R, S, Bl | |
| Dihydroflavonols | |||||||||||
| 53 | Dihydromyricetin 3-O-rhamnoside | C21H22O12 | 15.838 | [M − H]− | 466.1111 | 465.1038 | 465.1024 | −3.0 | 301 | T | |
| 54 | Dihydroquercetin | C15H12O7 | 28.746 | [M − H]− | 304.0583 | 303.0510 | 303.0496 | −4.6 | 285, 275, 151 | * T, O | |
| Dihydrochalcones | |||||||||||
| 55 | Phloretin 2′-O-xylosyl-glucoside | C26H32O14 | 21.931 | [M − H]− | 568.1792 | 567.1719 | 567.1696 | −4.1 | 437, 275, 169 | * R, S | |
| Anthocyanins | |||||||||||
| 56 | Cyanidin 3-O-(6″-p-coumaroyl-glucoside) | C30H27O13 | 20.896 | [M + H]+ | 595.1452 | 596.1525 | 596.1527 | 0.3 | 287 | * T, M, O | |
| 57 | Quercetin 3-O-xylosyl-glucuronide | C26H26O17 | 36.011 | [M + H]+ | 610.1170 | 611.1243 | 611.1247 | 0.7 | 679, 303, 285, 239 | * T, O | |
| Isoflavonoids | |||||||||||
| 58 | Dihydrobiochanin A | C16H14O5 | 4.146 | [M + H]+ | 286.0841 | 287.0914 | 287.0914 | 0.0 | 269, 203, 175 | O | |
| 59 | 3′,4′,5,7-Tetrahydroxyisoflavanone | C15H12O6 | 34.208 | [M − H]− | 288.0634 | 287.0561 | 287.0564 | 1.0 | 269, 259 | * T, O, Bl | |
| 60 | 5,6,7,3′,4′-Pentahydroxyisoflavone | C15H10O7 | 40.677 | [M − H]− | 302.0427 | 301.0354 | 301.0346 | −2.7 | 274, 200, 136 | * T, O, S, By | |
| 61 | 3′,4′,7-Trihydroxyisoflavanone | C15H12O5 | 45.000 | [M − H]− | 272.0685 | 271.0612 | 271.0609 | −1.1 | 177, 151, 119, 107 | * M, T, O | |
| 62 | Sativanone | C17H16O5 | 46.952 | [M − H]− | 300.0998 | 299.0925 | 299.0921 | −1.3 | 284, 269, 225 | S | |
| 63 | 3′-Hydroxydaidzein | C15H10O5 | 47.113 | [M − H]− | 270.0528 | 269.0455 | 269.0451 | −1.5 | 241, 224, 213, 181 | * R, T, M, Bl, O, S | |
| 64 | 4′-Methoxy-2′,3,7-trihydroxyisoflavanone | C16H14O6 | 47.278 | [M − H]− | 302.0790 | 301.0717 | 301.0704 | −4.3 | 283, 177 | R | |
| 65 | 3′-Hydroxymelanettin | C16H12O6 | 54.909 | [M − H]− | 300.0634 | 299.0561 | 299.0559 | −0.7 | 284 | * T, M, O, R, S | |
| Other polyphenols | |||||||||||
| Hydroxycoumarins | |||||||||||
| 66 | Esculin | C15H16O9 | 15.852 | [M − H]− | 340.0794 | 339.0721 | 339.0690 | −2.9 | 177 | * S, R | |
| 67 | Coumarin | C9H6O2 | 17.634 | [M + H]+ | 146.0368 | 147.0441 | 147.0428 | −4.8 | 103, 91 | O | |
| 68 | Esculetin | C9H6O4 | 20.473 | [M − H]− | 178.0266 | 177.0193 | 177.0196 | 0.7 | 149, 133, 105, 89 | * Bl, R, S, T | |
| 69 | Umbelliferone | C9H6O3 | 46.399 | ** [M − H]− | 162.0317 | 161.0244 | 161.0242 | −1.2 | 133, 117, 105 | * Bl, M, O, R, S | |
| Hydroxybenzoketones | |||||||||||
| 70 | 2-Hydroxy-4-methoxyacetophenone 5-sulfate | C9H10O7S | 12.281 | [M − H]− | 262.0147 | 261.0074 | 261.0076 | 0.8 | 181, 97 | * R, T | |
| Tyrosols | |||||||||||
| 71 | Oleoside 11-methylester | C17H24O11 | 14.451 | [M − H]− | 404.1319 | 403.1246 | 403.1238 | −2.0 | 223, 165 | R, S | |
| 72 | 3,4-DHPEA-AC | C10H12O4 | 85.689 | [M − H]− | 196.0736 | 195.0663 | 195.0656 | −3.6 | 135 | O | |
| Phenolic terpenes | |||||||||||
| 73 | Rosmanol | C20H26O5 | 53.370 | [M − H]− | 346.1780 | 345.1707 | 345.1693 | −4.1 | 301 | * R, S | |
| 74 | Carnosol | C20H26O4 | 79.943 | [M − H]− | 330.1831 | 329.1758 | 329.1742 | −4.9 | 285 | * R, T, S | |
| 75 | Carnosic acid | C20H28O4 | 85.366 | [M − H]− | 332.1988 | 331.1915 | 331.1907 | −2.4 | 287 | * R, S, Bl | |
| Alkylphenols | |||||||||||
| 76 | 3-Methylcatechol | C7H8O2 | 14.350 | [M − H]− | 124.0524 | 123.0455 | 123.0455 | 1.8 | 281, 187, 165 | * M, T | |
| Other Polyphenols | |||||||||||
| 77 | Salvianolic acid B | C36H30O16 | 32.130 | [M − H]− | 718.1534 | 717.1461 | 717.1442 | −2.8 | 519, 339, 321, 295 | * S, Bl | |
| 78 | Lithospermic acid | C27H22O12 | 35.122 | [M−H]− | 538.1111 | 537.1038 | 537.1013 | −4.7 | 493, 339, 295 | * T, O | |
| Lignans | |||||||||||
| 79 | Enterolactone | C18H18O4 | 4.786 | [M + H]+ | 298.1205 | 299.1278 | 299.1268 | −3.3 | 281, 187, 165 | O | |
| 80 | Sesamin | C20H18O6 | 18.227 | [M − H]− | 354.1103 | 353.1030 | 353.1021 | −2.5 | 338, 163 | P | |
| 81 | 7-Oxomatairesinol | C20H20O7 | 18.661 | [M + H]+ | 372.1209 | 373.1282 | 373.1286 | 1.1 | 358, 343, 328, 325 | D | |
| 82 | Secoisolariciresinol | C20H26O6 | 51.345 | [M − H]− | 362.1729 | 361.1656 | 361.1652 | −1.1 | 165, 121 | S | |
| 83 | Deoxyschisandrin | C24H32O6 | 84.114 | [M − H]− | 416.2199 | 415.2126 | 415.2107 | −4.6 | 402, 347, 361, 301 | S | |
| Stilbenes | |||||||||||
| 84 | Piceatannol | C14H12O4 | 8.718 | [M − H]− | 244.0736 | 243.0663 | 243.0662 | −0.4 | 225, 201, 175, 159 | * F, D | |
| Variables | TPC | TFC | TTC | DPPH | FRAP | ABTS | PMAP | RPA | FICC | •OH−RSA | Phenolic Acids | Flavonoids | Phenolic Terpenes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TFC | 0.62 | ||||||||||||
| TTC | 0.44 | 0.27 | |||||||||||
| DPPH | 0.72 | 0.77 ** | 0.35 | ||||||||||
| FRAP | 0.58 | 0.78 ** | 0.09 | 0.67 | |||||||||
| ABTS | 0.87 ** | 0.78 ** | 0.455 | 0.96 ** | 0.70 | ||||||||
| PMAP | 0.09 | 0.49 | 0.08 | 0.54 | 0.46 | 0.41 | |||||||
| RPA | 0.28 | 0.53 | −0.06 | 0.57 | 0.78 ** | 0.51 | 0.76 ** | ||||||
| FICC | 0.23 | 0.73 * | 0.07 | 0.46 | 0.64 | 0.48 | 0.20 | 0.37 | |||||
| •OH−RSA | 0.54 | 0.89 ** | 0.21 | 0.83 ** | 0.86 ** | 0.79 ** | 0.54 | 0.66 | 0.70 | ||||
| Phenolic acids | 0.45 | 0.60 | −0.19 | 0.52 | 0.90 ** | 0.53 | 0.53 | 0.88 | 0.41 | 0.66 | |||
| Flavonoids | 0.39 | 0.46 | −0.17 | 0.62 | 0.66 | 0.58 | 0.51 | 0.88 | 0.32 | 0.54 | 0.80 ** | ||
| Phenolic terpenes | 0.07 | 0.59 | −0.18 | 0.39 | 0.80 ** | 0.33 | 0.66 | 0.89 | 0.59 | 0.64 | 0.85 ** | 0.71 | |
| Stilbenoids | 0.07 | 0.59 | −0.18 | 0.39 | 0.80 ** | 0.33 | 0.66 | 0.89 | 0.59 | 0.64 | 0.85 ** | 0.71 | 1.00 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. https://doi.org/10.3390/antiox10111770
Ali A, Bashmil YM, Cottrell JJ, Suleria HAR, Dunshea FR. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants. 2021; 10(11):1770. https://doi.org/10.3390/antiox10111770
Chicago/Turabian StyleAli, Akhtar, Yasmeen M. Bashmil, Jeremy J. Cottrell, Hafiz A. R. Suleria, and Frank R. Dunshea. 2021. "LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential" Antioxidants 10, no. 11: 1770. https://doi.org/10.3390/antiox10111770
APA StyleAli, A., Bashmil, Y. M., Cottrell, J. J., Suleria, H. A. R., & Dunshea, F. R. (2021). LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants, 10(11), 1770. https://doi.org/10.3390/antiox10111770











