Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Isolation of Brain Mitochondria
2.4. Modeling Oxidative Stress in Mitochondria and Adding Metabolic Protectors In Vitro
2.5. Free and Protein-Bound TBARS Assay
2.6. Assay of TCA Enzymes Activity
2.7. Determination of the Thiol Redox State
2.8. Statistical Analysis
3. Results
Protective Effect of PL in tBHP-Induced Oxidative Stress of Mitochondria In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2525967. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, 125–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ratan, R.R. Oxidative stress and Huntington’s disease: The good, the bad, and the ugly. J. Huntingtons Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Vega, I.E.; Brundin, P. Oxidative stress in neurodegenerative disease: Causation or association. Oncotarget 2017, 8, 10778. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wuliji, O.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Shimohama, S.; Tanino, H.; Kawakami, N. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2000, 273, 5–9. [Google Scholar] [CrossRef]
- Hayashi, M. Oxidative stress in development of brain disorders. Neuropathology 2009, 29, 1–8. [Google Scholar] [CrossRef]
- Conrad, M.; Schick, J.; Friedmann, J.P. Angeli Glutathione and thioredoxin dependent systems in neurodegenerative disease: What can be learned from reverse genetics in mice. Neurochem. Int. 2013, 62, 738–749. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Aoyama, K.; Suh, S.W.; Hamby, A.M.; Liu, J.; Chan, W.Y.; Chen, Y.; Swanson, R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- McBean, G.J.; Aslan, M.; Griffiths, H.R.; Torrão, R.C. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015, 5, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenin, J. Alzheimer’s disease: Redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid. Redox Signal. 2012, 16, 974–1031. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. Biochim. Biophys. Acta 1998, 1366, 211–223. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lezi, E.; Swerdlow, R.H. Mitochondria in neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286. [Google Scholar]
- Mancuso, M. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J. Alzheimers Dis. 2006, 10, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef]
- Dringen, R.; Hirrlinger, J. Glutathione pathways in the brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Internat. J.Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef]
- Bains, J.S.; Shaw, C.H. Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Rev. 1997, 25, 335–358. [Google Scholar] [CrossRef]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Morgan, B.; Riemer, J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Signal. 2017, 27, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lipton, S.A. Cell death: Protein misfolding and neurodegenerative diseases. Apoptosis 2009, 14, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Colombo, G.; Gagliano, N.; Colombo, R.; Giustarini, D. S-glutathionylation in life and death decisions of the cell. Free Radic. Res. 2011, 45, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Butterfield, D. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J. Bioenerg. Biomembr. 2009, 41, 441–446. [Google Scholar] [CrossRef]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Han, D.; Sancheti, H.; Yap, L.P.; Kaplowitz, N.; Cadenas, E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J. Biol. Chem. 2010, 285, 39646–39654. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grintzalis, K.; Zisimopoulos, D.; Grune, T.; Weber, D.; Georgiou, C.D. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic. Biol. Med. 2013, 59, 27–35. [Google Scholar] [CrossRef]
- Zheng, W.; Ren, S.; Graziano, J.H. Manganese inhibits mitochondrial aconitase: A mechanism of manganese neurotoxicity. Brain Res. 1998, 799, 334–342. [Google Scholar] [CrossRef]
- Singer, T.P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem. Anal. 1974, 22, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Dekker, E.E. Evidence for the identity and some comparative properties of alpha-ketoglutarate and 2-keto-4-hydroxyglutarate dehydrogenase activity. J. Biol. Chem. 1980, 255, 1107–1112. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Georgiou, C.D. Determination of the thiol redox state of organisms: New oxidative stress indicators. Anal. Bioanal. Chem. 2004, 378, 1783–1792. [Google Scholar] [CrossRef]
- Menon, D.; Board, P.G. A fluorometric method to quantify protein glutathionylation using glutathione derivatization with 2,3-naphthalenedicarboxaldehyde. Anal. Biochem. 2013, 433, 132–136. [Google Scholar] [CrossRef]
- Gabuzda, D.; Busciglio, J.; Chen, L.B.; Matsudaira, P.; Yankner, B.A. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J. Biol. Chem. 1994, 269, 13623–13628. [Google Scholar] [CrossRef]
- Velliquette, R.A.; O’Connor, T.; Vassar, R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: Possible early events in Alzheimer’s disease pathogenesis. J. Neurosci. 2005, 25, 10874–10883. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA mutations and bioenergetic defects in aging and degenerative diseases. In The Molecular and Genetic Basis of Neurological Diseases, 2nd ed.; Rosenberg, R.N., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1997; pp. 237–269. [Google Scholar]
- Brouillet, E.; Guyot, M.C.; Mittoux, V.; Altairac, S.; Conde, F.; Palfi, S.; Hantraye, P. Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J. Neurochem. 1998, 70, 794–805. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, H.; Choi, H.-J.; Lee, S.; Kim, K. Protein Glutathionylation in the Pathogenesis of Neurodegenerative Diseases. Oxid. Med. Cell. Long. 2017, 2017, 2818565. [Google Scholar] [CrossRef]
- Newman, S.F.; Sultana, R.; Perluigi, M.; Coccia, R.; Cai, J.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Butterfield, D.A. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J. Neurosci. Res. 2007, 85, 1506–1514. [Google Scholar] [CrossRef]
- Young, A.; Gill, R.; Mailloux, R.J. Protein S-glutathionylation: The linchpin for the transmission of regulatory information on redox buffering capacity in mitochondriaChem. Biol. Int. 2019, 299, 151–162. [Google Scholar] [CrossRef]
- Herrero-Mendez, A.; Almeida, A.; Fernández, E.; Maestre, C.; Moncada, S.; Bolaños, J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009, 11, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef] [PubMed]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef]
- Liedhegner, E.A.S.; Gao, X.H.; Mieyal, J.J. Mechanisms of altered redox regulation in neurodegenerative diseases—Focus on S-glutathionylation. Antioxid. Redox Signal. 2012, 16, 543–566. [Google Scholar] [CrossRef]
- Townsend, D.M. S-glutathionylation: Indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007, 7, 313–324. [Google Scholar] [CrossRef]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharm. 2017, 174, 1750–1770. [Google Scholar] [CrossRef]
- Zarubina, I.V.; Lukk, M.V.; Shabanov, P.D. Antihypoxic and antioxidant effects of exogenous succinic acid and aminothiol succinate-containing antihypoxants. Bull. Exp. Biol. Med. 2012, 153, 336–339. [Google Scholar] [CrossRef]
- Pozdnyakov, D.I.; Zolotych, D.S.; Larsky, M.V. Correction of mitochondrial dysfunction by succinic acid derivatives under experimental cerebral ischemia conditions. Curr. Issues Pharm. Med. Sci. 2021, 34, 42–48. [Google Scholar] [CrossRef]
- Pizova, N.V. Succinic acid derivatives in therapy for cerebrovascular disease. Neurol. Neuropsychiatry Psychosom. 2010, 2, 67–68. [Google Scholar] [CrossRef][Green Version]
- Moiseenok, A.G.; Komar, V.I.; Khomich, T.I.; Kanunnikova, N.P.; Slyshenkov, V.S. Pantothenic acid in maintaining thiol and immune homeostasis. Biofactors 2000, 11, 53–55. [Google Scholar] [CrossRef]
- Slyshenkov, V.S.; Dymkowska, D.; Wojtczak, L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004, 569, 169–172. [Google Scholar] [CrossRef]
- Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef]
- Semenovich, D.S.; Kanunnikova, N.P.; Moiseenok, A.G. Oxidative stress in mitochondria of the brain tissue with chronic aluminum neurotoxicosis and applying of glutathione and coenzyme A biosynthesis modulators. Dokl. Nat. Acad. Sci. Belarus 2020, 64, 78–85. [Google Scholar] [CrossRef]
- Kanunnikova, N.P.; Bashun, N.Z.; Moiseenok, A.G. Use of CoA biosynthesis modulators and selenoprotein model substances in correction of brain ischemic and reperfusion injuries. Lipid Peroxidation 2012, 492–513. [Google Scholar]
- Semenovich, D.S.; Lukiyenko, E.P.; Titko, O.V.; Kanunnikova, N.P. Panthenol and succinate as modulators of changes of redox balance and energy metabolism in the experimental model of Parkinson’s disease. Ind. J. Appl. Res. 2018, 8, 436–438. [Google Scholar]
- Lejay, A.; Paradis, S.; Lambert, A. N-Acetyl Cysteine Restores Limb Function, Improves Mitochondrial Respiration, and Reduces Oxidative Stress in a Murine Model of Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 730–738. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharm. Toxicol. 2009, 105, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Aluise, C.D.; Joshi, G.; Sultana, R.; St Clair, D.K.; Markesbery, W.R.; Butterfield, D.A. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: Toward therapeutic modulation of mild cognitive impairment. J. Neurosci. Res. 2010, 88, 2618–2629. [Google Scholar] [CrossRef]
- Sandhir, R.; Sood, A.; Mehrotra, A.; Kamboj, S.S. N-Acetylcysteine reverses mitochondrial dysfunctions and behavioral abnormalities in 3-nitropropionic acid-induced Huntington’s disease. Neurodegener. Dis. 2012, 9, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Semenovich, D.S.; Lukienko, E.P.; Kanunnikova, N.P. Modulating Oxidative Stress Indices and Thiol-Disulfide Balance in the Brain Structures by Pantothenic Acid Derivatives in an Experimental Model of Parkinson’s Disease. Neurochem. J. 2021, 15, 24–29. [Google Scholar] [CrossRef]
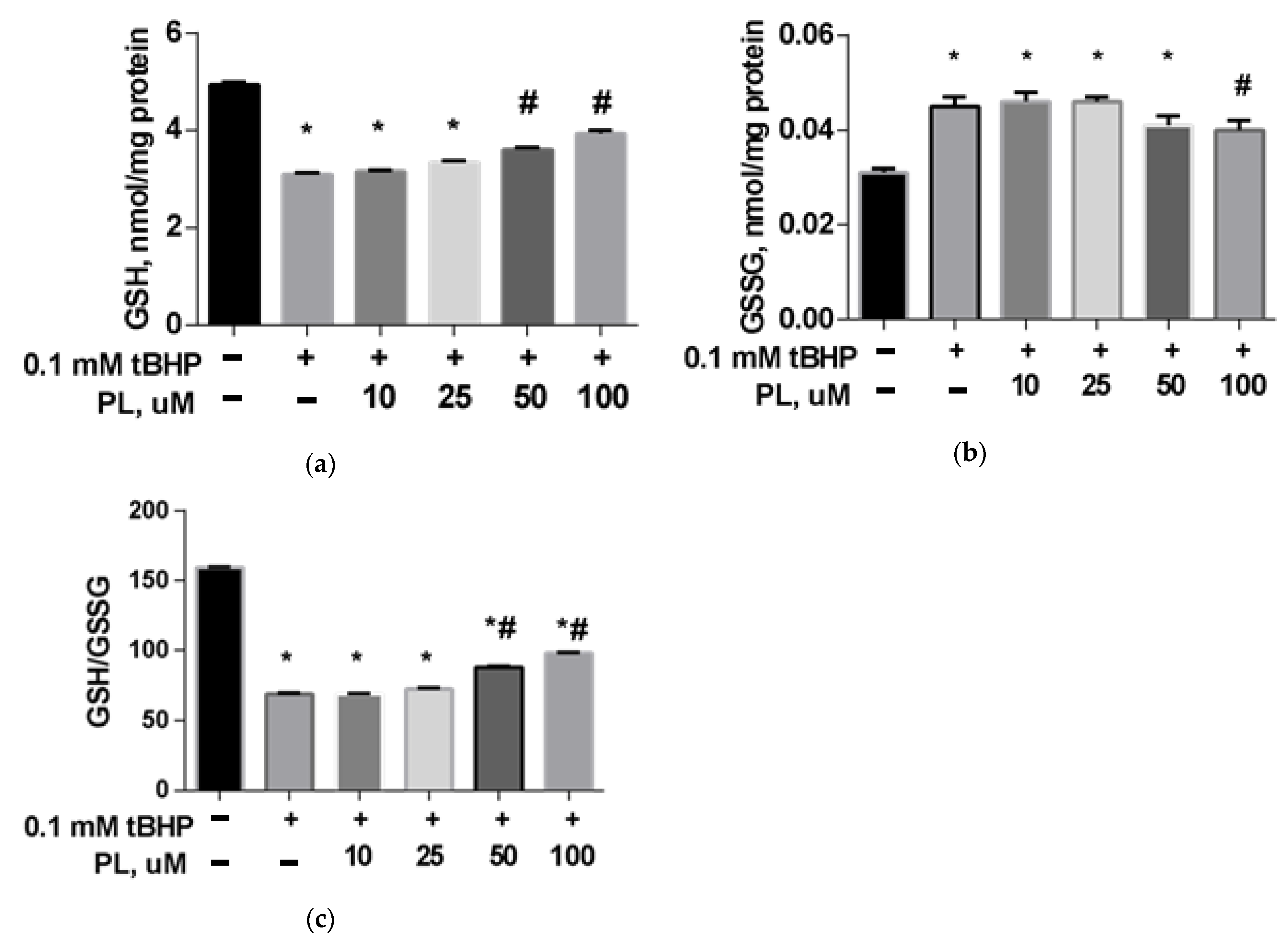
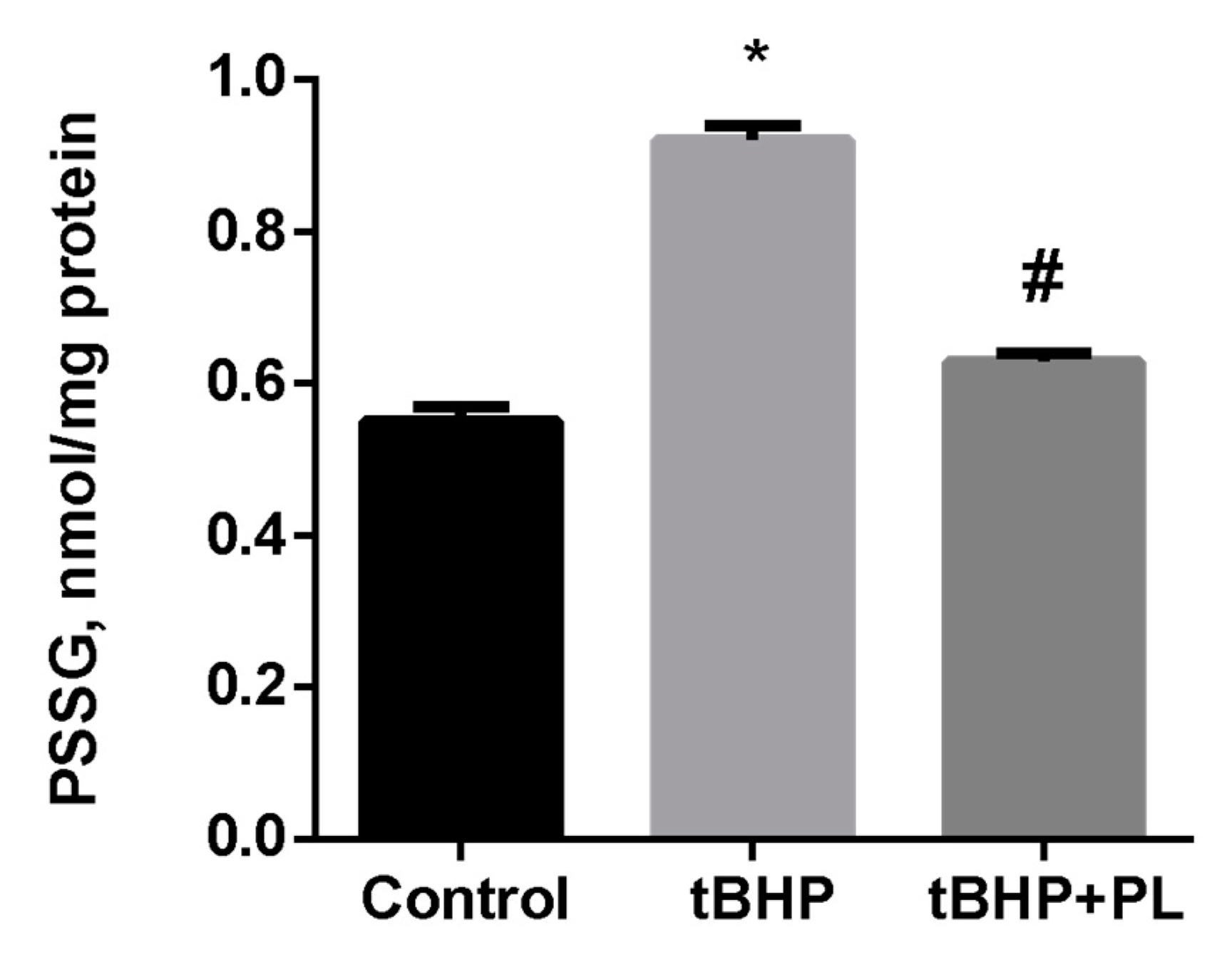


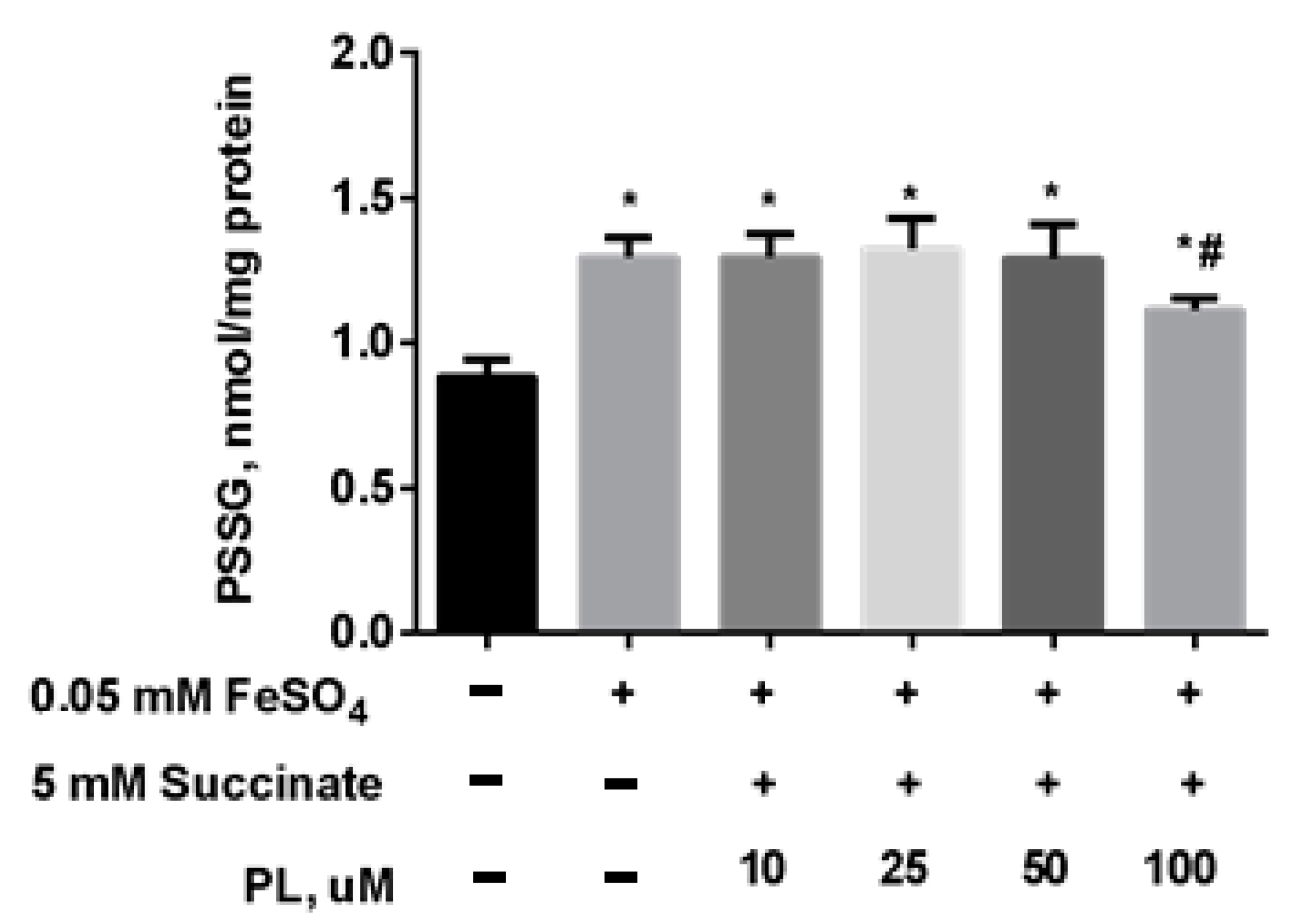
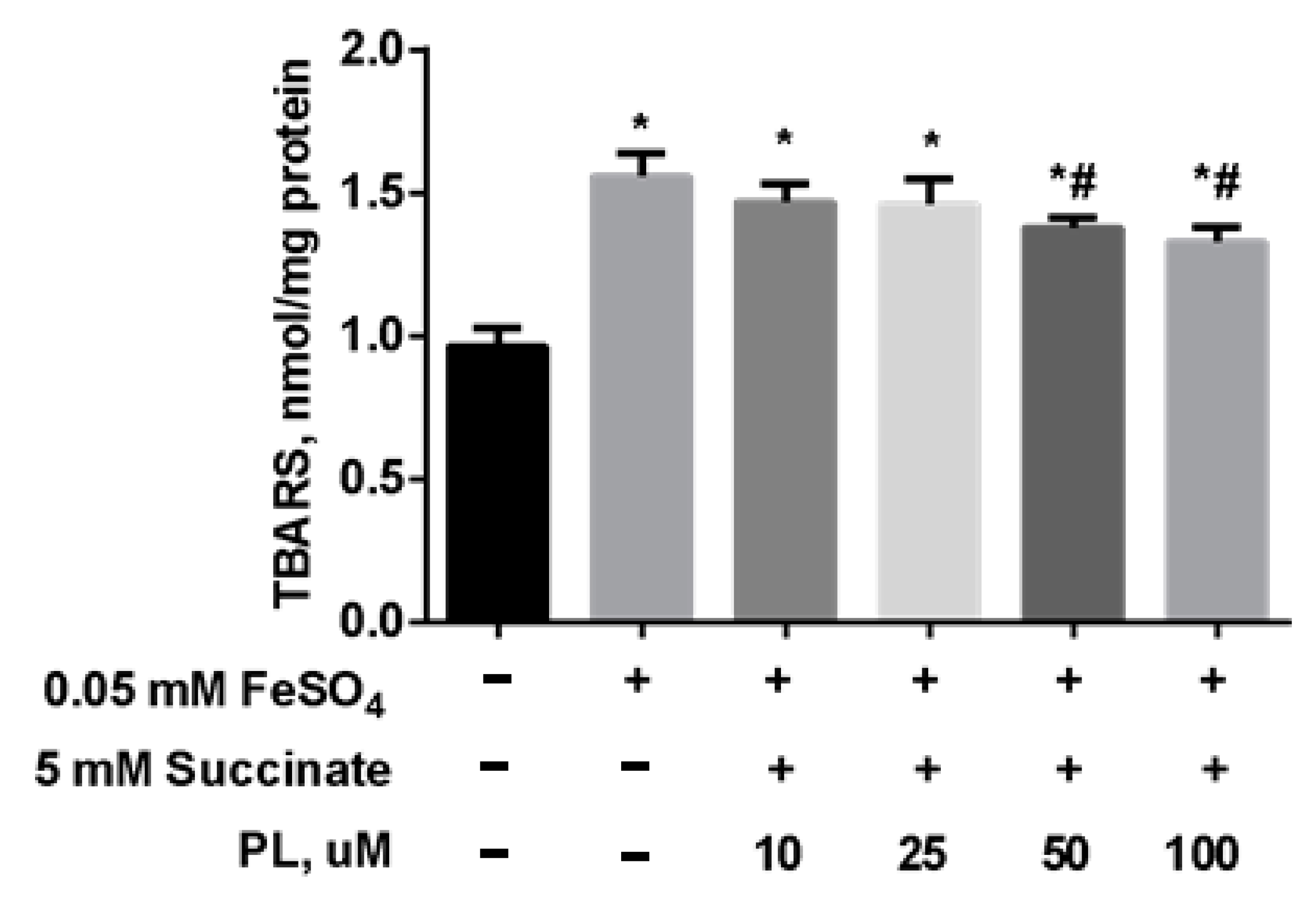
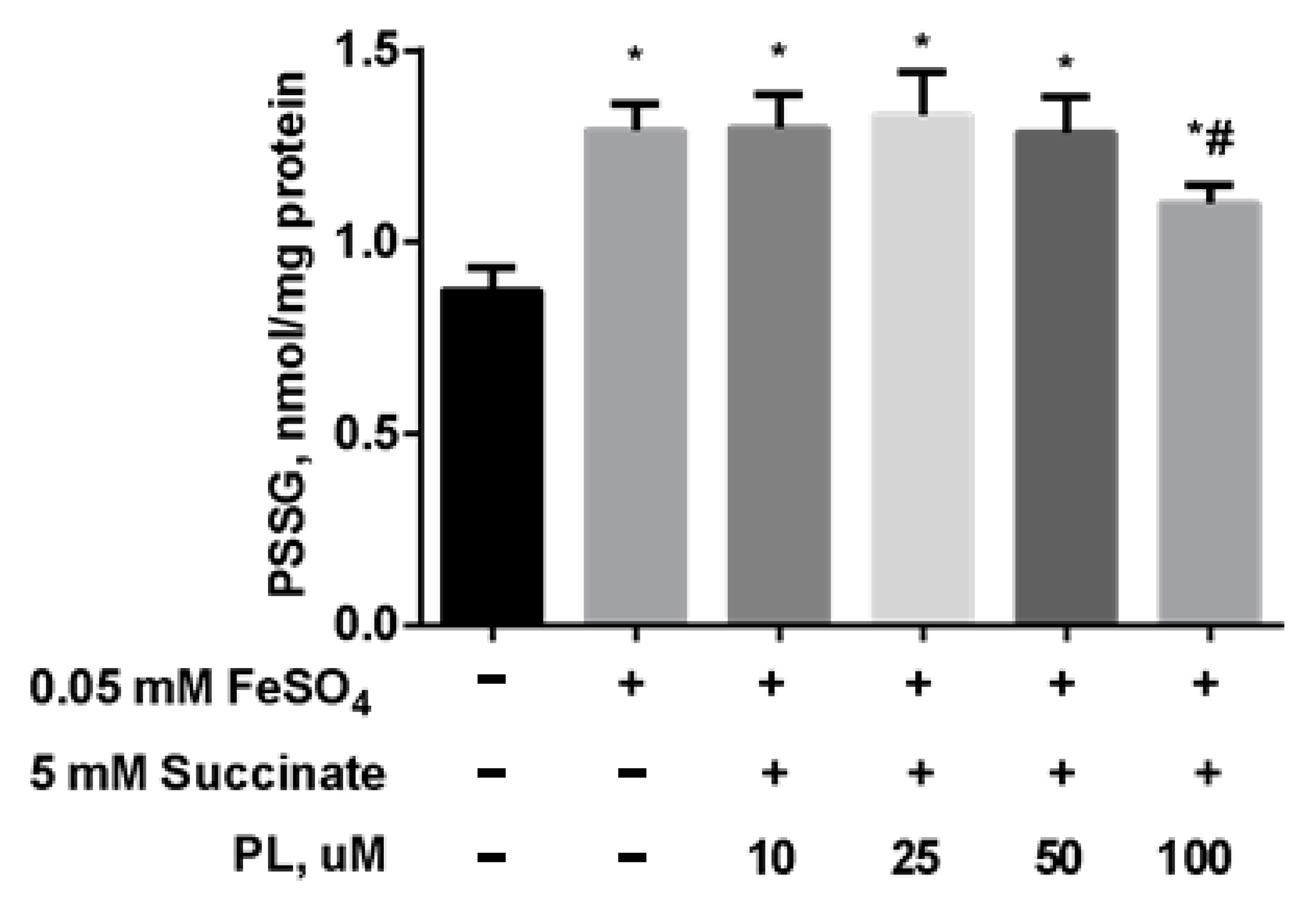
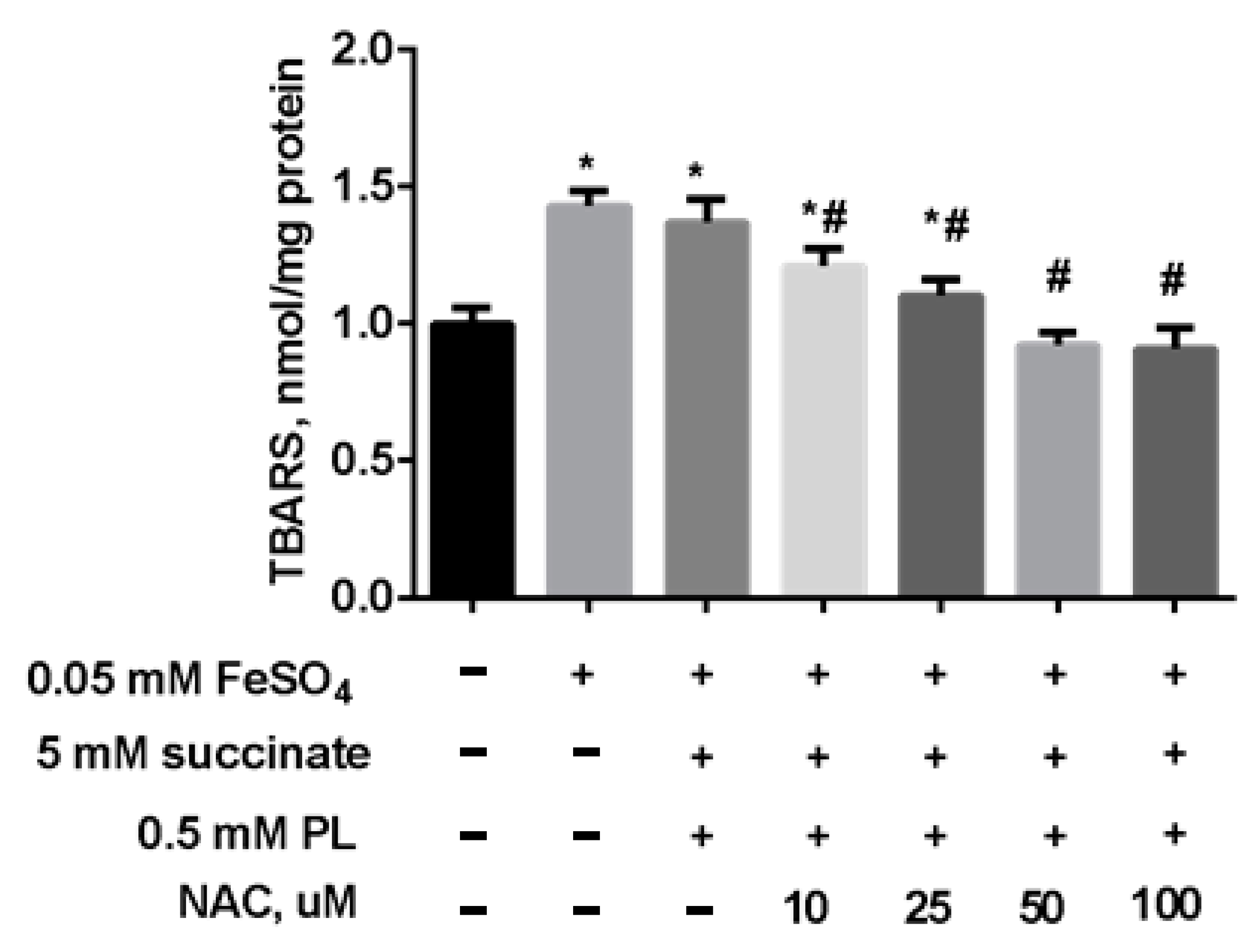


| Groups | Free TBARS | Protein-Bound TBARS |
|---|---|---|
| Control | 0.56 ± 0.02 | 10.26 ± 0.20 |
| tBHP | 1.65 ± 0.03 * | 14.12 ± 0.18 * |
| tBHP + 10 µM PL | 1.46 ± 0.02 *# | 12.16 ± 0.19 *# |
| tBHP + 25 µM PL | 1.21 ± 0.03 *# | 12.38 ± 0.20 *# |
| tBHP + 50 µM PL | 1.10 ± 0.04 *# | 12.10 ± 0.21 *# |
| tBHP + 100 µM PL | 0.98 ± 0.02 *# | 11.08 ± 0.20 *# |
| Groups | SDH | OGDH | Aconitase |
|---|---|---|---|
| Control | 84.47 ± 0.82 | 4.08 ± 0.12 | 14.78 ± 0.20 |
| tBHP | 59.99 ± 0.83 * | 3.08 ± 0.19 * | 10.46 ± 0.12 * |
| tBHP + 50 µM PL | 76.49 ± 1.38 *# | 3.22 ± 0.02 *# | 13.14 ± 0.09 *# |
| tBHP + 100 µM PL | 74.03 ± 0.54 *# | 3.78 ± 0.04 *# | 14.23 ± 0.20 *# |
| Groups | PSH |
|---|---|
| Control | 152.75 ± 0.98 |
| tBHP | 137.86 ± 1.05 * |
| tBHP + 10 µM PL | 138.94 ± 1.10 * |
| tBHP + 25 µM PL | 139.28 ± 0.98 * |
| tBHP + 50 µM PL | 144.15 ± 0.95 *# |
| tBHP + 100 µM PL | 145.93 ± 0.96 *# |
| Groups | GSH | GSSG | GSH/GSSG |
|---|---|---|---|
| Control | 2.59 ± 0.50 | 0.13 ± 0.07 | 20.20 ± 2.05 |
| FeSO4 | 1.63 ± 0.65 * | 0.14 ± 0.08 | 11.96 ± 3.22 * |
| FeSO4 + succinate + 0.1 mM PL | 1.69 ± 0.38 * | 0.14 ± 0.09 | 12.09 ± 3.34 * |
| FeSO4 + succinate + 0.25 mM PL | 1.55 ± 0.40 * | 0.15 ± 0.08 | 10.58 ± 3.15 * |
| FeSO4 + succinate + 0.5 mM PL | 1.89 ± 0.32 * | 0.13 ± 0.07 | 14.15 ± 2.80 * |
| FeSO4 + succinate + 1 mM PL | 2.15 ± 0.26 *# | 0.14 ± 0.07 | 15.08 ± 3.60 * |
| Groups | GSH | GSSG | GSH/GSSG |
|---|---|---|---|
| Control | 2.33 ± 0.12 | 0.17 ± 0.07 | 13.78 ± 1.24 |
| FeSO4 | 1.51 ± 0.10 * | 0.20 ± 0.04 | 7.85 ± 2.20 * |
| FeSO4 + succinate + PL | 1.63 ± 0.14 * | 0.21 ± 0.05 | 7.96 ± 2.15 * |
| FeSO4 + succinate + PL + 0.1 mM NAC | 1.74 ± 0.33 * | 0.23 ± 0.06 | 7.65 ± 2.08 * |
| FeSO4 + succinate + PL + 0.25 mM NAC | 1.89 ± 0.15 *# | 0.20 ± 0.07 | 9.50 ± 3.10 * |
| FeSO4 + succinate + PL + 0.5 mM NAC | 2.04 ± 0.10 *# | 0.18 ± 0.05 | 11.43 ± 2.05 *# |
| FeSO4 + succinate + PL + 1 mM NAC | 2.43 ± 0.11 *# | 0.19 ± 0.04 | 12.88 ± 1.93 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenovich, D.S.; Plotnikov, E.Y.; Titko, O.V.; Lukiyenko, E.P.; Kanunnikova, N.P. Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro. Antioxidants 2021, 10, 1699. https://doi.org/10.3390/antiox10111699
Semenovich DS, Plotnikov EY, Titko OV, Lukiyenko EP, Kanunnikova NP. Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro. Antioxidants. 2021; 10(11):1699. https://doi.org/10.3390/antiox10111699
Chicago/Turabian StyleSemenovich, Dmitry S., Egor Yu. Plotnikov, Oksana V. Titko, Elena P. Lukiyenko, and Nina P. Kanunnikova. 2021. "Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro" Antioxidants 10, no. 11: 1699. https://doi.org/10.3390/antiox10111699
APA StyleSemenovich, D. S., Plotnikov, E. Y., Titko, O. V., Lukiyenko, E. P., & Kanunnikova, N. P. (2021). Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro. Antioxidants, 10(11), 1699. https://doi.org/10.3390/antiox10111699







