Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
- Control: 25/15 °C (12 h/12 h day/night), 700 μmol m−2s−1 light intensity, and 65–70% relative humidity until maturity;
- Non-acclimated, cold-stressed: 25/15 °C (12 h/12 h day/night), 700 μmol m−2s−1 light intensity, and 65–70% relative humidity for one day; temperature then reduced to 13/7 °C (12 h/12 h day/night) over 4 days to avoid lethal shock, where it remained at this temperature until maturity; and
- Cold-acclimated, cold-stressed: 25/18 °C (12 h/12 h day/night), 700 μmol m−2s−1 light intensity, and 65–70% relative humidity for one day, followed by 42 d of cold acclimation, involving 7 d exposure at each decreasing temperature beginning with 23/15 °C, 21/13 °C, 20/12 °C, 20/10 °C, 18/8 °C, 15/8 °C (12 h/12 h day/night) before exposing the plants to cold stress at 13/7 °C (12 h/12 h day/night; 700 μmol m−2s−1 light intensity, and 65–70% relative humidity). Thereafter the temperature remained at 13/7 °C until maturity.
2.2. Stress Injury
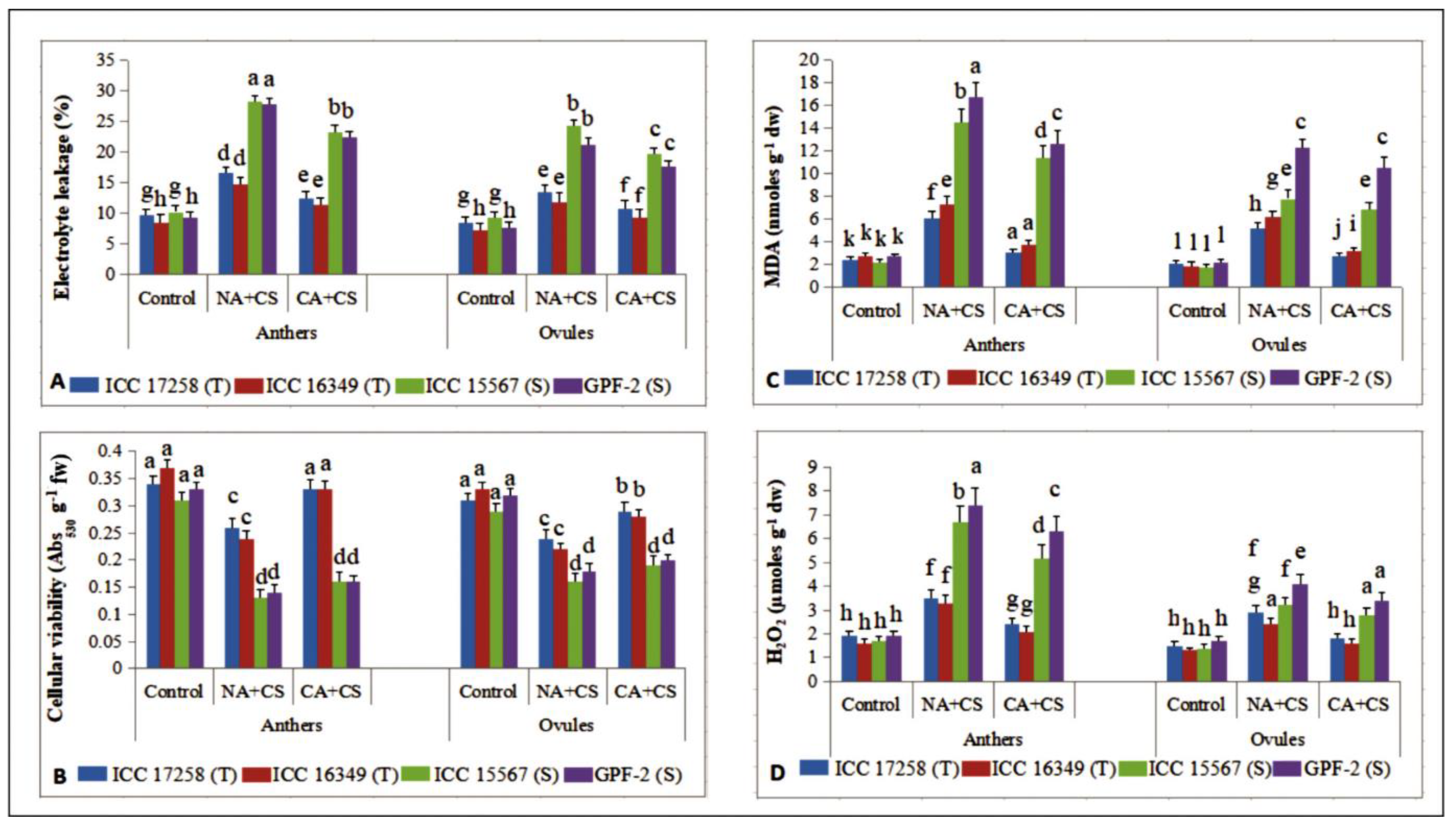
2.2.1. Membrane Damage
2.2.2. Cellular Oxidizing Ability
2.2.3. Relative Leaf Water Content
2.2.4. Stomatal Conductance
2.2.5. Photochemical Efficiency
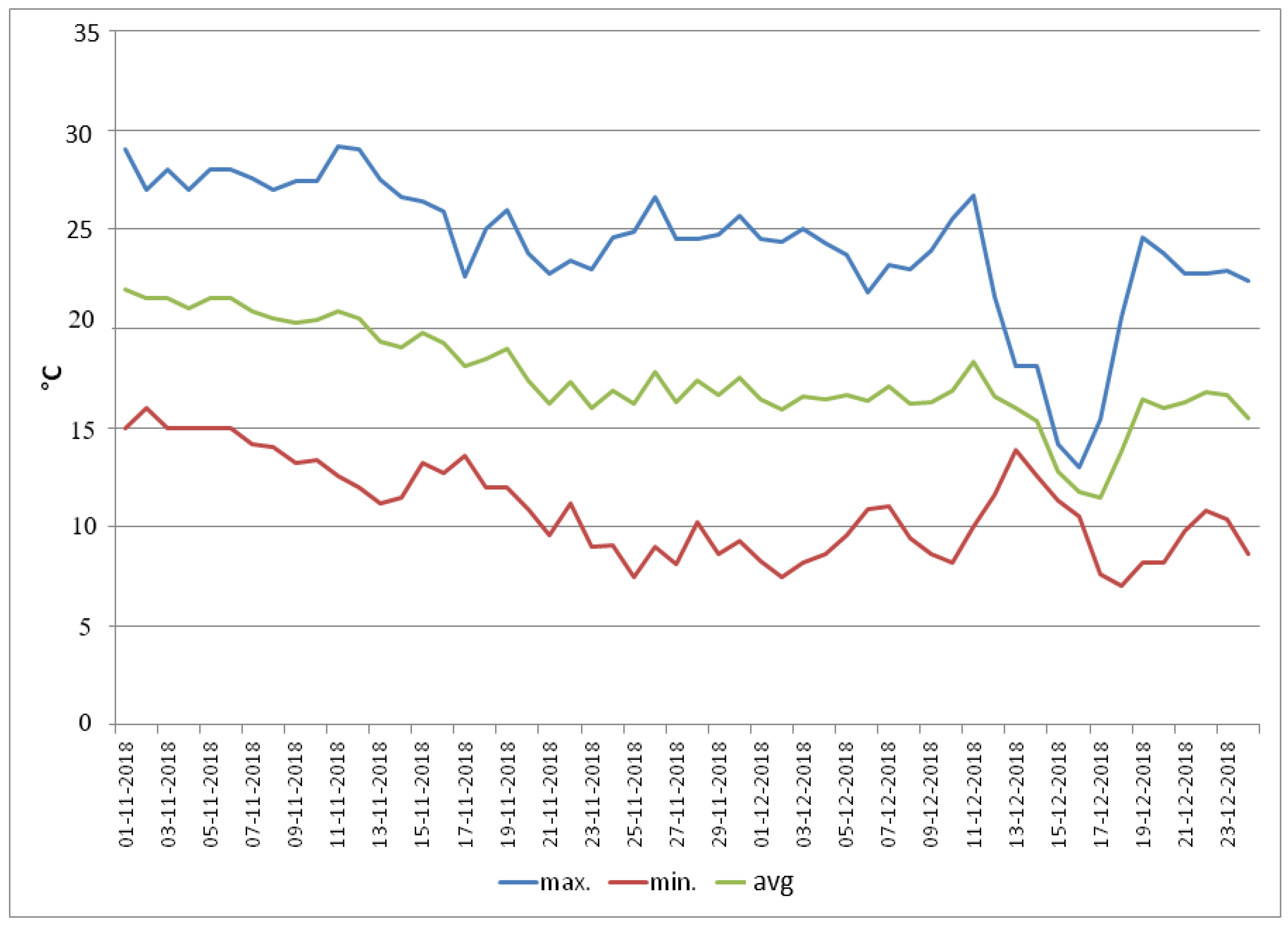
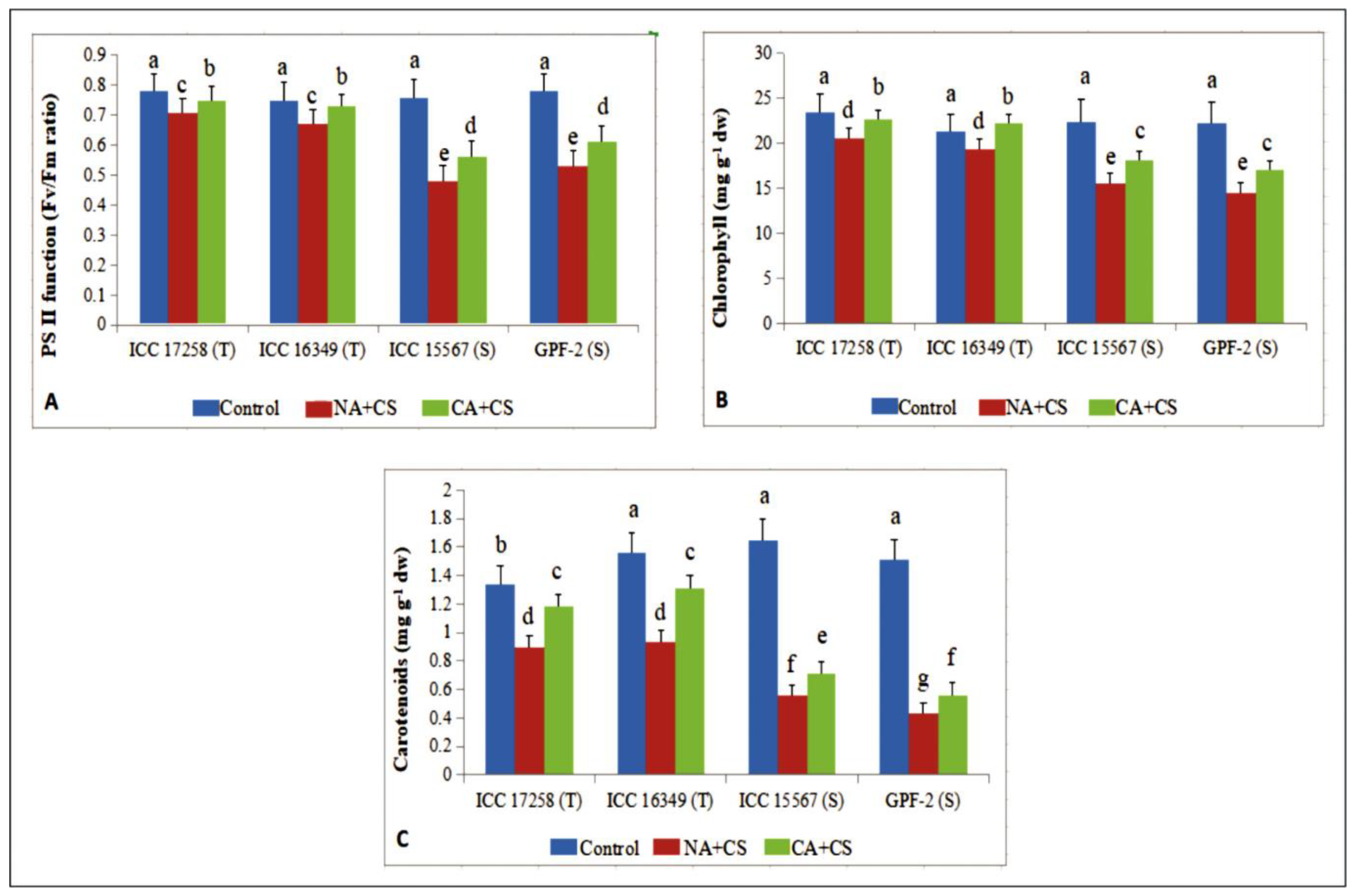
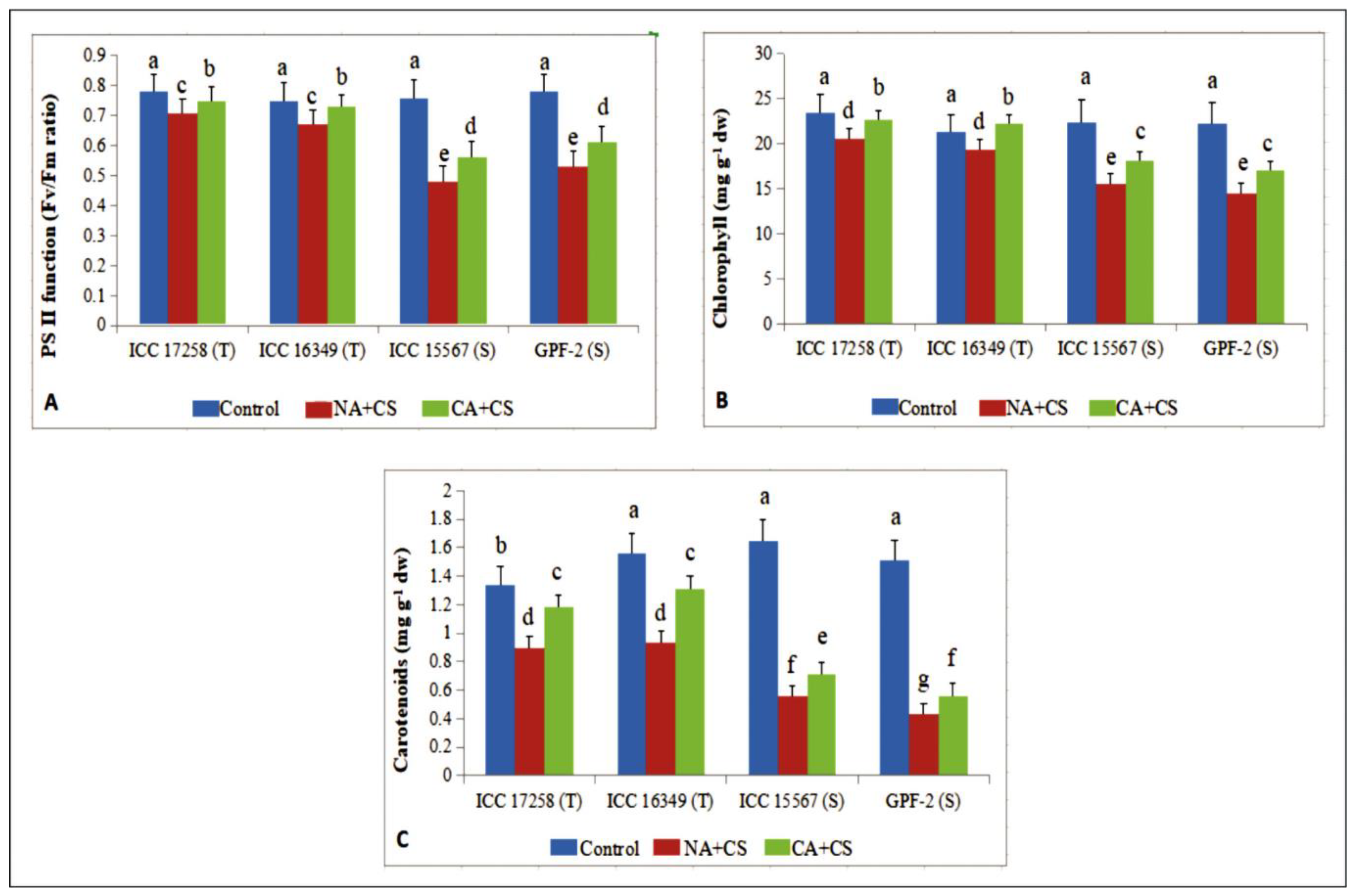
2.2.6. Chlorophyll and Carotenoids
2.3. Reproductive Traits
2.3.1. Pollen Germination
2.3.2. Pollen Viability
2.3.3. Stigma Receptivity
2.3.4. Ovule Viability
2.4. Oxidative Stress and Antioxidants
2.4.1. Malondialdehyde
2.4.2. Hydrogen Peroxide
2.4.3. Superoxide Dismutase
2.4.4. Catalase
2.4.5. Ascorbate Peroxidase
2.4.6. Glutathione Reductase
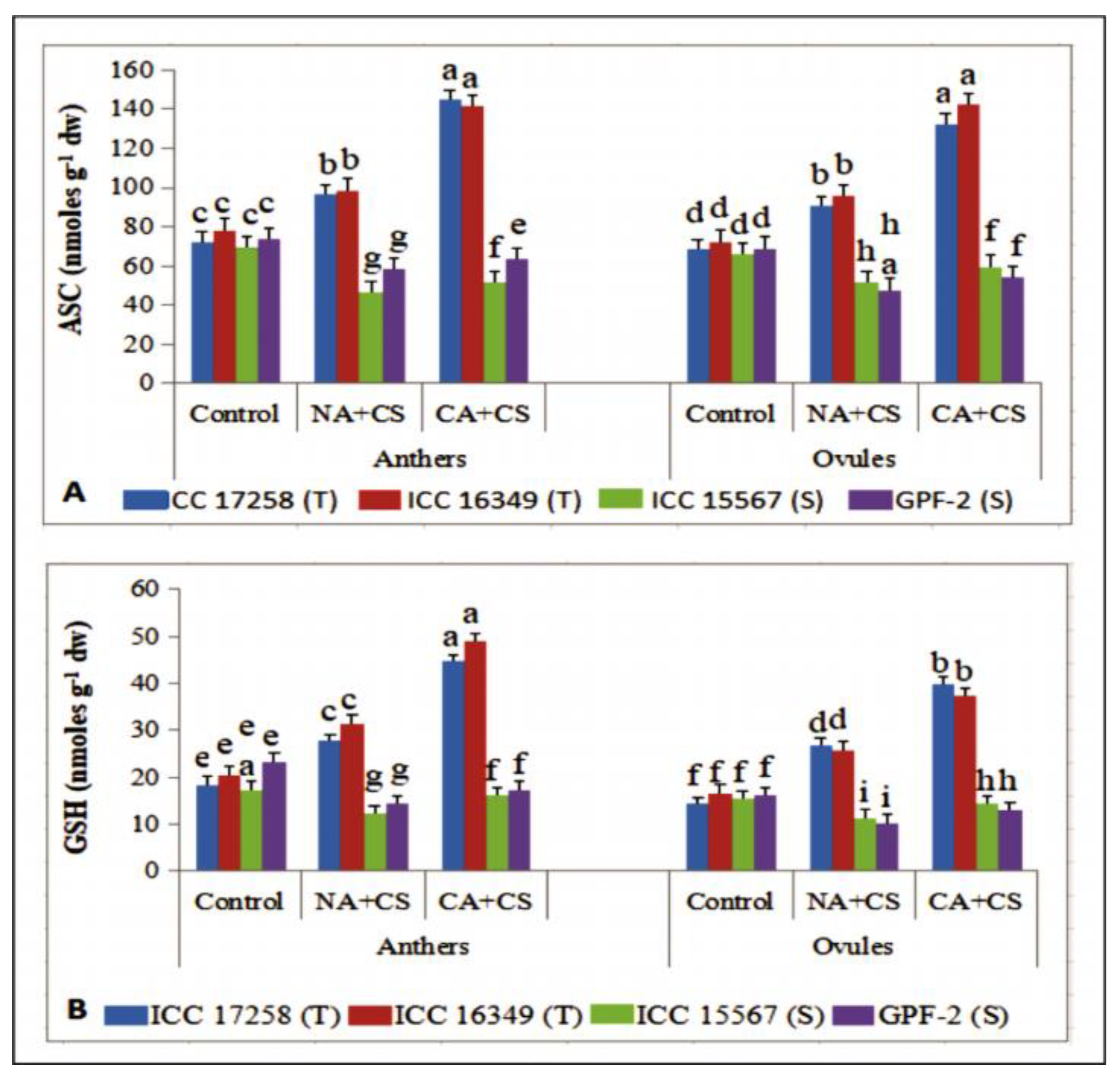
2.4.7. Ascorbic Acid
2.4.8. Glutathione
2.5. Soluble Proteins
2.6. Solutes
2.6.1. Proline
2.6.2. Endogenous γ-Aminobutyric Acid
2.6.3. Trehalose
2.6.4. Sucrose
2.6.5. In-Vitro Pollen Germination
2.7. Statistical Analysis
3. Results
3.1. Stress Injury to Leaves
3.1.1. Membrane Damage
3.1.2. Relative Leaf Water Content
3.1.3. Stomatal Conductance
3.1.4. Photosystem II Function
3.1.5. Photosynthetic Pigments
3.2. Reproductive Traits
3.2.1. Pollen Germination
3.2.2. Stigma Receptivity
3.2.3. Pollen Viability
3.2.4. Ovule Viability
3.2.5. Tissue Damage to Anthers and Ovules
3.3. Oxidative Stress and Antioxidants
3.3.1. Malondialdehyde
3.3.2. Hydrogen Peroxide
3.3.3. Superoxide Dismutase
3.3.4. Ascorbate Peroxidase
3.3.5. Catalase
3.3.6. Glutathione Reductase
3.3.7. Ascorbate
3.3.8. Glutathione
3.4. Cryoprotective Solutes
3.4.1. Proline
3.4.2. Sucrose
3.4.3. γ-. Aminobutyric Acid
3.4.4. Trehalose
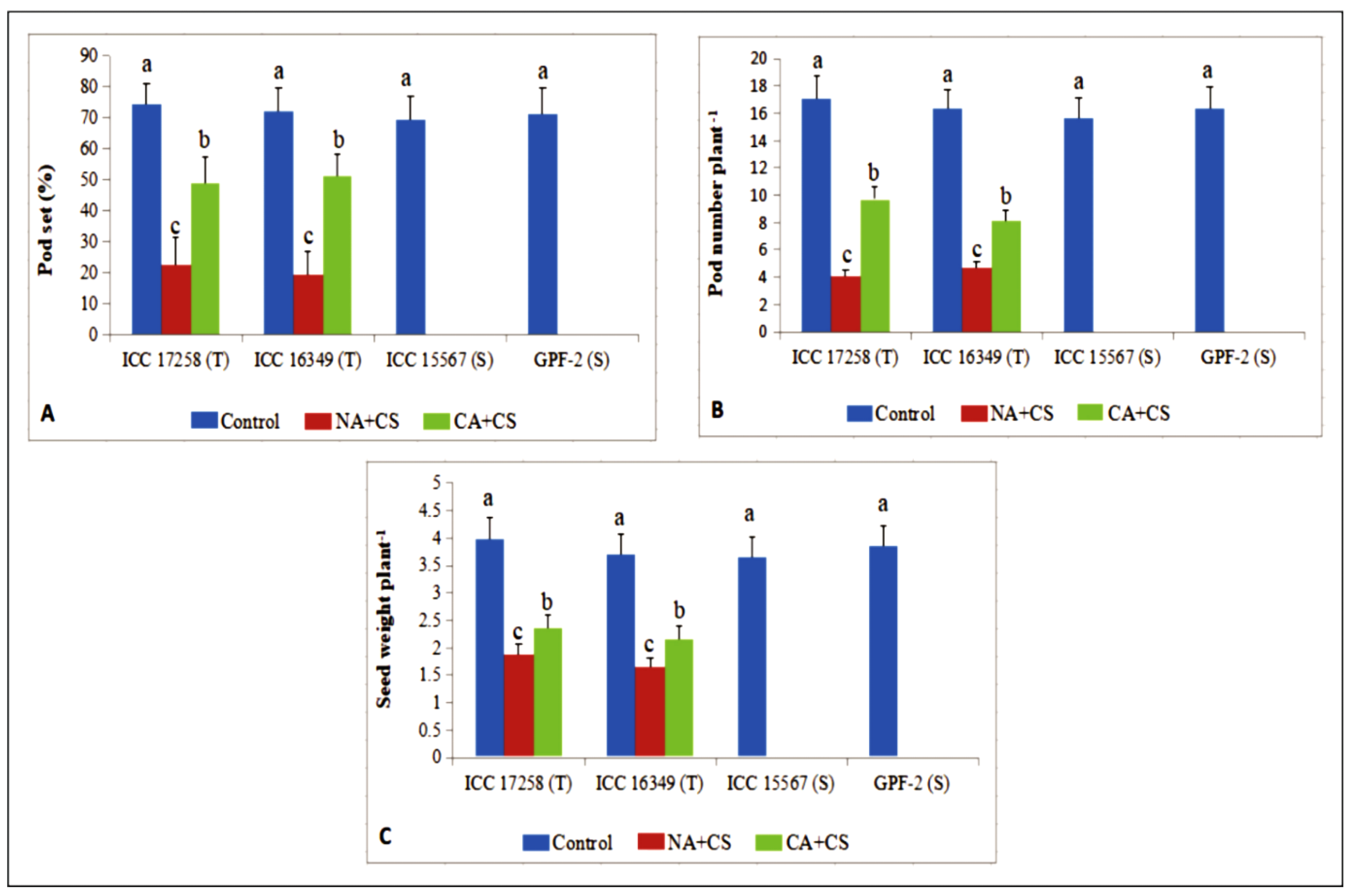
3.5. Yield Traits
3.6. Effect of Cryoprotective Solutes and Antioxidants on In-Vitro Pollen Germination
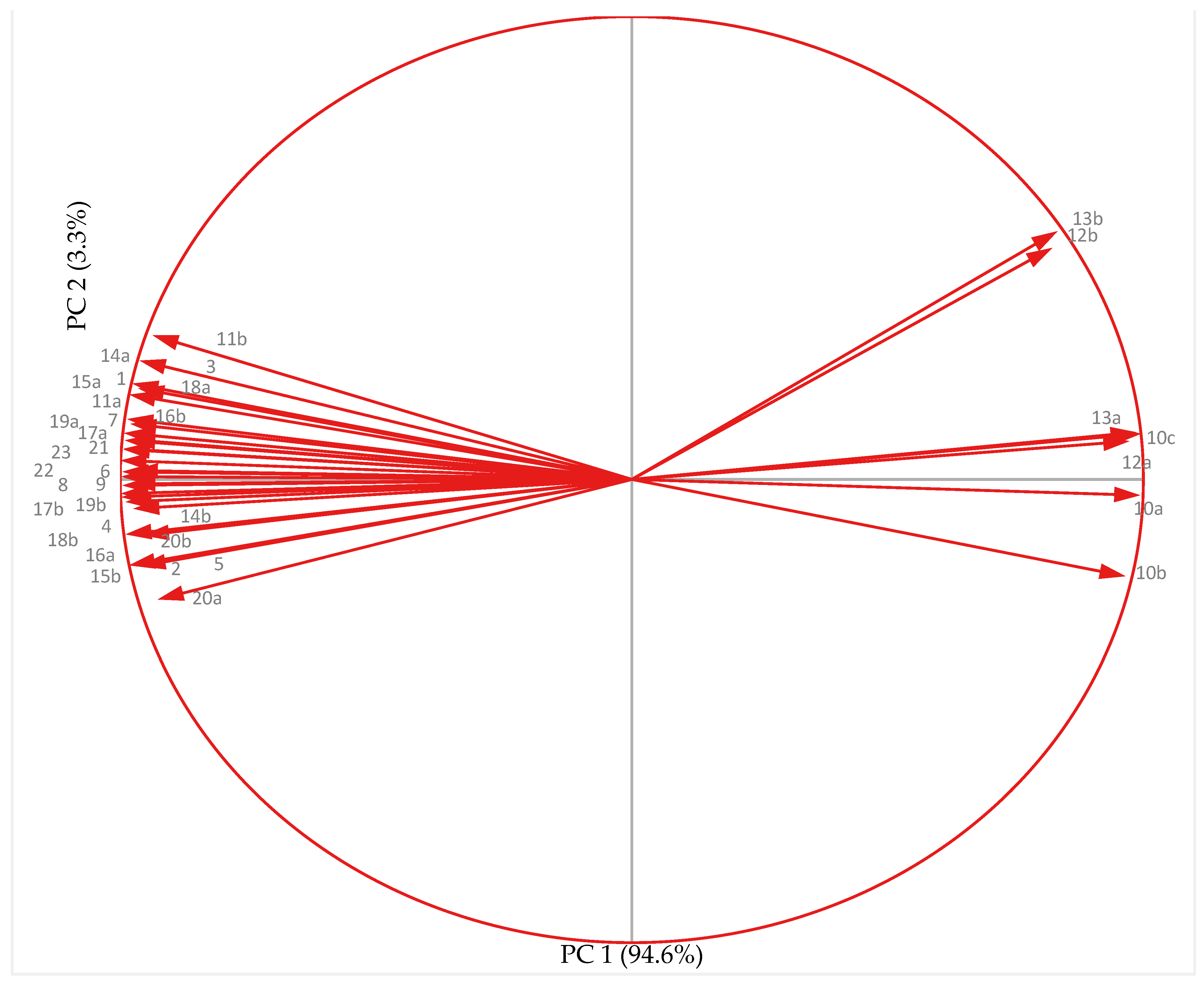
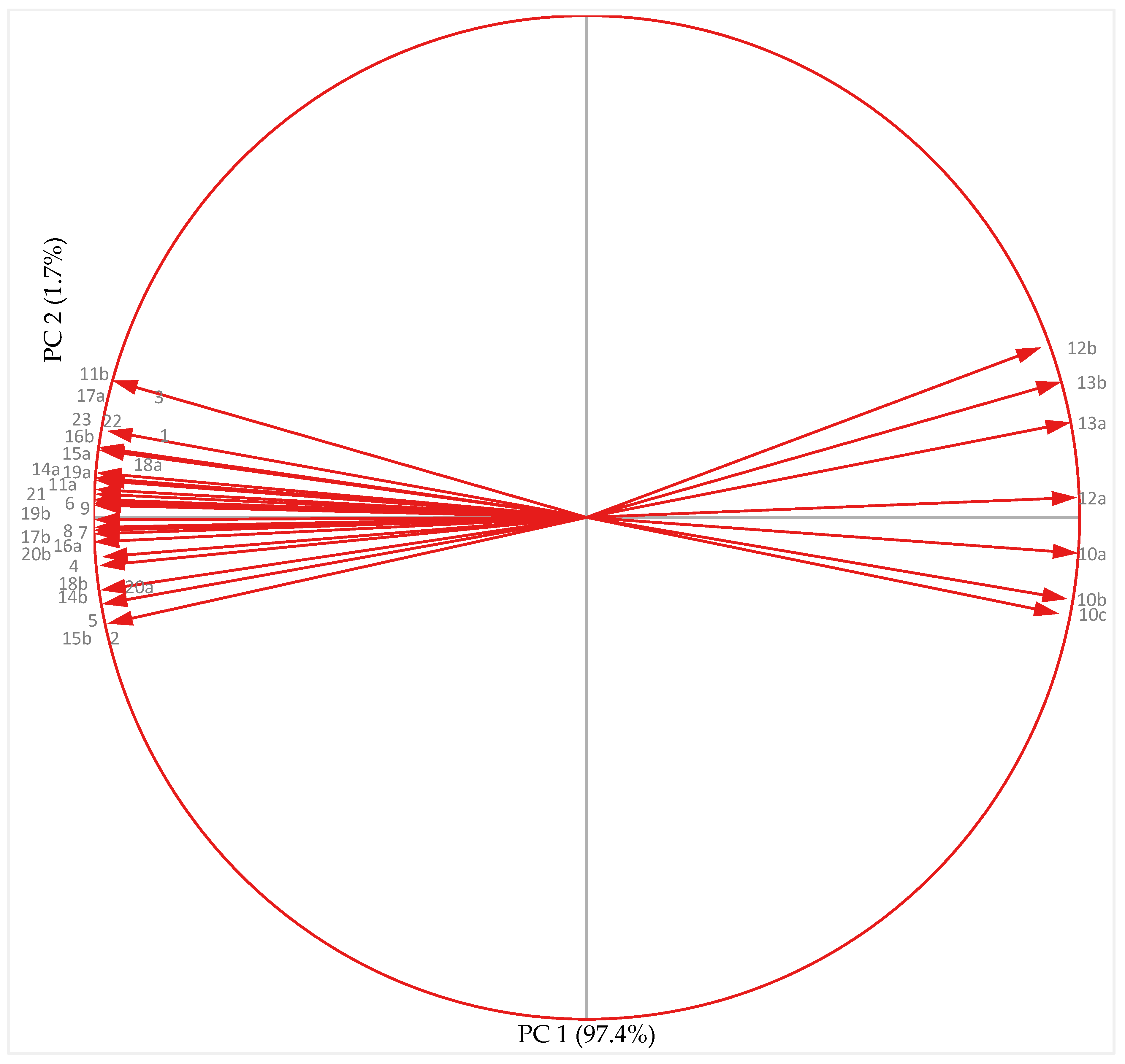
3.7. Principal Component Analysis
3.7.1. Non-Acclimated (NA) Plants
3.7.2. Cold-Acclimated Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croser, J.S.; Clarke, H.J.; Siddique, K.H.M.; Khan, T.N. Low-temperature stress: Implications for chickpea (Cicer arietinum L.) improvement. Crit. Rev. Plant. Sci. 2003, 22, 185–219. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Malhotra, R.S.; Halila, M.H.; Knights, E.J.; Verma, M.M. Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 1993, 73, 137–149. [Google Scholar] [CrossRef]
- Kiran, A.; Sharma, P.N.; Awasthi, R.; Nayyar, H.; Seth, R.; Chandel, S.S.; Sharma, K.D. Disruption of carbohydrate and proline metabolism in anthers under low temperature causes pollen sterility in chickpea. Environ. Exp. Bot. 2021, 188, 104500. [Google Scholar] [CrossRef]
- Mir, A.H.; Bhat, M.A.; Dar, S.A.; Sofi, P.A.; Bhat, N.A.; Mir, R.R. Assessment of cold tolerance in chickpea (Cicer spp.) grown under cold/freezing weather conditions of North-Western Himalayas of Jammu and Kashmir, India. Physiol. Mol. Biol. Plants 2021, 27, 1105–1118. [Google Scholar] [CrossRef]
- Srinivasan, A.; Johansen, C.; Saxena, N.P. Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): Characterization of stress and genetic variation in pod set. Field Crop. Res. 1998, 57, 181–193. [Google Scholar] [CrossRef]
- Clarke, H.J.; Siddique, K.H.M. Response of chickpea genotypes to low temperature stress during reproductive development. Field Crop. Res. 2004, 90, 323–334. [Google Scholar] [CrossRef]
- Nayyar, H.; Bains, T.; Kumar, S. Low temperature induced floral abortion in chickpea: Relationship to abscisic acid and cryoprotectants in reproductive organs. Environ. Exp. Bot. 2005, 53, 39–47. [Google Scholar] [CrossRef]
- Nayyar, H.; Bains, T.S.; Kumar, S. Chilling stressed chickpea seedlings: Effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ. Exp. Bot. 2005, 54, 275–285. [Google Scholar] [CrossRef]
- Berger, J.D.; Kumar, S.; Nayyar, H.; Street, K.A.; Sandhu, J.S.; Henzell, J.M.; Clarke, H.C. Temperature-stratified screening of chickpea (Cicer arietinum L.) genetic resource collections reveals very limited reproductive chilling tolerance compared to its annual wild relatives. Field. Crop. Res. 2012, 126, 119–129. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Saltveit, M.E. Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol. Plant. 2001, 113, 548–556. [Google Scholar] [CrossRef]
- Pearce, R.S. Molecular analysis of acclimation to cold. Plant Growth Regul. 1999, 29, 47–76. [Google Scholar] [CrossRef]
- Rife, C.L.; Zeinali, H. Cold tolerance in oilseed rape over varying acclimation durations. Crop Sci. 2003, 43, 96–100. [Google Scholar] [CrossRef]
- Burchett, S.; Niven, S.; Fuller, M.P. The effect of cold-acclimation on the water relations and freezing tolerance of Hordeum vulgare L. Cryo. Letters 2006, 27, 295–303. [Google Scholar]
- Mishra, K.B.; Mishra, A.; Kubasek, J.; Urban, O.; Heyer, A.G. Low temperature induced modulation of photosynthetic induction in non-acclimated and cold-acclimated Arabidopsis thaliana: Chlorophyll a fluorescence and gas-exchange measurements. Photosyn. Res. 2019, 139, 123–143. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116. [Google Scholar]
- Turan, O.; Ekmekçi, Y. Chilling tolerance of Cicer arietinum lines evaluated by photosystem II and antioxidant activities. Turk. J. Bot. 2014, 38, 499–510. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.; Nayyar, H. 2013 Heat-stress-induced reproductive failures in chickpea (Cicer arietinum L.) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and non aborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Mattison, O.; Knox, R.B.; Heslop-Harrison, J.; Heslop-Harrison, Y. Protein pellicle of stigmatic papillae as a probable recognition site in incompatible reactions. Nature 1974, 247, 298–300. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defense against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase activities of hydrocarbon-utilizing Candida yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Mavis, R.D.; Stellwagen, E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J. Biol. Chem. 1968, 243, 809–814. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Identification of high-temperature tolerant lentil (Lens culinaris Medik.) genotypes through leaf and pollen traits. Front. Plant Sci. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant. Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Ezura, H. Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. J. Jpn. Soc. Hort. Sci. 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. 1. Fractionation and micro determination of cell carbohydrates. Biochem. J. 1952, 50, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushal, N.; Nayyar, H.; Gaur, P. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol. Plant. 2012, 34, 1651–1658. [Google Scholar] [CrossRef]
- Jones, M.G.; Outlaw, W.H.; Lowry, O.H. Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977, 60, 379–383. [Google Scholar] [CrossRef]
- Turan, O.; Ekmekçi, Y. Activities of photosystem II and antioxidant enzymes in chickpea (Cicer arietinum L.) cultivars exposed to chilling temperatures. Acta Physiol. Plant. 2011, 33, 67–78. [Google Scholar] [CrossRef]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant cell walls throughout evolution: Towards a molecular understanding of their design principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef]
- Frank, H.A.; Brudvig, G.W. Redox functions of carotenoids in photosynthesis. Biochemistry 2004, 43, 8607–8615. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Irigoyen, J.J.; Sanchez-Diaz, M. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol. Plant. 2003, 117, 540–549. [Google Scholar] [CrossRef]
- Lee, S.H.; Singh, A.P.; Chung, G.C.; Ahn, S.J.; Noh, E.K.; Steudle, E. Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol. Plant. 2004, 120, 413–420. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S.; Lopez-Gomez., M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kari-man, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387. [Google Scholar]
- Kiran, A.; Kumar, S.; Nayyar, H.; Sharma, K.D. Low temperature-induced aberrations in male and female reproductive organ development cause flower abortion in chickpea. Plant Cell Environ. 2019, 42, 2075–2089. [Google Scholar]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef]
- Chaki, T.; Hirata, N.; Yoshikawa, Y.; Tachibana, S.; Tokinaga, Y.; Yamakage, M. Lipid emulsion, but not propofol, induces skeletal muscle damage and lipid peroxidation. J. Anesth. 2019, 33, 628–635. [Google Scholar] [CrossRef]
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef]
- Camejo, D.; Jimenez, A.; Alarcon, J.J.; Torres, W.; Gomez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006, 33, 177–187. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhyaya, H.D. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative compo-nents of seeds. J. Agron. Crop Sci. 2008, 194, 457–464. [Google Scholar]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Saxena, N.P.; Johansen, C. Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): Genetic variation in gamete development and function. Field Crop. Res. 1999, 60, 209–222. [Google Scholar] [CrossRef]
- Fu, G.F.; Jian, S.O.N.G.; Xiong, J.; Li, Y.R.; Chen, H.Z.; Le, M.K.; Tao, L.X. Changes of oxidative stress and soluble sugar in anthers involve in rice pollen abortion under drought stress. Agric. Sci. China 2011, 10, 1016–1025. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wan, C.; Kong, J.; Zhang, Z.; Li, Y.; Zhu, Y. Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Funct. Plant Biol. 2004, 31, 369–376. [Google Scholar] [CrossRef]
- Wan, Z.; Jing, B.; Tu, J.; Ma, C.; Shen, J.; Yi, B.; Fu, T. Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theor. Appl. Genet. 2008, 116, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Nanculao, G.D.; Herrera, M.L.; Carcamo, M.P.; Velasquez, V.B. Relative expression of genes related with cold tolerance in temperate rice at the seedling stage. Afr. J. Biotechnol. 2014, 13, 2506–2512. [Google Scholar]
- Soengas, P.; Rodriguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243. [Google Scholar] [CrossRef]
- Dai, K.; Peng, T.; Ke, D.; Wei, B. Photocatalytic hydrogen generation using a nanocomposite of multi-walled carbon nanotubes and TiO2 nanoparticles under visible light irradiation. Nanotechnology 2009, 20, 125603. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Amiri, R.M.; Mehraban, F.H.; Khaneghah, H.Z. Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russian J. Plant Physiol. 2012, 59, 183–189. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Role of tocopherol (vitamin E) in plants: Abiotic stress tolerance and beyond. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: London, UK, 2014; pp. 267–289. [Google Scholar]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Wodtke, E. Temperature adaptation of biological membranes. The effects of acclimation temperature on the unsaturation of the main neutral and charged phospholipids in mitochondrial membranes of the carp (Cyprinus carpio L.). Biochim. Biophys. Acta Biomembr. 1981, 640, 698–709. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Cox, S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia× hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Chang, Q.; Gu, C.; Song, A.; Chen, S.; Chen, F. Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 2014, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Landry, E.J.; Fuchs, S.J.; Bradley, V.L.; Johnson, R.C. The effect of cold acclimation on the low molecular weight carbohydrate composition of safflower. Heliyon 2017, 3, e00402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzucotell, E.; Belloni, S.; Marone, D.; De Leonardis, A.M.; Guerra, D.; Di Fonzo, N.; Cattivelli, L.; Mastrangelo, A.M. The E3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef]
- Bhandari, K.; Sharma, K.D.; Rao, B.H.; Siddique, K.H.; Gaur, P.; Agrawal, S.K.; Nayyar, H. Temperature sensitivity of food legumes: A physiological insight. Acta Physiol. Plant. 2017, 39, 68. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Vera-Hernandez, P.; Ortega Ramirez, M.A.; Martinez Nunez, M.; Ruiz-Rivas, M.; Rosas-Cárdenas, F.D.F. Proline as a probable biomarker of cold stress tolerance in sorghum (Sorghum bicolor). Mex. J. Biotechnol. 2018, 3, 77–86. [Google Scholar]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [PubMed]
- Malekzadeh, P.; Khara, J.; Heydari, R. Alleviating effects of exogenous Gamma-aminobutyric acid on tomato seedling under chilling stress. Physiol. Mol. Biol. Plants 2014, 20, 133–137. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. 2019 Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- Tabaei-Aghdaei, S.R.; Pearce, R.S.; Harrison, P. Sugars regulate cold-induced gene expression and freezing-tolerance in barley cell cultures. J. Exp. Bot. 2003, 54, 1565–1575. [Google Scholar] [PubMed]
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA 1986, 83, 2422–2426. [Google Scholar] [CrossRef]
- Parish, R.W.; Phan, H.A.; Iacuone, S.; Li, S.F. Tapetal development and abiotic stress: A centre of vulnerability. Funct. Plant Biol. 2012, 39, 553–559. [Google Scholar] [CrossRef]
- Pressman, E.; Harel, D.; Zamski, E.; Shaked, R.; Althan, L.; Rosenfeld, K.; Firon, N. The effect of high temperatures on the expression and activity of sucrose-cleaving enzymes during tomato (Lycopersicon esculentum) anther development. J. Hort. Sci. Biotechnol. 2006, 81, 341–348. [Google Scholar] [CrossRef]




| Treatment | ICC 17258 (T) | ICC 16348 (T) | ICC 15567 (S) | GPF2 (S) |
|---|---|---|---|---|
| Control | 85.6 ± 5.9 a | 88.1 ± 5.1 a | 82.1 ± 4.8 a | 79.6 ± 5.5 a |
| Cold-stressed (CS) (13/7 °C;12 h/12 h; 1 d) | 35.6 ± 4.9 d | 31.3 ± 3.6 d | 8.9 ± 2.1 f | 6.8 ±1.6 f |
| CS + Proline (1mM) | 53.5 ± 4.8 cd | 52.4 ± 5.4 d | 21.9 ± 3.4 ef | 23.4 ± 2.5 ef |
| CS + GABA (1 mM) | 61.3 ± 4.3 bc | 60.1 ± 4.7 bc | 33.5 ± 2.3 e | 31.5 ± 2.1 e |
| CS + Sucrose (1 mM) | 65.6 ± 4.2 b | 62.4 ± 4.6 b | 31.3 ± 2.1 e | 34.2 ± 3.2 e |
| CS + Trehalose (1 mM) | 54.5 ± 3.5 d | 52.4 ± 4.4 d | 29.5 ± 3.1 e | 31.2 ± 3.3 e |
| CS + Ascorbate (1 mM) | 53.8 ± 3.2 d | 56.1 ± 3.7 cd | 28.7 ± 2.7 e | 31.3 ± 2.9 e |
| CS + Reduced Glutathione (1 mM) | 56.4 ± 3.5 cd | 53.2 ± 2.9 d | 26.3 ± 2.4 ef | 29.6 ± 2.2 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, A.; Kiran, A.; Sharma, K.D.; Prasad, P.V.V.; Jha, U.C.; Siddique, K.H.M.; Nayyar, H. Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules. Antioxidants 2021, 10, 1693. https://doi.org/10.3390/antiox10111693
Rani A, Kiran A, Sharma KD, Prasad PVV, Jha UC, Siddique KHM, Nayyar H. Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules. Antioxidants. 2021; 10(11):1693. https://doi.org/10.3390/antiox10111693
Chicago/Turabian StyleRani, Anju, Asha Kiran, Kamal Dev Sharma, P. V. Vara Prasad, Uday C. Jha, Kadambot H. M. Siddique, and Harsh Nayyar. 2021. "Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules" Antioxidants 10, no. 11: 1693. https://doi.org/10.3390/antiox10111693
APA StyleRani, A., Kiran, A., Sharma, K. D., Prasad, P. V. V., Jha, U. C., Siddique, K. H. M., & Nayyar, H. (2021). Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules. Antioxidants, 10(11), 1693. https://doi.org/10.3390/antiox10111693








