Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Animals
2.2. Growth Performance Analysis
2.3. Blood Pressure Measurement
2.4. Blood Biochemistry Assay
2.5. Histological Analysis of the Aorta
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of FE Supplementation on the Growth Performance
3.2. Effect of FE Supplementation on the Blood Biochemical Parameters
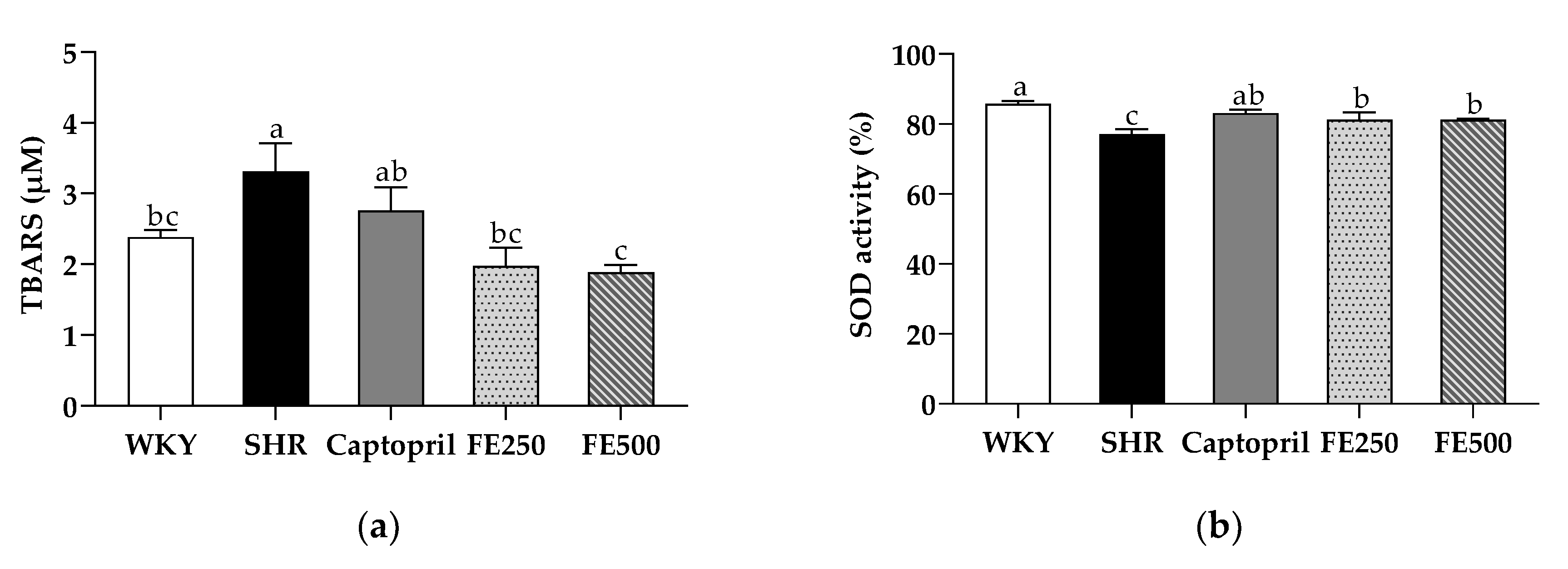
3.3. Effects of FE Supplementation on the Antioxidant Capacity, including the TBARS Concentration and SOD Activity
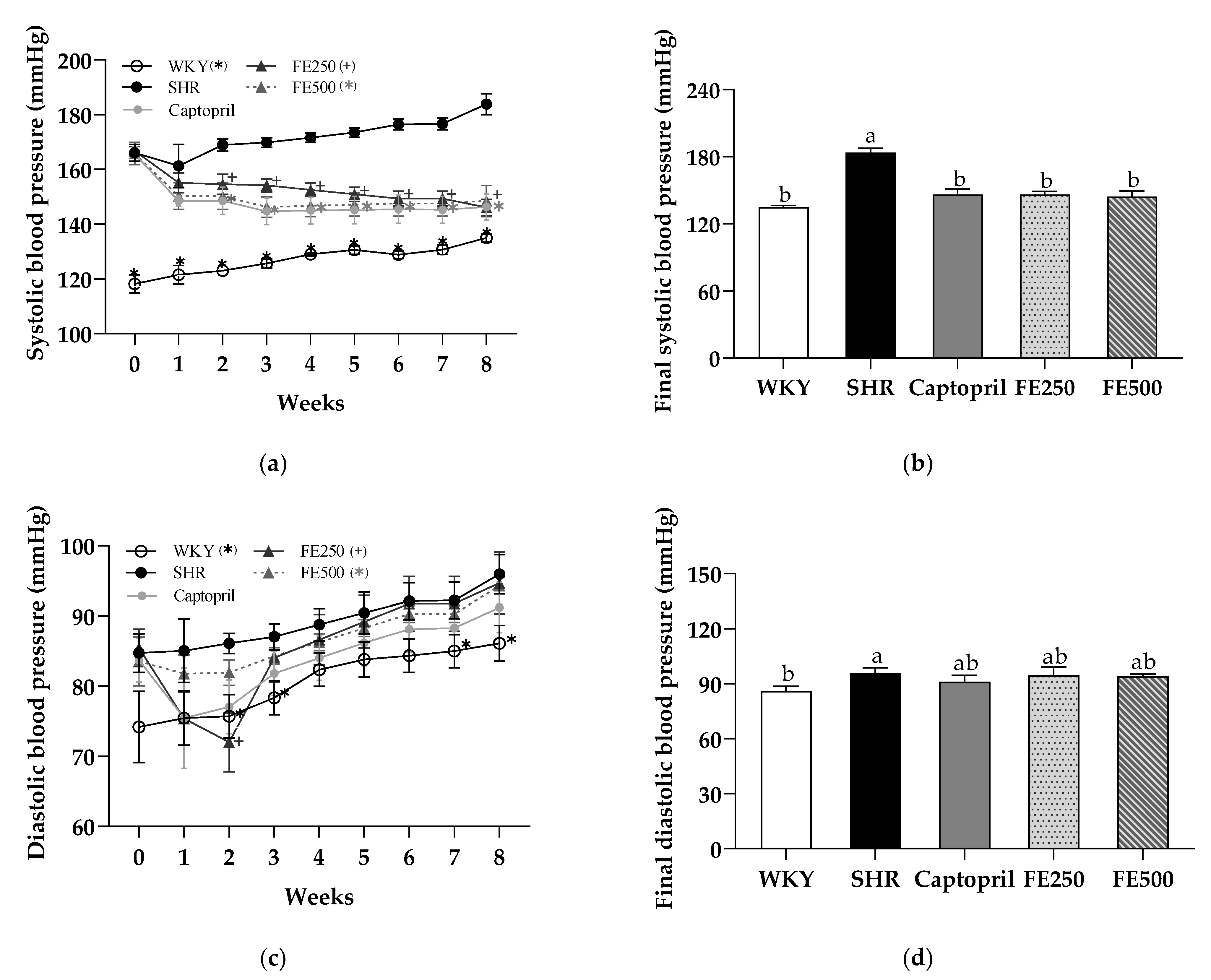
3.4. Effects of FE Supplementation on the Blood Pressure
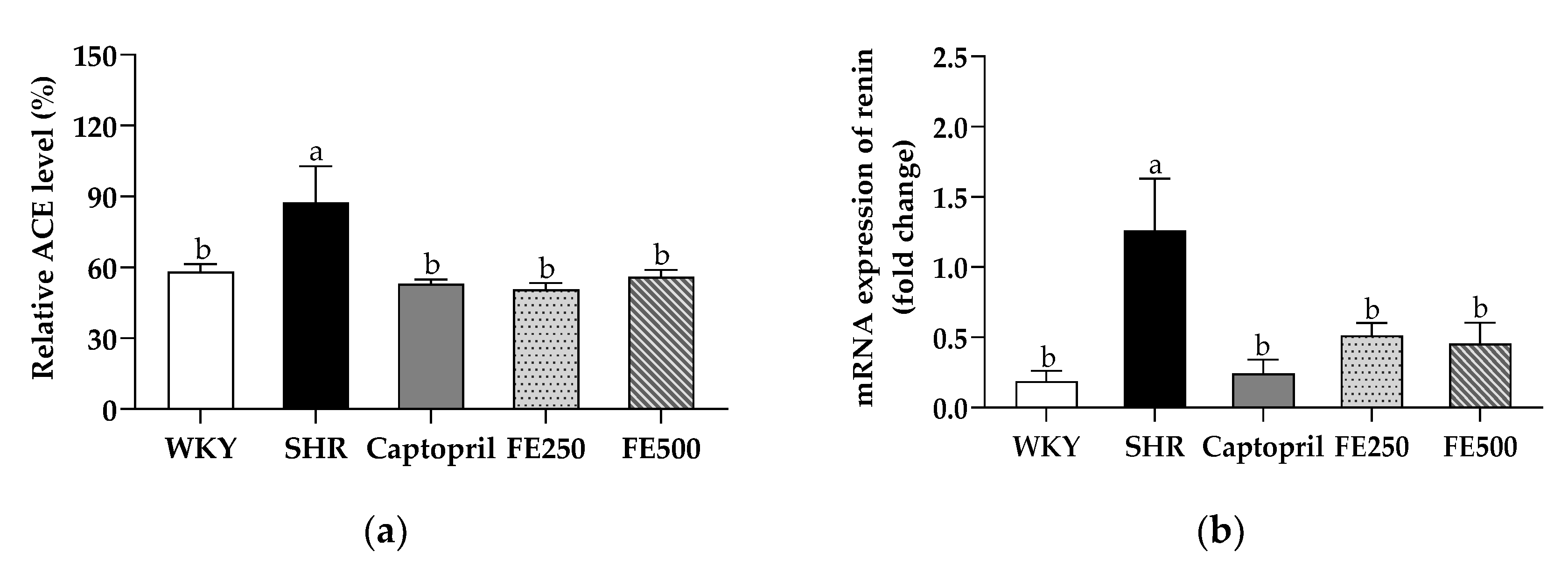
3.5. Effects of FE Supplementation on the ACE Level and Renin Activity
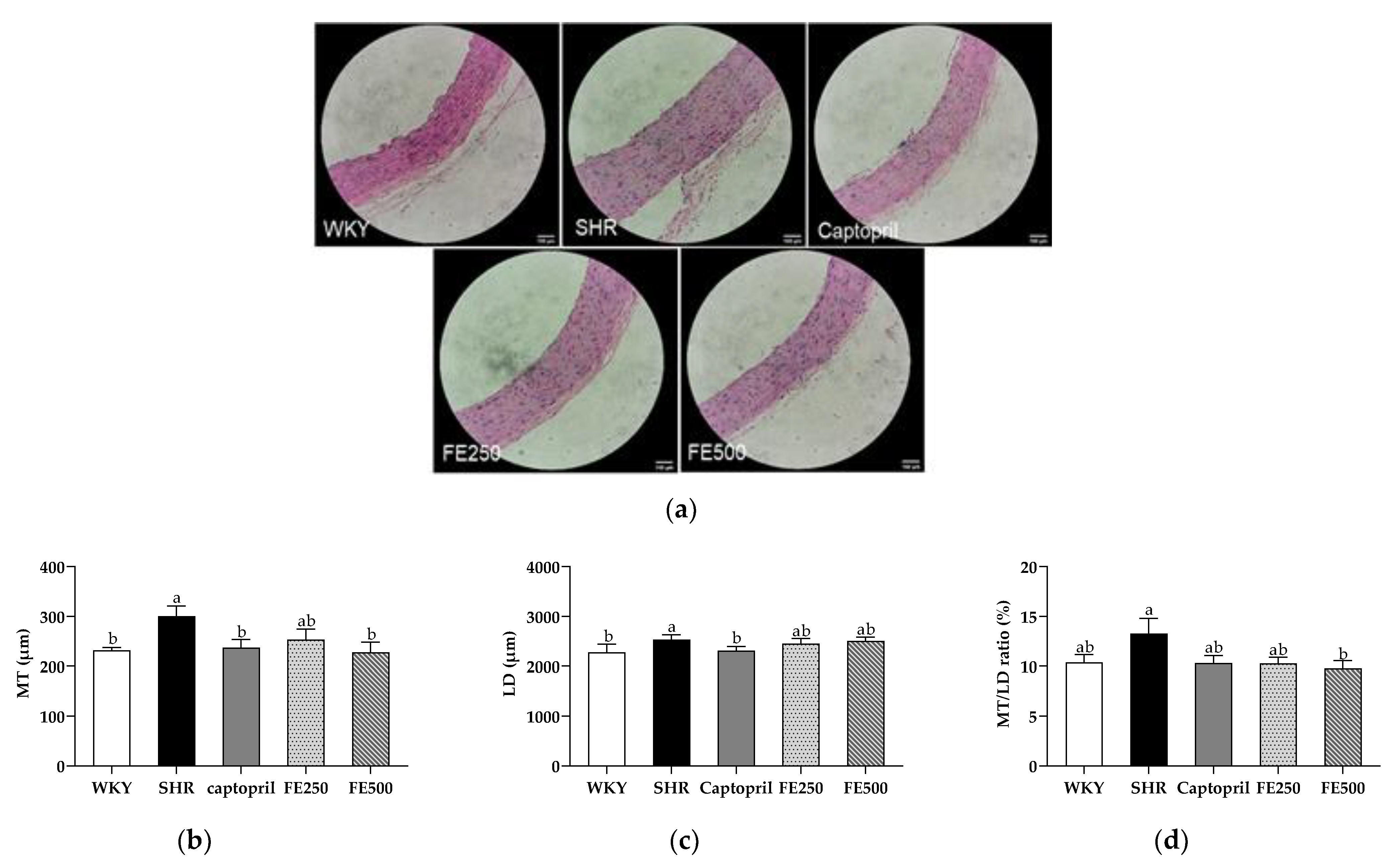
3.6. Effect of FE Supplementation on Vascular Remodeling in the SHRs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Liang, Y.; Gao, H.; Wang, J.; Wang, Q.; Zhao, S.; Zhang, J.; Qiu, J. Alleviative effect of grape seed proanthocyanidin extract on small artery vascular remodeling in spontaneous hypertensive rats via inhibition of collagen hyperplasia. Mol. Med. Rep. 2017, 15, 2643–2652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vriz, O.; Magne, J.; Jarosh, J.; Bossone, E.; Aboyans, V.; Palatini, P. Local carotid arterial stiffness is an independent determinant of left ventricular remodeling in never-treated hypertensive patients. Blood Press 2019, 28, 23–33. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; Sorlie, P.; Wolf, P.A. Components of blood pressure and risk of atherothrombotic brain infarction: The framingham study. Stroke 1976, 7, 327–331. [Google Scholar] [CrossRef]
- Manning, R.D., Jr.; Tian, N.; Meng, S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Akpaffiong, M.J.; Taylor, A.A. Antihypertensive and vasodilator actions of antioxidants in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 1450–1460. [Google Scholar] [CrossRef]
- Bader, M.; Ganten, D. Update on tissue renin-angiotensin systems. J. Mol. Med. 2008, 86, 615–621. [Google Scholar] [CrossRef]
- Parish, R.C.; Miller, L.J. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. Drug Saf. 1992, 7, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Bwai, M.; Afolayan, M.; Odukomaiya, D.; Orishadipe, A. Proximate composition, mineral and phytochemical constituents of Eleusine coracana (finger millet). Int. J. Adv. Chem. 2014, 2, 171–174. [Google Scholar] [CrossRef]
- J Jayawardana, S.A.S.; Samarasekera, J.K.R.R.; Hettiarachchi, G.H.C.M.; Gooneratne, J.; Choudhary, M.I.; Jabeen, A. Anti-inflammatory and antioxidant properties of finger Millet (Eleusine coracana (L.) Gaertn.) varieties cultivated in Sri Lanka. BioMed Res. Int. 2021, 2021, 7744961. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Chandra, S.; Pallavi; Sharma, A.K. Review of finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Kumari, D.; Madhujith, T.; Chandrasekara, A. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Sci. Nutr. 2017, 5, 474–485. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Nithya, M.; Rose, C.; Chandra, T.S. The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim. Biophys. Acta 2004, 1689, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Rajasekaran, N.S.; Chandra, T.S. Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats. Nutr. Res. 2005, 25, 1109–1120. [Google Scholar] [CrossRef]
- Mushtaq, M.N.; Akhtar, M.S. Alamgeer Blood pressure lowering effect of Pennisetum glaucum in rats. Bangladesh J. Pharmacol. 2015, 10, 494–499. [Google Scholar] [CrossRef]
- Okamoto, K.; Tabei, R.; Fukushima, M.; Nosaka, S.; Yamori, Y.; Ichijima, K.; Haebara, H.; Matsumoto, M.; Maruyama, T.; Suzuki, Y.; et al. Further observations of the development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1966, 30, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yang, S.C.; Chen, J.R.; Tzeng, Y.H.; Han, B.C. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br. J. Nutr. 2004, 92, 507–512. [Google Scholar] [CrossRef]
- Gattone, V.H., 2nd. Body weight of the spontaneously hypertensive rat during the suckling and weanling periods. Jpn. Heart J. 1986, 27, 881–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shido, O.; Nagasaka, T. Metabolic and blood pressure response to feeding activity is enhanced in freely moving spontaneously hypertensive rats. Jpn. J. Physiol. 1989, 39, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2017, 56, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A. Understanding and application of liver function tests. Korean J. Med. 2009, 76, 163–168. [Google Scholar]
- Gole, M.K.; Dasgupta, S. Role of plant metabolites in toxic liver injury. Asia Pac. J. Clin. Nutr. 2002, 11, 48–50. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, S.; Haque, T.; Kathak, R.R.; Ali, N. Association between serum liver enzymes and hypertension: A cross-sectional study in Bangladeshi adults. BMC Cardiovasc. Disord. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Tsuchikura, S.; Iida, H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp. Anim. 2004, 53, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.; Meng, X.; Yao, S.; Jin, L.; Yang, J.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016, 6, 20848–20860. [Google Scholar] [CrossRef]
- Su, M.L.; He, Y.; Li, Q.S.; Zhu, B.H. Efficacy of acetylshikonin in preventing obesity and hepatic steatosis in db/db mice. Molecules 2016, 21, 976. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Pan, T.M. Red mold dioscorea decreases blood pressure when administered alone or with amlodipine and is a potentially safe functional food in SHR and WKY rats. J. Funct. Foods 2013, 5, 1456–1465. [Google Scholar] [CrossRef]
- Huang, X.; Yang, J.; Song, B.; Wang, N.; Ma, M.; Wang, H.; Wang, S.; Hao, S.; Cheng, G. Caduet enhances connexin 43 phosphorylation in left ventricular and thoracic aorta of SH model rats. Exp. Ther. Med. 2020, 20, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.G.; Zahradka, P.; Taylor, C.G. Lentil-based diets attenuate hypertension and large-artery remodelling in spontaneously hypertensive rats. Br. J. Nutr. 2014, 111, 690–698. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Hirooka, Y.; Araki, S.; Nozoe, M.; Kishi, T.; Sunagawa, K. High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens. Res. 2008, 31, 2075–2083. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liang, K.; Ding, Y. Increased nitric oxide inactivation by reactive oxygen species in lead-induced hypertension. Kidney Int. 1999, 56, 1492–1498. [Google Scholar] [CrossRef]
- Kumar, K.V.; Das, U.N. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic. Res. Commun. 1993, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef]
- Sripriya, G.; Chandrasekharan, K.; Murty, V.S.; Chandra, T.S. ESR spectroscopic studies on free radical quenching action of finger millet (Eleusine coracana). Food Chem. 1996, 57, 537–540. [Google Scholar] [CrossRef]
- Viswanath, V.; Urooj, A.; Malleshi, N.G. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana). Food Chem. 2009, 114, 340–346. [Google Scholar] [CrossRef]
- Wei, S.; Cheng, D.; Yu, H.; Wang, X.; Song, S.; Wang, C. Millet-enriched diets attenuate high salt-induced hypertension and myocardial damage in male rats. J. Funct. Foods 2018, 44, 304–312. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Signal. 2014, 20, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Ookawara, T.; Imazeki, N.; Matsubara, O.; Kizaki, T.; Oh-Ishi, S.; Nakao, C.; Sato, Y.; Ohno, H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am. J. Physiol. 1998, 275, C840–C847. [Google Scholar] [CrossRef]
- Ikarashi, N.; Toda, T.; Hatakeyama, Y.; Kusunoki, Y.; Kon, R.; Mizukami, N.; Kaneko, M.; Ogawa, S.; Sugiyama, K. Anti-hypertensive effects of acacia polyphenol in spontaneously hypertensive rats. Int. J. Mol. Sci. 2018, 19, 700. [Google Scholar] [CrossRef]
- Dickhout, J.G.; Lee, R.M.K.W. Blood pressure and heart rate development in young spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H794–H800. [Google Scholar] [CrossRef]
- Kim, K.J.; Hwang, E.S.; Kim, M.J.; Park, J.H.; Kim, D.O. Antihypertensive effects of polyphenolic extract from korean red pine (Pinus densiflora sieb. et zucc.) bark in spontaneously hypertensive rats. Antioxidants 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Massy, Z.A.; Azizi, M.; Bakris, G.; Ritz, E.; Covic, A.; Goldsmith, D.; Heine, G.H.; Jager, K.J.; Kanbay, M.; et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015, 386, 1588–1598. [Google Scholar] [CrossRef]
- Gradman, A.H.; Vivas, Y. New drugs for hypertension: What do they offer? Curr. Hypertens. Rep. 2006, 8, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kitami, Y.; Hiwada, K.; Kokubu, T. Kidney renin gene expression in spontaneously hypertensive rats. J. Hypertens. 1989, 7, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Chamiot-Clerc, P.; Renaud, J.F. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension 2001, 37, 313–321. [Google Scholar]
- Man, S.; Yang, L.; Xiang, H.; Lu, G.; Wang, Y.; Liu, C.; Gao, W. Antihypertensive and renal protective effect of Shunaoxin pill combined with captopril on spontaneous hypertension rats. Biomed. Pharmacother. 2020, 125, 109977–109984. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wang, Y.; Li, Y.; Zhao, L.; Gong, Y.; Wang, M.; Wang, M.; Fu, G.; Zhang, W. Rosuvastatin reverses hypertension-induced changes in the aorta structure and endothelium-dependent relaxation in rats through suppression of apoptosis and inflammation. J. Cardiovasc. Pharmacol. 2020, 75, 584–595. [Google Scholar] [CrossRef]
- Liu, F.; Li, H.; Li, Z.; Shan, S. Treatment of peroxidase derived from foxtail millet bran attenuates atherosclerosis by inhibition of cd36 and stat3 in vitro and in vivo. J. Agric. Food Chem. 2020, 68, 1276–1285. [Google Scholar] [CrossRef]
| WKY | SHR | Captopril | FE250 | FE500 | |
|---|---|---|---|---|---|
| Final body weight (g) | 377.1 ± 2.73 a | 344.7 ± 6.09 b | 350.5 ± 6.10 b | 352.9 ± 8.52 b | 346.7 ± 3.78 b |
| Weight gain (g) | 17.58 ± 0.53 a | 12.78 ± 0.18 c | 14.05 ± 0.70 bc | 15.50 ± 0.70 b | 15.48 ± 0.52 b |
| Food intake (g/week) | 74.19 ± 0.74 c | 79.21 ± 0.92 b | 79.42 ± 1.05 b | 82.91 ± 0.66 a | 81.84 ± 0.41 a |
| WKY | SHR | Captopril | FE250 | FE500 | |
|---|---|---|---|---|---|
| AST level (IU/L) | 18.57 ± 1.87 b | 24.71 ± 1.94 a | 17.97 ± 1.57 b | 15.94 ± 2.65 b | 13.85 ± 1.08 b |
| ALT level (IU/L) | 4.285 ± 0.76 | 33.27 ± 14.1 | 8.045 ± 1.16 | 19.59 ± 6.45 | 14.95 ± 2.41 |
| TG level (mg/dL) | 42.51 ± 5.91 c | 135.0 ± 7.24 a | 74.46 ± 6.85 b | 91.98 ± 18.1 b | 83.41 ± 2.54 b |
| TC level (mg/dL) | 88.36 ± 2.18 b | 95.55 ± 1.20 a | 77.74 ± 0.88 d | 80.68 ± 1.29 c,d | 83.41 ± 2.54 b,c |
| LDL-C level (mg/dL) | 8.95 ± 0.25 b | 9.84 ± 0.38 a | 7.73 ± 0.15 c | 8.35 ± 0.39 b,c | 7.54 ± 0.11 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Jeong, E.W.; Yang, Y.S.; Kim, H.-J.; Go, G.-w.; Lee, H.G. Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 1766. https://doi.org/10.3390/antiox10111766
Park SY, Jeong EW, Yang YS, Kim H-J, Go G-w, Lee HG. Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in Spontaneously Hypertensive Rats. Antioxidants. 2021; 10(11):1766. https://doi.org/10.3390/antiox10111766
Chicago/Turabian StylePark, Se Yeong, Eun Woo Jeong, Yun Sun Yang, Hyun-Joo Kim, Gwang-woong Go, and Hyeon Gyu Lee. 2021. "Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in Spontaneously Hypertensive Rats" Antioxidants 10, no. 11: 1766. https://doi.org/10.3390/antiox10111766
APA StylePark, S. Y., Jeong, E. W., Yang, Y. S., Kim, H.-J., Go, G.-w., & Lee, H. G. (2021). Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in Spontaneously Hypertensive Rats. Antioxidants, 10(11), 1766. https://doi.org/10.3390/antiox10111766







