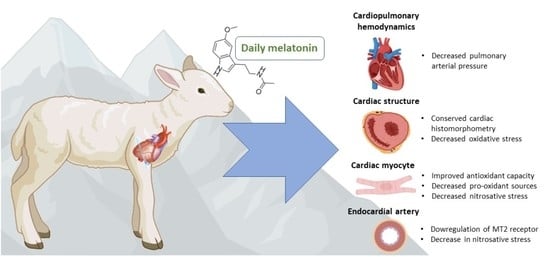
Melatonin Reduces Oxidative Stress in the Right Ventricle of Newborn Sheep Gestated under Chronic Hypoxia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Cardiopulmonary Measurements
2.3. Cardiac Histomorphometry
2.4. Antioxidant Enzyme Expression and Activity in the Right Ventricle
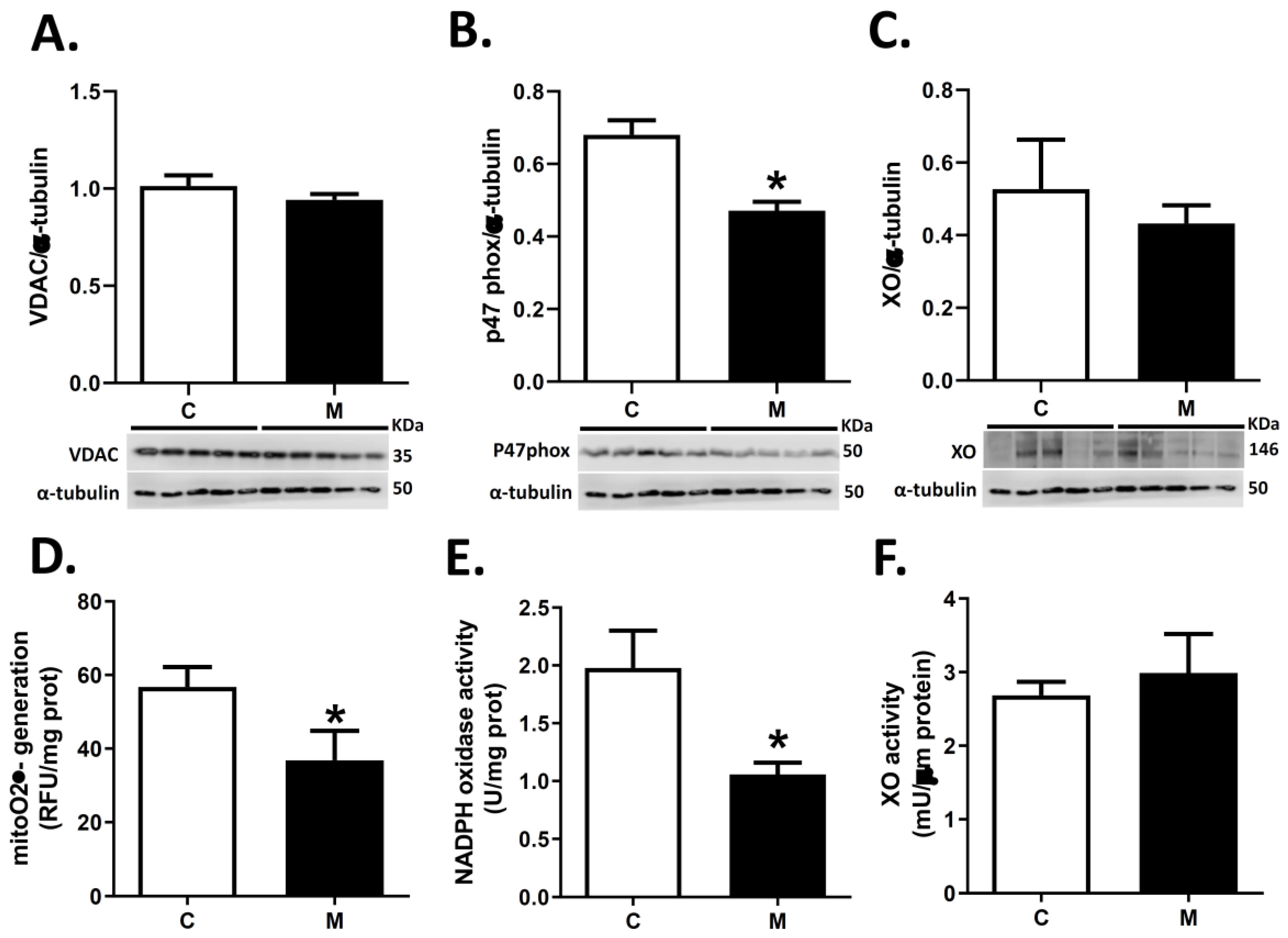
2.5. Quantification of Pro-Oxidant Sources in the Right Ventricle
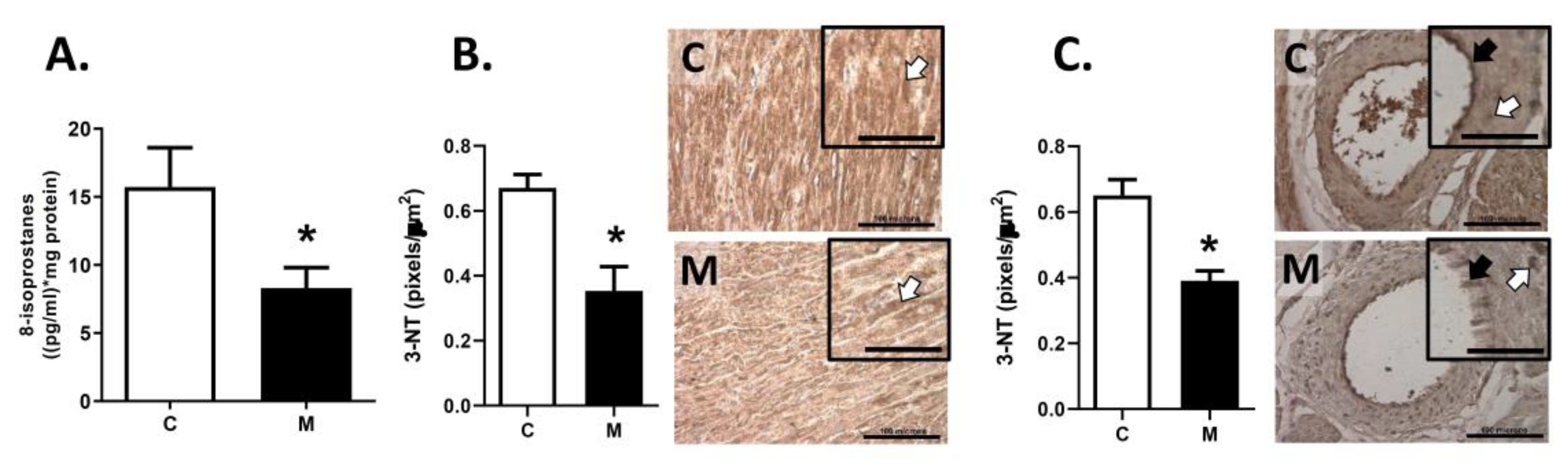
2.6. Oxidative Stress Markers
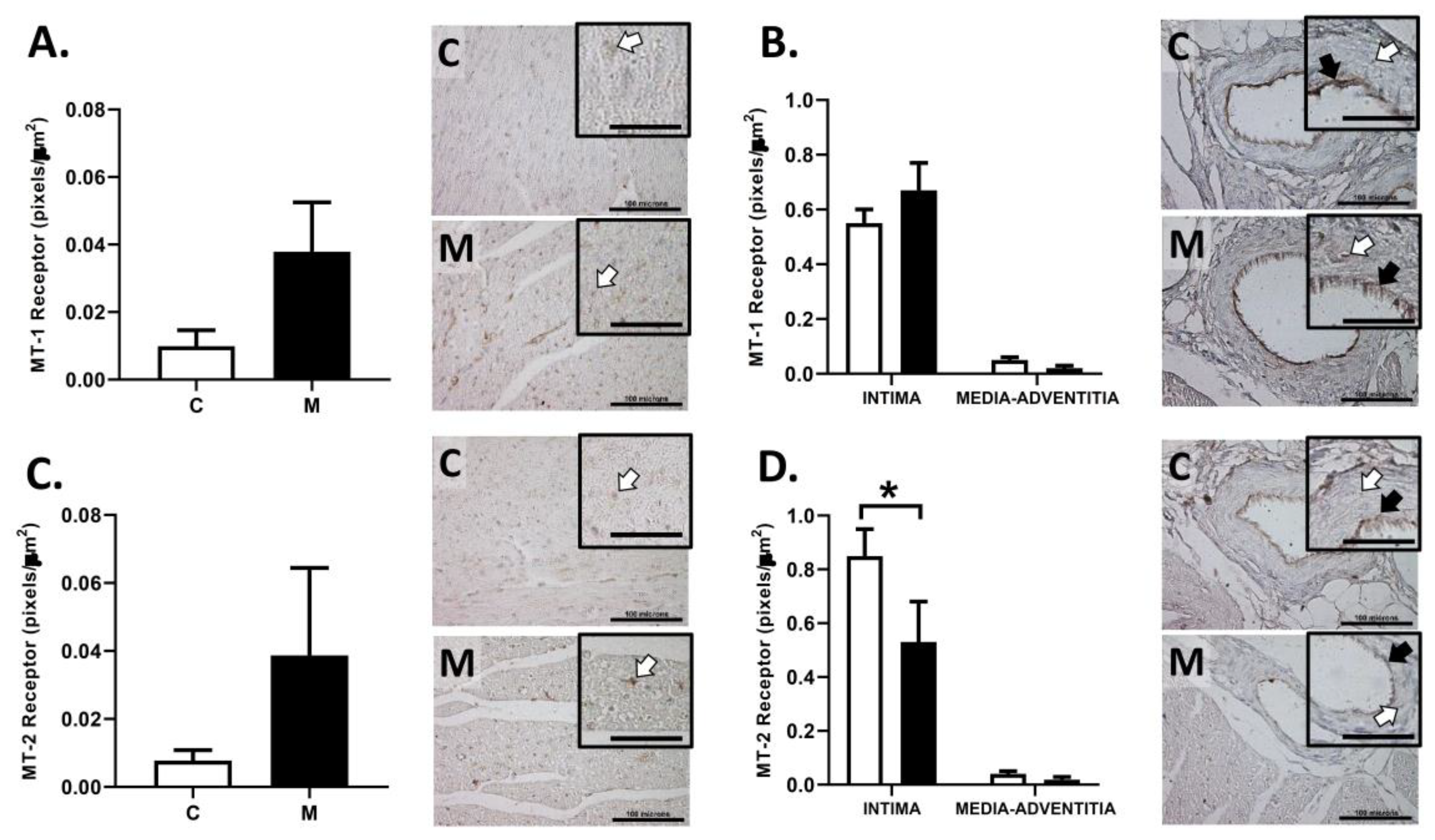
2.7. Right Ventricle Immunohistochemistry
2.8. Statistical Analyses
3. Results
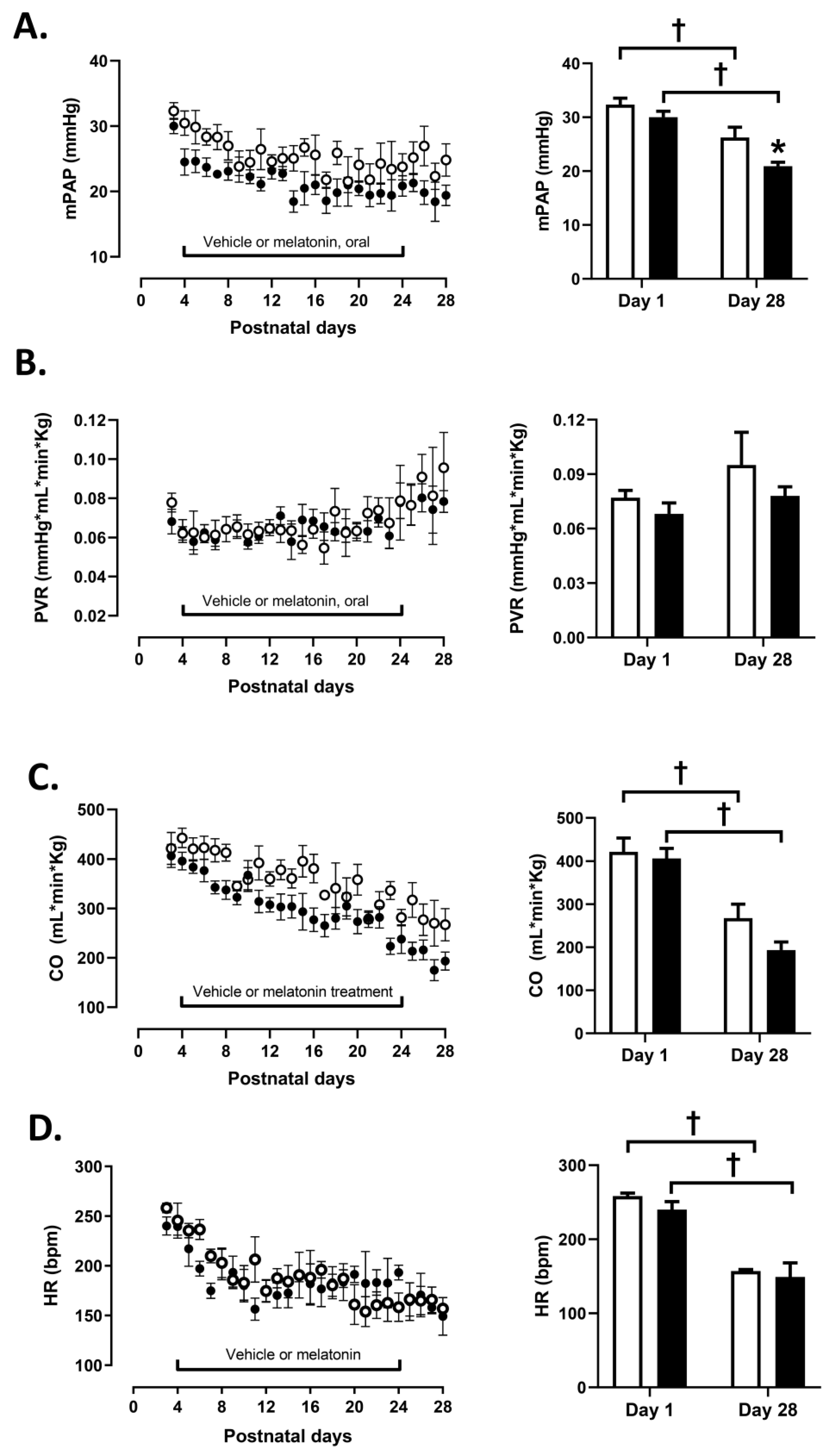
3.1. Cardiopulmonary Variables
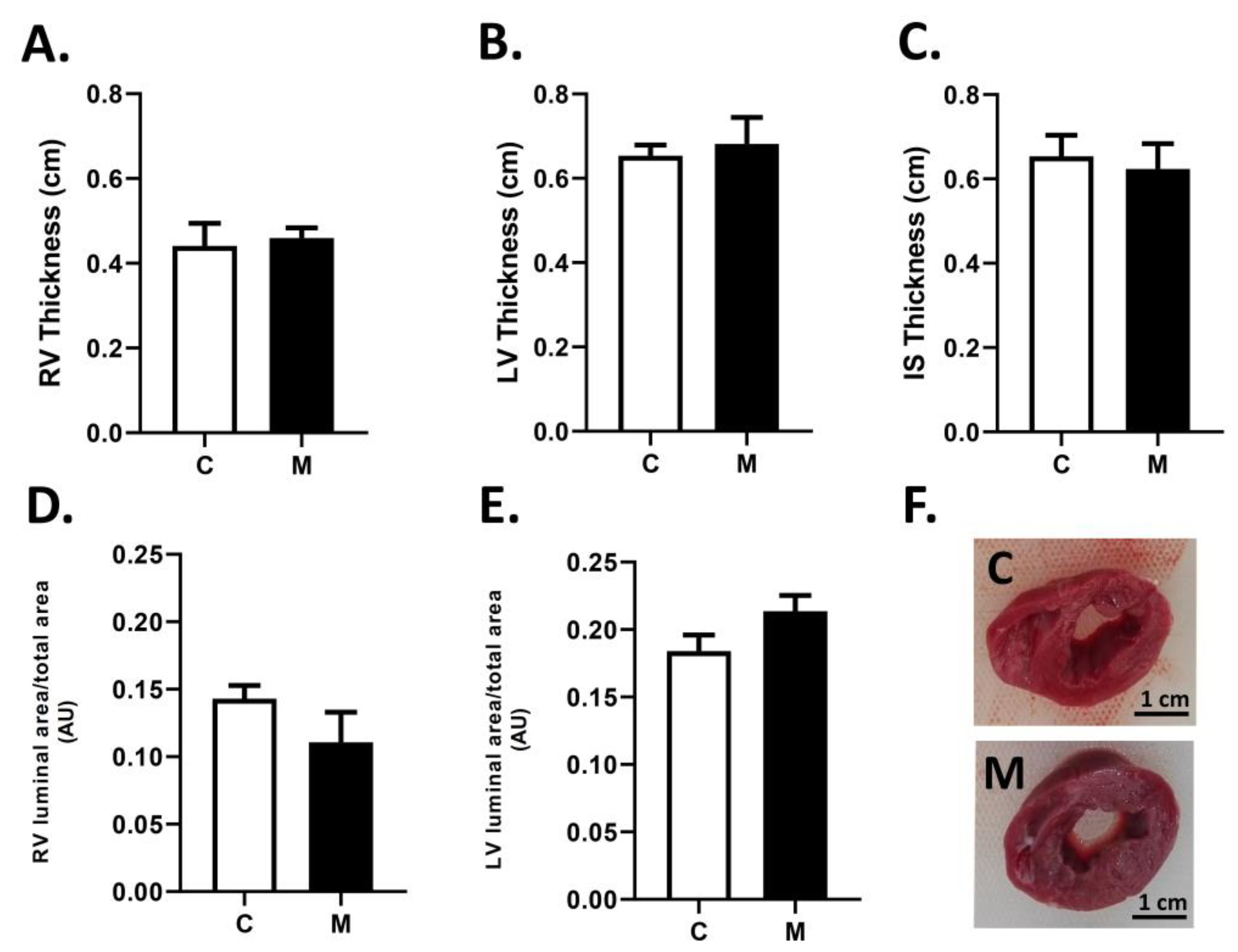
3.2. Cardiac Biometry
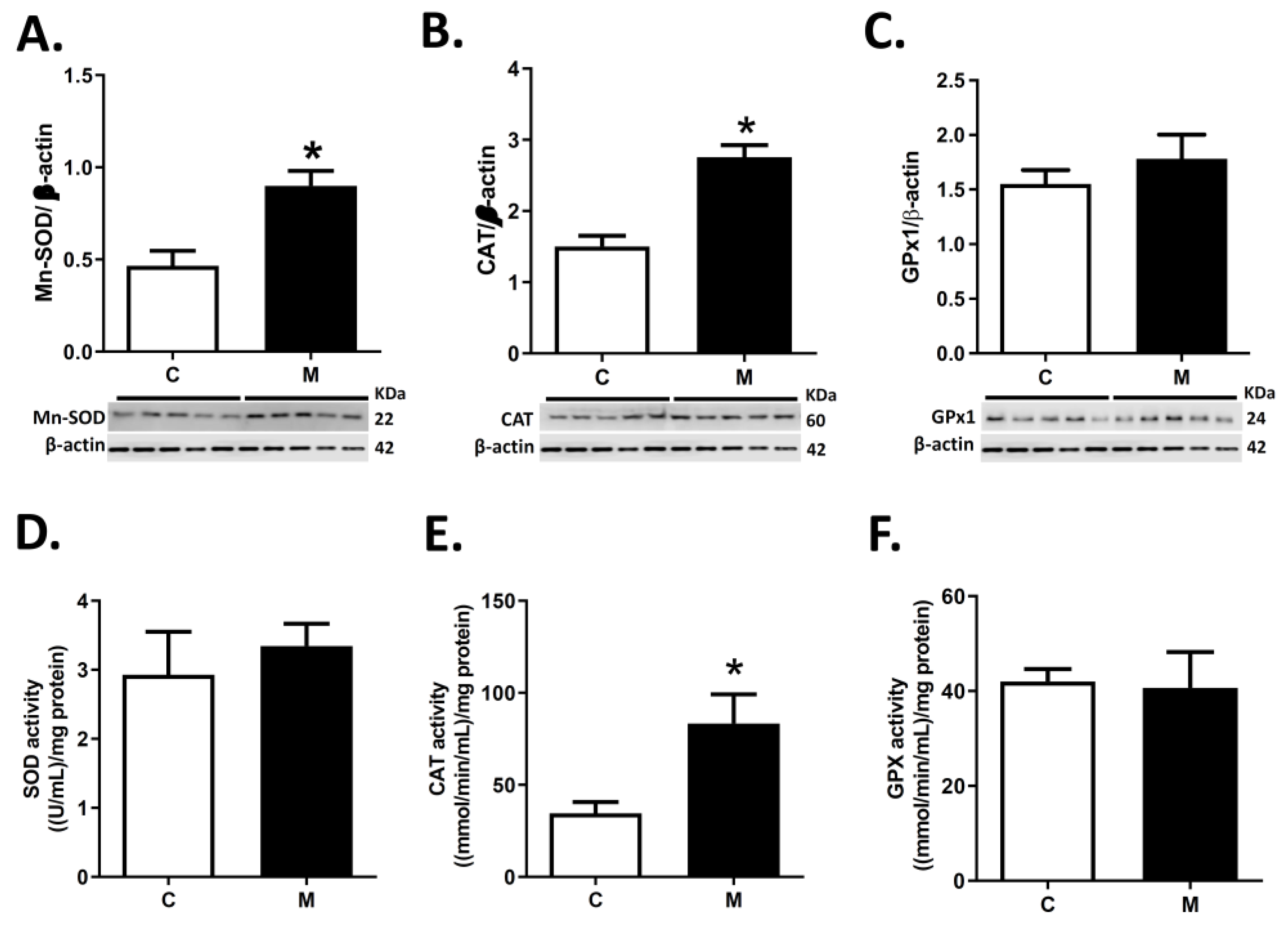
3.3. Antioxidant Enzymes Expression and Activity in RV
3.4. ROS Sources in RV
3.5. Oxidative Stress in RV
3.6. Immunolocalization of Melatonin Receptors in the RV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Herrera, E.A.; Krause, B.; Ebensperger, G.; Reyes, R.V.; Casanello, P.; Parra-Cordero, M.; Llanos, A.J. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front. Pharmacol. 2014, 24, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Raj, U. Hypoxic Pulmonary Hypertension of the Newborn. Compr. Physiol. 2011, 1, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.G.; Shriver, M.; Bemis, L.; Hickler, B.; Wilson, M.; Brutsaert, T.; Parra, E.; Vargas, E. Maternal adaptation to high-altitude pregnancy: An experiment of nature—A review. Placenta 2004, 25 (Suppl. A), S60–S71. [Google Scholar] [CrossRef]
- Herrera, E.A.; Farías, J.G.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Castillo, R.L. Pharmacological approaches in either intermittent or permanent hypoxia: A tale of two exposures. Pharmacol. Res. 2015, 101, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef] [Green Version]
- Kocken, J.M.; da Costa Martins, P.A. Epigenetic Regulation of Pulmonary Arterial Hypertension-Induced Vascular and Right Ventricular Remodeling: New Opportunities. Int. J. Mol. Sci. 2020, 21, 8901. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [Green Version]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Bogaard, H.J.; Abe, K.; Vonk, N.A.; Voelkel, N.F. The right ventricle under pressure: Cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009, 135, 794–804. [Google Scholar] [CrossRef] [Green Version]
- Mital, S.; Chung, W.K.; Colan, S.D.; Sleeper, L.A.; Manlhiot, C.; Arrington, C.B.; Cnota, J.F.; Graham, E.M.; Mitchell, M.E.; Goldmuntz, E.; et al. Pediatric Heart Network Investigators. Renin-angiotensin-aldosterone genotype influences ventricular remodeling in infants with single ventricle. Circulation 2011, 123, 2353–2362. [Google Scholar] [CrossRef] [Green Version]
- Karamlou, T.; Giraud, G.D.; McKeogh, D.; Jonker, S.S.; Shen, I.; Ungerleider, R.M.; Thornburg, K.L. Right ventricular remodeling in response to volume overload in fetal sheep. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H985–H991. [Google Scholar] [CrossRef]
- Yokota, N.; Bruneau, B.G.; Fernandez, B.E.; de Bold, M.L.; Piazza, L.A.; Eid, H.; de Bold, A.J. Dissociation of cardiac hypertrophy, myosin heavy chain isoform expression, and natriuretic peptide production in DOCA-salt rats. Am. J. Hypertens. 1995, 8, 301–310. [Google Scholar] [CrossRef]
- Bello-Klein, A.; Mancardi, D.; Araujo, A.S.; Schenkel, P.C.; Turck, P.; de Lima Seolin, B.G. Role of redox homeostasis and inflammation in the pathogenesis of pulmonary arterial hypertension. Curr. Med. Chem. 2018, 25, 1340–1351. [Google Scholar] [CrossRef]
- Reddy, S.; Bernstein, D. Molecular mechanisms of right ventricular failure. Circulation 2015, 132, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Shults, N.V.; Melnyk, O.; Suzuki, D.I.; Suzuki, Y.J. Redox biology of right-sided heart failure. Antioxidants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.; Boet, A.; Rucker-Martin, C.; Lambert, M.; Gomez, A.M.; Benitah, J.P.; Perros, F.; Humbert, M.; Antigny, F. Ca2+ handling remodeling and STIM1L/Orai1/TRPC1/TRPC4 upregulation in monocrotaline-induced right ventricular hypertrophy. J. Mol. Cell. Cardiol. 2018, 118, 208–224. [Google Scholar] [CrossRef]
- Pechánová, O.; Varga, Z.V.; Cebová, M.; Giricz, Z.; Pacher, P.; Ferdinandy, P. Cardiac NO signalling in the metabolic syndrome. Br. J. Pharmacol. 2015, 172, 1415–1433. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.C.; Ma, H.P.; Jing, L.L.; Li, L.; Jia, Z.P. The antioxidative effect of a novel free radical scavenger 4′-hydroxyl-2-substituted phenylnitronyl nitroxide in acute high-altitude hypoxia mice. Biol. Pharm. Bull. 2013, 36, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlow-Walden, L.R. Melatonin--a highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Candia, A.; Veliz, M.; Carrasco-Pozo, C.; Castillo, R.L.; Cárdenas, J.C.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 2019, 22, 101128. [Google Scholar] [CrossRef] [PubMed]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Liu, L.; Jockers, R. Melatonin receptor structures shed new light on melatonin research. J. Pineal. Res. 2019, 67, e12606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ma, Q. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal. Res. 2018, 64, e12471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, M.; Liu, Z.; Zhang, B.; Jing, L.; Li, B.; Li, K.; Chen, X.; Zhang, M.; Yu, B.; Ren, K.; et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1β: In vivo and in vitro studies. J. Pineal. Res. 2017, 63. [Google Scholar] [CrossRef]
- Weekley, L.B. Effects of melatonin on isolated pulmonary artery and vein: Role of the vascular endothelium. Pulm. Pharmacol. 1993, 6, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; González-Candia, A. Comment on Melatonin as a potential adjuvant treatment for COVID-19. Life. Sci. 2020, 253, 117739. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E. Cardiovascular Effects of Melatonin. In Melatonin: Biological Basis of Its Function in Health and Disease; Pandi-Perumal, S.R., Cardinali, D.P., Eds.; Eurekah.com/Landes Bioscience: Georgetown, TX, USA, 2005. [Google Scholar]
- Gonzaléz-Candia, A.; Candia, A.A.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. The newborn sheep translational model for pulmonary arterial hypertension of the neonate at high altitude. J. Dev. Orig. Health Dis. 2020, 11, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Berry, M.J.; Botting, K.J.; Darby, J.R.T.; Frasch, M.G.; Gatford, K.L.; Giussani, D.A.; Gray, C.L.; Harding, R.; Herrera, E.A.; et al. Improving pregnancy outcomes in humans through studies in sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1123–R1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzaléz-Candia, A.; Candia, A.A.; Figueroa, E.G.; Feixes, E.; Gonzalez-Candia, C.; Aguilar, S.A.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin long-lasting beneficial effects on pulmonary vascular reactivity and redox balance in chronic hypoxic ovine neonates. J. Pineal. Res. 2020, 68, e12613. [Google Scholar] [CrossRef]
- Herrera, E.A.; Pulgar, V.M.; Riquelme, R.A.; Sanhueza, E.M.; Reyes, R.V.; Ebensperger, G.; Parer, J.T.; Valdéz, E.A.; Giussani, D.A.; Blanco, C.E.; et al. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2234–R2240. [Google Scholar] [CrossRef] [Green Version]
- Torres, F.; González-Candia, A.; Montt, C.; Ebensperger, G.; Chubretovic, M.; Serón-Ferré, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J. Pineal. Res. 2015, 58, 362–373. [Google Scholar] [CrossRef]
- Østergaard, K.H.; Baandrup, U.T.; Wang, T.; Bertelsen, M.F.; Andersen, J.B.; Smerup, M.; Nyengaard, J.R. Left ventricular morphology of the giraffe heart examined by stereological methods. Anat. Rec. Hoboken 2013, 296, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; de la Jara, N.; Peña, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid. Med. Cell. Longev. 2018, 7239123. [Google Scholar] [CrossRef]
- Whidden, M.A.; McClung, J.M.; Falk, D.J.; Hudson, M.B.; Smuder, A.J.; Nelson, W.B.; Powers, S.K. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J. Appl. Physiol. 2009, 106, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczyk, G.; Bełtowski, J.; Marciniak, A.; Kotarski, J. Serum isoprostanes levels in patients after abdominal hysterectomy. Rocz. Akad. Med. Bialymst. 2005, 50, 322–324. [Google Scholar]
- Astorga, C.R.; González-Candia, A.; Candia, A.A.; Figueroa, E.G.; Cañas, D.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin Decreases Pulmonary Vascular Remodeling and Oxygen Sensitivity in Pulmonary Hypertensive Newborn Lambs. Front. Physiol. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, E.A.; Rojas, R.T.; Krause, B.J.; Ebensperger, G.; Reyes, R.V.; Giussani, D.A.; Parer, J.T.; Llanos, A.J. Cardiovascular function in term fetal sheep conceived, gestated and studied in the hypobaric hypoxia of the Andean altiplano. J. Physiol. 2016, 594, 1231–1245. [Google Scholar] [CrossRef] [Green Version]
- Peñaloza, D.; Arias-Stella, J. The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation 2007, 115, 1132–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, T.M.; Peabody, J.; Baddoura, P.; Fallica, J.; Mock, J.R.; Singer, B.D.; D’Alessio, F.R.; Damarla, M.; Damico, R.L.; Hassoun, P.M. Right Ventricular Angiogenesis is an Early Adaptive Response to Chronic Hypoxia-Induced Pulmonary Hypertension. Microcirculation 2015, 22, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Siebenmann, C.; Lundby, C. Regulation of cardiac output in hypoxia. Scan. J. Med. Sci. Sports 2015, 25 (Suppl. 4), 53–59. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.R.; Sinkin, R.A. Transition from fetus to newborn. Pediatr. Clin. N. Am. 2015, 62, 329–343. [Google Scholar] [CrossRef]
- Morton, S.U.; Brodsky, D. Fetal Physiology and the Transition to Extrauterine Life. Clin. Perinatol. 2016, 43, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Farías, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; McNamara, P.J. Persistent pulmonary hypertension of the newborn: Advances in diagnosis and treatment. Semin. Fetal. Neonatal. Med. 2015, 20, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef]
- Azizi, M.; Pasbakhsh, P.; Nadji, S.A.; Pourabdollah, M.; Mokhtari, T.; Sadr, M.; Omidi, N.; Kashani, I.R.; Zendehdel, A. Therapeutic effect of perinatal exogenous melatonin on behavioral and histopathological changes and antioxidative enzymes in neonate mouse model of cortical malformation. Int. J. Dev. Neurosci. 2018, 68, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal. Res. 2013, 55, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Minc, E.; de Coppet, P.; Masson, P.; Thiery, L.; Dutertre, S.; Amor-Guéret, M.; Jaulin, C.J. The human copper-zinc superoxide dismutase gene (SOD1) proximal promoter is regulated by Sp1, Egr-1, and WT1 via non-canonical binding sites. Biol. Chem. 1999, 274, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free. Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Ann. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Martín, M.; Macías, M.; Escames, G.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal. Res. 2001, 30, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, J.; Tian, F.; Hu, S.; Chen, Y.; Fu, Z. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal. Res. 2019, 66, e12542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1–42. J. Pineal. Res. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Zhang, W.X.; He, B.M.; Wu, Y.; Qiao, J.F.; Peng, Z.Y. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019, 217, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; San Martín, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Hou, L.; Sun, F.; Huang, R.; Sun, W.; Zhang, D.; Wang, Q. Inhibition of NADPH oxidase by apocynin prevents learning and memory deficits in a mouse Parkinson’s disease model. Redox Biol. 2019, 22, 101134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lu, Y.; Ping, N.N.; Li, X.; Lin, Y.X.; Li, C.F. Apocynin ameliorates pressure overload-induced cardiac remodeling by inhibiting oxidative stress and apoptosis. Physiol. Res. 2017, 66, 741–752. [Google Scholar] [CrossRef]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal. Res. 2017, 63, e12413. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, M.; Herrmann, J.; Tolle, M.; van der Giet, M. Xanthine Oxidase and its Role as Target in Cardiovascular Disease: Cardiovascular Protection by Enzyme Inhibition? Curr. Pharm. Des. 2017, 23, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Tain, Y.L. Postnatal dexamethasone-induced programmed hypertension is related to the regulation of melatonin and its receptors. Steroids 2016, 108, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91. [Google Scholar] [CrossRef]
- Blanco, S.; Hernández, R.; Franchelli, G.; Ramos-Álvarez, M.M.; Peinado, M.Á. Melatonin influences NO/NOS pathway and reduces oxidative and nitrosative stress in a model of hypoxic-ischemic brain damage. Nitric Oxide 2017, 62, 32–43. [Google Scholar] [CrossRef]
- Aguilar, S.A.; Arias, P.V.; Canquil, I.; Ebensperger, G.; Llanos, A.J.; Reyes, R.V.; González-Candia, A.; Herrera, E.A. El tratamiento postnatal con melatonina modula la expresión de agentes prostanoides en pulmón de neonatos de oveja con hipertensión pulmonar [Melatonin modulates the expression of pulmonary prostanoids]. Rev. Med. Chile 2019, 147, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Rodriguez, A. Melatonin in cardiovascular disease. Expert Opin. Investig. Drugs 2012, 21, 1593–1596. [Google Scholar] [CrossRef]
- Baltatu, O.C.; Senar, S.; Campos, L.A.; Cipolla-Neto, J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. Int. J. Mol. Sci. 2019, 20, 4342. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Maronde, E.; Wicht, H.; Taskén, K.; Genieser, H.G.; Dehghani, F.; Olcese, J.; Korf, H.W. CREB phosphorylation and melatonin biosynthesis in the rat pineal gland: Involvement of cyclic AMP dependent protein kinase type II. J. Pineal. Res. 1999, 27, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Bae, J.H.; Lee, J.H.; Kim, Y.N.; Kim, D.K. The Melatonin Signaling Pathway in a Long-Term Memory In Vitro Study. Molecules 2018, 23, 737. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Kim, M.O. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, L.P.; Turan, S.; Aberdeen, G.W. Sex differences and the effects of intrauterine hypoxia on growth and in vivo heart function of fetal guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R243–R254. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Chen, L.; Polster, B.M.; Pinkas, G.; Song, H. Prenatal hypoxia impairs cardiac mitochondrial and ventricular function in guinea pig offspring in a sex-related manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1232–R1241. [Google Scholar] [CrossRef]
- Lumbroso, D.; Joseph, V. Impaired acclimatization to chronic hypoxia in adult male and female rats following neona-tal hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R421–R427. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzaléz-Candia, A.; Arias, P.V.; Aguilar, S.A.; Figueroa, E.G.; Reyes, R.V.; Ebensperger, G.; Llanos, A.J.; Herrera, E.A. Melatonin Reduces Oxidative Stress in the Right Ventricle of Newborn Sheep Gestated under Chronic Hypoxia. Antioxidants 2021, 10, 1658. https://doi.org/10.3390/antiox10111658
Gonzaléz-Candia A, Arias PV, Aguilar SA, Figueroa EG, Reyes RV, Ebensperger G, Llanos AJ, Herrera EA. Melatonin Reduces Oxidative Stress in the Right Ventricle of Newborn Sheep Gestated under Chronic Hypoxia. Antioxidants. 2021; 10(11):1658. https://doi.org/10.3390/antiox10111658
Chicago/Turabian StyleGonzaléz-Candia, Alejandro, Pamela V. Arias, Simón A. Aguilar, Esteban G. Figueroa, Roberto V. Reyes, Germán Ebensperger, Aníbal J. Llanos, and Emilio A. Herrera. 2021. "Melatonin Reduces Oxidative Stress in the Right Ventricle of Newborn Sheep Gestated under Chronic Hypoxia" Antioxidants 10, no. 11: 1658. https://doi.org/10.3390/antiox10111658
APA StyleGonzaléz-Candia, A., Arias, P. V., Aguilar, S. A., Figueroa, E. G., Reyes, R. V., Ebensperger, G., Llanos, A. J., & Herrera, E. A. (2021). Melatonin Reduces Oxidative Stress in the Right Ventricle of Newborn Sheep Gestated under Chronic Hypoxia. Antioxidants, 10(11), 1658. https://doi.org/10.3390/antiox10111658