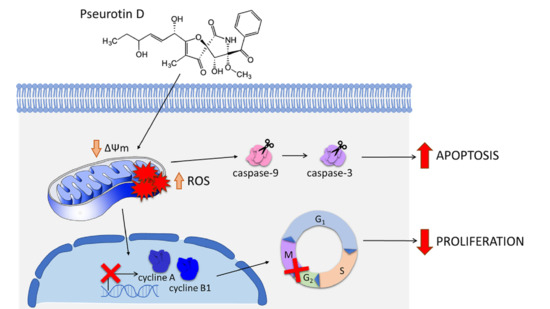
Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability and Cytotoxicity
2.4. Apoptosis of Cells
2.5. Cell Cycle Analysis
2.6. Western Blot Analysis
2.7. Measurement of Metabolic Activity by BIOLOG MitoPlates
2.8. Measurement of Mitochondrial Potential
2.9. Measurement of Mitochondrial ROS Production
2.10. Measurement of Metabolic Activity by Seahorse Assay
2.11. Statistical Analysis
3. Results
3.1. Pseurotin D Decreased Cell Viability but Did Not Induce Acute Cytotoxicity in MEC-1 Cells
3.2. Pseurotin D Induced Apoptosis of MEC-1 Cells
3.3. Pseurotin D Arrested MEC-1 Cells in the G2/M Cell Cycle Phase
3.4. Pseurotin D Decreased the Mitochondrial Respiration and Activity of MEC-1 Cells
3.5. Pseurotin D Downregulated the Phosphorylation of Key Signaling Pathways in the Proliferation of Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parikh, S.A.; Meacham, P.J.; Zent, C.S.; Evans, A.G. Multiple B cell malignancies in patients with chronic lymphocytic leukemia: Epidemiology, pathology, and clinical implications. Leuk. Lymphoma 2020, 61, 1037–1051. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2017, 92, 946–965. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, G.; Montserrat, E. Critical molecular pathways in CLL therapy. Mol. Med. 2018, 24, 9. [Google Scholar] [CrossRef]
- Kipps, T.J.; Choi, M.Y. Targeted Therapy in Chronic Lymphocytic Leukemia. Cancer J. 2019, 25, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Banerji, V. Targeting Mitochondrial Bioenergetics as a Therapeutic Strategy for Chronic Lymphocytic Leukemia. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J. Oxidative Stress by Targeted Agents Promotes Cytotoxicity in Hematologic Malignancies. Antioxidants Redox Signal. 2009, 11, 1123–1137. [Google Scholar] [CrossRef] [Green Version]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Nairismägi, M.-L.; Gerritsen, M.E.; Li, Z.M.; Wijaya, G.C.; Chia, B.; Laurensia, Y.; Lim, J.Q.; Yeoh, K.W.; Yao, X.S.; Pang, W.L.; et al. Oncogenic activation of JAK3-STAT signaling confers clinical sensitivity to PRN371, a novel selective and potent JAK3 inhibitor, in natural killer/T-cell lymphoma. Leukemia 2018, 32, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.; Bishayee, A. Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in Cancer Metastasis and Translational Advances. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.A.; Larner, A.C. Toward a new STATe: The role of STATs in mitochondrial function. Semin. Immunol. 2014, 26, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.A.; Hyun, M.; Cantwell, M.; Raza, A.; Mertens, C.; Raje, V.; Sisler, J.; Tracy, E.; Torres-Odio, S.; Gispert, S.; et al. Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production. Sci. Signal. 2017, 10, eaag2588. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, X.; Song, H.; Feng, D.; Jiang, Y.; Wu, S.; Geng, J. The STAT-ROS cycle extends IFN-induced cancer cell apoptosis. Int. J. Oncol. 2017, 52, 305–313. [Google Scholar] [CrossRef]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vašíček, O.; Fedr, R.; Skoroplyas, S.; Chalupa, D.; Sklenář, M.; Tharra, P.R.; Švenda, J.; Kubala, L. Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells. Phytomedicine 2020, 69, 153194. [Google Scholar] [CrossRef] [PubMed]
- Ando, O.; Satake, H.; Nakajima, M.; Sato, A.; Nakamura, T.; Kinoshita, T.; Furuya, K.; Haneishi, T. Synerazol, a new antifungal antibiotic. J. Antibiot. 1991, 44, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komagata, D.; Fujita, S.; Yamashita, N.; Saito, S.; Morino, T. Novel Neuritogenic Activities of Pseurotin A and Penicillic Acid. J. Antibiot. 1996, 49, 958–959. [Google Scholar] [CrossRef] [Green Version]
- Asami, Y.; Kakeya, H.; Komi, Y.; Kojima, S.; Nishikawa, K.; Beebe, K.; Neckers, L.; Osada, H. Azaspirene, a fungal product, inhibits angiogenesis by blocking Raf-1 activation. Cancer Sci. 2008, 99, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, Y.; Yabuta, Y.; Sekine, A.; Fujii, K.; Harada, K.-I.; Oikawa, T.; Sato, M.; Furumai, T.; Oki, T. Directed Biosynthesis of Fluorinated Pseurotin A, Synerazol and Gliotoxin. J. Antibiot. 2004, 57, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Asami, Y.; Kakeya, H.; Onose, R.; Yoshida, A.; Matsuzaki, A.H.; Osada, H. Azaspirene: A Novel Angiogenesis Inhibitor Containing a 1-Oxa-7-azaspiro [4.4]non-2-ene-4,6-dione Skeleton Produced by the FungusNeosartoryasp. Org. Lett. 2002, 4, 2845–2848. [Google Scholar] [CrossRef]
- Anjum, K.; Bi, H.; Chai, W.; Lian, X.-Y.; Zhang, Z. Antiglioma pseurotin A from marine Bacillus sp. FS8D regulating tumour metabolic enzymes. Nat. Prod. Res. 2017, 32, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, O.; Rubanova, D.; Chytkova, B.; Kubala, L. Natural pseurotins inhibit proliferation and inflammatory responses through the inactivation of STAT signaling pathways in macrophages. Food Chem. Toxicol. 2020, 141, 111348. [Google Scholar] [CrossRef] [PubMed]
- Rubanova, D.; Dadova, P.; Vasicek, O.; Kubala, L. Pseurotin D Inhibits the Activation of Human Lymphocytes. Int. J. Mol. Sci. 2021, 22, 1938. [Google Scholar] [CrossRef] [PubMed]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef]
- Georgiev, Y.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.; Vasicek, O.; Rise, F.; Denev, P.; Lojek, A.; Batsalova, T.; et al. Tilia tomentosa pectins exhibit dual mode of action on phagocytes as β-glucuronic acid monomers are abundant in their rhamnogalacturonans I. Carbohydr. Polym. 2017, 175, 178–191. [Google Scholar] [CrossRef]
- Kudová, J.; Vašíček, O.; Číž, M.; Kubala, L. Melatonin promotes cardiomyogenesis of embryonic stem cells via inhibition of HIF-1α stabilization. J. Pineal Res. 2016, 61, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Binó, L.; Kučera, J.; Štefková, K.; Šindlerová, L.; Lánová, M.; Kudová, J.; Kubala, L.; Pacherník, J. The stabilization of hypoxia inducible factor modulates differentiation status and inhibits the proliferation of mouse embryonic stem cells. Chem. Interact. 2016, 244, 204–214. [Google Scholar] [CrossRef]
- Vasicek, O.; Lojek, A.; Číž, M. Serotonin and its metabolites reduce oxidative stress in murine RAW264.7 macrophages and prevent inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef]
- Crowley, L.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Gibellini, L.; Bianchini, E.; Nasi, M.; Pinti, M.; Salvioli, S.; Cossarizza, A. Quantification of Mitochondrial Reactive Oxygen Species in Living Cells by Using Multi-Laser Polychromatic Flow Cytometry. Cytom Part A 2016, 89a, 1106–1110. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gong, M.-W.; Peng, Z.-F.; Zhou, T.; Ying, M.-G.; Zheng, Q.-H.; Liu, Q.-Y.; Zhang, Q.-Q. The Marine Fungal Metabolite, Dicitrinone B, Induces A375 Cell Apoptosis through the ROS-Related Caspase Pathway. Mar. Drugs 2014, 12, 1939–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fan, D. Ginsenoside Rg5 induces G2/M phase arrest, apoptosis and autophagy via regulating ROS-mediated MAPK pathways against human gastric cancer. Biochem. Pharmacol. 2019, 168, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, Y.; Yang, C.; Zhang, H.; Li, Y.; Wu, B.; Huang, J.; Zhou, X.; Huang, B.; Yang, K.; et al. Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis. 2017, 8, e3024. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacarelli, T.; Azar, A.; Sell, C. Aberrant mTOR activation in senescence and aging: A mitochondrial stress response? Exp. Gerontol. 2014, 68, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.B.; Yu, M.-R.; Yang, Y.; Jiang, Z.; Ha, H. Reactive Oxygen Species-Regulated Signaling Pathways in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, S241–S245. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.V.; Lancaster, J.R.; Meszaros, A.T.; Weidinger, A. Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol. 2017, 13, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, A.; Aboud, N.; Kolch, W.; Matallanas, D. Hidden Targets in RAF Signalling Pathways to Block Oncogenic RAS Signalling. Genes 2021, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.; Mouton-Liger, F.; Paquet, C.; Hugon, J. Modulation of oxidative stress and tau phosphorylation by the mTOR activator phosphatidic acid in SH-SY5Y cells. FEBS Lett. 2011, 585, 1801–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Qiu, P.; Yuan, Y.; Zheng, L.; He, J.; Wang, C.; Guo, Q.; Kenny, J.; Liu, Q.; Zhao, J.; et al. Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species. Theranostics 2019, 9, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- A Helal, G.; A Ahmed, F.; Askora, A.; Saber, T.M.; Rady, S.M. PSEUROTIN A FROM Aspergillus fumigatus Fr. Aumc 8002 Exhibits anticancer activity against hepatocellular carcinoma in vitro and in vivo. Slov. Veter-Res. 2019, 56. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahed, K.S.; Siddique, A.B.; Mohyeldin, M.M.; Qusa, M.H.; Goda, A.A.; Singh, S.S.; Ayoub, N.M.; King, J.A.; Jois, S.D.; El Sayed, K.A. Pseurotin A as a novel suppressor of hormone dependent breast cancer progression and recurrence by inhibiting PCSK9 secretion and interaction with LDL receptor. Pharmacol. Res. 2020, 158, 104847. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosejová, E.; Bosnjakovic, R.; Kubala, L.; Vašíček, O. Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells. Antioxidants 2021, 10, 1576. https://doi.org/10.3390/antiox10101576
Mosejová E, Bosnjakovic R, Kubala L, Vašíček O. Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells. Antioxidants. 2021; 10(10):1576. https://doi.org/10.3390/antiox10101576
Chicago/Turabian StyleMosejová, Eva, Rebeka Bosnjakovic, Lukáš Kubala, and Ondřej Vašíček. 2021. "Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells" Antioxidants 10, no. 10: 1576. https://doi.org/10.3390/antiox10101576
APA StyleMosejová, E., Bosnjakovic, R., Kubala, L., & Vašíček, O. (2021). Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells. Antioxidants, 10(10), 1576. https://doi.org/10.3390/antiox10101576