Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Metabolic Analysis Profiling Using LC/ESI-TOF-MS/MS
2.1.1. Plant Collection and Extraction
2.1.2. Metabolic Analysis Profiling Using LC/ESI-TOF-MS/MS
2.2. Isolation of Methyl Gallate and Quercetin and HPTLC Analysis
2.2.1. Instrumentation
2.2.2. General Experimental Procedure
2.2.3. Isolation and Purification of Methyl Gallate and Quercetin
2.2.4. HPTLC Analysis
Preparation of a Standard Solution of Methyl Gallate
Calibration Graph
Plant Sample Assay
2.3. In Vivo Study
2.3.1. Animals
2.3.2. Induction of Diabetes
2.3.3. Study Design
2.3.4. Induction of Hind Limb Ischemia
2.3.5. Collection of Tissue Samples
2.3.6. Histopathological Investigation
2.3.7. Biochemical Analysis
Determination of Levels of the Oxidative Stress and Angiogenesis Markers in the Hind Limb Tissue
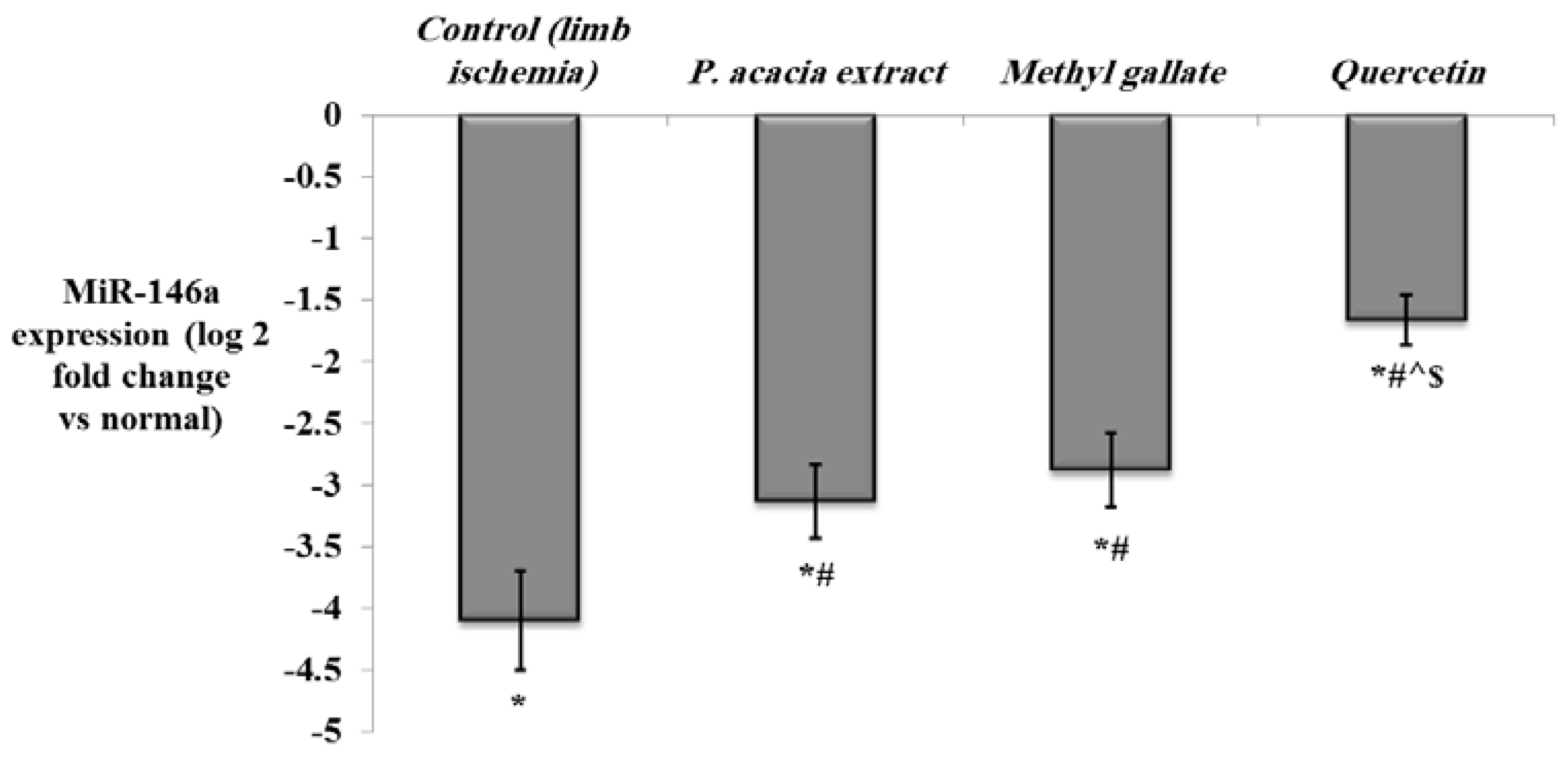
Determination of the Expression of miRNA-146a, NF-κb, HIF-1α, VEGF, and FGF-2 in the Hind Limb Tissue by Quantitative Real-Time PCR
2.4. Statistical Analysis
3. Results and Discussion
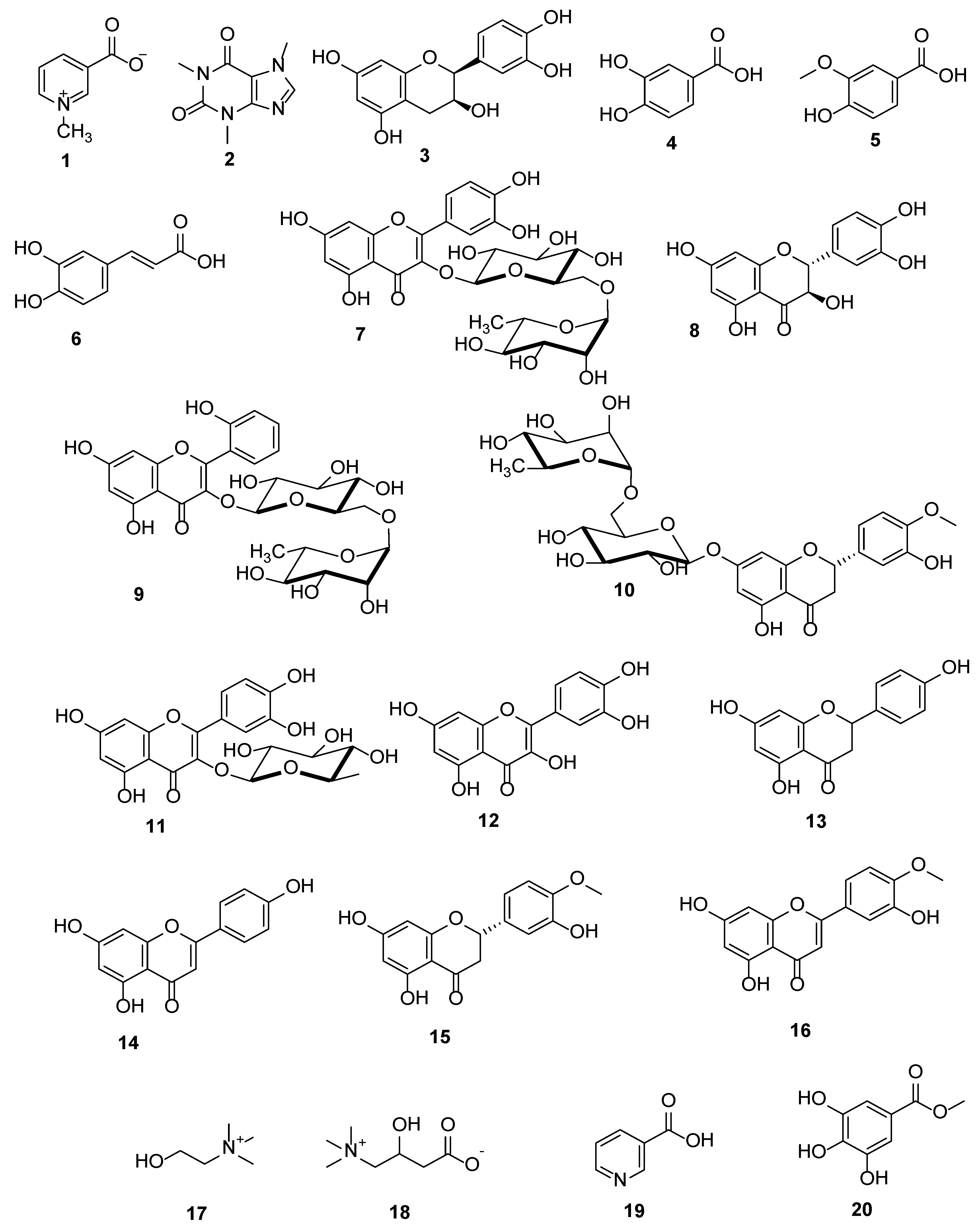
3.1. Metabolic Analysis Profiling using LC/ESI-TOF-MS/MS
3.2. Identification of the Isolated Compounds
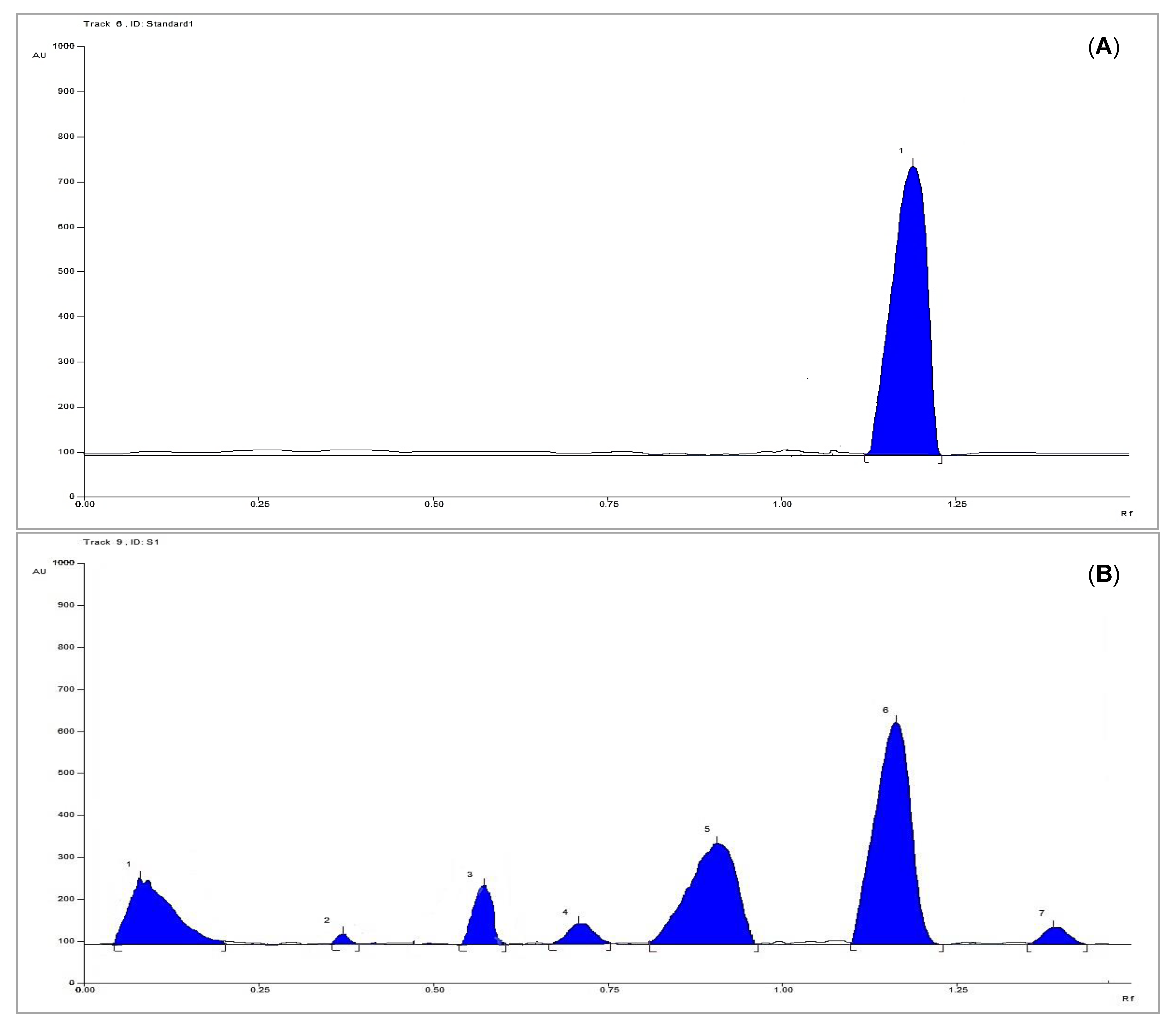
3.3. HPTLC Analysis for Quantification of Methyl Gallate
3.3.1. Linearity
3.3.2. System Precision
3.3.3. Method Precision
3.3.4. Accuracy
3.3.5. Limits of Detection and Quantification
3.3.6. Analytical Solution Stability
3.3.7. Sample Analysis
3.4. In Vivo Study
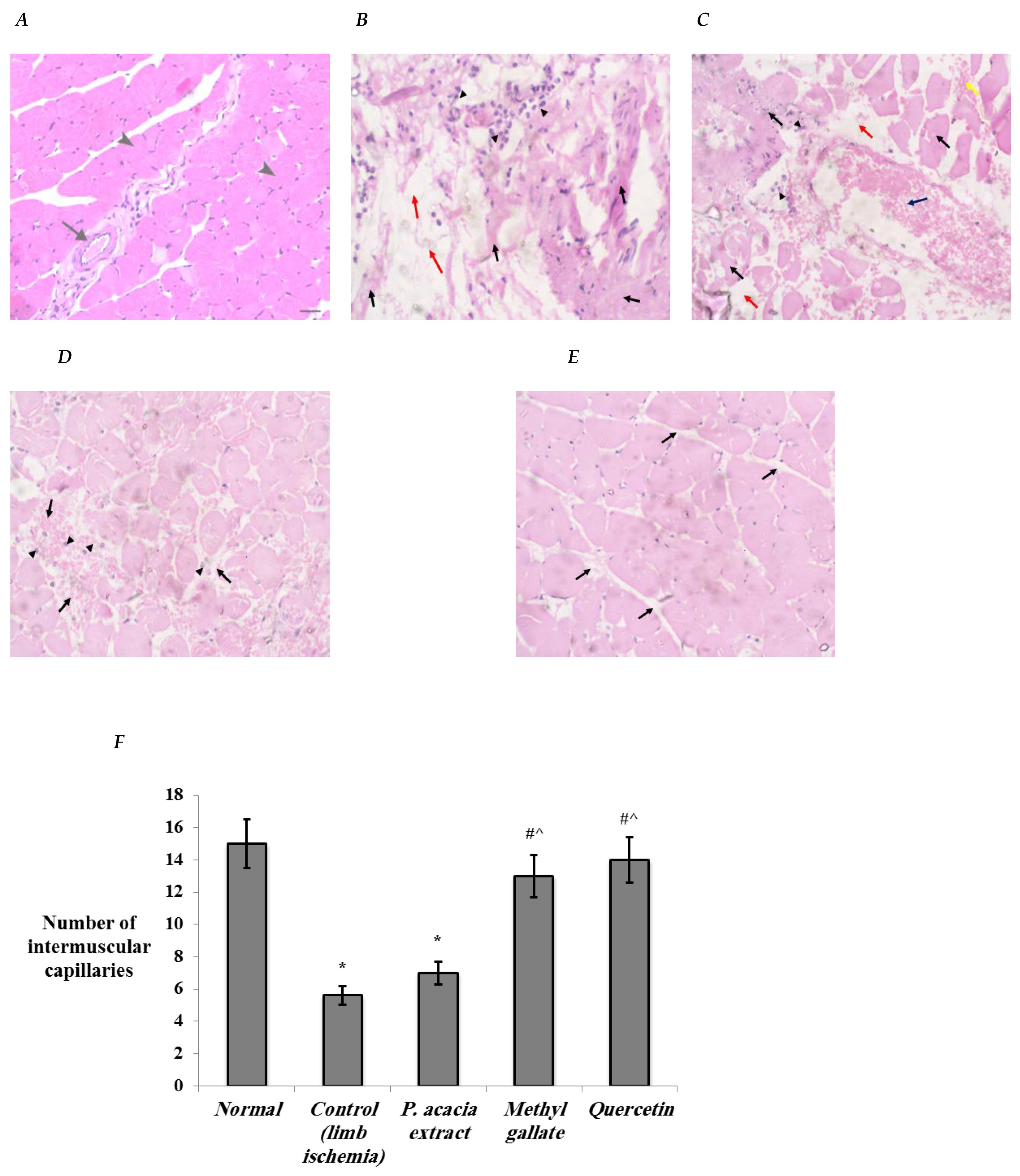
3.4.1. Histopathological Changes in the Study Groups
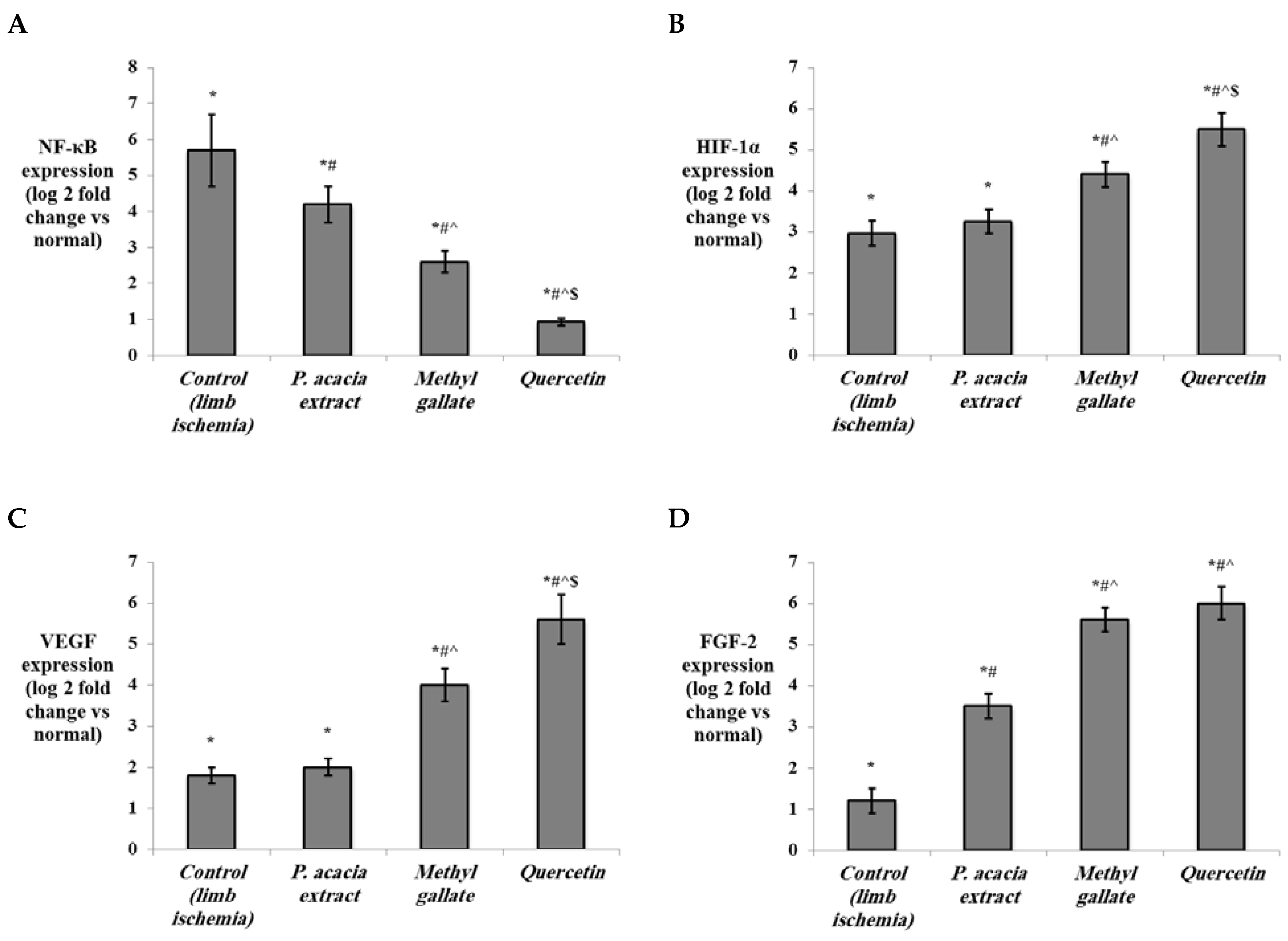
3.4.2. Effect of Treatment with the P. acacia Extract, Methyl Gallate and Quercetin on the Investigated Biochemical Parameters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, A.; Komada, M.R.; Sane, D.C. Abnormal angiogenesis in diabetes mellitus. Med. Res. Rev. 2003, 23, 117–145. [Google Scholar] [CrossRef]
- Olin, J.W.; Sealove, B.A. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo Clin. Proc. 2010, 85, 678–692. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Lüders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Eitenmüller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell. Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- Al-Khaldi, A.; Al-Sabti, H.; Galipeau, J.; Lachapelle, K. Therapeutic angiogenesis using autologous bone marrow stromal cells: Improved blood flow in a chronic limb ischemia model. Ann. Thorac. Surg. 2003, 75, 204–209. [Google Scholar] [CrossRef]
- Ozawa, C.R.; Banfi, A.; Glazer, N.L.; Thurston, G.; Springer, M.L.; Kraft, P.E.; McDonald, D.M.; Blau, H.M. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004, 113, 516–527. [Google Scholar] [CrossRef]
- Wang, C.P.; Shi, Y.W.; Tang, M.; Zhang, X.C.; Gu, Y.; Liang, X.M.; Wang, Z.W.; Ding, F. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol. Neurobiol. 2017, 54, 2126–2142. [Google Scholar] [CrossRef]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; deMuinck, E.D.; Zhuang, Z.; Drinane, M.; Kauser, K.; Rubanyi, G.M.; Qian, H.S.; Murata, T.; Escalante, B.; Sessa, W.C. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA 2005, 102, 10999–11004. [Google Scholar] [CrossRef]
- Foresti, R.; Motterlini, R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic. Res. 1999, 31, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Hajrezaei, M.; Abdul Kadir, H.; Zandi, K. Loranthus micranthus Linn.: Biological activities and phytochemistry. Evid. Based Complement. Altern. Med. 2013, 2013, 273712. [Google Scholar] [CrossRef] [PubMed]
- Deeni, Y.Y.; Sadiq, N.M. Antimicrobial properties and phytochemical constituents of the leaves of African mistletoe (Tapinanthus dodoneifolius (DC) Danser) (Loranthaceae): An ethnomedicinal plant of Hausaland, Northern Nigeria. J. Ethnopharmacol. 2002, 83, 235–240. [Google Scholar] [CrossRef]
- Costa, R.M.; Vaz, A.F.; Oliva, M.L.; Coelho, L.C.; Correia, M.T.; Carneiro-daCunha, M.G. A new mistletoe Phthirusa pyrifolia leaf lectin with antimicrobial properties. Process. Biochem. 2010, 45, 526–533. [Google Scholar] [CrossRef]
- Obatomi, D.K.; Bikomo, E.O.; Temple, V.J. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 1994, 43, 13–17. [Google Scholar] [CrossRef]
- Osadebe, P.O.; Okide, G.B.; Akabogu, I.C. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus (Linn.) sourced from five different host trees. J. Ethnopharmacol. 2004, 95, 133–138. [Google Scholar] [CrossRef]
- Moreno-Salazara, S.F.; Robles-Zepedab, R.E.; Johnsona, D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia 2008, 79, 132–141. [Google Scholar] [CrossRef]
- Elegami, A.A.; Elnima, E.I.; Muddathir, A.K.; Omer, M.E. Antimicrobial activity of Plicosepalus acaciae. Fitoterapia 2001, 72, 431–434. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Hanafy, A.; Labib, G.S.; Badr, G.M. Antihyperglycemic Activities of Extracts of the Mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z. Naturforsch. C J. Biosci. 2014, 69, 391–398. [Google Scholar] [CrossRef]
- Badr, J.M.; Shaala, L.A.; Youssef, D.T.A. Loranthin: A new polyhydroxylated flavanocoumarin from Plicosepalus acacia with significant free radical scavenging and antimicrobial activity. Phytochem. Lett. 2013, 6, 113–117. [Google Scholar] [CrossRef]
- Bamane, F.H.; Badr, J.M.; Amin, O.A. Antioxidant activities and flavonoid contents of selected plants belonging to family Loranthaceae. Afr. J. Biotechnol. 2012, 11, 14380–14385. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some african plants belonging to Kalanchoe species: Antitrypanosomal, cytotoxic, antitopoisomerase activities and chemical profiling using ultra-performance liquid chromatography/ quadrupole-time-of-flight mass spectrometer. Pharmacogn. Mag. 2021, 17, 73. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical profiling, in vitro and in silico anti-microbial and anti-cancer activity evaluations and Staph GyraseB and h-TOP-IIβ receptor-docking studies of major constituents of Zygophyllum coccineum L. Aqueous-ethanolic extract and its subsequent fractions: An approach to validate traditional phytomedicinal knowledge. Molecules 2021, 26, 577. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.S.; Khanfar, M.S. Validation of an RP-HPLC Method for the Determination of Asenapine Maleate in Dissolution Media and Application to Study In Vitro Release from Co-Crystals. Sci. Pharm. 2021, 89, 14. [Google Scholar] [CrossRef]
- Taniyama, Y.; Morishita, R.; Aoki, M.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindlimb ischemia models: Preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A.; Yamamoto, Y.; Tanaka, K.; Matsubara, K.; Sugitani, M.; Fujihara, S.; Harada, S.; Kaetsu, Y.; Yoshida, A.; Hisatome, I. Fenofibrate enhances neovascularization in a murine ischemic hindlimb model. J. Cardiovasc. Pharmacol. 2009, 54, 399–404. [Google Scholar] [CrossRef]
- Köksoy, C.; Oziş, E.; Cakmak, A.; Yazgan, U.; Okcu-Heper, A.; Köksoy, A.; Demirpençe, E.; Deniz Dinçer, U. Simvastatin pretreatment reduces the severity of limb ischemia in an experimental diabetes model. J. Vasc. Surg. 2007, 45, 590–596. [Google Scholar] [CrossRef][Green Version]
- Jokinen, M.P.; Lieuallen, W.G.; Boyle, M.C.; Johnson, C.L.; Malarkey, D.E.; Nyska, A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol. Pathol. 2011, 39, 850–860. [Google Scholar] [CrossRef]
- Lang, R.; Yagar, E.F.; Eggers, R.; Hofmann, T. Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J. Agric. Food Chem. 2008, 56, 11114–11121. [Google Scholar] [CrossRef]
- Mendes, V.M.; Coelho, M.; Tomé, A.R.; Cunha, R.A.; Manadas, B. Validation of an LC-MS/MS method for the quantification of caffeine and theobromine using non-matched matrix calibration curve. Molecules 2019, 24, 2863. [Google Scholar] [CrossRef]
- Cai, R.J.; Yin, X.L.; Liu, J.; Qin, D.X.; Zhao, G.Z. Characterization and identification of in vitro metabolites of (-)-epicatechin using ultra-high performance liquid chromatography-mass spectrometry. Trop. J. Pharm. Res. 2018, 16, 2985. [Google Scholar] [CrossRef]
- Wang, X.; Yan, K.; Ma, X.; Li, W.; Chu, Y.; Guo, J.; Li, S.; Zhou, Y.; Liu, C. Simultaneous determination and pharmacokinetic study of protocatechuic aldehyde and its major active metabolite protocatechuic acid in rat plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. Sci. 2016, 54, 697–705. [Google Scholar] [CrossRef]
- Grieman, M.M.; Greaves, J.; Saltzman, E.S. A method for analysis of vanillic acid in polar ice cores. Clim. Past 2015, 11, 227–232. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, Z.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by uplc-qqq-ms/ms. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef]
- Yang, C.-J.; Wang, Z.-B.; Mi, Y.-Y.; Gao, M.-J.; Lv, J.-N.; Meng, Y.-H.; Yang, B.-Y.; Kuang, H.-X. UHPLC-MS/MS Determination, pharmacokinetic, and bioavailability study of taxifolin in rat plasma after oral administration of its nanodispersion. Molecules 2016, 21, 494. [Google Scholar] [CrossRef]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. LC-MS/MS Analysis of flavonoid compounds from Zanthoxylum zanthoxyloides extracts and their antioxidant activities. Nat. Prod. Commun. 2017, 12, 1865–1868. [Google Scholar] [CrossRef]
- Lee, J.-T.; Pao, L.-H.; Hsieh, C.-D.; Huang, P.-W.; Hu, O.Y.-P. Development and validation of an LC-MS/MS method for simultaneous quantification of hesperidin and hesperetin in rat plasma for pharmacokinetic studies. Anal. Methods 2017, 9, 3329–3337. [Google Scholar] [CrossRef]
- Šibul, F.; Orčić, D.; Berežni, S.; Anačkov, G.; Mimica-Dukić, N. HPLC–MS/MS profiling of wild-growing scentless chamomile. Acta Chromatogr. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, D.; Gao, J.; Zhu, Y.; Sun, H.; Bi, K. Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 58, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.A.; Escolar, M.; Perez, G.; Garcia-Quetglas, E.; Sadaba, B.; Azanza, J.R. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 875–881. [Google Scholar] [CrossRef]
- Bruce, S.J.; Guy, P.A.; Rezzi, S.; Ross, A.B. Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J. Agric. Food Chem. 2010, 58, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, D.J.; Shippar, J.J.; Gilmore, J.M. Determination of free and total choline and carnitine in infant formula and adult/pediatric nutritional formula by liquid chromatography/tandem mass spectrometry (LC/MS/MS): Single-Laboratory Validation, First Action 2015.10. J. AOAC Int. 2016, 99, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chernonosov, A.A.; Karpova, E.A.; Lyakh, E.M. Identification of phenolic compounds in Myricaria bracteata leaves by high-performance liquid chromatography with a diode array detector and liquid chromatography with tandem mass spectrometry. Rev. Bras. Farmacogn. 2017, 27, 576–579. [Google Scholar] [CrossRef]
- Noman, O.M.; Mothana, R.A.; Al-Rehaily, A.J.; Al Qahtani, A.S.; Nasr, F.A.; Khaled, J.M.; Alajmi, M.F.; Al-Said, M.S. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. Grown in Saudi Arabia. Saudi Pharm. J. 2019, 27, 724–730. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Goda, M.S.; Fahim, J.R.; Hassanean, H.A.; Eltamany, E.E.; Ibrahim, A.K.; AboulMagd, A.M.; Fayez, S.; El-kader, A.M.A.; et al. Thalassosterol, a new cytotoxic aromatase inhibitor ergosterol derivative from the Red Sea seagrass Thalassodendron ciliatum. Mar. Drugs 2020, 18, 354. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Chidambara-Murthy, K.N. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Vieira, A.J.S.C.; Gaspar, E.M.; Santos, P.M.P. Mechanisms of potential antioxidant activity of caffeine. Radiat. Phys. Chem. 2020, 174, 108968. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of antioxidant properties of a conjugate of taxifolin with glyoxylic acid and selected flavonoids. Antioxidants 2021, 10, 1262. [Google Scholar] [CrossRef]
- Tan, Y.; Tam, C.C.; Rolston, M.; Alves, P.; Chen, L.; Meng, S.; Hong, H.; Chang, S.K.C.; Yokoyama, W. Quercetin ameliorates insulin resistance and restores gut microbiome in mice on high-fat diets. Antioxidants 2021, 10, 1251. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Huang, H.; Song, L.; Zhang, H.; Zhang, H.; Zhang, J.; Zhao, W. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: A systematic review and meta-analysis. Kidney Blood Press. Res. 2013, 38, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dardik, A. A Murine Model of Hind Limb Ischemia to Study Angiogenesis and Arteriogenesis. Methods Mol. Biol. 2018, 1717, 135–143. [Google Scholar] [CrossRef]
- Taniyama, Y.; Morishita, R.; Hiraoka, K.; Aoki, M.; Nakagami, H.; Yamasaki, K.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: Molecular mechanisms of delayed angiogenesis in diabetes. Circulation 2001, 104, 2344–2350. [Google Scholar] [CrossRef]
- Ferraro, B.; Cruz, Y.L.; Baldwin, M.; Coppola, D.; Heller, R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010, 17, 763–769. [Google Scholar] [CrossRef]
- So, K.; Tei, Y.; Zhao, M.; Miyake, T.; Hiyama, H.; Shirakawa, H.; Imai, S.; Mori, Y.; Nakagawa, T.; Matsubara, K.; et al. Hypoxia-induced sensitisation of TRPA1 in painful dysesthesia evoked by transient hindlimb ischemia/reperfusion in mice. Sci. Rep. 2016, 6, 23261. [Google Scholar] [CrossRef]
- Kuroda, Y.; Togashi, H.; Uchida, T.; Haga, K.; Yamashita, A.; Sadahiro, M. Oxidative stress evaluation of skeletal muscle in ischemia-reperfusion injury using enhanced magnetic resonance imaging. Sci. Rep. 2020, 10, 10863. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Sasaki, H.; Fukuda, S.; Otani, H.; Zhu, L.; Yamaura, G.; Engelman, R.M.; Das, D.K.; Maulik, N. Hypoxic preconditioning triggers myocardial angiogenesis: A novel approach to enhance contractile functional reserve in rat with myocardial infarction. J. Mol. Cell. Cardiol. 2002, 34, 335–348. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of NADPH oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar] [CrossRef]
- Colavitti, R.; Pani, G.; Bedogni, B.; Anzevino, R.; Borrello, S.; Waltenberger, J.; Galeotti, T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002, 277, 3101–3108. [Google Scholar] [CrossRef]
- Birukova, A.A.; Lee, S.; Starosta, V.; Wu, T.; Ho, T.; Kim, J.; Berliner, J.A.; Birukov, K.G. A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS ONE 2012, 7, e30957. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Philippova, M.; Oskolkova, O.; Kadl, A.; Furnkranz, A.; Karabeg, E.; Afonyushkin, T.; Gruber, F.; Breuss, J.; Minchenko, A.; et al. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 2006, 99, 900–908. [Google Scholar] [CrossRef]
- Hutter, R.; Speidl, W.S.; Valdiviezo, C.; Sauter, B.; Corti, R.; Fuster, V.; Badimon, J.J. Macrophages transmit potent proangiogenic effects of oxLDL in vitro and in vivo involving HIF-1α activation: A novel aspect of angiogenesis in atherosclerosis. J. Cardiovasc. Transl. Res. 2013, 6, 558–569. [Google Scholar] [CrossRef]
- Wu, Q.; Finley, S.D. Mathematical Model Predicts Effective Strategies to Inhibit VEGF-eNOS Signaling. J. Clin. Med. 2020, 9, 1255. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef]
- Mutoh, A.; Ueda, S. Peroxidized unsaturated fatty acids stimulate Toll-like receptor 4 signaling in endothelial cells. Life Sci. 2013, 92, 984–992. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Spirig, R.; Djafarzadeh, S.; Regueira, T.; Shaw, S.G.; von Garnier, C.; Takala, J.; Jakob, S.M.; Rieben, R.; Lepper, P.M. Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS ONE 2010, 5, e0010983. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.C.; Chung, C.Y.; Chang, Y.Y.; Lin, C.K.; Sheu, J.R.; Huang, C.J. Salutary Effects of Cepharanthine against Skeletal Muscle and Kidney Injuries following Limb Ischemia/Reperfusion. Evid. Based Complement. Alternat. Med. 2015, 2015, 504061. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, Y.; Yu, P.; Liu, J.; Mei, X.; Meng, J. Protective Effect of Hydrogen Gas on Mice Hind Limb Ischemia-Reperfusion Injury. J. Surg. Res. 2021, 266, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Schmidt, C.A.; Green, T.D.; Spangenburg, E.E.; Neufer, P.D.; McClung, J.M. Targeted Expression of Catalase to Mitochondria Protects Against Ischemic Myopathy in High-Fat Diet-Fed Mice. Diabetes 2016, 65, 2553–2568. [Google Scholar] [CrossRef]
- Zaghloul, N.; Patel, H.; Codipilly, C.; Marambaud, P.; Dewey, S.; Frattini, S.; Huerta, P.T.; Nasim, M.; Miller, E.J.; Ahmed, M. Overexpression of extracellular superoxide dismutase protects against brain injury induced by chronic hypoxia. PLoS ONE 2014, 9, e108168. [Google Scholar] [CrossRef]
- Crispo, J.A.; Ansell, D.R.; Piche, M.; Eibl, J.K.; Khaper, N.; Ross, G.M.; Tai, T.C. Protective effects of polyphenolic compounds on oxidative stress-induced cytotoxicity in PC12 cells. Can. J. Physiol. Pharmacol. 2010, 88, 429–438. [Google Scholar] [CrossRef]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef]
- Wang, C.R.; Zhou, R.; Ng, T.B.; Wong, J.H.; Qiao, W.T.; Liu, F. First report on isolation of methyl gallate with antioxidant, anti-HIV-1 and HIV-1 enzyme inhibitory activities from a mushroom (Pholiota adiposa). Environ. Toxicol. Pharmacol. 2014, 37, 626–637. [Google Scholar] [CrossRef]
- Farhoosh, R.; Nyström, L. Antioxidant potency of gallic acid, methyl gallate and their combinations in sunflower oil triacylglycerols at high temperature. Food Chem. 2018, 244, 29–35. [Google Scholar] [CrossRef]
- Rahman, N.; Jeon, M.; Kim, Y.S. Methyl gallate, a potent antioxidant inhibits mouse and human adipocyte differentiation and oxidative stress in adipocytes through impairment of mitotic clonal expansion. Biofactors 2016, 42, 716–726. [Google Scholar] [CrossRef]
- Ahmed, A.Z.; Satyam, S.M.; Shetty, P.; D’Souza, M.R. Methyl Gallate Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by Suppressing Oxidative Stress. Scientifica 2021, 2021, 6694340. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Kinaci, M.K.; Erkasap, N.; Kucuk, A.; Koken, T.; Tosun, M. Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp. Ther. Med. 2012, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhong, T.; Wu, H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. Sci. 2015, 11, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Akkoyun, D.C.; Akyuz, A.; Dogan, M.; Erboga, M.; Aktas, C.; Caglar, V.; Uygur, R.; Topcu, B.; Yilmaz, A.; Gurel, A. Quercetin Inhibits Heart Injury in Lipopolysaccharide-induced Endotoxemic Model by Suppressing the Effects of Reactive Oxygen Species. Anal. Quant. Cytopathol. Histopathol. 2016, 38, 183–188. [Google Scholar]
- Yan, J.; Tie, G.; Park, B.; Yan, Y.; Nowicki, P.T.; Messina, L.M. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: Roles of endothelial nitric oxide synthase and endothelial progenitor cells. J. Vasc. Surg. 2009, 50, 1412–1422. [Google Scholar] [CrossRef]
- Sorrentino, S.; Iaconetti, C.; De Rosa, S.; Polimeni, A.; Sabatino, J.; Gareri, C.; Passafaro, F.; Mancuso, T.; Tammè, L.; Mignogna, C.; et al. Hindlimb Ischemia Impairs Endothelial Recovery and Increases Neointimal Proliferation in the Carotid Artery. Sci. Rep. 2018, 8, 761. [Google Scholar] [CrossRef]
- Yang, C.; Hwang, H.H.; Jeong, S.; Seo, D.; Jeong, Y.; Lee, D.Y.; Lee, K. Inducing angiogenesis with the controlled release of nitric oxide from biodegradable and biocompatible copolymeric nanoparticles. Int. J. Nanomed. 2018, 13, 6517–6530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, X.; Li, H.; Feng, G.; Nie, Y.; Wei, Y.; Li, N.; Han, Z.; Han, Z.C.; Kong, D.; et al. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater. 2020, 113, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, Q.H.; Zhou, C.J.; Hu, M.Z.; Qian, H.X. Protective effect of eNOS overexpression against ischemia/reperfusion injury in small-for-size liver transplantation. Exp. Ther. Med. 2016, 12, 3181–3188. [Google Scholar] [CrossRef]
- Park, B.; Hoffman, A.; Yang, Y.; Yan, J.; Tie, G.; Bagshahi, H.; Nowicki, P.T.; Messina, L.M. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J. Vasc. Surg. 2010, 51, 165–173. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Li, P.G.; Sun, L.; Han, X.; Ling, S.; Gan, W.T.; Xu, J.W. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology 2012, 89, 220–228. [Google Scholar] [CrossRef]
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Kong, S.M.Y.; Tumanov, S.; Chen, W.; Cantley, J.; Ayer, A.; Maghzal, G.J.; Midwinter, R.G.; Chan, K.H.; Ng, M.K.C.; et al. Hmox1 (Heme Oxygenase-1) Protects Against Ischemia-Mediated Injury via Stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Thierry, A.; Delbauve, S.; Preyat, N.; Soares, M.P.; Roumeguère, T.; Leo, O.; Flamand, V.; Le Moine, A.; Hougardy, J.M. Specific expression of heme oxygenase-1 by myeloid cells modulates renal ischemia-reperfusion injury. Sci. Rep. 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE 2014, 9, e92246. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2008, 20, 1767–1812. [Google Scholar] [CrossRef]
- Selvaraju, V.; Parinandi, N.L.; Adluri, R.S.; Goldman, J.W.; Hussain, N.; Sanchez, J.A.; Maulik, N. Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid. Redox Signal. 2014, 20, 2631–2665. [Google Scholar] [CrossRef]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Fox-Talbot, K.; Steenbergen, C.; Bosch-Marcé, M.; Semenza, G.L. Adenoviral transfer of HIF-1 alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 18769–18774. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.V.; Arbeit, J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 13264–13269. [Google Scholar] [CrossRef]
- Ma, Y.; Zechariah, A.; Qu, Y.; Hermann, D.M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 2012, 90, 1873–1882. [Google Scholar] [CrossRef]
- Samura, M.; Hosoyama, T.; Takeuchi, Y.; Ueno, K.; Morikage, N.; Hamano, K. Therapeutic strategies for cell-based neovascularization in critical limb ischemia. J. Transl. Med. 2017, 15, 49. [Google Scholar] [CrossRef]
- Isner, J.M.; Pieczek, A.; Schainfeld, R.; Blair, R.; Haley, L.; Asahara, T.; Rosenfield, K.; Razvi, S.; Walsh, K.; Symes, J.F. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 1996, 348, 370–374. [Google Scholar] [CrossRef]
- Conte, C.; Riant, E.; Toutain, C.; Pujol, F.; Arnal, J.F.; Lenfant, F.; Prats, A.C. FGF2 translationally induced by hypoxia is involved in negative and positive feedback loops with HIF-1alpha. PLoS ONE 2008, 3, e3078. [Google Scholar] [CrossRef]
- Yamamoto, N.; Oyaizu, T.; Enomoto, M.; Horie, M.; Yuasa, M.; Okawa, A.; Yagishita, K. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep. 2020, 10, 2744. [Google Scholar] [CrossRef] [PubMed]
- Masaki, I.; Yonemitsu, Y.; Yamashita, A.; Sata, S.; Tanii, M.; Komori, K.; Nakagawa, K.; Hou, X.; Nagai, Y.; Hasegawa, M.; et al. Angiogenic gene therapy for experimental critical limb ischemia: Acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ. Res. 2002, 90, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tao, Q.; Li, G.; Xiang, L.; Zheng, X.; Zhang, T.; Wu, C.; Li, D. Fibroblast Growth Factor 2 Attenuates Renal Ischemia-Reperfusion Injury via Inhibition of Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2020, 8, 147. [Google Scholar] [CrossRef]
- Sumi, M.; Tateishi, N.; Shibata, H.; Ohki, T.; Sata, M. Quercetin glucosides promote ischemia-induced angiogenesis, but do not promote tumor growth. Life Sci. 2013, 93, 814–819. [Google Scholar] [CrossRef]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, W.; Chen, Y.; Du, Z.; Zhu, F.; Wang, T.; Jiang, B. Ischemic postconditioning ameliorates acute kidney injury induced by limb ischemia/reperfusion via transforming TLR4 and NF-κB signaling in rats. J. Orthop. Surg. Res. 2021, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.B.; Chen, B.H.; Tang, Y.N.; Weng, C.W.; Lin, L.N. Dexmedetomidine protects against lung injury induced by limb ischemia-reperfusion via the TLR4/MyD88/NF-κB pathway. Kaohsiung J. Med. Sci. 2019, 35, 672–678. [Google Scholar] [CrossRef]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Li, S.H.; Chen, L.; Pang, X.M.; Su, S.Y.; Zhou, X.; Chen, C.Y.; Huang, L.G.; Li, J.P.; Liu, J.L. Decreased miR-146a expression in acute ischemic stroke directly targets the Fbxl10 mRNA and is involved in modulating apoptosis. Neurochem. Int. 2017, 107, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kong, L.; Ni, Q.; Lu, Y.; Ding, W.; Liu, G.; Pu, L.; Tang, W.; Kong, L. miR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS ONE 2014, 9, e101530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, Y.; Gao, L.; Mao, C.; Zeng, H.; Wang, X.; Sun, Y.; Gu, J.; Wang, Y.; Chen, K.; et al. MicroRNA-146a protects against myocardial ischaemia reperfusion injury by targeting Med1. Cell. Mol. Biol. Lett. 2019, 24, 62. [Google Scholar] [CrossRef]
- He, X.; Zheng, Y.; Liu, S.; Shi, S.; Liu, Y.; He, Y.; Zhang, C.; Zhou, X. MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. J. Cell. Physiol. 2018, 233, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Bai, W.D.; Liu, J.Q.; Zheng, Z.; Guan, H.; Zhou, Q.; Su, L.L.; Xie, S.T.; Wang, Y.C.; Li, J.; et al. Up-regulation of FGFBP1 signaling contributes to miR-146a-induced angiogenesis in human umbilical vein endothelial cells. Sci. Rep. 2016, 6, 25272. [Google Scholar] [CrossRef]
- Zhu, K.; Pan, Q.; Zhang, X.; Kong, L.Q.; Fan, J.; Dai, Z.; Wang, L.; Yang, X.R.; Hu, J.; Wan, J.L.; et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis 2013, 34, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chu, A.; Feng, Y.; Chen, L.; Shao, Y.; Luo, Q.; Deng, X.; Wu, M.; Shi, X.; Chen, Y. MicroRNA-146a: A Comprehensive Indicator of Inflammation and Oxidative Stress Status Induced in the Brain of Chronic T2DM Rats. Front. Pharmacol. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, B.; Peng, X.; Wang, C.; Wang, K.; Han, F.; Xu, J. Quercetin suppresses migration and invasion by targeting miR-146a/GATA6 axis in fibroblast-like synoviocytes of rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2020, 42, 221–227. [Google Scholar] [CrossRef]




| Gene | Forward Primer | Reverse Primer | Annealing Temperature |
|---|---|---|---|
| NF-κb | 5’-CAATGGCTACACAGGACCA-3’ | 5′-CACTGTCACCTGGAACCAGA-3′ | 55 °C |
| HIF-1α | 5’-TGCTTGGTGCTGATTTGTGA-3’ | 5’-GGTCAGATGATCAGAGTCCA-3’ | 54 °C |
| VEGF | 5’-AAAAACGAAAGCGCAAGAAA-3’ | 5’-TTTCTCCGCTCTGAACAAGG-3’ | 51 °C |
| FGF-2 | 5’-GGCTCTACTGCAAGAACGGC-3’ | 5’-GAAACAGTATGGCCTTCTGTC-3’ | 53 °C |
| GAPDH | 5′-ATGACTCTACCCACGGCAAG−3’ | 5′-GATCTCGCTCCTGGAAGATG-3’ | 55 °C |
| No. | Polarity Mode | MZmine ID | Ret. Time (min) | Measured m/z | Calculated m/z | Mass Error (ppm) | Adduct | Molecular Formula | MS/MS Spectrum | Deduced Compound | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||||||
| 1 | Positive | 83 | 1.37 | 138.0543 | 138.0555 | −8.69 | [M + H] + | C7H7NO2 | 138, 94 | Trigonelline | [30] |
| 2 | Positive | 167 | 4.94 | 195.0876 | 195.0882 | −3.08 | [M + H] + | C8H10N4O2 | 195, 138 | Caffeine | [31] |
| Catechins | |||||||||||
| 3 | Negative | 272 | 4.63 | 289.0717 | 289.0712 | 1.73 | [M − H] − | C15H14O6 | 289, 245, 205, 179 | (−)−Epicatechin | [32] |
| Phenolic Acids | |||||||||||
| 4 | Negative | 108 | 1.21 | 153.0192 | 153.0188 | 2.61 | [M − H] − | C7H6O4 | 153, 109 | Protocatechuic acid | [33] |
| 5 | Negative | 326 | 6.86 | 167.0343 | 167.0344 | −0.60 | [M − H] − | C9H8O2 | 167, 152, 124, 108 | Vanillic acid | [34] |
| 6 | Positive | 657 | 9.36 | 181.0516 | 181.0501 | 8.82 | [M + H] + | C9H8O4 | 181, 163 | Caffeic acid | [35] |
| Flavonoids and Their Glycosides | |||||||||||
| 7 | Negative | 307 | 6.26 | 609.1453 | 609.1456 | −0.49 | [M − H] − | C27H30O16 | 609, 300 | Rutin | [35] |
| 8 | Negative | 314 | 6.50 | 303.0504 | 303.0505 | −0.33 | [M − H] − | C15H12O7 | 303, 285 | Taxifolin | [36] |
| 9 | Negative | 318 | 6.57 | 593.1569 | 593.1506 | 10.62 | [M − H] − | C27H29O15 | 593, 285 | Datiscin | [37] |
| 10 | Positive | 305 | 6.71 | 611.1911 | 611.1976 | −10.63 | [M + H] + | C28H34O15 | 611, 303 | Hesperidin | [38] |
| 11 | Negative | 339 | 7.22 | 447.0945 | 447.0927 | 4.03 | [M − H] − | C21H20O11 | 447, 300 | Quercetrin | [37] |
| 12 | Negative | 488 | 9.51 | 301.0359 | 301.0348 | 3.65 | [M − H] − | C15H9O7 | 301, 151 | Quercetin | [35] |
| 13 | Negative | 528 | 10.03 | 271.0607 | 271.0606 | 0.37 | [M − H] − | C15H12O5 | 271, 177, 151, 119 | Naringenin | [39] |
| 14 | Negative | 558 | 10.48 | 269.0465 | 269.0450 | 5.58 | [M − H] − | C15H10O5 | 269, 151 | Apigenin | [35] |
| 15 | Positive | 863 | 10.62 | 303.0886 | 303.0869 | 5.61 | [M + H] + | C16H14O6 | 303, 151 | Hesperetin | [40] |
| 16 | Positive | 975 | 11.42 | 301.0736 | 301.0712 | 7.97 | [M + H] + | C16H12O6 | 301, 286 | Diosmetin | [41] |
| Miscellaneous compounds | |||||||||||
| 17 | Positive | 33 | 1.19 | 104.1066 | 104.1070 | −3.84 | [M] + | C5H14NO | 104, 60 | Choline | [42] |
| 18 | Positive | 37 | 1.24 | 162.1115 | 162.1130 | −9.25 | [M + H] + | C7H15NO3 | 162, 103, 85 | Carnitine | [43] |
| 19 | Positive | 141 | 1.73 | 124.0386 | 124.0399 | −10.48 | [M + H] + | C6H5NO2 | 124, 80 | Nicotinic acid | [30] |
| 20 | Negative | 281 | 4.90 | 183.0309 | 183.0293 | 8.74 | [M − H] − | C8H8O5 | 183, 140, 124 | Methyl gallate | [44] |
| Parameter | Results |
|---|---|
| Linearity range (µg per band) | 4–40 |
| Correlation coefficient (R2) | 0.966 |
| Regression equation | Y = 910.69X + 20,008 |
| Limit of detection (µg per band) | 0.4893 |
| Limit of quantification (µg per band) | 1.6310 |
| System precision (%RSD) | 2.52 |
| Method precision (%RSD) | 1.14 |
| Histopathological Features | Normal | Control (Limb Ischemia) | P. acacia | Methyl Gallate | Quercetin |
|---|---|---|---|---|---|
| Degenerative changes | None | Marked (Grade 4) | Moderate (Grade 3) | None | None |
| Edema, hemorrhage | None | Marked (Grade 4) | Moderate (Grade 3) | Mild hemorrhage (Grade 2) | Minimal edema (Grade 1) |
| Inflammation | None | Marked (Grade 4) | Moderate (Grade 3) | Mild (Grade 2) | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Hamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling. Antioxidants 2021, 10, 1701. https://doi.org/10.3390/antiox10111701
Abdel-Hamed AR, Mehanna ET, Hazem RM, Badr JM, Abo-Elmatty DM, Abdel-Kader MS, Goda MS. Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling. Antioxidants. 2021; 10(11):1701. https://doi.org/10.3390/antiox10111701
Chicago/Turabian StyleAbdel-Hamed, Asmaa R., Eman T. Mehanna, Reem M. Hazem, Jihan M. Badr, Dina M. Abo-Elmatty, Maged S. Abdel-Kader, and Marwa S. Goda. 2021. "Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling" Antioxidants 10, no. 11: 1701. https://doi.org/10.3390/antiox10111701
APA StyleAbdel-Hamed, A. R., Mehanna, E. T., Hazem, R. M., Badr, J. M., Abo-Elmatty, D. M., Abdel-Kader, M. S., & Goda, M. S. (2021). Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling. Antioxidants, 10(11), 1701. https://doi.org/10.3390/antiox10111701







