The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Acid Whey
2.1.2. Fermented Bacon Production
2.2. Methods
2.2.1. Microbiological Analyses
2.2.2. Determination of the pH Value
2.2.3. Oxidative Reduction Potential (ORP) Measurement
2.2.4. TBARS Value
2.2.5. Fatty Acid Profile
2.2.6. Sensory Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Assessment
3.2. pH and ORP
3.3. TBARS Index
3.4. Fatty Acid Profile
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Li, J.; Zhou, T.; Li, J.; Yang, J.; Chen, W.; Xiong, Y.L. Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (NanxWudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Sci. 2016, 121, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.; Axelsson, L.; McLeod, A.; Rode, T.M.; Heir, E. Health and safety considerations of fermented sausages. J. Food Qual. 2017, 3, 9753894. [Google Scholar] [CrossRef]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Curr. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Hammes, W.P. Metabolism of nitrate in fermented meats: The characteristic feature of a specific group of fermented foods. Food Microbiol. 2012, 29, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Christieansa, S.; Picgirarda, L.; Parafitaa, E.; Lebertb, A.; Gregori, T. Impact of reducing nitrate/nitrite levels on the behavior of Salmonella Typhimurium and Listeria monocytogenes in French dry fermented sausages. Meat Sci. 2018, 137, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hospital, X.F.; Hierro, E.; Fernandez, M. Effect of reducing nitrate and nitrite added to dry fermented sausages on the survival of Salmonella Typhimurium. Food Res. Int. 2014, 62, 410–415. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, K.M.; Karwowska, M.; Dolatowski, Z.J. Use of acid whey and mustard seed to replace nitrites during cooked sausage production. Meat Sci. 2014, 96, 750–756. [Google Scholar] [CrossRef]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Stasiak, D.M. Effect of acid whey on physicochemical characteristics of dry-cured organic pork loins without nitrite. Int. J. Food Sci. Technol. 2016, 51, 970–977. [Google Scholar] [CrossRef]
- Horsch, A.M.; Sebranek, J.G.; Dickson, J.S.; Niebuhr, S.E.; Larson, E.M.; Lavieri, N.A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014, 96, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Riel, G.; Boulaaba, A.; Popp, J.; Klein, G. Effects of parsley extract powder as an alternative for the direct addition of sodium nitrite in the production of mortadella-type sausages—Impact on microbiological, physicochemical and sensory aspects. Meat Sci. 2017, 131, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.S.; Katsanidis, E.; Marantidou, S.; Bloukas, J.G. Effect of freeze-dried leek powder (FDLP) and nitrite level on processing and quality characteristics of fermented sausages. Meat Sci. 2011, 87, 140–145. [Google Scholar] [CrossRef]
- Łaszkiewicz, B.; Szymański, P.; Kołożyn-Krajewska, D. Application of Lactiplantibacillus plantarum SCH1 for the Bioconservation of Cooked Sausage Made from Mechanically Separated Poultry Meat. Appl. Sci. 2021, 11, 1576. [Google Scholar] [CrossRef]
- Łaszkiewicz, B.; Szymański, P.; Kołożyn-Krajewska, D. The effect of selected lactic acid bacterial strains on the technological and microbiological quality of mechanically separated poultry meat cured with a reduced amount of sodium nitrite. Poult. Sci. 2020, 100, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gøtterup, J.; Olsen, K.; Knöchel, S.; Tjener, K.; Stahnke, L.H.; Møller, J.K. Relationship between nitrate/nitrite reductase activities in meat associated staphylococci and nitrosylmyoglobin formation in a cured meat model system. Int. J. Food Microbiol. 2007, 120, 303–310. [Google Scholar] [CrossRef]
- Szymański, P.; Łaszkiewicz, B.; Siekierko, U.; Kern-Jędrychowska, A.; Kołożyn-Krajewska, D. The use of the mixed bacteria Limosilactobacillus fermentum and Staphylococcus carnosus in the meat curing process with a reduced amount of sodium nitrite. Appl. Sci. 2021, 11, 904. [Google Scholar] [CrossRef]
- Hospital, X.F.; Carballo, J.; Fernandez, M.; Arnau, J.; Gratacos, M.; Hierro, E. Technological implications of reducing nitrate and nitrite levels in dry-fermented sausages: Typical microbiota, residual nitrate and nitrite and volatile profile. Food Control. 2015, 57, 275–281. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Addition of acid whey improves organic dry-fermented sausage without nitrite production and its nutritional value. Int. J. Food Sci. Technol. 2018, 53, 246–253. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J. Shelf life of organic roast pork enriched with acid whey-plant extracts combination. J. Food Qual. 2016, 39, 171–180. [Google Scholar] [CrossRef]
- Karwowska, M.; Wójciak, K.M.; Dolatowski, Z.J. The influence of acid whey and mustard seed on lipid oxidation of organic fermented sausage without nitrite. J. Sci. Food Agric. 2015, 95, 628–634. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Montero, R.; Belloch, C.; Flores, M. Microbial changes and aroma profile of nitrate reduced dry sausages during vacuum storage. Meat Sci. 2019, 147, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.U.; Nam, K.C.; Lee, E.J. Lipid oxidation and flavor. Appl. Muscle Biol. Meat Sci. 2009, 12, 227–246. [Google Scholar]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of tyree modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 31, 1309–1313. [Google Scholar] [CrossRef]
- Skryplonek, K.; Jasińska, M. Quality of fermented probiotic beverages made from frozen acid whey and milk during refrigerated storage. Żywn. Nauka Technol. Jakość 2016, 1, 32–44. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese whey fermentation by its native microbiota: Proteolysis and bioactive peptides release with ACE-inhibitory activity. Fermentation 2020, 6, 19. [Google Scholar] [CrossRef]
- Rzepkowska, A.; Zielińska, D.; Ołdak, A.; Kołożyn-Krajewska, D. Safety assessment and antimicrobial properties of the lactic acid bacteria strains isolated from polish raw fermented meat products. Int. J. Food Prop. 2017, 20, 2736–2747. [Google Scholar] [CrossRef]
- Dinçer, E.; Kıvanç, M. Lipolytic Activity of Lactic Acid Bacteria Isolated from Turkish Pastırma. Anadolu Univ. J. Sci. Technol. C Life Sci. Biotechnol. 2018, 7, 12–19. [Google Scholar] [CrossRef]
- ISO 4833-2:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Pour Plate Technique; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 4833-1:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 21527-1:2008. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- ISO 21527-2:2008. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- PN-ISO 21528-2:2005. Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2. Colony Count Method; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- ISO 11290-1:2017. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and Others Listeria spp.—Part 1: Detection Method; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- EN ISO 12966-1: 2015-01:2015. Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- PN-EN ISO 13299:2016-05. Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile; ISO: Warsaw, Poland, 2016. [Google Scholar]
- Majou, D.; Christieans, S. Mechanisms of the bactericidal effects of nitrate and nitrite in cured meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kononiuk, A.D.; Karwowska, M. Bioactive compounds in fermented sausages prepared from beef and fallow deer meat with acid whey addition. Molecules 2020, 25, 2429. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of starter cultures on the safety of fermented meat products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, D.A.; Filippou, P.; De Smet, S.; De Vuyst, L.; Leroy, F. Effect of temperature and pH on the community dynamics of coagulase-negative staphylococci during spontaneous meat fermentation in a model system. Food Microbiol. 2018, 76, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.M.; Chen, L.; Zhou, G. Influence of sodium nitrite on protein oxidation and nitrosation of sausages subjected to processing and storage. Meat Sci. 2016, 116, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Bolotin, A.; Mauger, S.; Malarme, K.; Ehrlich, S.D.; Sorokin, A. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek 1999, 76, 27–76. [Google Scholar] [CrossRef]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissen-Bach, J.; Ehrlich, S.D.; Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef]
- Igarashi, T.; Kono, Y.; Tanaka, K. Molecular cloning of manganese catalase from Lactobacillus plantarum. J. Biol. Chem. 1996, 271, 29521–29524. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Libera, J.; Karwowska, M.; Stasiak, D.M.; Dolatowski, Z.J. Microbiological and physicochemical properties of dry-cured neck inoculated with probiotic of Bifidobacterium animalis ssp. lactis BB-12. Int. J. Food Sci. Technol. 2015, 50, 1560–1566. [Google Scholar] [CrossRef]
- Wang, X.H.; Ren, H.Y.; Liu, D.Y.; Zhu, W.Y.; Wang, W. Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control 2013, 32, 591–596. [Google Scholar] [CrossRef]
- Coutron-Gambotti, C.; Gandemer, G. Lipolysis and oxidation in subcutaneous adipose tissue during dry-cured ham processing. Food Chem. 1999, 64, 95–101. [Google Scholar] [CrossRef]
- Sebranek, J.G. Basic curing ingredients. In Ingredients in Meat Products: Properties, Functionality, and Applications; Tarte, R., Ed.; Springer: New York, NY, USA, 2009; pp. 1–24. [Google Scholar]
- Wójciak, K.M.; Dolatowski, Z.J. Evaluation of natural preservatives in combination with acid whey for use in fermented sausage. Sci. Agric. 2016, 73, 125–133. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Karwowska, M.; Dolatowski, Z.J. Fatty acid profile, color and lipid oxidation of organic fermented sausage during chilling storage as influenced by acid whey and probiotic strains addition. Sci. Agric. 2015, 2, 124–131. [Google Scholar] [CrossRef]
- Karwowska, M.; Dolatowski, Z.J. Effect of acid whey and freeze-dried cranberries on lipid oxidation and fatty acid composition of nitrite-/nitrate-free fermented sausage made from deer meat. Asian-Australas. J. Anim. Sci. 2017, 30, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Najjari, A.; Boumaiza, M.; Jaballah, S.; Boudabous, A.; Ouzari, H.I. Application of isolated Lactobacillus sakei and Staphylococcus xylosus strains as a probiotic starter culture during the industrial manufacture of Tunisian dry-fermented sausages. Food Sci. Nutr. 2020, 8, 4172–4184. [Google Scholar] [CrossRef]
- Arief, I.I.; Afiyah, D.N.; Wulandari, Z.; Budiman, C. Physicochemical properties, fatty acid profiles, and sensory characteristics of fermented beef sausage by probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. J. Food Sci. 2016, 81, M2761–M2769. [Google Scholar] [CrossRef]
- Nikkilä, P.; Johansson, T.; Toivanen, L.; Rosenqvist, H. Theeffect of tween 80 on the fatty acid composition of Lactobacillus buchneri and Lactobacillus brevis. J. Gen. Appl. Microbiol. 1995, 41, 327–332. [Google Scholar] [CrossRef][Green Version]
- Lauret, R.; Morel-Deville, F.; Berthier, F.; Champomier-Vergès, M.; Postma, P.; Ehrlich, S.D.; Zagorec, M. Carbohydrate utilization in Lactobacillus sake. Appl. Environ. Microbiol. 1996, 62, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Partanen, L.; Marttinen, N.; Alatossava, T. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst. Appl. Microbiol. 2001, 24, 500–506. [Google Scholar] [CrossRef] [PubMed]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Suutari, M.; Laakso, S. Temperature adaptation in Lactobacillus fermentum: Interconversions of oleic, vaccenic and dihydrosterulic acids. J. Gen. Microbiol. 1992, 138, 445–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valencia, I.; Ansorena, D.; Astiasara, I. Nutritional and sensory properties of dry fermented sausages enriched with n-3 PUFAs. Meat Sci. 2006, 72, 727–733. [Google Scholar] [CrossRef]
- Bak, K.H.; Richards, M.P. Hexanal as a predictor of development of oxidation flavor in cured and uncured deli meat products as affected by natural antioxidants. Foods 2021, 10, 152. [Google Scholar] [CrossRef]
- Popova, T.; Marinova, P.; Vasileva, V.; Gorinov, Y.; Lidji, K. Oxidative changes in lipids and proteins in beef during storage. Arch. Zootech. 2009, 12, 30–38. [Google Scholar]
- Hęś, M.; Korczak, J. The influence of different factors on the kinetics of the lipid oxidation in meat. Nauka Przyr. Technol. 2007, 1, 3. [Google Scholar]
- Neffe-Skocińska, K.; Okoń, A.; Kołożyn-Krajewska, D.; Dolatowski, Z. Amino acid profile and sensory characteristics of dry fermented pork loins produced with a mixture of probiotic starter cultures. J. Sci. Food Agric. 2017, 97, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Rebecchi, A.; Dallolio, M.; Braceschi, G.; Domínguez, R.; Dallolio, G.; Trevisan, M.; Lorenzo, J.M.; Lucini, L. Changes in the chemical and sensory profile of ripened Italian salami following the addition of different microbial starters. Meat Sci. 2021, 180, 108584. [Google Scholar] [CrossRef]
- Hunt, M.; King, A. AMSA Meat Color. Measurement Guidelines; American Meat Science Association: Savoi, IL, USA, 2012. [Google Scholar]
- Jaworska, D.; Przybylski, W. The effect of selected factors on sensory quality of pork. Żywn. Nauka Technol. Jakość 2014, 5, 21–35. [Google Scholar] [CrossRef]
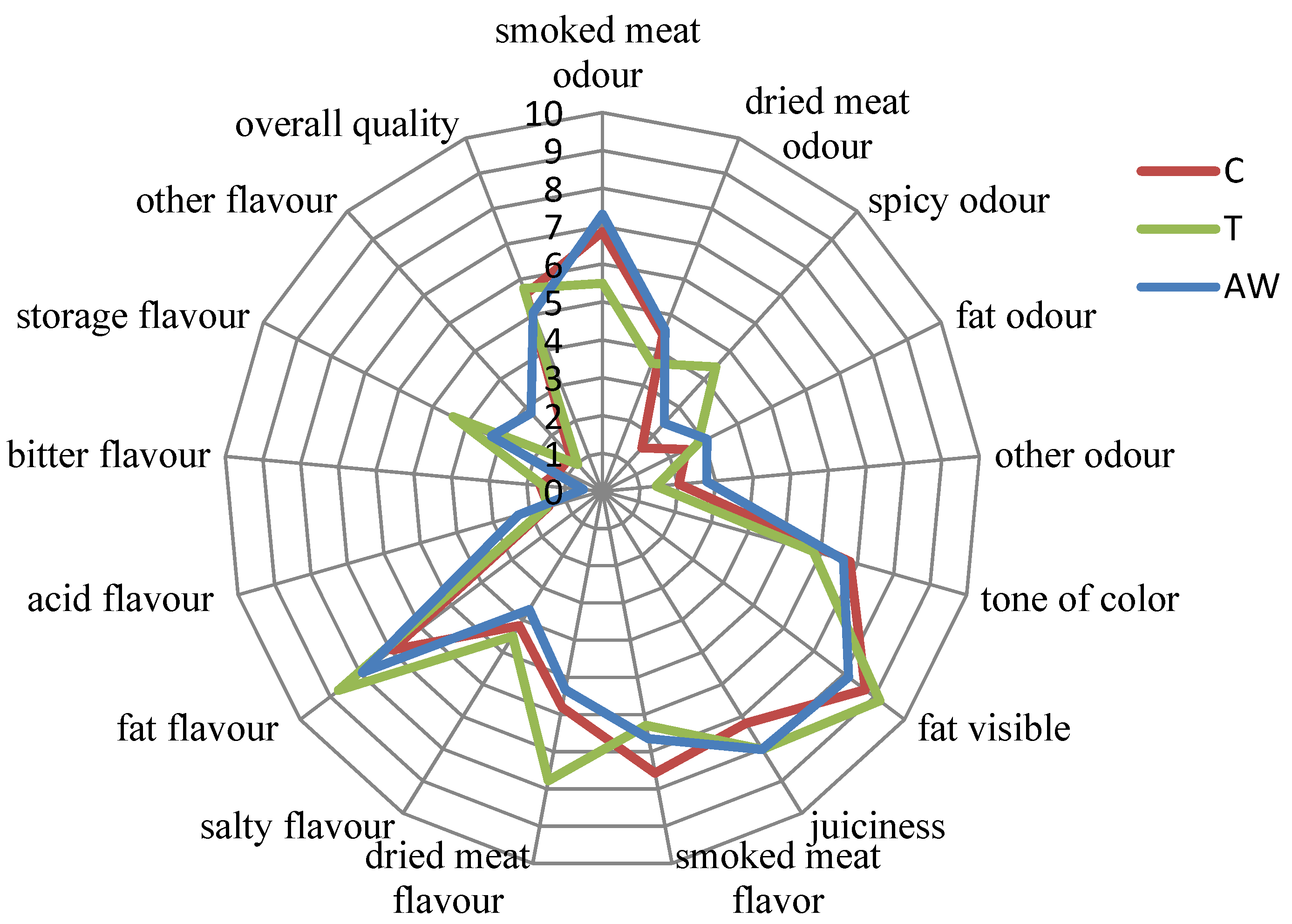
| Discriminants | Definition | Boundary Terms |
|---|---|---|
| Flavor | ||
| Smoked meat | Intensity flavor of volatile organic compounds from the thermal decomposition of wood | 0 = not intensive–10 = very intensive |
| Dried meat | Characteristic of dry fermented meat | 0 = not intensive–10 = very intensive |
| Salty | Intensity of salty flavor | 0 = not intensive–10 = very intensive |
| Fatty | Intensity of a fatty flavor | 0 = not intensive–10 = very intensive |
| Acid | Intensity of an acid flavor | 0 = not intensive–10 = very intensive |
| Bitter | Intensity of a bitter flavor | 0 = not intensive–10 = very intensive |
| Stored | Lack of freshness | 0 = not intensive–10 = very intensive |
| Other | Other sensations not on the list | 0 = not intensive–10 = very intensive |
| Odor | ||
| Smoked meat | Intensity of the smoked odor | 0 = not intensive–10 = very intensive |
| Dried meat | Intensity of a dried meat odor | 0 = not intensive–10 = very intensive |
| Spicy | Intensity of an irritating impression when smelling | 0 = not intensive–10 = very intensive |
| Fatty | Intensity of a fatty odor | 0 = not intensive–10 = very intensive |
| Other | Other sensations, not on the list | 0 = not intensive–10 = very intensive |
| Appearance | ||
| Tone of color | Intensity of the red color associated to meat tissue (cured meat color) | 0 = light pink–10 = dark pink |
| Fat visible | The amount of fat visible in a cross-section of the product | 0 = low amount–10 = high amount |
| Juiciness | The appearance of juiciness in the product, which is a combination of fat and water | 0 = not very juicy–10 = very juicy |
| Overall quality | Attribute of the total quality of dry fermented pork bacon | 0 = low–10 = very high |
| Treatment | Number of Microorganisms (log CFU/g) | Treatment | Time of Storage | Treatment × Time of Storage | ||
|---|---|---|---|---|---|---|
| Storage time (weeks) | 0 | 4 | p | p | p | |
| TVC | C | 6.40 ± 0.17 aD | 6.36 ± 0.21 bD | *** | *** | *** |
| T | 6.48 ± 0.22 aD | 7.32 ± 0.14 cE | ||||
| AW | 6.23 ± 0.25 aD | 6.70 ± 0.09 aE | ||||
| LAB | C | 5.34 ± 0.26 aD | 6.08 ± 0.15 aE | *** | *** | * |
| T | 6.00 ± 0.41 bD | 7.18 ± 0.08 bE | ||||
| AW | 6.72 ± 0.17 cD | 6.90 ± 0.23 cD | ||||
| ENT | C | <1.00 | <1.00 | *** | *** | *** |
| T | <1.00 | <1.00 | ||||
| AW | <1.00 | 2.78 ± 0.32 aD | ||||
| Y&M | C | 6.54 ± 0.21 bE | 5.30 ± 0.43 aD | * | *** | * |
| T | 5.70 ± 0.09 aD | 5.60 ± 0.41 aD | ||||
| AW | 6.58 ± 0.18 bE | 5.90 ± 0.24 aD | ||||
| STA | C | <1.00 | <1.00 | NS | NS | NS |
| T | <1.00 | <1.00 | ||||
| AW | <1.00 | <1.00 | ||||
| LIST | C | nd | nd | NS | NS | NS |
| T | nd | nd | ||||
| AW | nd | nd | ||||
| Treatment | Storage Time (Weeks) | Treatment | Time of Storage | Treatment × Time of Storage | ||
|---|---|---|---|---|---|---|
| 0 | 4 | p | p | p | ||
| pH | C | 5.89 ± 0.01 bD | 5.90 ± 0.01 bD | *** | *** | *** |
| T | 6.06 ± 0.01 cE | 6.00 ± 0.04 cD | ||||
| AW | 5.78 ± 0.01 aE | 5.67 ± 0.01 aD | ||||
| ORP (mV) | C | 282.60 ± 1.87 aD | 300.50 ± 1.65 aE | *** | *** | *** |
| T | 287.87 ± 1.42 bD | 304.33 ± 0.68 bE | ||||
| AW | 363.13 ± 1.57 cE | 347.00 ± 3.32 cD | ||||
| Treatment | Storage Time (Weeks) | Treatment | Time of Storage | Treatment × Time of Storage | ||
|---|---|---|---|---|---|---|
| 0 | 4 | p | p | p | ||
| TBARS (mg/kg) | C | 0.96 ± 0.03 bE | 0.70 ± 0.03 aD | *** | *** | *** |
| T | 0.71 ± 0.05 aD | 1.62 ± 0.03 bE | ||||
| AW | 1.68 ± 0.05 cE | 0.73 ± 0.02 aD | ||||
| Treatment | Storage Time (Weeks) | Treatment | Time of Storage | Treatment × Time of Storage | ||
|---|---|---|---|---|---|---|
| 0 | 4 | p | p | p | ||
| Ʃ SFA | C | 34.77 ± 0.02 aD | 36.80 ± 0.04 bE | *** | *** | *** |
| T | 39.02 ± 0.22 bE | 35.55 ± 0.03 aD | ||||
| AW | 39.00 ± 0.01 bE | 36.70 ± 0.07 bD | ||||
| Ʃ MUFA | C | 51.37 ± 0.02 abE | 50.35 ± 0.09 aD | *** | *** | *** |
| T | 50.90 ± 0.10 aD | 56.80 ± 0.07 cE | ||||
| AW | 51.55 ± 0.03 bE | 51.15 ± 0.05 bD | ||||
| Ʃ PUFA | C | 13.75 ± 0.01 Be | 12.70 ± 0.03 cD | *** | *** | *** |
| T | 9.75 ± 0.02 aE | 7.60 ± 0.00 aD | ||||
| AW | 9.55 ± 0.01 aD | 11.80 ± 0.02 bE | ||||
| n-3 | C | 1.30 ± 0.01 cE | 1.20 ± 0.02 cD | *** | *** | *** |
| T | 0.83 ± 0.02 aE | 0.60 ± 0.03 aD | ||||
| AW | 0.90 ± 0.02 bD | 1.10 ± 0.01 bE | ||||
| n-6 | C | 11.75 ± 0.05 cE | 10.90 ± 0.02 cD | *** | *** | *** |
| T | 8.38 ± 0.15 bE | 6.40 ± 0.05 aD | ||||
| AW | 8.05 ± 0.05 aD | 10.20 ± 0.03 bE | ||||
| Ʃ PUFA/Ʃ SFA | C | 0.40 ± 0.02 bE | 0.35 ± 0.02 bD | *** | NS | *** |
| T | 0.25 ± 0.01 aE | 0.21 ± 0.01 aD | ||||
| AW | 0.24 ± 0.04 aD | 0.32 ± 0.03 bE | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoń, A.; Szymański, P.; Zielińska, D.; Szydłowska, A.; Siekierko, U.; Kołożyn-Krajewska, D.; Dolatowski, Z.J. The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage. Antioxidants 2021, 10, 1711. https://doi.org/10.3390/antiox10111711
Okoń A, Szymański P, Zielińska D, Szydłowska A, Siekierko U, Kołożyn-Krajewska D, Dolatowski ZJ. The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage. Antioxidants. 2021; 10(11):1711. https://doi.org/10.3390/antiox10111711
Chicago/Turabian StyleOkoń, Anna, Piotr Szymański, Dorota Zielińska, Aleksandra Szydłowska, Urszula Siekierko, Danuta Kołożyn-Krajewska, and Zbigniew J. Dolatowski. 2021. "The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage" Antioxidants 10, no. 11: 1711. https://doi.org/10.3390/antiox10111711
APA StyleOkoń, A., Szymański, P., Zielińska, D., Szydłowska, A., Siekierko, U., Kołożyn-Krajewska, D., & Dolatowski, Z. J. (2021). The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage. Antioxidants, 10(11), 1711. https://doi.org/10.3390/antiox10111711







