Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
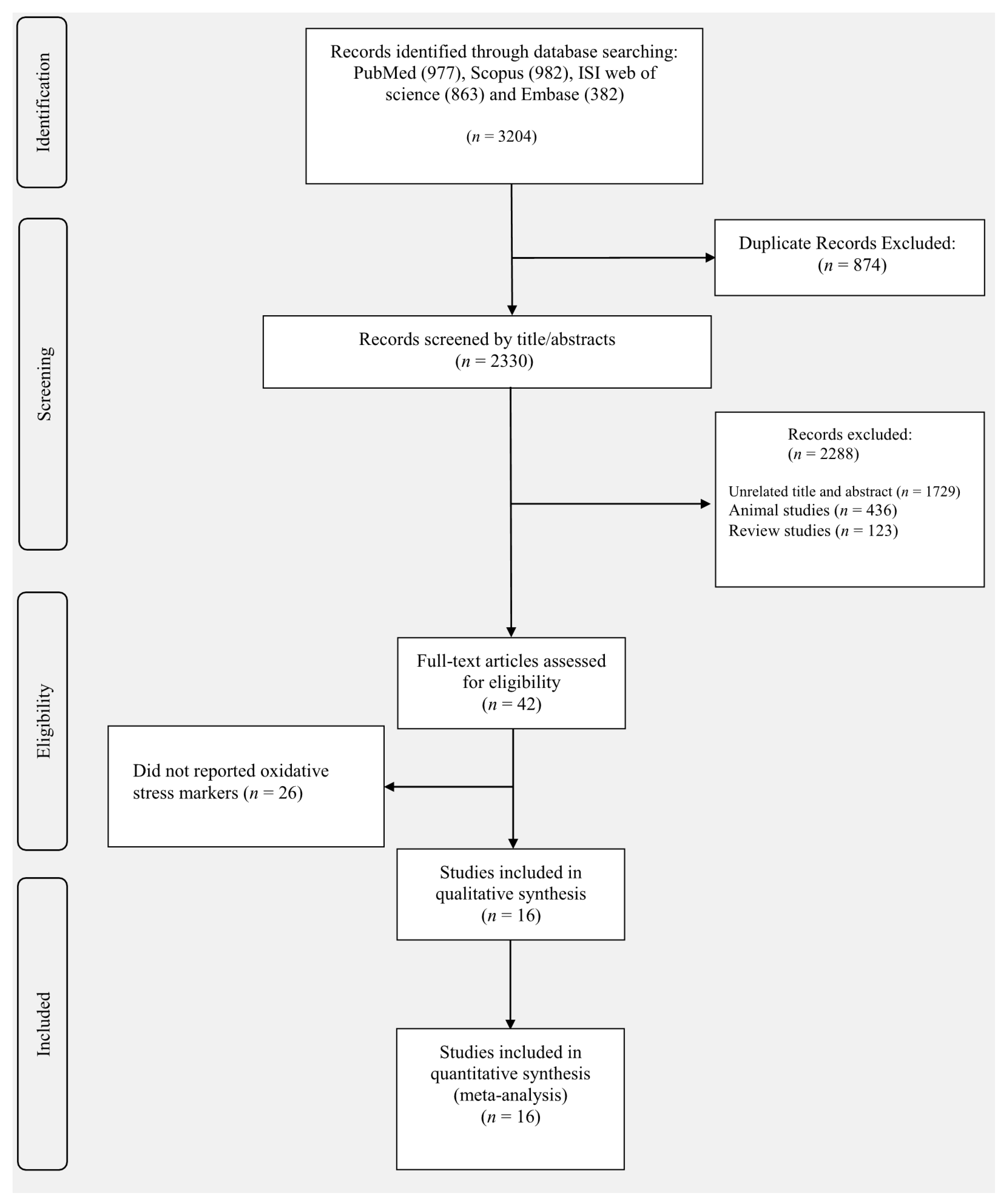
3.1. Study Selection
3.2. Study Characteristics
3.3. Meta-Analysis
3.3.1. Effect of Green Tea Supplementation on TAC
3.3.2. Effect of Green Tea on MDA
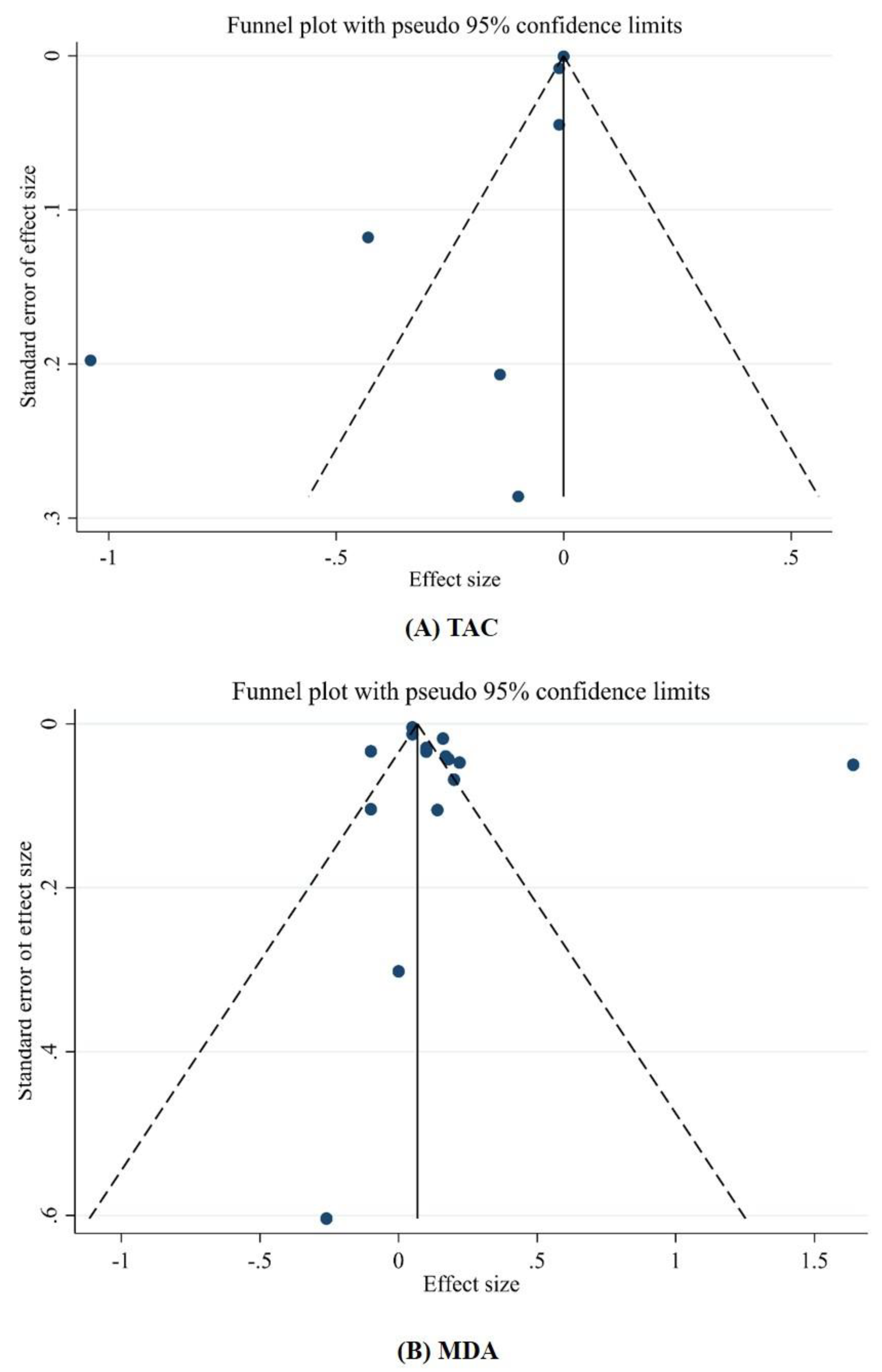
3.3.3. Publication Bias and Sensitivity Analysis
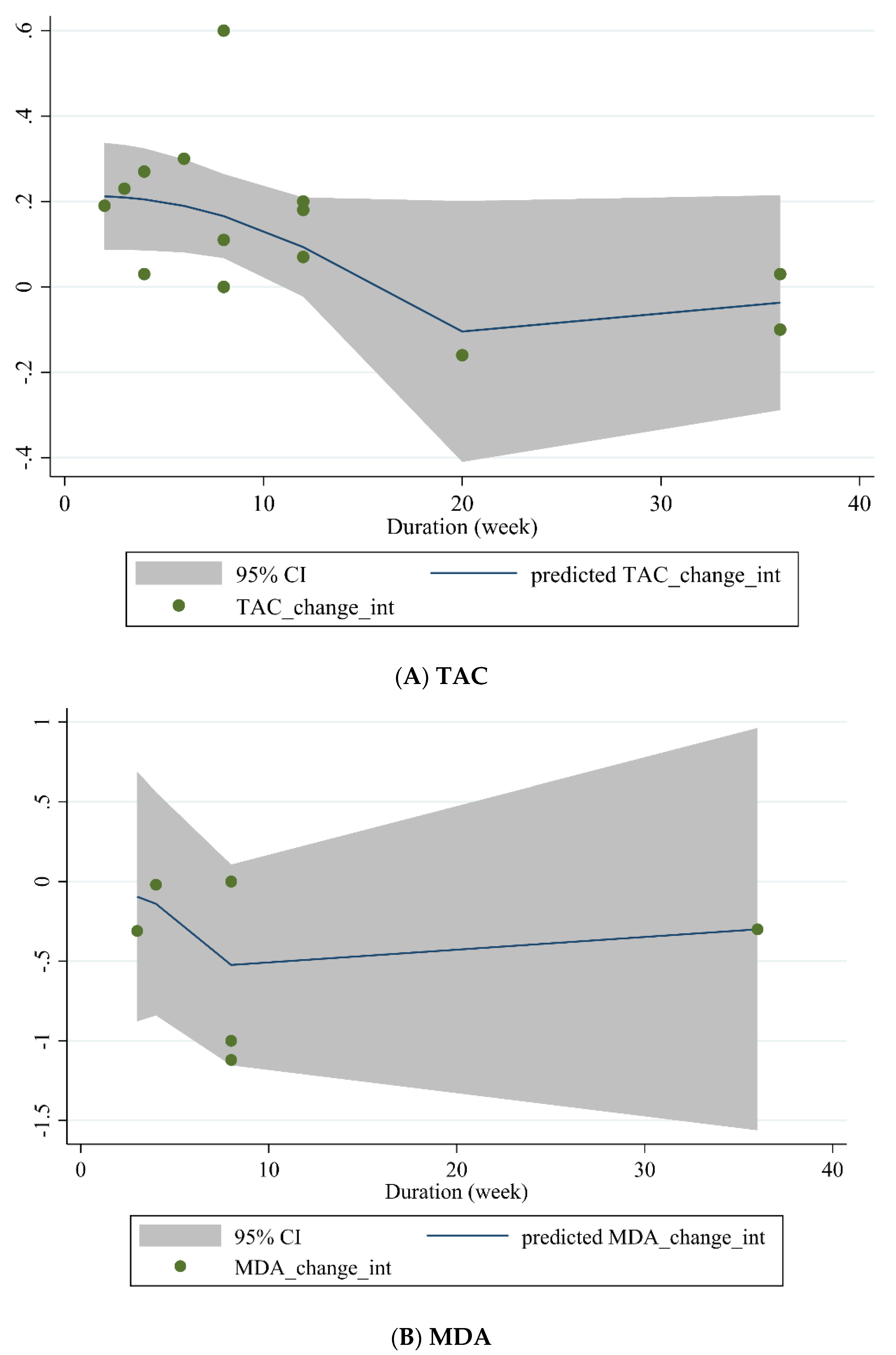
3.3.4. Non-Linear Dose-Response between Dose and Duration of Green Tea Supplementation and Antioxidant Status
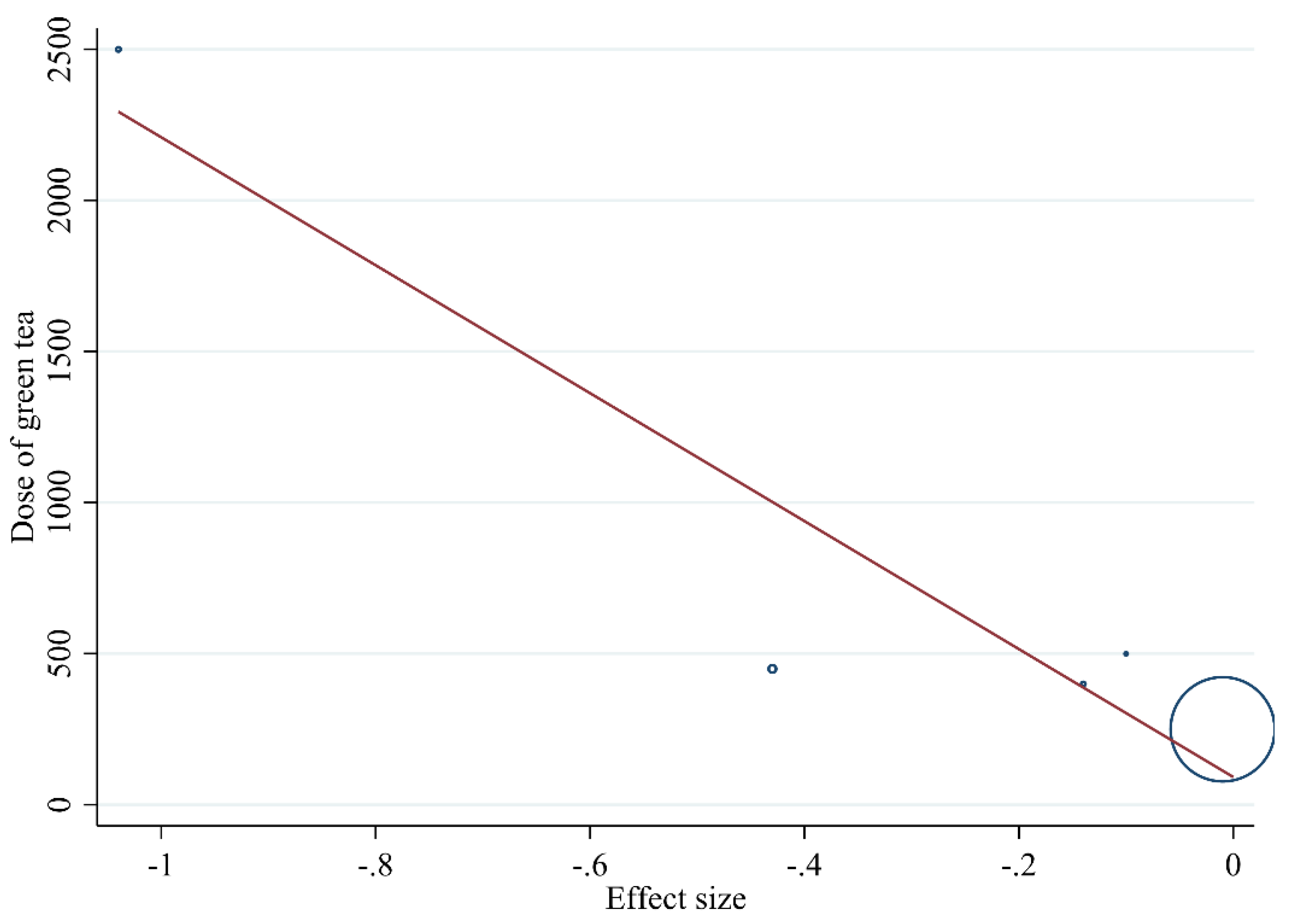
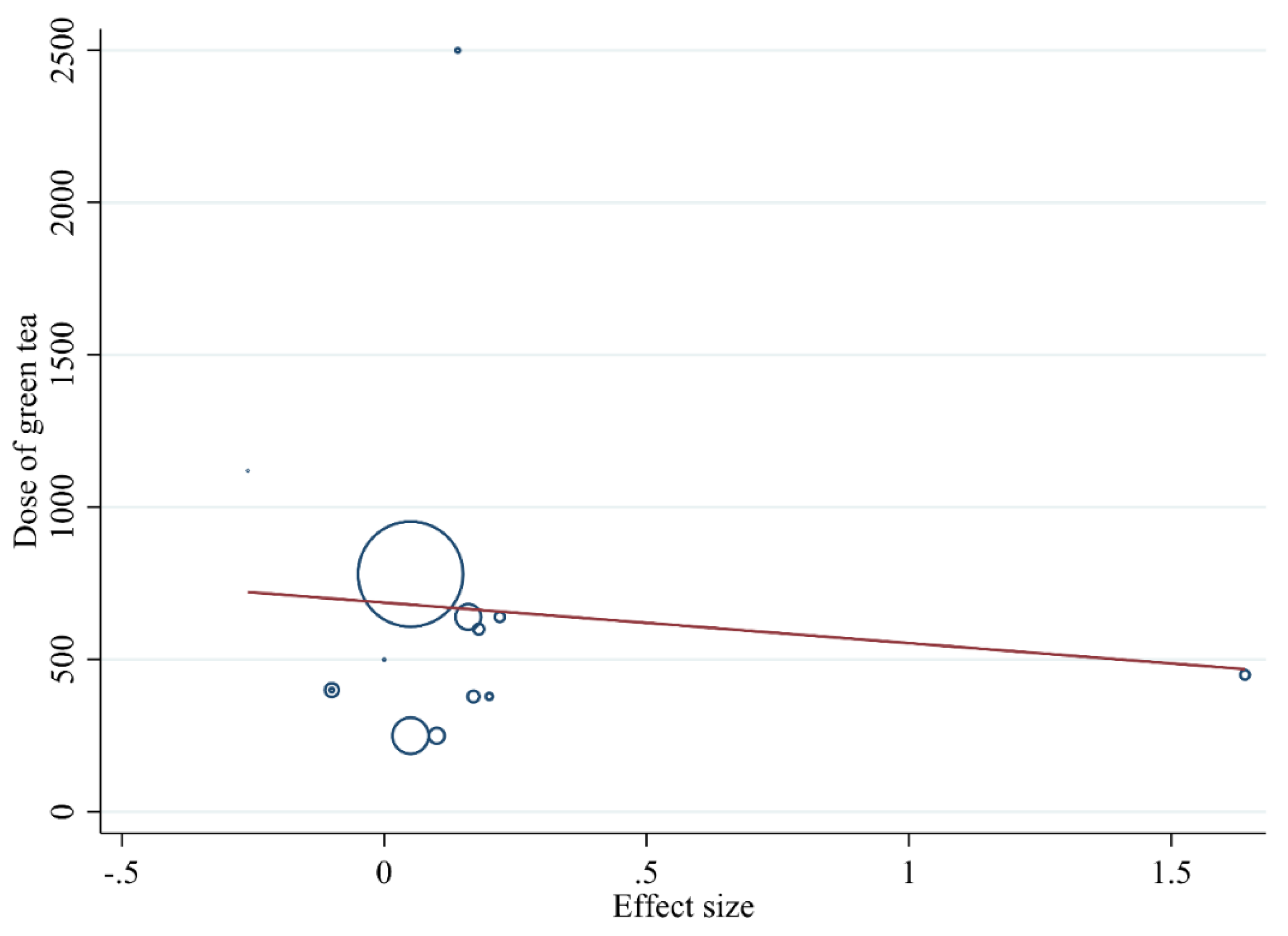
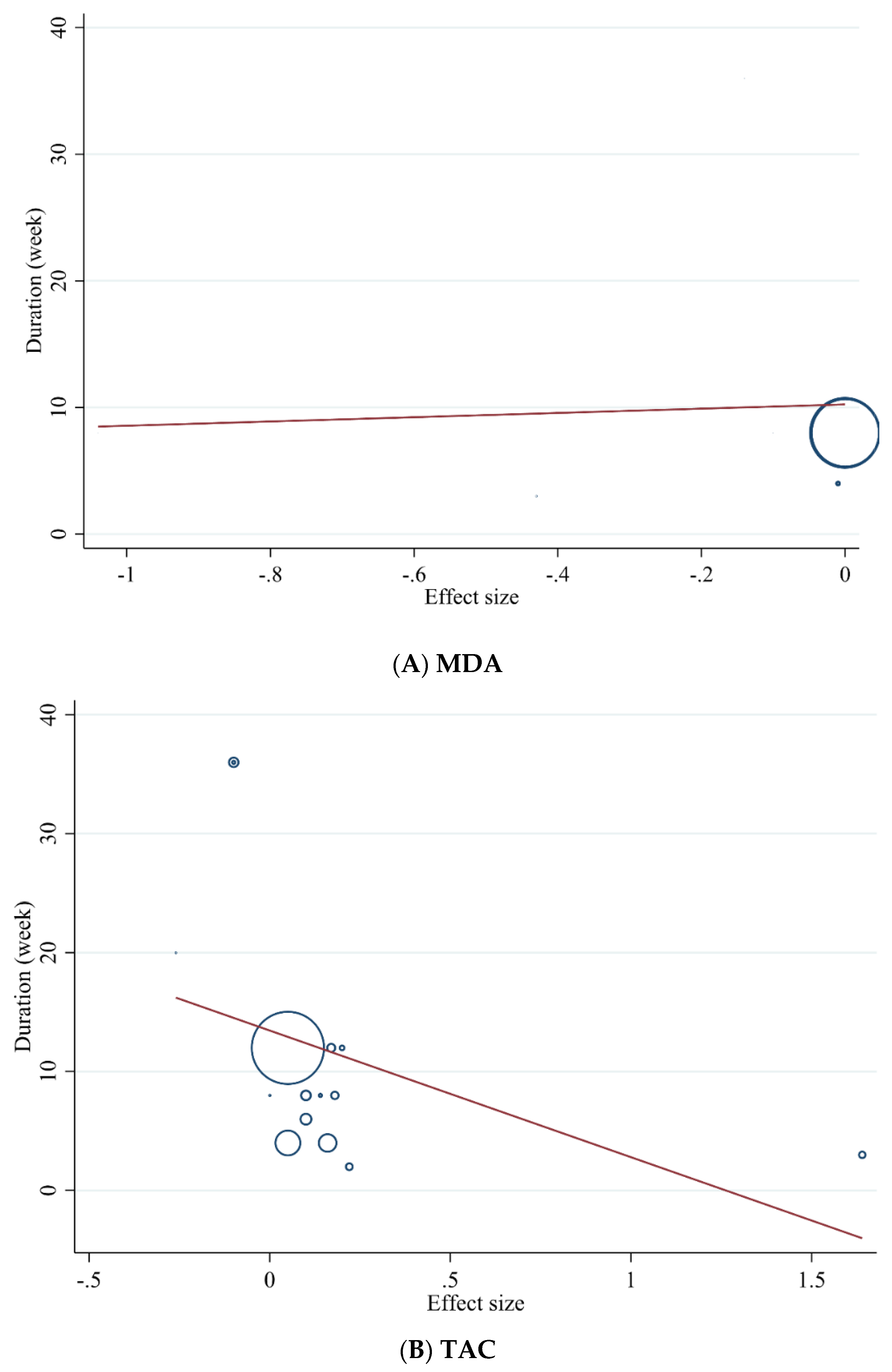
3.3.5. Meta-Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aiyegoro, O.A.; Okoh, A.I. Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Jówko, E.; Sacharuk, J.; Bałasinska, B.; Wilczak, J.; Charmas, M.; Ostaszewski, P.; Charmas, R. Effect of a Single Dose of Green Tea Polyphenols on the Blood Markers of Exercise-Induced Oxidative Stress in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 486–496. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Jówko, E.; Sacharuk, J.; Bałasinska, B.; Ostaszewski, P.; Charmas, M.; Charmas, R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr. Res. 2011, 31, 813–821. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Kafeshani, M.; Karimian, J.; Maracy, M.R.; Entezari, M. The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes. J. Diet. Suppl. 2017, 14, 346–357. [Google Scholar] [CrossRef]
- Wright, E., Jr.; Scism-Bacon, J.; Glass, L. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Atabek, M.E.; Vatansev, H.; Erkul, I. Oxidative stress in childhood obesity. J. Pediatr. Endocrinol. Metab. 2004, 17, 1063–1068. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Azizbeigi, K.; Stannard, S.R.; Atashak, S. Green Tea Supplementation During Resistance Training Minimally Affects Systemic Inflammation and Oxidative Stress Indices in Obese Men. Jundishapur J. Nat. Pharm. Prod. in press. 2019. [Google Scholar] [CrossRef]
- Vaz, S.R.; de Amorim, L.M.N.; de Nascimento, P.V.F.; Veloso, V.S.P.; Nogueira, M.S.; Castro, I.A.; Mota, J.F.; Botelho, P.B. Effects of green tea extract on oxidative stress and renal function in diabetic individuals: A randomized, double-blinded, controlled trial. J. Funct. Foods 2018, 46, 195–201. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Kim, H.W.; Kim, W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4456. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Lasaite, L.; Spadiene, A.; Savickiene, N.; Skesters, A.; Silova, A. The Effect of Ginkgo biloba and Camellia sinensis Extracts on Psychological State and Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nat. Prod. Commun. 2014, 9, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A. Dietary Polyphenols and Human Health. Nutrition 2020, 12, 2893. [Google Scholar] [CrossRef]
- Thielecke, F.; Boschmann, M. The potential role of green tea catechins in the prevention of the metabolic syndrome—A review. Phytochemistry 2009, 70, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Phung, O.J.; Baker, W.; Matthews, L.J.; Lanosa, M.; Thorne, A.; Coleman, C. Effect of green tea catechins with or without caffeine on anthropometric measures: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 91, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Vahabzadeh, Z.; Molodi, M.; Nikkho, B.; Saghebjoo, M.; Saedmocheshi, S.; Zamani, F.; Roshani, Y.; Babanzadeh, S. Aerobic training and hydroalcoholic extracts of green tea improve pro-oxidant-antioxidant balance and histopathological score in the N-methyl-N-nitrosourea-induced prostate cancer model of rat. EXCLI J. 2020, 19, 762–772. [Google Scholar]
- Sirichaiwetchakoon, K.; Lowe, G.M.; Eumkeb, G. The Free Radical Scavenging and Anti-Isolated Human LDL Oxidation Activities of Pluchea indica (L.) Less. Tea Compared to Green Tea (Camellia sinensis). BioMed Res. Int. 2020, 2020, 4183643. [Google Scholar] [CrossRef]
- Ye, Q.; Ye, L.; Xu, X.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complement. Altern. Med. 2012, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Ayissi, V.B.O.; Ebrahimi, A.; Schluesenner, H. Epigenetic effects of natural polyphenols: A focus on SIRT1-mediated mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32. [Google Scholar] [CrossRef]
- Panza, V.S.P.; Wazlawik, E.; Schütz, G.R.; Comin, L.; Hecht, K.C.; da Silva, E.L. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition 2008, 24, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Mulugeta, A.; Tong, C.; Newman, E.; Lyons, T.J. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr. Res. 2013, 33, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, A.; Vafa, M.; Neyestani, T.R.; Khamseh, M.; Hoseini, F. The effects of green tea consumption on metabolic and anthropometric indices in patients with Type 2 diabetes. J. Res. Med. Sci. 2013, 18, 1080–1086. [Google Scholar]
- Koutelidakis, A.E.; Rallidis, L.; Koniari, K.; Panagiotakos, D.; Komaitis, M.; Zampelas, A.; Anastasiou-Nana, M.; Kapsokefalou, M. Effect of green tea on postprandial antioxidant capacity, serum lipids, C-reactive protein and glucose levels in patients with coronary artery disease. Eur. J. Nutr. 2013, 53, 479–486. [Google Scholar] [CrossRef]
- Noronha, N.Y.; Pinhel, M.A.; Nicoletti, C.F.; Quinhoneiro, D.C.G.; Pinhanelli, V.C.; de Oliveira, B.A.; Nonino, C.B. Green tea supplementation improves oxidative stress biomarkers and modulates IL-6 circulating levels in obese women. Nutr. Hosp. Organo Of. Soc. Española Nutr. Parenter. Enter. 2019, 36, 583–588. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Soeizi, E.; Rafraf, M.; Asghari-Jafarabadi, M.; Ghaffari, A.; Rezamand, A.; Doostan, F. Effects of green tea on serum iron parameters and antioxidant status in patients with β-thalassemia major. Pharm. Sci. 2017, 23, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jabłecka, A. Effects of Green Tea Supplementation on Elements, Total Antioxidants, Lipids, and Glucose Values in the Serum of Obese Patients. Biol. Trace Element Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Spadiene, A.; Savickiene, N.; Ivanauskas, L.; Jakštas, V.; Skesters, A.; Silova, A.; Rodovicius, H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J. Food Drug Anal. 2014, 22, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Tafti, M.F. The Effect of Green Tea versus Sour Tea on Insulin Resistance, Lipids Profiles and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Iran. J. Med. Sci. 2014, 39, 424–432. [Google Scholar]
- Kuo, Y.-C.; Lin, J.-C.; Bernard, J.R.; Liao, Y.-H. Green tea extract supplementation does not hamper endurance-training adaptation but improves antioxidant capacity in sedentary men. Appl. Physiol. Nutr. Metab. 2015, 40, 990–996. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.-F.; Cheng, J.-C.; Chang, Y.-H.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Tsai, K.-S.; Wang, C.-K. Comparative studies on the hypolipidemic, antioxidant and hepatoprotective activities of catechin-enriched green and oolong tea in a double-blind clinical trial. Food Funct. 2018, 9, 1205–1213. [Google Scholar] [CrossRef]
- Sobhani, V.; Mehrtash, M.; Shirvani, H.; Fasihi-Ramandi, M. Effects of Short-Term Green Tea Extract Supplementation on VO2 Max and Inflammatory and Antioxidant Responses of Healthy Young Men in a Hot Environment. Int. J. Prev. Med. 2020, 11, 170. [Google Scholar]
- Bazyar, H.; Hosseini, S.A.; Saradar, S.; Mombaini, D.; Allivand, M.; Labibzadeh, M.; Alipour, M. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med. 2021, 18, 405–411. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H.R. References; Wiley Online Library: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Moura, B.M.; Panza, V.P.; Brunetta, H.S.; Tamborindeguy, A.C.; De Oliveira, M.V.; Sakugawa, R.L.; Nunes, E.A.; Da Silva, E.L.; Diefenthaeler, F. Effect of mate tea consumption on rapid force production after eccentric exercise: A randomized, controlled, crossover study. Sport Sci. Health 2020, 16, 571–581. [Google Scholar] [CrossRef]
- Cazzola, R.; Rondanelli, M. N-Oleoyl-Phosphatidyl-Ethanolamine and Epigallo Catechin-3-Gallate Mitigate Oxidative Stress in Overweight and Class I Obese People on a Low-Calorie Diet. J. Med. Food 2020, 23, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Prasad, R.; Jain, A. Effect of green tea extract (catechins) in reducing oxidative stress seen in patients of pulmonary tuberculosis on DOTS Cat I regimen. Phytomedicine 2010, 17, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Vlachopoulos, C.; Aznaouridis, K.; Baou, K.; Vasiliadou, C.; Pietri, P.; Xaplanteris, P.; Stefanadi, E.; Stefanadis, C. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 300–305. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-F.; Lin, T.-Y.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Improvement of green tea polyphenol with milk on skin with respect to antioxidation in healthy adults: A double-blind placebo-controlled randomized crossover clinical trial. Food Funct. 2016, 7, 893–901. [Google Scholar] [CrossRef]
- Arab, H.; Mahjoub, S.; Hajian-Tilaki, K.; Moghadasi, M. The effect of green tea consumption on oxidative stress markers and cognitive function in patients with Alzheimer’s disease: A prospective intervention study. Casp. J. Intern. Med. 2016, 7, 188–194. [Google Scholar]
- Erba, D.; Riso, P.; Bordoni, A.; Foti, P.; Biagi, P.; Testolin, G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005, 16, 144–149. [Google Scholar] [CrossRef]
- Freese, R.; Basu, S.; Hietanen, E.; Nair, J.; Nakachi, K.; Bartsch, H.; Mutanen, M. Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high-linoleic acid diet in healthy females. Eur. J. Nutr. 1999, 38, 149–157. [Google Scholar] [CrossRef]
- Hirano-Ohmori, R.; Takahashi, R.; Momiyama, Y.; Taniguchi, H.; Yonemura, A.; Tamai, S.; Umegaki, K.; Nakamura, H.; Kondo, K.; Ohsuzu, F. Green Tea Consumption and Serum Malondialdehyde-Modified LDL Concentrations in Healthy Subjects. J. Am. Coll. Nutr. 2005, 24, 342–346. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, C.-Y.O.; Aldini, G.; Johnson, E.J.; Rasmussen, H.; Yoshida, Y.; Niki, E.; Blumberg, J.B.; Russell, R.M.; Yeum, K.-J. Supplementation with lutein or lutein plus green tea extracts does not change oxidative stress in adequately nourished older adults. J. Nutr. Biochem. 2010, 21, 544–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhang, M.; Xie, X.; Jin, J.; Holman, C.D.J. Green tea consumption and glutathione S-transferases genetic polymorphisms on the risk of adult leukemia. Eur. J. Nutr. 2015, 56, 603–612. [Google Scholar] [CrossRef]
- Young, J.F.; Dragstedt, L.O.; Haraldsdóttir, J.; Daneshvar, B.; Kall, M.A.; Loft, S.; Nilsson, L.; Nielsen, S.E.; Mayer, B.; Skibsted, L.H.; et al. Green tea extract only affects markers of oxidative status postprandially: Lasting antioxidant effect of flavonoid-free diet. Br. J. Nutr. 2002, 87, 343–355. [Google Scholar] [CrossRef]
- Narotzki, B.; Reznick, A.Z.; Mitki, T.; Aizenbud, D.; Levy, Y. Green Tea Drinking Improves Erythrocytes and Saliva Oxidative Status in the Elderly. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 832, pp. 25–33. [Google Scholar]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Sami, G.; Khoshraftar, E.; Shayesteh, T.H.; Ranjbar, A. The effects of Chamomile tea on antioxidative biomarkers in operating room staff. J. HerbMed Pharmacol. 2015, 4, 98–101. [Google Scholar]
- Sapper, T.N.; Mah, E.; Ahn-Jarvis, J.; McDonald, J.D.; Chitchumroonchokchai, C.; Reverri, E.J.; Vodovotz, Y.; Bruno, R.S. A green tea-containing starch confection increases plasma catechins without protecting against postprandial impairments in vascular function in normoglycemic adults. Food Funct. 2016, 7, 3843–3853. [Google Scholar] [CrossRef]
- Lowe, G.M.; Gana, K.; Rahman, K. Dietary supplementation with green tea extract promotes enhanced human leukocyte activity. J. Complement. Integr. Med. 2015, 12, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Suraphad, P.; Suklaew, P.O.; Ngamukote, S.; Adisakwattana, S.; Mäkynen, K. The Effect of Isomaltulose Together with Green Tea on Glycemic Response and Antioxidant Capacity: A Single-Blind, Crossover Study in Healthy Subjects. Nutrition 2017, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Hrishi, T.; Kundapur, P.; Naha, A.; Thomas, B.; Kamath, S.; Bhat, G. Effect of adjunctive use of green tea dentifrice in periodontitis patients—A Randomized Controlled Pilot Study. Int. J. Dent. Hyg. 2015, 14, 178–183. [Google Scholar] [CrossRef]
- Da Silva, W.; Machado, Á.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef]
- Vinson, J.A.; Dabbagh, Y.A.; Serry, M.M.; Jang, J. Plant Flavonoids, Especially Tea Flavonols, Are Powerful Antioxidants Using an in Vitro Oxidation Model for Heart Disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Capacity and Polyphenols Components of Teas: Implications for Altering In Vivo Antioxidant Status. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Li, C.-W.; Shih, Y.-T.; Chuang, L.-T. Antioxidant and Anti-Inflammatory Properties of Lower-Polymerized Polyphenols in Oolong Tea. Int. J. Food Prop. 2013, 17, 752–764. [Google Scholar] [CrossRef]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch. Toxicol. 2008, 83, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.Y.; Kim, C.H. Epigallocatechin-3-gallate Regulates Inducible Nitric Oxide Synthase Expression in Human Umbilical Vein Endothelial Cells. Lab. Anim. Res. 2011, 27, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Tayama, Y.; Sugihara, K.; Sanoh, S.; Miyake, K.; Morita, S.; Kitamura, S.; Ohta, S. Effect of Tea Beverages on Aldehyde Oxidase Activity. Drug Metab. Pharmacokinet. 2011, 26, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, S.; Castro, E.; Rocha-Pereira, P.; Rebelo, I.; Rocha, S.; Santos-Silva, A. The effect of green tea in oxidative stress. Clin. Nutr. 2006, 25, 790–796. [Google Scholar] [CrossRef]
- Heijnen, C.; Haenen, G.; van Acker, F.; van der Vijgh, W.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. Vitr. 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Kim, W.; Yang, H.J.; Youn, H.; Yun, Y.J.; Seong, K.M.; Youn, B. Myricetin inhibits Akt survival signaling and induces Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated HaCaT human immortalized keratinocytes. J. Radiat. Res. 2010, 51, 285–296. [Google Scholar] [CrossRef] [Green Version]


| Author | Publication Year | Country | Study Design | Participant | Sex | Trial Duration (Week) | Means Age | Means BMI | Intervention | Sample Size | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | Treatment Group | Green Tea Dose (mg) | Control Group | IG | CG | |||||||
| Azizbeigi et al. 2009 | 2009 | Iran | RCT | Obese men | M | 8 | 23.9 | 22.8 | 31.8 | 30.8 | Green tea extract | 500 mg | 500 mg sucrose | 10 | 10 |
| Jowko et al. 2011 | 2011 | Poland | DB/R/PC | Healthy individuals | M | 4 | 21.5 | 21.2 | 23.85 | 23.31 | Green tea extract | 640 mg of polyphenols | Maltodextrin | 17 | 18 |
| Bogdanski et al. 2012 | 2012 | Poland | DB/R/PC | Obese, hypertensive patients | F/M | 12 | 49.2 | 51.5 | 32.5 | 33.9 | Green tea extract | 379 mg | Pure microcrystalline cellulose | 28 | 28 |
| Suliburska et al. 2012 | 2012 | Poland | DB/R/PC | Obese patients | F/M | 12 | 48.56 | 52.26 | 32.07 | 33.45 | Green tea extract | 379 mg | Pure microcrystalline cellulose | 23 | 23 |
| Mousavi et al. 2013 (A) | 2013 | Iran | RCT | T2DM patients | F/M | 8 | 54.6 | 52 | 27.4 | 28.1 | Green tea | 4 cup = 10,000 mg | - | 26 | 14 |
| Mousavi et al. 2013 (B) | 2013 | Iran | RCT | T2DM patients | F/M | 8 | 56.2 | 52 | 28.1 | 28.1 | Green tea | 2 cup = 5000 mg | - | 25 | 14 |
| Lasaite et al. 2014 (A) | 2014 | Lithuania | DB/R/PC | T2DM patients | F/M | 36 | 57.2 | 56.8 | NR | NR | Green tea extract | 400 | Microcrystalline cellulose | 17 | 14 |
| Lasaite et al. 2014 (B) | 2014 | Lithuania | DB/R/PC | T2DM patients | F/M | 36 | 57.2 | 56.8 | NR | NR | Green tea extract | 600 | Microcrystalline cellulose | 17 | 14 |
| Spadiene et al. 2014 (A) | 2014 | Lithuania | DB/R/PC | T2DM patients | nr | 36 | 62.18 | 62.18 | 35.23 | 34.98 | Green tea extract | 400 | Microcrystalline cellulose | 20 | 25 |
| Spadiene et al. 2014 (B) | 2014 | Lithuania | DB/R/PC | T2DM patients | nr | 36 | 62.18 | 62.18 | 35.23 | 34.98 | Green tea extract | 600 | Microcrystalline cellulose | 20 | 25 |
| Mozaffari-Khosravi et al. 2014 | 2014 | Iran | RCT | Type 2 Diabetes Mellitus | F/M | 4 | 52.2 | 52.1 | 28 | 28.3 | Green tea | 450 mL | Sour tea | 48 | 46 |
| Kuo et al. 2015 | 2015 | Taiwan | DB/PC | Healthy individuals | M | 4 | 20 | 20 | 21.95 | 23.55 | Green tea extract | 250 mg | Starch capsule | 10 | 10 |
| Hadi et al. 2016 | 2016 | Iran | DB/R/PC | Soccer players | M | 3 | 22.6 | 22.82 | Green tea extract | 450 mg | 450 mg of maltodextrin | 18 | 18 | ||
| Soeizi et al. 2017 | 2017 | Iran | SB/R/PC | β–Thalassemia Major | F/M | 8 | 23.1 | 24.2 | 20.9 | 19.42 | Green tea | 2500 mg | Warm water | 26 | 26 |
| Ribeiro Vaz et al. 2018 | 2018 | Brazil | DB/R/PC | type 1 DM or type 2 DM | F/M | 20 | 46.48 | 52.29 | 27.58 | 25.6 | Green tea extract | 1120 mg | cellulose capsules | 27 | 28 |
| Venkatakrishnan et al. 2018 | 2018 | Taiwan | DB/R/PC | Hypercholesterolemic subjects | F | 12 | 45 | 45 | 31.39 | 28.81 | Green tea | 600 mL (780.6 mg of catechin) | Tea flavor with very less concentration of catechin and caffeine | 20 | 20 |
| Sadowska-Krepa et al. 2019 | 2019 | Poland | RCT | Male students of the physical education | M | 6 | 22 | 23.1 | 23.73 | 24.72 | Green tea extract | 250 mg | Microcrystalline cellulose, magnesium stearate and maltodextrin | 8 | 8 |
| Sobhani et al. 2020 | 2020 | Iran | RCT | Healthy individuals | M | 2 | 26.12 | 24 | 23.85 | 24.62 | Green tea extract | 640 mg of polyphenols | Maltodextrin | 8 | 7 |
| Bazyar et al. 2020 | 2020 | Iran | RCT | T2DM patients | F/M | 8 | 51.75 | 52.61 | 29.46 | 29.28 | Green tea extract | 600 mg of EGCG | 600 mg of wheat flour | 22 | 22 |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|
| Mousavi et al. (2013) | U | H | H | U | L | L | U |
| Lasaite et al. (2014) | U | L | L | U | L | L | L |
| Spadiene et al. (2014) | U | L | L | U | L | L | L |
| Jówkoa et al. (2011) | U | L | L | U | U | L | U |
| Sadowska-Krępa et al. (2019) | U | L | H | U | L | L | U |
| Bogdanski et al. (2012) | U | L | L | U | L | L | L |
| Hadi et al. (2016) | U | L | L | U | L | L | L |
| Mozaffari-Khosravi et al. (2014) | U | H | U | U | U | L | U |
| Azizbeigi et al. (2019) | U | U | H | U | U | L | U |
| Ribeiro Vaza et al. (2018) | U | L | L | U | H | L | L |
| Suliburska et al. (2012) | L | L | L | L | L | L | L |
| Kuo et al. (2014) | U | L | L | L | L | L | L |
| Soeizi et al. (2017) | L | L | L | L | L | L | L |
| Venkatakrishnan et al. (2018) | U | L | L | U | L | L | L |
| Sobhani et al. (2020) | U | L | L | U | L | L | U |
| Bazyar et al. (2020) | U | L | L | U | L | L | L |
| NO | WMD (95%CI) | p | P Heterogeneity | I2 | |
|---|---|---|---|---|---|
| Subgroup analyses of green tea supplementation on TAC concentrations. | |||||
| Overall effect | 16 | 0.20 (0.09, 0.30) | <0.001 | <0.001 | 98.6% |
| Trial duration (week) | |||||
| <8 | 5 | 0.43 (0.10, 0.75) | 0.010 | <0.001 | 99.6% |
| ≥8 | 11 | 0.08 (0.02, 0.13) | 0.004 | <0.001 | 80.1% |
| Type of green tea | |||||
| Green tea extract | 12 | 0.23 (0.03, 0.42) | 0.019 | <0.001 | 98.9% |
| Brewed green tea | 4 | 0.07 (0.03, 0.10)_ | <0.001 | 0.169 | 40.5% |
| Sex | |||||
| Both | 8 | 0.12 (0.08, 0.17) | <0.001 | 0.173 | 32.0% |
| Male | 6 | 0.38 (0.07, 0.69) | 0.003 | <0.001 | 99.5% |
| Female | 1 | 0.05 (0.04, 0.05) | 0.015 | - | - |
| Intervention dose (mg/d) | |||||
| ≤400 | 6 | 0.06 (−0.01, 0.13) | 0.132 | <0.001 | 87.1% |
| >400 | 10 | 0.29 (0.09, 0.49) | 0.004 | <0.001 | 99.1% |
| Health status | |||||
| Healthy | 11 | 0.08 (0.02, 0.13) | 0.004 | <0.001 | 80.1% |
| Unhealthy | 5 | 0.43 (0.10, 0.75) | 0.010 | <0.001 | 99.6% |
| Baseline BMI (kg/m2) | |||||
| 18.5–24.9 | 6 | 0.38 (0.08, 0.68) | 0.011 | <0.001 | 99.5% |
| 25–29.9 | 4 | 0.11 (0.07, 0.15) | <0.001 | 0.387 | 1.1% |
| ≥30 | 5 | 0.06 (−0.03, 0.16) | 0.176 | <0.001 | 88.2% |
| Subgroup analyses of green tea supplementation on MDA concentrations | |||||
| Overall effect | 8 | −0.00 (−0.00, 0.00) | 0.636 | <0.001 | 84.1% |
| Trial duration (week) | |||||
| <8 | 3 | −0.08 (−0.21, 0.04) | 0.185 | 0.002 | 84.2% |
| ≥8 | 5 | −0.00 (−0.00, 0.00) | 0.860 | <0.001 | 86.3% |
| Type of green tea | |||||
| Green tea extract | 4 | −0.17 (−0.43, 0.09) | 0.202 | 0.004 | 77.1% |
| Brewed green tea | 4 | −0.00 (−0.00, 0.00) | 0.860 | <0.001 | 89.5% |
| Sex | |||||
| Both | 4 | −0.00 (−0.00, 0.00) | 0.860 | <0.001 | 89.5% |
| Male | 3 | −0.18 (−0.51, 0.15) | 0.499 | 0.002 | 84.3% |
| Intervention dose | |||||
| ≤400 | 2 | −0.01 (−0.02, 0.00) | 0.215 | 0.530 | 0.0% |
| >400 | 6 | −0.00 (−0.00, 0.00) | 0.821 | <0.001 | 88.1% |
| Health status | |||||
| Healthy | 6 | −0.00 (−0.00, 0.00) | 0.853 | <0.001 | 82.9% |
| Unhealthy | 2 | −0.20 (−0.61, 0.20) | 0.331 | <0.001 | 92.1% |
| BMI | |||||
| 18.5–24.9 | 3 | −0.46 (−0.98, 0.05) | 0.081 | <0.001 | 95.0% |
| 25–29.9 | 3 | −0.00 (−0.00, 0.00) | 0.493 | 0.609 | 0.0% |
| ≥30 | 2 | −0.12 (−0.45, 0.20) | 0.451 | 0.910 | 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasaei, N.; Asbaghi, O.; Samadi, M.; Setayesh, L.; Bagheri, R.; Gholami, F.; Soveid, N.; Casazza, K.; Wong, A.; Suzuki, K.; et al. Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants 2021, 10, 1731. https://doi.org/10.3390/antiox10111731
Rasaei N, Asbaghi O, Samadi M, Setayesh L, Bagheri R, Gholami F, Soveid N, Casazza K, Wong A, Suzuki K, et al. Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants. 2021; 10(11):1731. https://doi.org/10.3390/antiox10111731
Chicago/Turabian StyleRasaei, Niloufar, Omid Asbaghi, Mahsa Samadi, Leila Setayesh, Reza Bagheri, Fatemeh Gholami, Neda Soveid, Krista Casazza, Alexei Wong, Katsuhiko Suzuki, and et al. 2021. "Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Antioxidants 10, no. 11: 1731. https://doi.org/10.3390/antiox10111731
APA StyleRasaei, N., Asbaghi, O., Samadi, M., Setayesh, L., Bagheri, R., Gholami, F., Soveid, N., Casazza, K., Wong, A., Suzuki, K., & Mirzaei, K. (2021). Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants, 10(11), 1731. https://doi.org/10.3390/antiox10111731








