Saccharomyces cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Protein Expression and Purification
2.3. Crystallization, Data Collection, and Structure Determination
2.4. Protein LC-MS/MS Analysis
2.5. RS2 Analysis of Thiosulfate and Proteins
2.6. Thiosulfate:Cyanide Sulfurtransferase Activity Assay
2.7. Thiosulfate:GSH Sulfurtransferase Activity Assay
2.8. HPLC Analysis of the Products Generated by Thiosulfate Decomposition
2.9. Detection of Sulfane Sulfurs Using SSP4
2.10. Bioinformatics Analysis and Protein Structure Modeling
3. Results
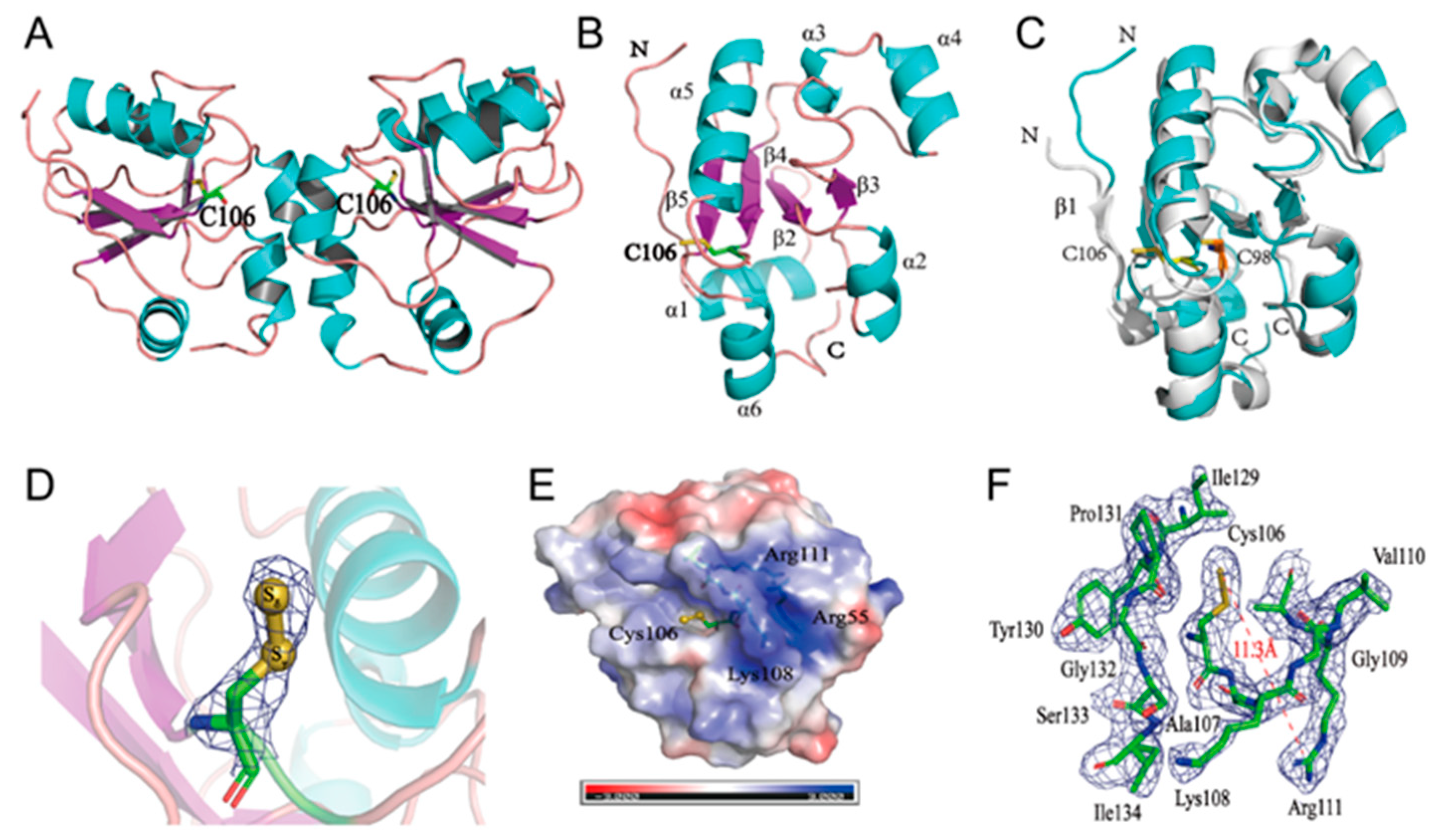
3.1. Crystal Structure of RDL2
3.2. Tandem MS Analysis of Thiosulfate Reacted-RDL2
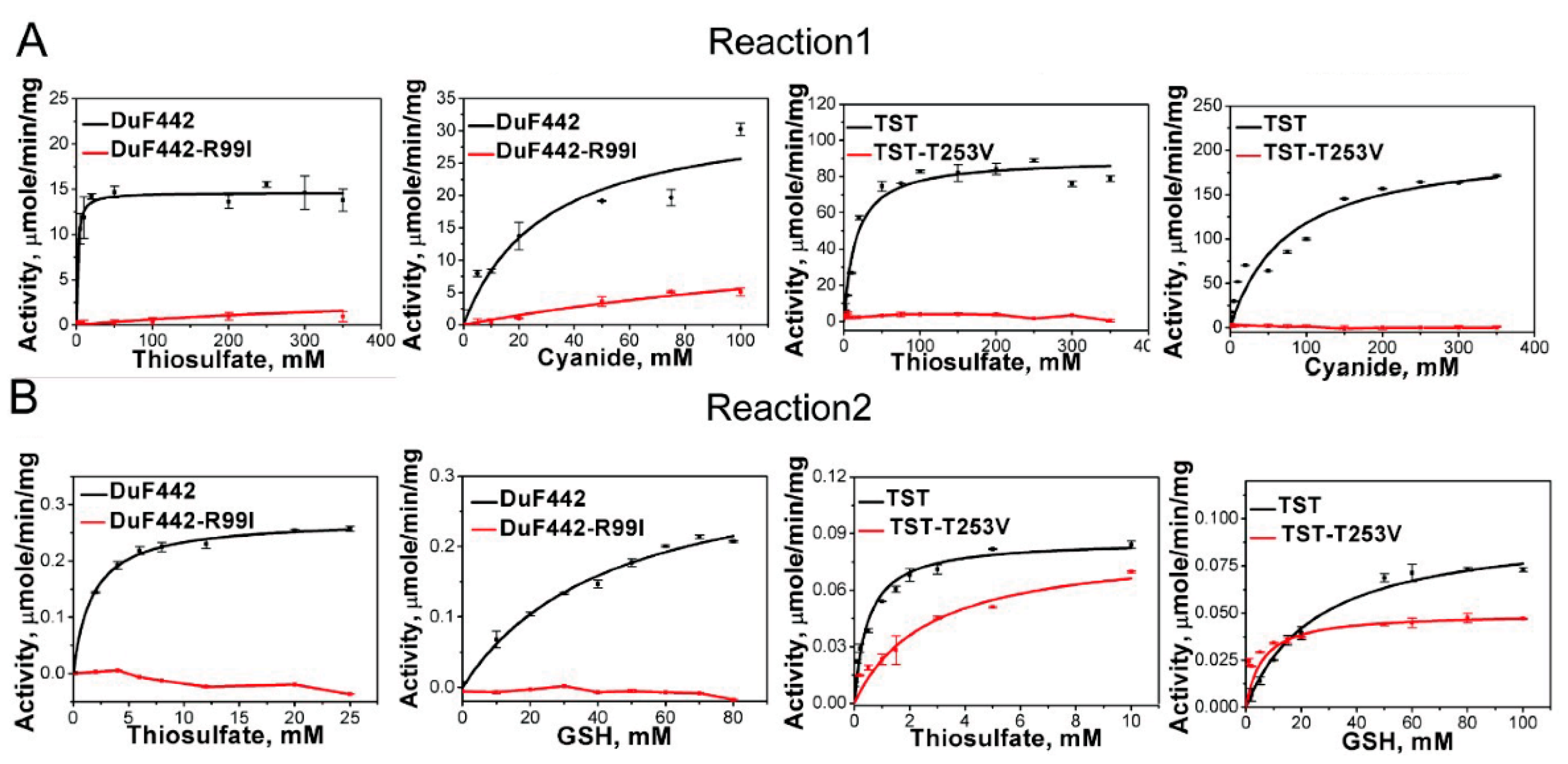
3.3. Activity Assay of RDL2
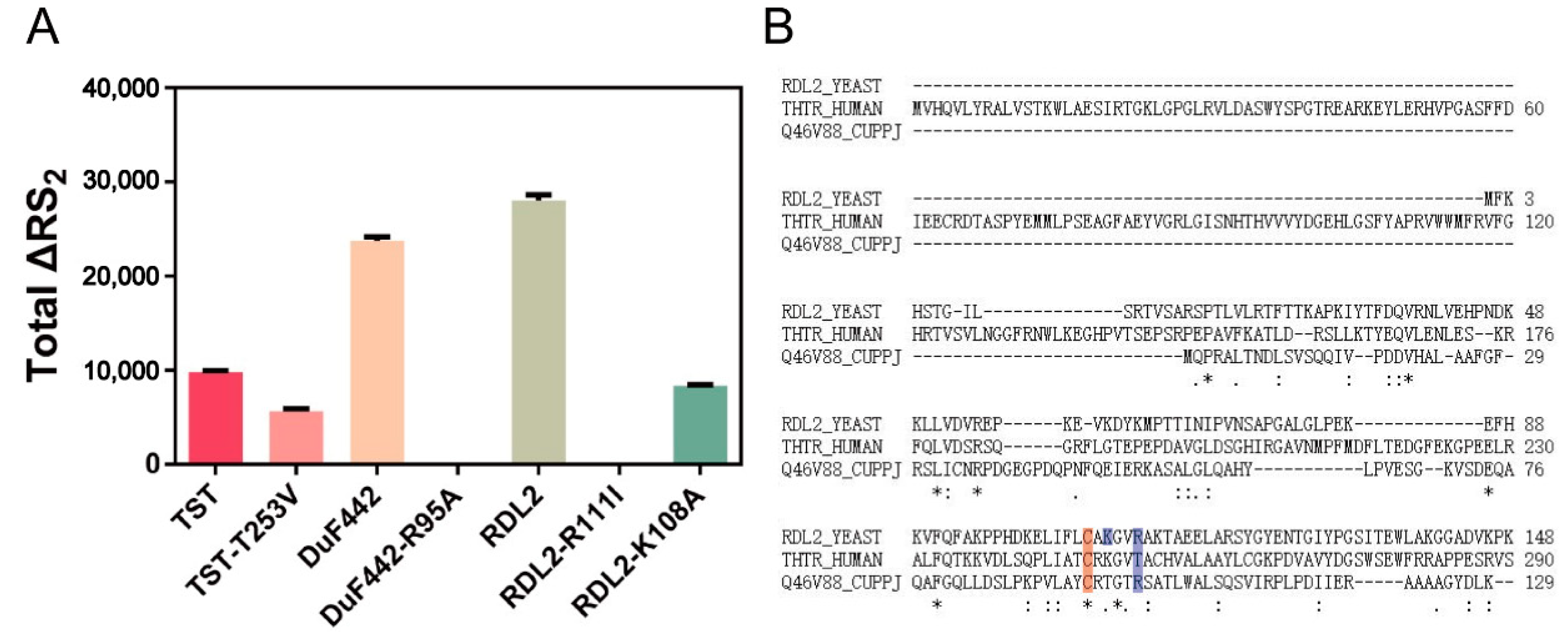
3.4. Effect of Arg111 on RDL2 Activity
3.5. Effect of Lys108 on RDL2 Activity
3.6. Effects of Arg99 on DUF442 Activity
3.7. Effect of the Loop-End Thr253 on TST Activity
3.8. Analysis of Active-Site Loops and Positive Electrostatic Fielding-Pockets of Structured Rhodaneses
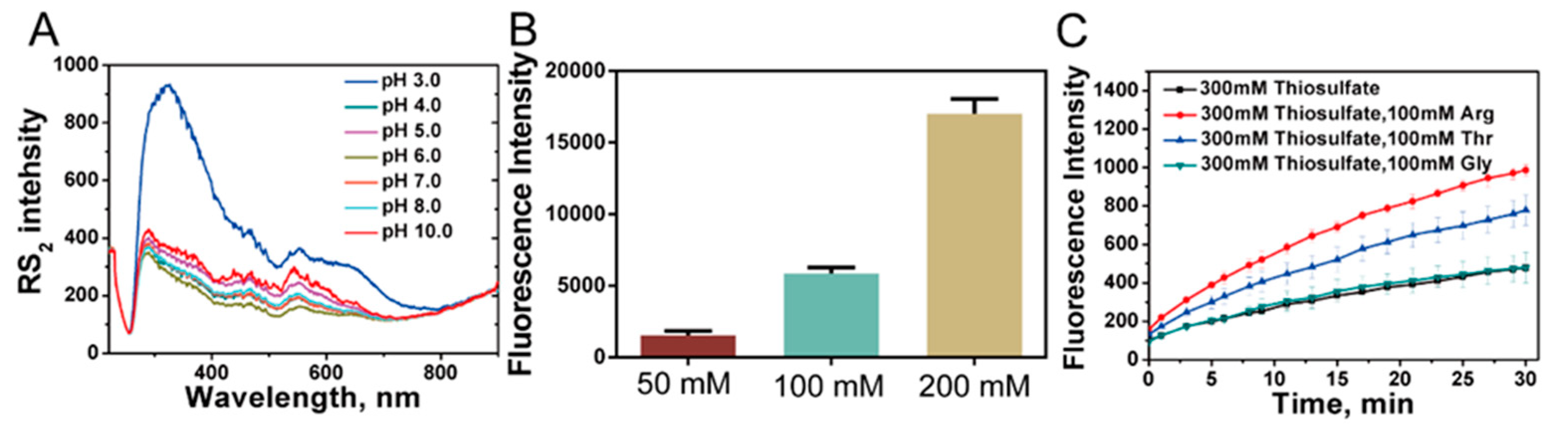
3.9. Effects of Arg and Thr on Acidic Decomposition of Thiosulfate
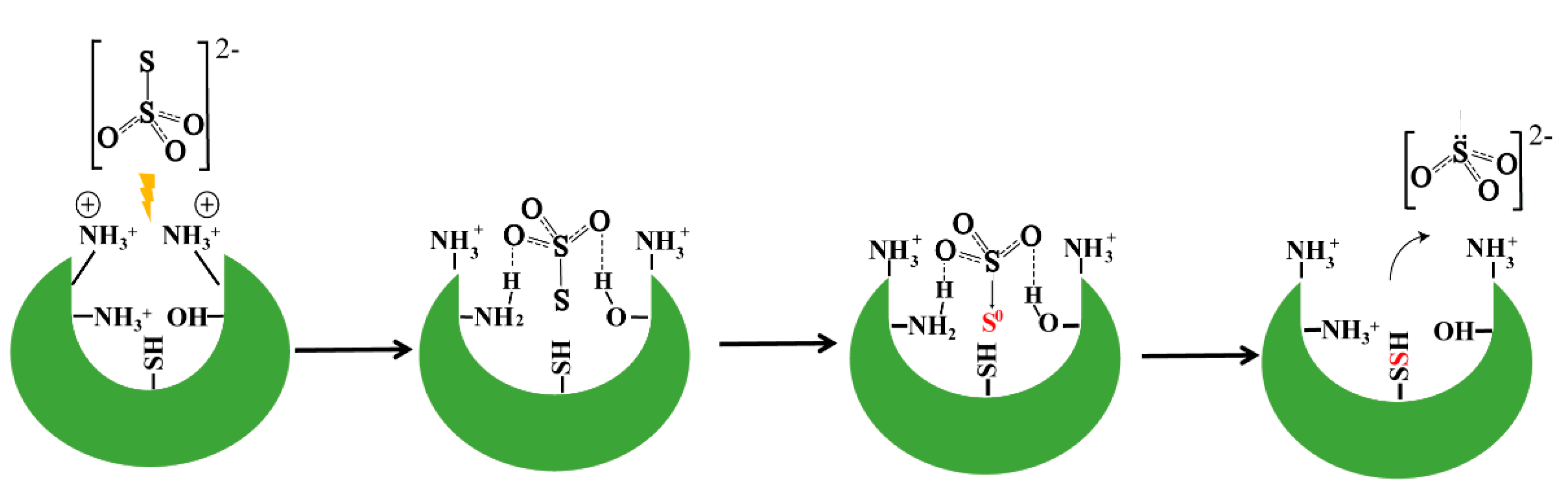
3.10. A Proposed Model for Rhodaneses Catalyzed Thiosulfate Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Walsh, B.J.C.; Flores-Mireles, A.L.; Peng, H.; Zhang, Y.; Zhang, Y.; Trinidad, J.C.; Hultgren, S.J.; Giedroc, D.P. Hydrogen sulfide sensing through reactive sulfur species (RSS) and nitroxyl (HNO) in Enterococcus faecalis. ACS Chem. Biol. 2018, 13, 1610–1620. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Dinegar, R.H.; Smellie, R.H.; Mer, V.K.L. Kinetics of the acid decomposition of sodium thiosulfate in dilute solutions. J. Am. Chem. Soc. 1951, 73, 2050–2054. [Google Scholar] [CrossRef]
- Rolia, E.; Chakrabarti, C.L. Kinetics of decomposition of tetrathionate, trithionate, and thiosulfate in alkaline media. Environ. Sci. Technol. 1982, 16, 852–857. [Google Scholar] [CrossRef]
- Hopfinger, M.; Zischka, F.; Seifert, M.; Kornath, A.J. Preparation and characterization of pure thiosulfuric acid. Z. Anorg. Allg. Chem. 2018, 644, 574–579. [Google Scholar] [CrossRef]
- Miaskiewicz, K.; Steudel, R. The structures of thiosulfuric acid H2S2O3 and its monoanion HS2O3−. Angew. Chem. Int. Ed. 1992, 31, 58–59. [Google Scholar] [CrossRef]
- Toohey, J.I.; Cooper, A.J. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef]
- Shen, J.; Peng, H.; Zhang, Y.; Trinidad, J.C.; Giedroc, D.P. Staphylococcus aureus sqr encodes a type II sulfide: Quinone oxidoreductase and impacts reactive sulfur speciation in cells. Biochemistry 2016, 55, 6524–6534. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Keithly, M.E.; Armstrong, R.N.; Higgins, K.A.; Edmonds, K.A.; Giedroc, D.P. Staphylococcus aureus CstB is a novel multidomain persulfide dioxygenase-sulfurtransferase involved in hydrogen sulfide detoxification. Biochemistry 2015, 54, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Riglar, D.T.; Giessen, T.W.; Baym, M.; Kerns, S.J.; Niederhuber, M.J.; Bronson, R.T.; Kotula, J.W.; Gerber, G.K.; Way, J.C.; Silver, P.A. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat. Biotechnol. 2017, 35, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Westley, J. Rhodanese. Adv. Enzymol. Relat. Areas. Mol. Biol. 1973, 39, 327–368. [Google Scholar]
- Arijs, I.; Vanhove, W.; Rutgeerts, P.; Schuit, F.; Preter, V.D. Decreased mucosal sulfide detoxification capacity in patients with crohn’s disease. Inflamm. Bowel Dis. 2013, 19, E70–E72. [Google Scholar] [CrossRef]
- Smirnov, A.; Comte, C.; Mager-Heckel, A.M.; Addis, V.; Krasheninnikov, I.A.; Martin, P.R.; Entelis, N.; Tarassov, I. Mitochondrial enzyme rhodanese is essential for 5 S ribosomal RNA import into human mitochondria. J. Biol. Chem. 2010, 285, 30792–30803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, X.; Li, H.; Liu, H.; Xia, Y.; Xun, L. The complete pathway for thiosulfate utilization in Saccharomyces cerevisiae. Appl. Environ. Microb. 2018, 84, e01241-18. [Google Scholar] [CrossRef] [Green Version]
- Libiad, M.; Motl, N.; Akey, D.L.; Sakamoto, N.; Fearon, E.R.; Smith, J.L.; Banerjee, R. Thiosulfate sulfurtransferase-like domain–containing 1 protein interacts with thioredoxin. J. Biol. Chem. 2018, 293, 2675–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libiad, M.; Sriraman, A.; Banerjee, R. Polymorphic Variants of Human Rhodanese Exhibit Differences in Thermal Stability and Sulfur Transfer Kinetics. J. Biol. Chem. 2015, 290, 23579–23588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliubich, F.; Gazerro, M.; Zanotti, G.; Delbono, S.; Bombieri, G.; Berni, R. Active site structural features for chemically modified forms of Rhodanese. J. Biol. Chem. 1996, 271, 21054–21061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallarossa, A.; Donahue, J.L.; Larson, T.J.; Bolognesi, M.; Bordo, D. Escherichia coli GlpE is a prototype sulfurtransferase for the single-domain Rhodanese homology superfamily. Structure 2001, 9, 1117–1252. [Google Scholar] [CrossRef] [Green Version]
- Cipollone, R.; Ascenzi, P.; Visca, P. Common themes and variations in the Rhodanese superfamily. IUBMB Life 2007, 59, ra72. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Myers, C.L.; Huttenhower, C.; Hibbs, M.A.; Hayes, A.P.; Paw, J.; Clore, J.J.; Mendoza, R.M.; Luis, B.S.; Nislow, C.; et al. Computationally driven, quantitative experiments discover genes required for mitochondrial biogenesis. PLoS Genet. 2009, 5, e1000407. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Li, Z.; Zhan, W.; Iyer, V.R.; Marcotte, E.M. Mechanisms of cell cycle control revealed by a systematic and quantitative overexpression screen in S. cerevisiae. PLoS Genet. 2008, 4, e1000120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.X.; Horowitz, P.M. The sulfurtransferase activity and structure of Rhodanese are affected by site-directed replacement of Arg-186 or Lys-249. J. Biol. Chem. 1994, 269, 8220–8225. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Chen, Z.; Zhao, R.; Wang, Q.; Ran, M.; Xia, Y.; Hu, X.; Liu, J.; Xian, M.; et al. Using resonance synchronous spectroscopy to characterize the reactivity and electrophilicity of biologically relevant sulfane sulfur. Redox Biol. 2019, 24, 101179. [Google Scholar] [CrossRef]
- Xia, Y.; Li, K.; Li, J.; Wang, T.; Gu, L.; Xun, L. T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis. Nucleic Acids Res. 2018, 47, e13. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chu, W.; Qi, Q.; Xun, L. New insights into the QuikChange™ process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 2015, 43, e12. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, J.; Lü, C.; Xia, Y.; Xin, Y.; Liu, H.; Xun, L.; Liu, H. FisR activates σ54 -dependent transcription of sulfide-oxidizing genes in Cupriavidus pinatubonensis JMP134. Mol. Microbiol. 2017, 105, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Sörbor, B.H. Rhodanese. Acta Chem. Scand. 1953, 7, 1137–1145. [Google Scholar]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial H2S oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamyshny, A., Jr.; Goifman, A.; Gun, J.; Rizkov, D.; Lev, O. Equilibrium distribution of polysulfide Ions in aqueous solutions at 25 °C: A new approach for the study of polysulfides’ equilibria. Environ. Sci. Technol. 2004, 38, 6633–6644. [Google Scholar] [CrossRef] [PubMed]


| Data Collection | |
|---|---|
| Space group | I 41 |
| Cell dimensions | |
| a, b, c (Å) | 79.753, 79.753, 110.382 |
| α, β, γ (°) | 90.00, 90.00, 90.00 |
| Wavelength (Å) | 0.9791 |
| Resolution (Å) | 50.00–2.47 (2.51–2.47) |
| 〈I/σ (I)〉 | 24.69 (3) |
| Completeness (%) | 100.0 (99.7) |
| Redundancy | 13.1 (10.3) |
| CC1/2 | 0.990 (0.976) |
| Rpim | 0.028 (0.230) |
| Refinement | |
| Resolution (Å) | 27.595–2.471 |
| No. reflections | 11,448 |
| Rwork/Rfree (%) | 20.77/24.72 |
| No. atoms | |
| Protein | 1952 |
| Water | 57 |
| B-factors | |
| Protein | 35.37 |
| Water | 32.55 |
| RMS deviations | |
| Bond lengths (Å) | 0.002 |
| Bond angles (°) | 0.516 |
| Ramachandran Plot | |
| favored | 99.15% |
| allowed | 0.85% |
| outliers | 0.00% |
| Rhodanese | Km Donor mM | Km Acc. mM | Vmax μmol min−1 mg−1 | kcat s−1 | kcat/Km Donor M−1 s−1 | Kcat/Km Acc. M−1 s−1 | |
|---|---|---|---|---|---|---|---|
| Reaction 1 | RDL2 | 32.5 ± 3.50 | 5.85 ± 2.18 | 55.04 ± 3.27 | 15.32 | 0.47 × 103 | 2.62 × 103 |
| RDL2-R111I | 100.66 ± 55.95 | 60.12 ± 33.78 | 3.40 ± 0.61 | 0.95 | 9.43 | 15.8 | |
| RDL2-K108A | 93.23 ± 2.67 | 9.13 ± 3.78 | 31.31 ± 2.21 | 8.71 | 93.42 | 0.95 × 103 | |
| Reaction 2 | RDL2 | 2.54 ± 0.27 | 50.01 ± 8.73 | 0.357 ± 0.031 | 0.099 | 38.98 | 1.98 |
| RDL2-R111I | -- | -- | -- | -- | -- | -- | |
| RDL2-K108A | 4.79 ± 0.57 | 69.41 ± 13.51 | 0.153 ± 0.016 | 0.042 | 8.77 | 0.61 |
| Rhodanese | Km Donor mM | Km Acc. mM | Vmax μmol min−1 mg−1 | kcat s−1 | kcat/Km Donor M−1 s−1 | Kcat/Km Acc. M−1 s−1 | |
|---|---|---|---|---|---|---|---|
| Reaction 1 | DUF442 | 1.61 ± 0.45 | 13.27 ± 0.91 | 21.18 ± 0.86 | 4.89 | 3.04 × 103 | 0.37 × 103 |
| DUF442-R99I | 227.68 ± 95.64 | 132.2 ± 28.42 | 8.12 ± 0.76 | 1.88 | 6.77 | 14.22 | |
| TST | 15.68 ± 2.86 | 26.59 ± 2.34 | 176.90 ± 3.39 | 101.73 | 6.49 × 103 | 3.83 × 103 | |
| TST-T253V | -- | -- | -- | -- | -- | -- | |
| Reaction 2 | DUF442 | 1.53 ± 0.35 | 44.7 ± 7.41 | 0.334 ± 0.026 | 0.078 | 50.98 | 1.74 |
| DUF442-R99I | -- | -- | -- | -- | -- | -- | |
| TST | 0.49 ± 0.10 | 25.62 ± 3.94 | 0.096 ± 0.005 | 0.055 | 0.11 × 103 | 2.15 | |
| TST-TV | 2.43 ± 0.70 | 1.77 ± 0.53 | 0.044 ± 0.002 | 0.025 | 10.29 | 14.12 |
| Cluster | Name | Organism | PDB ID | Loop Sequence |
|---|---|---|---|---|
| Arg | TSTD1 | Homo sapiens | 6BEV | CQMGKR |
| RDL1 | Saccharomyces cerevisiae | 3D1p | CASGKR | |
| YgaP | Escherichia coli | 5HPA | CQAGKR | |
| YnjE | Escherichia coli | 2WLR | CGTGWR | |
| SACOL1807 | Staphylococcus aureus | 3IWH | CAGGVR | |
| Bphyt_4191 | Paraburkholderia phytofirmans | 5VE3 | CRAGGR | |
| Rv0390 | Mycobacterium tuberculosis | 2FSX | CRSGNR | |
| TVG0868615 | Thermoplasma volcanium | 3GK5 | CAHGNR | |
| Thr | MST | Homo sapiens | 4JGT | CGSGVT |
| MST | Leishmania major | 1OKG | CGSGVT | |
| TST | Bos taurus | 1BOH | CRKGVT | |
| Ser | GlpE | Escherichia coli | 1GMX | CYHGNS |
| TUM1 | Saccharomyces cerevisiae | 3UTN | CGTGVS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, H.; Xia, Y.; Xun, L.; Liu, H. Saccharomyces cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition. Antioxidants 2021, 10, 1525. https://doi.org/10.3390/antiox10101525
Wang Q, Li H, Xia Y, Xun L, Liu H. Saccharomyces cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition. Antioxidants. 2021; 10(10):1525. https://doi.org/10.3390/antiox10101525
Chicago/Turabian StyleWang, Qingda, Huanjie Li, Yongzhen Xia, Luying Xun, and Huaiwei Liu. 2021. "Saccharomyces cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition" Antioxidants 10, no. 10: 1525. https://doi.org/10.3390/antiox10101525
APA StyleWang, Q., Li, H., Xia, Y., Xun, L., & Liu, H. (2021). Saccharomyces cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition. Antioxidants, 10(10), 1525. https://doi.org/10.3390/antiox10101525






