Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrieval of Published Studies
2.2. Exclusion and Inclusion Criteria
2.3. Data Extraction
2.4. Meta-Analysis
3. Results
3.1. Data Mining
3.2. Evaluation of the Nephroprotective Effect of the Antioxidant Compounds Tested
3.3. Assessment of Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carriazo, S.; Ortiz, A. European East-West Divide in Kidney Disease: The Need to Understand the Drivers of Chronic Kidney Disease Outcomes. Clin. Kidney J. 2021, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; Nahas, M.E.L.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The Definition, Classification, and Prognosis of Chronic Kidney Disease: A KDIGO Controversies Conference Report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef]
- Choi, B.; Kang, K.-S.; Kwak, M.-K. Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease. Molecules 2014, 19, 12727–12759. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Chronic Kidney Disease Basics|Chronic Kidney Disease Initiative|CDC. Available online: https://www.cdc.gov/kidneydisease/basics.html (accessed on 8 April 2021).
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The Global Burden of Kidney Disease and the Sustainable Development Goals. Bull. World Health Organ. 2018, 96, 414D–422D. [Google Scholar] [CrossRef]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative Stress, Anti-Oxidant Therapies and Chronic Kidney Disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased Prevalence of Oxidant Stress and Inflammation in Patients with Moderate to Severe Chronic Kidney Disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative Stress in Chronic Kidney Disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.-L. Oxidative Stress in Chronic Kidney Disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, Comparison, and Validation of Meta-Essentials: A Free and Simple Tool for Meta-Analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef]
- Adema, A.Y.; van Ittersum, F.J.; Hoenderop, J.G.; de Borst, M.H.; Nanayakkara, P.W.; Wee, P.M.T.; Heijboer, A.C.; Vervloet, M.G.; Consortium, N. Reduction of Oxidative Stress in Chronic Kidney Disease Does Not Increase Circulating α-Klotho Concentrations. PLoS ONE 2016, 11, e0144121. [Google Scholar] [CrossRef]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; de Zoysa, J.R.; et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef]
- de Boer, I.H.; Zelnick, L.R.; Ruzinski, J.; Friedenberg, G.; Duszlak, J.; Bubes, V.Y.; Hoofnagle, A.N.; Thadhani, R.; Glynn, R.J.; Buring, J.E.; et al. Effect of Vitamin D and Omega-3 Fatty Acid Supplementation on Kidney Function in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2019, 322, 1899–1909. [Google Scholar] [CrossRef]
- Chin, M.P.; Bakris, G.L.; Block, G.A.; Chertow, G.M.; Goldsberry, A.; Inker, L.A.; Heerspink, H.J.L.; O’Grady, M.; Pergola, P.E.; Wanner, C.; et al. Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am. J. Nephrol. 2018, 47, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Saiki, A.; Yamaguchi, T.; Sakuma, K.; Sasaki, H.; Ban, N.; Kawana, H.; Nagayama, D.; Nagumo, A.; Ohira, M.; et al. Probucol Suppresses Initiation of Chronic Hemodialysis Therapy and Renal Dysfunction-Related Death in Diabetic Nephropathy Patients: Sakura Study. J. Atheroscler. Thromb. 2013, 20, 494–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef]
- Goicoechea, M.; Garcia de Vinuesa, S.; Verdalles, U.; Verde, E.; Macias, N.; Santos, A.; Pérez de Jose, A.; Cedeño, S.; Linares, T.; Luño, J. Allopurinol and Progression of CKD and Cardiovascular Events: Long-Term Follow-up of a Randomized Clinical Trial. Am. J. Kidney Dis. 2015, 65, 543–549. [Google Scholar] [CrossRef]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.-I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A Phase IIb Randomized Controlled Trial Investigating the Effects of Tocotrienol-Rich Vitamin E on Diabetic Kidney Disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef]
- Nanayakkara, P.W.B.; van Guldener, C.; Ter Wee, P.M.; Scheffer, P.G.; van Ittersum, F.J.; Twisk, J.W.; Teerlink, T.; van Dorp, W.; Stehouwer, C.D.A. Effect of a Treatment Strategy Consisting of Pravastatin, Vitamin E, and Homocysteine Lowering on Carotid Intima-Media Thickness, Endothelial Function, and Renal Function in Patients with Mild to Moderate Chronic Kidney Disease: Results from the Anti-Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch. Intern. Med. 2007, 167, 1262–1270. [Google Scholar] [CrossRef]
- Nangaku, M.; Kanda, H.; Takama, H.; Ichikawa, T.; Hase, H.; Akizawa, T. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study). Kidney Int. Rep. 2020, 5, 879–890. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Chahin, J.; Méndez, M.L.; Gallego, E.; Macía, M.; del Castillo, N.; Rivero, A.; Getino, M.A.; et al. Effect of Pentoxifylline on Renal Function and Urinary Albumin Excretion in Patients with Diabetic Kidney Disease: The PREDIAN Trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nicola, L.D.; Bellizzi, V.; Minutolo, R.; Andreucci, M.; Capuano, A.; Garibotto, G.; Corso, G.; Andreucci, V.E.; Cianciaruso, B. Randomized, Double-Blind, Placebo-Controlled Study of Arginine Supplementation in Chronic Renal Failure. Kidney Int. 1999, 56, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Larczyński, W.; Aleksandrowicz, E.; Łysiak-Szydłowska, W.; Rutkowski, B. The Effect of N-Acetylcysteine on Proteinuria and Markers of Tubular Injury in Non-Diabetic Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2008, 31, 404–410. [Google Scholar] [CrossRef]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Neuwelt, A.; Larczyński, W.; Ziętkiewicz, M.; Aleksandrowicz, E.; Lysiak-Szydłowska, W.; Rutkowski, B. Atorvastatin Improves Tubular Status in Non-Diabetic Patients with Chronic Kidney Disease—Placebo Controlled, Randomized, Cross-over Study. Acta Biochim. Pol. 2010, 57, 547–552. [Google Scholar] [CrossRef]
- Ruggiero, B.; Trillini, M.; Tartaglione, L.; Rotondi, S.; Perticucci, E.; Tripepi, R.; Aparicio, C.; Lecchi, V.; Perna, A.; Peraro, F.; et al. Effects of Sevelamer Carbonate in Patients With CKD and Proteinuria: The ANSWER Randomized Trial. Am. J. Kidney Dis. 2019, 74, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian Green Propolis on Proteinuria and Renal Function in Patients with Chronic Kidney Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Nephrol. 2019, 20, 140. [Google Scholar] [CrossRef]
- Tayebi Khosroshahi, H.; Vaziri, N.D.; Abedi, B.; Asl, B.H.; Ghojazadeh, M.; Jing, W.; Vatankhah, A.M. Effect of High Amylose Resistant Starch (HAM-RS2) Supplementation on Biomarkers of Inflammation and Oxidative Stress in Hemodialysis Patients: A Randomized Clinical Trial. Hemodial. Int. 2018, 22, 492–500. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Zhang, L.; Coombes, J.; Pascoe, E.M.; Badve, S.V.; Dalziel, K.; Cass, A.; Clarke, P.; Ferrari, P.; McDonald, S.P.; Morrish, A.T.; et al. The Effect of Pentoxifylline on Oxidative Stress in Chronic Kidney Disease Patients with Erythropoiesis-Stimulating Agent Hyporesponsiveness: Sub-Study of the HERO Trial. Redox Rep. 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Abbate, M.; Remuzzi, G. Progression of Chronic Kidney Disease: Insights from Animal Models. Curr. Opin. Nephrol. Hypertens. 2006, 15, 250–257. [Google Scholar] [CrossRef] [PubMed]
- López-Novoa, J.M.; Rodríguez-Peña, A.B.; Ortiz, A.; Martínez-Salgado, C.; López Hernández, F.J. Etiopathology of Chronic Tubular, Glomerular and Renovascular Nephropathies: Clinical Implications. J. Transl. Med. 2011, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- López-Novoa, J.M.; Martínez-Salgado, C.; Rodríguez-Peña, A.B.; López-Hernández, F.J. Common Pathophysiological Mechanisms of Chronic Kidney Disease: Therapeutic Perspectives. Pharmacol. Ther. 2010, 128, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Guerrero-Romero, F. Efficacy of Pentoxifylline in the Management of Microalbuminuria in Patients with Diabetes. Curr. Diabetes Rev. 2008, 4, 55–62. [Google Scholar] [CrossRef]
- Leporini, C.; Pisano, A.; Russo, E.; D’Arrigo, G.; de Sarro, G.; Coppolino, G.; Bolignano, D. Effect of Pentoxifylline on Renal Outcomes in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Pharmacol. Res. 2016, 107, 315–332. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.B.; Sydor, A.; Akbari, A.; Fergusson, D.; Doucette, S.; Knoll, G. The Effect of Pentoxifylline on Proteinuria in Diabetic Kidney Disease: A Meta-Analysis. Am. J. Kidney Dis. 2008, 52, 454–463. [Google Scholar] [CrossRef] [PubMed]
- DiPetrillo, K.; Gesek, F.A. Pentoxifylline Ameliorates Renal Tumor Necrosis Factor Expression, Sodium Retention, and Renal Hypertrophy in Diabetic Rats. Am. J. Nephrol. 2004, 24, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Milena, F.J.; Mora, C.; León, C.; García, J. Renal Pro-Inflammatory Cytokine Gene Expression in Diabetic Nephropathy: Effect of Angiotensin-Converting Enzyme Inhibition and Pentoxifylline Administration. Am. J. Nephrol. 2006, 26, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Chiang, W.-C.; Lin, S.-L.; Tsai, T.-J. Therapeutic Efficacy of Pentoxifylline on Proteinuria and Renal Progression: An Update. J. Biomed. Sci. 2017, 24, 84. [Google Scholar] [CrossRef]
- Kanda, H.; Yamawaki, K. Bardoxolone Methyl: Drug Development for Diabetic Kidney Disease. Clin. Exp. Nephrol. 2020, 24, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Chertow, G.M.; Hebbar, S.; Vaziri, N.D.; Ward, K.W.; Meyer, C.J. Bardoxolone Methyl Decreases Megalin and Activates Nrf2 in the Kidney. J. Am. Soc. Nephrol. 2012, 23, 1663–1673. [Google Scholar] [CrossRef]
- Shelton, L.M.; Kevin Park, B.; Copple, I.M. Role of Nrf2 in Protection against Acute Kidney Injury. Kidney Int. 2013, 84, 1090–1095. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and Cubilin: Synergistic Endocytic Receptors in Renal Proximal Tubule. Am. J. Physiol.-Ren. Physiol. 2001, 280, F562–F573. [Google Scholar] [CrossRef]
- Ito, M.; Nangaku, M. Increased albuminuria in bardoxolone methyl–treated type 2 diabetes patients: Mere reflection of eGFR improvement? Kidney Int. 2019, 96, 823–825. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Nava, V.; Locatelli, M.; Abbate, M.; Gaspari, F.; Carrara, F.; Sangalli, F.; Remuzzi, G.; Benigni, A. Analogs of Bardoxolone Methyl Worsen Diabetic Nephropathy in Rats with Additional Adverse Effects. Am. J. Physiol.-Ren. Physiol. 2012, 304, F808–F819. [Google Scholar] [CrossRef]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for Chronic Kidney Disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental Factors and Unhealthy Lifestyle Influence Oxidative Stress in Humans—An Overview. Environ. Sci. Pollut. Res. 2013, 20, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- McKeegan, K.; Mason, S.A.; Trewin, A.J.; Keske, M.A.; Wadley, G.D.; Della Gatta, P.A.; Nikolaidis, M.G.; Parker, L. Reactive oxygen species in exercise and insulin resistance: Working towards personalized antioxidant treatment. Redox Biol. 2021, 44, 102005. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidant supplementation, redox deficiencies and exercise performance: A falsification design. Free Radic. Biol. Med. 2020, 158, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]

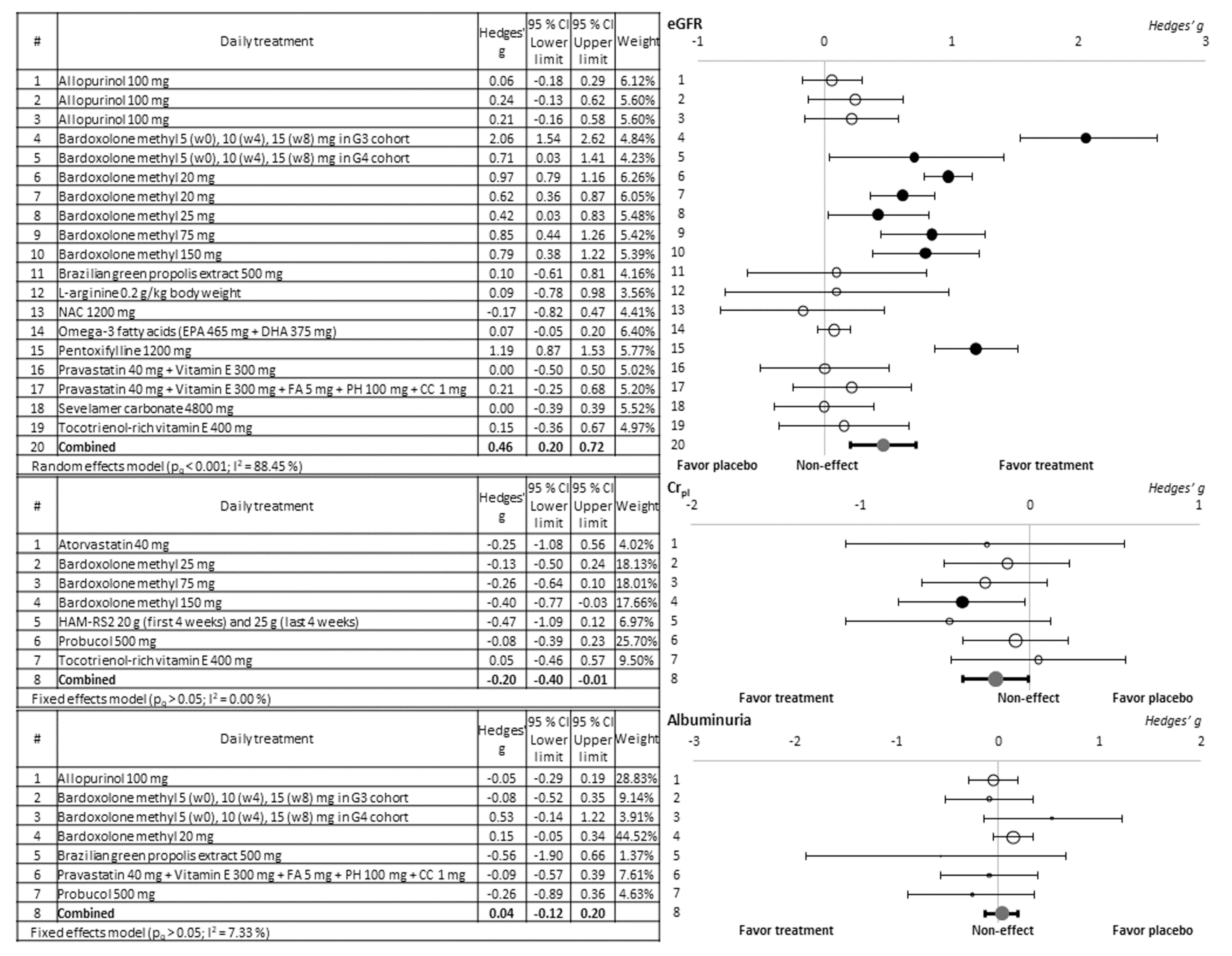
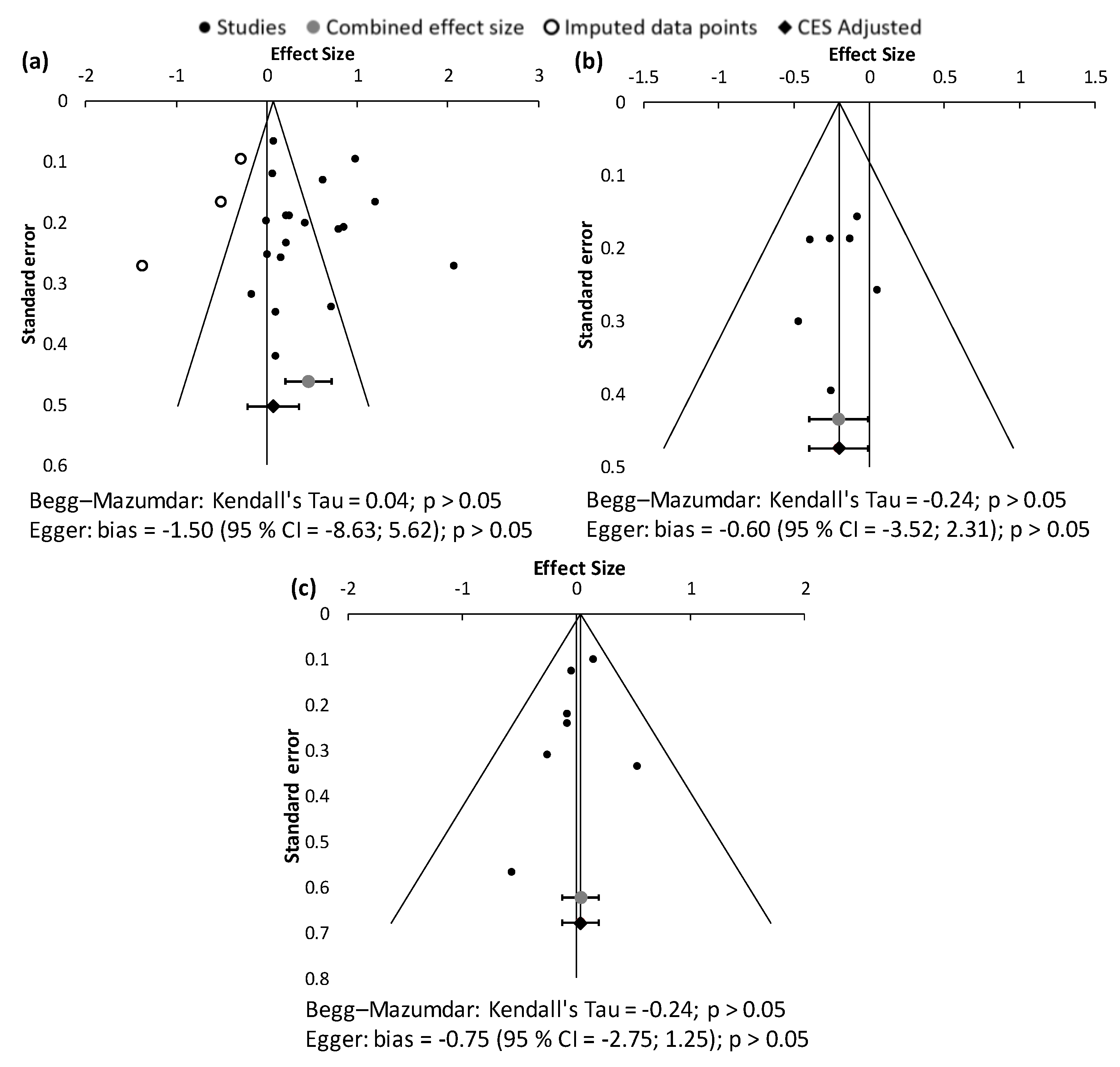

| Study Identification | Design | Location | Duration of Recruitment | Population | Number of Patients Initially Included (Treatment/Control or Placebo Group) | Nephroprotective Treatment | Jadad Score | Renal Function Biomarkers Available | ||
|---|---|---|---|---|---|---|---|---|---|---|
| eGFR | Crpl | AU | ||||||||
| Adema et al., 2016 [16] | Prospective, randomized, double-blind trial | The Netherlands | May 2001–December 2002 | Non-diabetic patients with mild–moderate chronic renal failure who had no manifest arterial occlusive disease | 34/28 | Pravastatin 40 mg/day (from baseline) + Vitamin E 300 mg/day (from month 6) p.o. for 12 months | 5 | Yes | No | No |
| Badve et al., 2020 [17] | Prospective, randomized, double-blind trial | Australia and New Zealand | March 2014–December 2016 | Adults with stage 3 or 4 chronic kidney disease and no history of gout | 182/181 | Allopurinol 100 mg/day (the first 12 weeks) and up to 300 mg/day (until the end of the study) p.o. for 2 years | 4 | Yes | No | Yes |
| de Boer et al., 2019 [18] | Prospective, randomized, double-blind trial | USA | November 2011–March 2014 | Patients with type 2 diabetes at baseline to ascertain CKD outcomes | 289/320 | Omega-3 fatty acids (Eicosapentaenoic acid 465 mg/day + Docosahexaenoic acid 375 mg/day) p.o. for 5 years | 5 | Yes | No | No |
| Chin et al., 2018 [19] | Prospective, randomized, double-blind trial | USA, European Union, Australia, Canada, Israel, and Mexico | June 2011–September 2012 | Patients with type 2 diabetes mellitus and stage 4 chronic kidney disease | 1097/1088 | Bardoxolone methyl 20 mg/day p.o. for 48 weeks | 4 | Yes | No | No |
| Endo et al., 2013 [20] | Prospective, randomized, open-label trial | Japan | October 2001–September 2004 | Patients with type 2 diabetes mellitus and albuminuria | 80/82 | Probucol 500 mg/day for 5 years | 3 | No | Yes | Yes |
| Goicoechea et al., 2010 [21] | Prospective, randomized, open-label trial | Spain | January 2007–May 2007 | Patients with chronic kidney disease | 57/56 | Allopurinol 100 mg/day for 24 months | 5 | Yes | No | No |
| Goicoechea et al., 2015 [22] | Prospective, randomized, open-label trial | Spain | May 2007–May 2012 | Patients with chronic kidney disease | 57/56 | Allopurinol 100 mg/day for 5 years | 5 | Yes | No | No |
| Koay et al., 2021 [23] | Prospective, randomized, double-blind trial | Malaysia | March 2019–September 2020 | Patients with diabetic kidney disease | 31/30 | Tocotrienol-rich vitamin E 400 mg/day p.o. for 12 months | 4 | Yes | Yes | No |
| Nanayakkara et al., 2007 [24] | Prospective, randomized, double-blind trial | The Netherlands | May 2001–December 2002 | Non-diabetic patients with chronic renal failure who had no manifest arterial occlusive disease | 47/46 | Pravastatin 40 mg/day (from baseline) + Vitamin E 300 mg/day (from month 6) + Folic acid 5 mg/day, pyridoxine hydrochloride 100 mg/day and cyanocobalamin 1 mg/day (from month 12) p.o. for 24 months | 5 | Yes | No | Yes |
| Nangaku et al., 2020 [25] | Prospective, randomized, double-blind trial | Japan | December 2014–September 2017 | Patients with with type 2 diabetes and stage 3 (cohort G3) and stage 4 CKD (cohort G4) | 41/41 (G3) 24/14 (G4) | Bardoxolone methyl mg/day, followed by dose escalation, as tolerated, to 10 mg/day at week 4 and 15 mg/day at week 8 p.o. for 8 weeks | 4 | Yes | No | Yes |
| Navarro-González et al., 2015 [26] | Prospective, randomized, open-label trial | Spain | January 2008–December 2008 | Patients with type 2 diabetes mellitus and diabe tic nephropathy | 82/87 | Pentoxifylline 1200 mg/day p.o. for 2 years | 4 | Yes | No | No |
| de Nicola et al., 1999 [27] | Prospective, randomized, double-blind trial | Italy | Not specified | Patients with proteinuria aged 18 to 60 years with a moderate to medium degree of chronic renal failure | 11/10 | L-arginine 0.2 g/kg body weight/day p.o. for 6 months | 3 | Yes | No | No |
| Pergola et al., 2011 [28] | Prospective, randomized, double-blind trial | USA | Not specified | Adults with moderate to severe CKD and type 2 diabetes | 57/57/56/57 | Bardoxolone methyl 25 mg/day or Bardoxolone methyl 75 mg/day or Bardoxolone methyl 150 mg/day p.o. for 52 weeks | 4 | Yes | Yes | No |
| Renke et al., 2008 [29] | Prospective, randomized, open-label, cross-over trial | Poland | Not specified | Non-diabetic patients aged 18 to 65 with chronic kidney disease | 20/20 | N-acetylcysteine 1200 mg/day p.o. for 8 weeks | 3 | Yes | No | No |
| Renke et al., 2010 [30] | Prospective, randomized, cross-over trial | Poland | Not specified | Non-diabetic patients aged 18 to 65 with chronic kidney disease | 14/14 | Atorvastatin 40 mg/day p.o. for 12 weeks | 3 | No | Yes | No |
| Ruggiero et al., 2019 [31] | Prospective, randomized, open-label, cross-over trial | Italy | November 2013–December 2014 | Adults with CKD and proteinuria | 53/53 | Sevelamer carbonate 4800 mg/day p.o. for 3 months | 3 | Yes | No | No |
| Silveira et al., 2019 [32] | Prospective, randomized, double-blind trial | Brazil | Not specified | Patients aged 18 to 90 with chronic kidney disease and proteinuria | 18/16 | Brazilian green propolis extract 500 mg/day (35.5 mg total flavonoids + 77.96 mg total phenolic compounds) p.o. for 12 months | 5 | Yes | No | Yes |
| Tayebi-Khosroshahi et al., 2018 [33] | Prospective, randomized, double-blind, parallel trial | Iran | November 2016–February 2017 | End-stage renal disease patients maintained on chronic hemodialysis | 22/22 | HAM-RS2 20 g/day (the first 4 weeks) and 25 g/day (until the end of the study) p.o. for 8 weeks | 4 | No | Yes | No |
| Zeeuw et al., 2013 [34] | Prospective, randomized, double-blind, parallel trial | USA, European Union, Australia, Canada, Israel, and Mexico | June 2011–September 2012 | Patients with type 2 diabetes mellitus and stage 4 chronic kidney disease | 1088/1097 | Bardoxolone methyl 20 mg/day p.o. for 56 weeks | 4 | Yes | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, A.G.; López-Hernández, F.J.; Vicente-Vicente, L.; Morales, A.I. Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease? Antioxidants 2021, 10, 1669. https://doi.org/10.3390/antiox10111669
Casanova AG, López-Hernández FJ, Vicente-Vicente L, Morales AI. Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease? Antioxidants. 2021; 10(11):1669. https://doi.org/10.3390/antiox10111669
Chicago/Turabian StyleCasanova, Alfredo G., Francisco J. López-Hernández, Laura Vicente-Vicente, and Ana I. Morales. 2021. "Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease?" Antioxidants 10, no. 11: 1669. https://doi.org/10.3390/antiox10111669
APA StyleCasanova, A. G., López-Hernández, F. J., Vicente-Vicente, L., & Morales, A. I. (2021). Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease? Antioxidants, 10(11), 1669. https://doi.org/10.3390/antiox10111669








