Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Pumpkin Samples and Preparation
2.3. Pumpkin Pulp Extracts and Absorbance Spectra
2.4. Determination of Antioxidant Capacity (ABTS, FRAP)
2.5. Lipidomic Profiling by UHPLC-Orbitrap Mass Spectrometry
2.6. Carotenoid Analysis and Quantification by HPLC-DAD
2.7. Statistical Analysis
3. Results
3.1. Absorbance Spectra of Pumpkin Pulp Extracts
3.2. In Vitro Antioxidant Capacity of Pumpkin Pulp Extracts
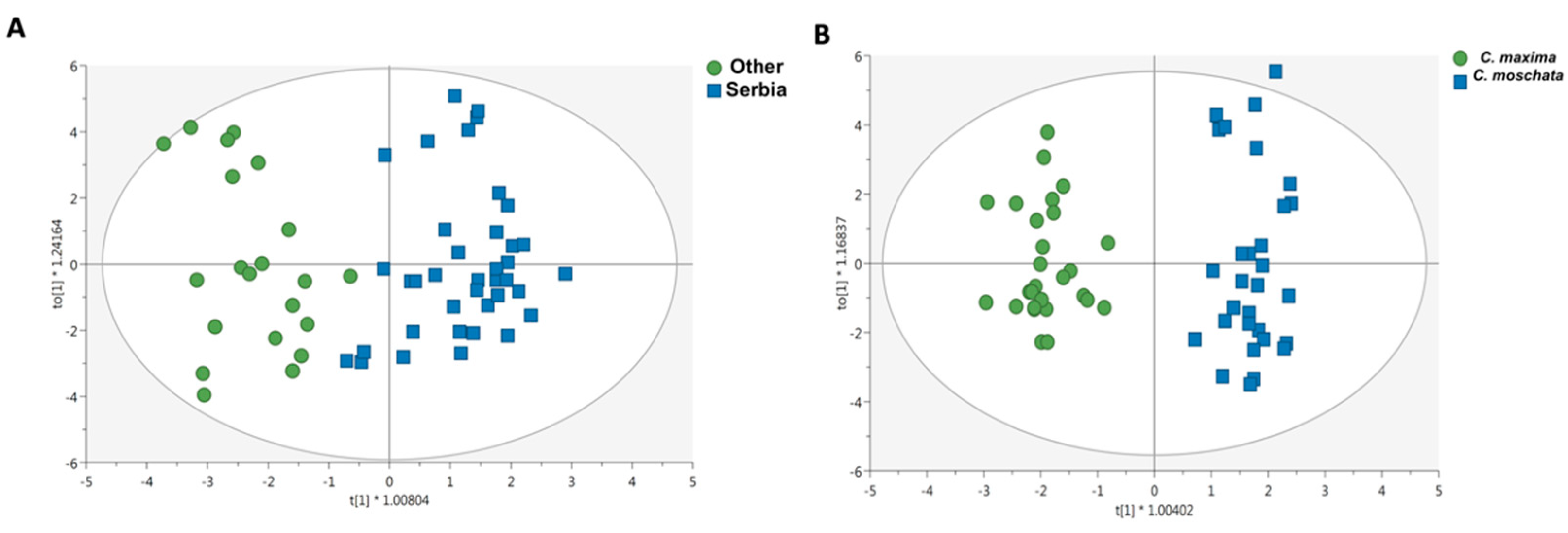
3.3. Untargeted Lipidomic Profiling and Discrimination of Pumpkin Pulp Extracts
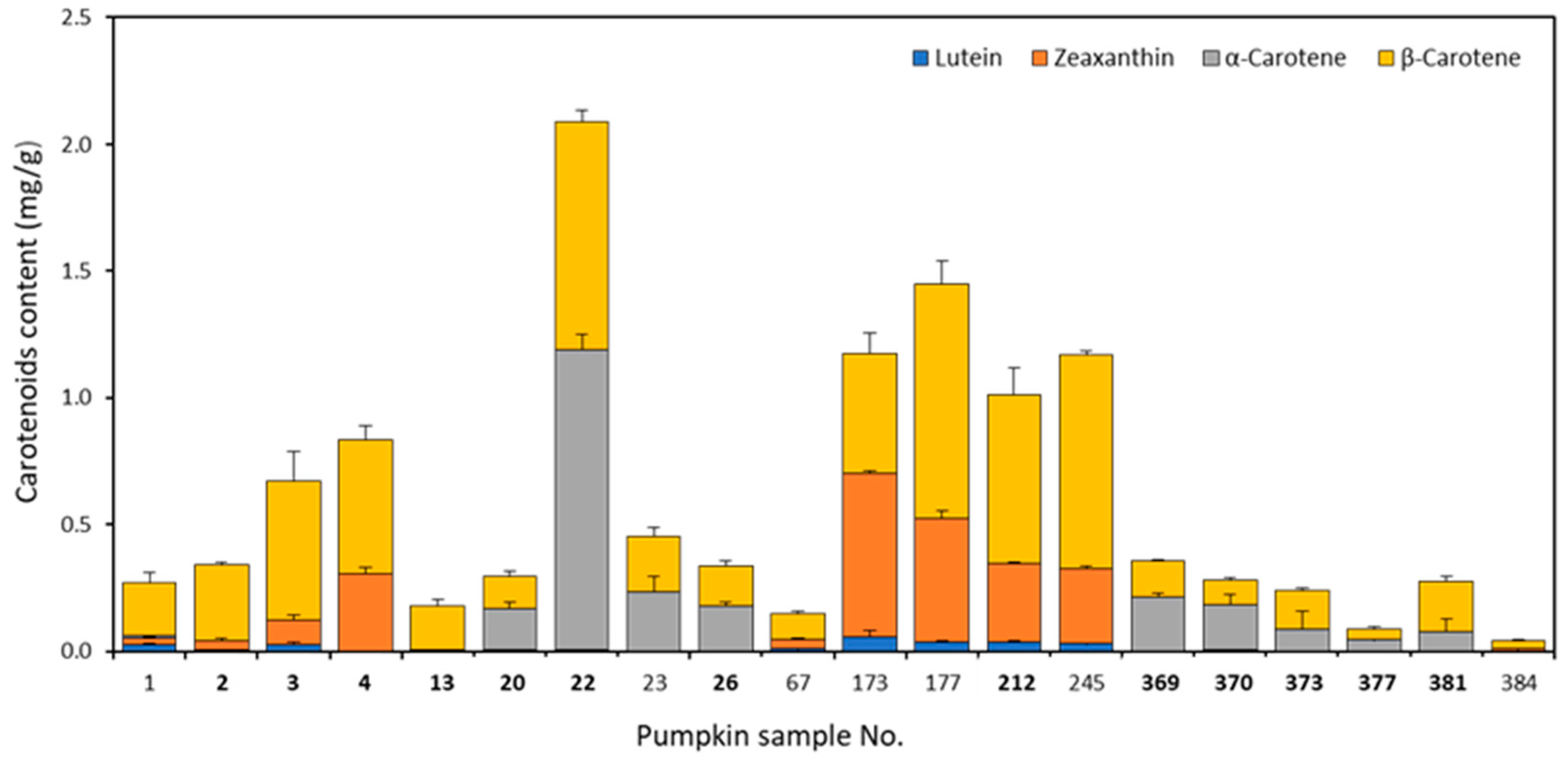
3.4. Carotenoid Contents in Pumpkin Pulp Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, G.; Khan, A.A. Pumpkin: Horticultural Importance and Its Roles in Various Forms; A Review. Int. J. Hortic. Agric. 2019, 4, 1–6. [Google Scholar]
- Bratsch, A. Specialty Crop Profile: Pumpkins. Va. Coop. Ext. 2009, 100, 1–8. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Effect of industrial and domestic processing on antioxidant properties of pumpkin pulp. LWT Food Sci. Technol. 2013, 53, 382–385. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Kandil, E.; Abo-Zeid, M.A.; Mohammed, S.E.; Ahmed, E.K. Anti-inflammatory activity of two varieties of pumpkin seed oil in an adjuvant arthritis model in rats. Grasas Aceites 2017, 68, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chari, K.Y.; Polu, P.R.; Shenoy, R.R. An Appraisal of Pumpkin Seed Extract in 1, 2-Dimethylhydrazine Induced Colon Cancer in Wistar Rats. J. Toxicol. 2018, 2018, 6086490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossell-Williams, M.; Davis, A.; O’Connor, N. Inhibition of testosterone-induced hyperplasia of the prostate of sprague-dawley rats by pumpkin seed oil. J. Med. Food 2006, 9, 284–286. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Medghalchi, M.; Nazemiyeh, H.; Asgharian, P.; Shadvar, K.; Hamishehkar, H. Effect of Cucurbita Maxima on Control of Blood Glucose in Diabetic Critically Ill Patients. Adv. Pharm. Bull. 2018, 8, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Muruganantham, N.; Solomon, S.; Senthamilselvi, M.M. Antimicrobial activity of Cucurbita maxima flowers (Pumpkin). J. Pharmacogn. Phytochem. 2016, 5, 15. [Google Scholar]
- Sindi, A.M.; Hosny, K.M. Preparation and evaluation of protective e ffect of pumpkin seed oil based self nanoemulsifying oral delivery system against ibuprofen-induced peptic ulcer. J. Drug Deliv. Sci. Technol. 2019, 52, 415–420. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef]
- Huang, H.; Yu, T.; Li, J.; Qu, S.; Wang, M.; Wu, T. Characterization of Cucurbita maxima Fruit Metabolomic Profiling and Transcriptome to Reveal Fruit Quality and Ripening Gene Expression Patterns. J. Plant Biol. 2019, 62, 203–216. [Google Scholar] [CrossRef]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2019, 50, 384–401. [Google Scholar] [CrossRef]
- Nawirska-Olszanska, A.; Biesiada, A.; Sokol-Letowska, A.; Kucharska, A.Z. Characteristics of organic acids in the fruit of different pumpkin species. Food Chem. 2014, 148, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rao, R. Nutritional quality characteristics of pumpkin fruit as revealed by its biochemical analysis. Int. Food Res. J. 2013, 20, 2309–2316. [Google Scholar]
- Zdunić, G.M.; Menković, N.R.; Jadranin, M.; Novaković, M.; Šavikin, K.P.; Živković, J. Phenolic compounds and carotenoids in pumpkin fruit and related traditional products. Hem. Ind. 2016, 70, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Dissanayake, D.M.R.H.; Deraniyagala, S.A.; Hettiarachchi, C.M.; Thiripuranathar, G. The Study of Antioxidant and Antibacterial Properties of Skin, Seeds and Leaves of The Sri Lankan Variety of Pumpkin. IOSR J. Pharm. 2018, 8, 43–48. [Google Scholar]
- Kulaitiene, J.; Černiauskiene, J.; Jariene, E.; Danilčenko, H.; Levickiene, D. Antioxidant activity and other quality parameters of cold pressing pumpkin seed oil. Not. Bot. Horti Agrobot. 2018, 46, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Malkanthi, H.; Umadevi, S.; Jamuna, K. Glycemic response and antioxidant activity of pumpkin seed powder (Cucurbita maxima) blended biscuits. J. Pharmacogn. Phytochem. 2018, 7, 1877–1882. [Google Scholar]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-ŁȨtowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Abbas, H.M.K.; Huang, H.X.; Huang, W.J.; Xue, S.D.; Yan, S.J.; Wu, T.Q.; Li, J.X.; Zhong, Y.J. Evaluation of Metabolites and Antioxidant Activity in Pumpkin Species. Nat. Prod. Commun. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Bemfeito, C.M.; Carneiro, J.D.D.S.; Carvalho, E.E.N.; Coli, P.C.; Pereira, R.C.; Vilas Boas, E.V.D.B. Nutritional and functional potential of pumpkin (Cucurbita moschata) pulp and pequi (Caryocar brasiliense Camb.) peel flours. J. Food Sci. Technol. 2020, 57, 3920–3925. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and Health-Beneficial Nutrients in Fruits of Eighteen Cucurbita Cultivars: Analysis of Diversity and Dietary Implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Cent. J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bergantin, C.; Maietti, A.; Tedeschi, P.; Font, G.; Manyes, L.; Marchetti, N. HPLC-UV/Vis-APCI-MS/MS Determination of Major Carotenoids and Their Bioaccessibility from “Delica” (Cucurbita maxima) and “Violina” (Cucurbita moschata) Pumpkins as Food Traceability Markers. Molecules 2018, 23, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulczynski, B.; Gramza-Michalowska, A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita maxima Duchesne) Cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid content in different varieties of pumpkins. J. Food Compos. Anal. 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 77–106. [Google Scholar]
- Damiani, E.; Carloni, P.; Rocchetti, G.; Senizza, B.; Tiano, L.; Joubert, E.; de Beer, D.; Lucini, L. Impact of Cold versus Hot Brewing on the Phenolic Profile and Antioxidant Capacity of Rooibos (Aspalathus linearis) Herbal Tea. Antioxidants 2019, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Castellon, J.; Rocchetti, G.; Vallverdu-Queralt, A.; Illan, M.; Torrado-Prat, X.; Lamuela-Raventos, R.M.; Lucini, L. New vacuum cooking techniques with extra-virgin olive oil show a better phytochemical profile than traditional cooking methods: A foodomics study. Food Chem. 2021, 362, 130194. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 2019, 5, e02994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Masanori, A. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7947–7958. [Google Scholar] [CrossRef] [PubMed]
- Morais, H.; Ramos, A.C.; Cserháti, T.; Forgács, E. Effects of fluorescent light and vacuum packaging on the rate of decomposition of pigments in paprika (Capsicum annuum) powder determined by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2001, 936, 139–144. [Google Scholar] [CrossRef]
- Kevrešan, Z.S.; Mastilović, J.S.; Mandicć, A.I.; Torbica, A.M. Effect of different ripening conditions on pigments of pepper for paprika production at green stage of maturity. J. Agric. Food Chem. 2013, 61, 9125–9130. [Google Scholar] [CrossRef]
- Frank, H.A.; Christensen, R.L. Excited electronic states and the photochemistry and photophysics of carotenoids. In The Carotenoids: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhauser Verlag: Basel, Switzerland, 2008; Volume 4, pp. 167–188. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC-MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [Green Version]
- Perez-Galvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017, 20, 3090–3101. [Google Scholar] [CrossRef] [Green Version]
- Courraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2020, 1–51. [Google Scholar] [CrossRef]
- Abdel-Aal el, S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Pereira, A.M.; Krumreich, F.D.; Ramos, A.H.; Krolow, A.C.R.; Santos, R.B.; Gularte, M.A. Physicochemical characterization, carotenoid content and protein digestibility of pumpkin access flours for food application. Food Sci. Technol. 2020, 40, 691–698. [Google Scholar] [CrossRef]
- Kulaitiene, J.; Jariene, E.; Danilcenko, H.; Cerniauskiene, J.; Wawrzyniak, A.; Hamulka, J.; Jukneviciene, E. Chemical composition of pumpkin (Cucurbita maxima D.) flesh flours used for food. J. Food Agric. Environ. 2014, 12, 61–64. [Google Scholar]
- Norshazila, S.; Irwandi, J.; Othman, R.; Zuhanis, Y. Carotenoid content in different locality of pumpkin (Cucurbita moschata) in Malaysia. Int. J. Pharm. Pharm. Sci. 2014, 6, 29–32. [Google Scholar]
- De Carvalho, L.M.J.; Gomes, P.B.; de Oliviera Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Armesto, J.; Rocchetti, G.; Senizza, B.; Pateiro, M.; Barba, F.J.; Dominguez, R.; Lucini, L.; Lorenzo, J.M. Nutritional characterization of Butternut squash (Cucurbita moschata D.): Effect of variety (Ariel vs. Pluto) and farming type (conventional vs. organic). Food Res. Int. 2020, 132, 109052. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Wang, M.; Wang, Y.; Xu, W.; Han, H.; Wang, Z.; Zhong, Y.; Huang, H.; Qu, S. Accumulation of Carotenoids and Expression of CarotenoidBiosynthesis Genes in Fruit Flesh During Fruit Development in TwoCucurbita maxima Inbred Lines. Hortic. Plant J. 2020. In Press. [Google Scholar] [CrossRef]
- Perez Guttierez, R.M. Review of Cucurbita pepo (Pumpkin) its Phytochemistry and Pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Gry, J.; Soborg, I.; Andersson, H.C. Cucurbitacins in Plant Food; Nordic Council of Ministers, Ed.; Temanord: Copenhagen, Denmark, 2006; Volume 556, pp. 1–68. [Google Scholar]




| VIP Marker (OPLS-DA) | VIP Score (OPLS-DA) | Log2(FC) Serbian vs. Other |
|---|---|---|
| Myrigalone A | 1.77 ± 0.41 | −0.46 |
| Steviol | 1.70 ± 0.53 | −1.64 |
| Sterebin A | 1.69 ± 0.36 | 0.81 |
| Ginsenoside Rh4 | 1.64 ± 0.76 | 0.26 |
| Cucurbitacin E | 1.58 ± 0.80 | ns |
| Oxysolavetivone | 1.44 ± 0.79 | −0.96 |
| ent-15-Kaurene-17,19-dioic acid | 1.44 ± 0.49 | 0.46 |
| 12'-Apo-b-carotene-3,12'-diol | 1.36 ± 0.82 | −0.69 |
| Apo-14'-zeaxanthinal | 1.32 ± 0.42 | −0.26 |
| Epioxylubimin | 1.30 ± 0.44 | 1.44 |
| (8'R)-Neochrome | 1.25 ± 0.62 | 0.19 |
| (9E)-Valenciaxanthin | 1.19 ± 1.07 | −0.22 |
| Lubiminol | 1.18 ± 0.77 | 1.01 |
| beta-Carotene | 1.14 ± 0.74 | −0.77 |
| 19'-Hexanoyloxymytiloxanthin | 1.11 ± 0.66 | ns |
| 4-Methoxycinnamoyloleanolic acid methyl ester | 1.08 ± 0.44 | −0.49 |
| 7(14)-Bisabolene-2,3,10,11-tetrol | 1.04 ± 0.91 | ns |
| Geranyl benzoate | 1.04 ± 0.54 | ns |
| Violaxanthin | 1.01 ± 0.54 | ns |
| Cincassiol B | 1.01 ± 0.42 | 0.61 |
| Momordicoside C | 0.99 ± 1.12 | ns |
| Methyl geranate | 0.95 ± 1.73 | −0.46 |
| Furanofukinin | 0.89 ± 0.50 | ns |
| 4,5-Dihydrovomifoliol | 0.85 ± 0.69 | −0.39 |
| VIP Marker (OPLS-DA) | VIP Score (OPLS-DA) | Log2(FC) C. maxima vs. C. moschata |
|---|---|---|
| Epioxylubimin | 2.23 ± 0.42 | −2.57 |
| 8alpha-8-Hydroxy-12-oxo-13-abieten-18-oic acid | 1.77 ± 0.48 | −0.71 |
| Lubiminol | 1.73 ± 0.41 | −1.93 |
| 4-Methoxycinnamoyloleanolic acid methyl ester | 1.63 ± 0.62 | 1.32 |
| Sterebin A | 1.53 ± 0.33 | −0.67 |
| 12'-Apo-b-carotene-3,12'-diol | 1.53 ± 0.73 | 1.11 |
| Ginsenoside Rh4 | 1.36 ± 0.36 | ns |
| ent-15-Kaurene-17,19-dioic acid | 1.35 ± 1.21 | ns |
| Geranyl benzoate | 1.18 ± 0.94 | 0.39 |
| Apo-14'-zeaxanthinal | 1.14 ± 0.60 | 1.19 |
| (8'R)-Neochrome | 1.13 ± 0.85 | ns |
| Steviol | 1.10 ± 1.06 | 0.69 |
| Cucurbitacin E | 1.09 ± 1.36 | 2.06 |
| beta-Carotene | 1.00 ± 1.27 | 0.93 |
| Apo-10'-violaxanthal | 0.96 ± 0.84 | 1.19 |
| Momordicoside C | 0.95 ± 0.77 | ns |
| Myrigalone A | 0.94 ± 0.71 | 0.47 |
| 7(14)-Bisabolene-2,3,10,11-tetrol | 0.90 ± 0.69 | −0.16 |
| Apo-12'-violaxanthal | 0.90 ± 0.56 | 0.48 |
| Furanofukinin | 0.89 ± 0.80 | 0.5 |
| Glandulone B | 0.88 ± 1.27 | 0.49 |
| Methyl (9Z)-6'-oxo-6,5'-diapo-6-carotenoate | 0.86 ± 1.28 | ns |
| 4,5-Dihydrovomifoliol | 0.84 ± 0.58 | 0.39 |
| Methyl geranate | 0.83 ± 0.65 | 0.61 |
| Ganoderiol C | 0.82 ± 1.21 | ns |
| Oxysolavetivone | 0.81 ± 0.54 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljić, M.; Rocchetti, G.; Krstić, S.; Mišan, A.; Brdar-Jokanović, M.; Marcheggiani, F.; Martinelli, E.; Lucini, L.; Damiani, E. Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection. Antioxidants 2021, 10, 1580. https://doi.org/10.3390/antiox10101580
Miljić M, Rocchetti G, Krstić S, Mišan A, Brdar-Jokanović M, Marcheggiani F, Martinelli E, Lucini L, Damiani E. Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection. Antioxidants. 2021; 10(10):1580. https://doi.org/10.3390/antiox10101580
Chicago/Turabian StyleMiljić, Milorad, Gabriele Rocchetti, Sanja Krstić, Aleksandra Mišan, Milka Brdar-Jokanović, Fabio Marcheggiani, Erika Martinelli, Luigi Lucini, and Elisabetta Damiani. 2021. "Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection" Antioxidants 10, no. 10: 1580. https://doi.org/10.3390/antiox10101580
APA StyleMiljić, M., Rocchetti, G., Krstić, S., Mišan, A., Brdar-Jokanović, M., Marcheggiani, F., Martinelli, E., Lucini, L., & Damiani, E. (2021). Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection. Antioxidants, 10(10), 1580. https://doi.org/10.3390/antiox10101580












