Effects of Hypothermia and Allopurinol on Oxidative Status in a Rat Model of Hypoxic Ischemic Encephalopathy
Abstract
:1. Introduction
2. Materials and Methods
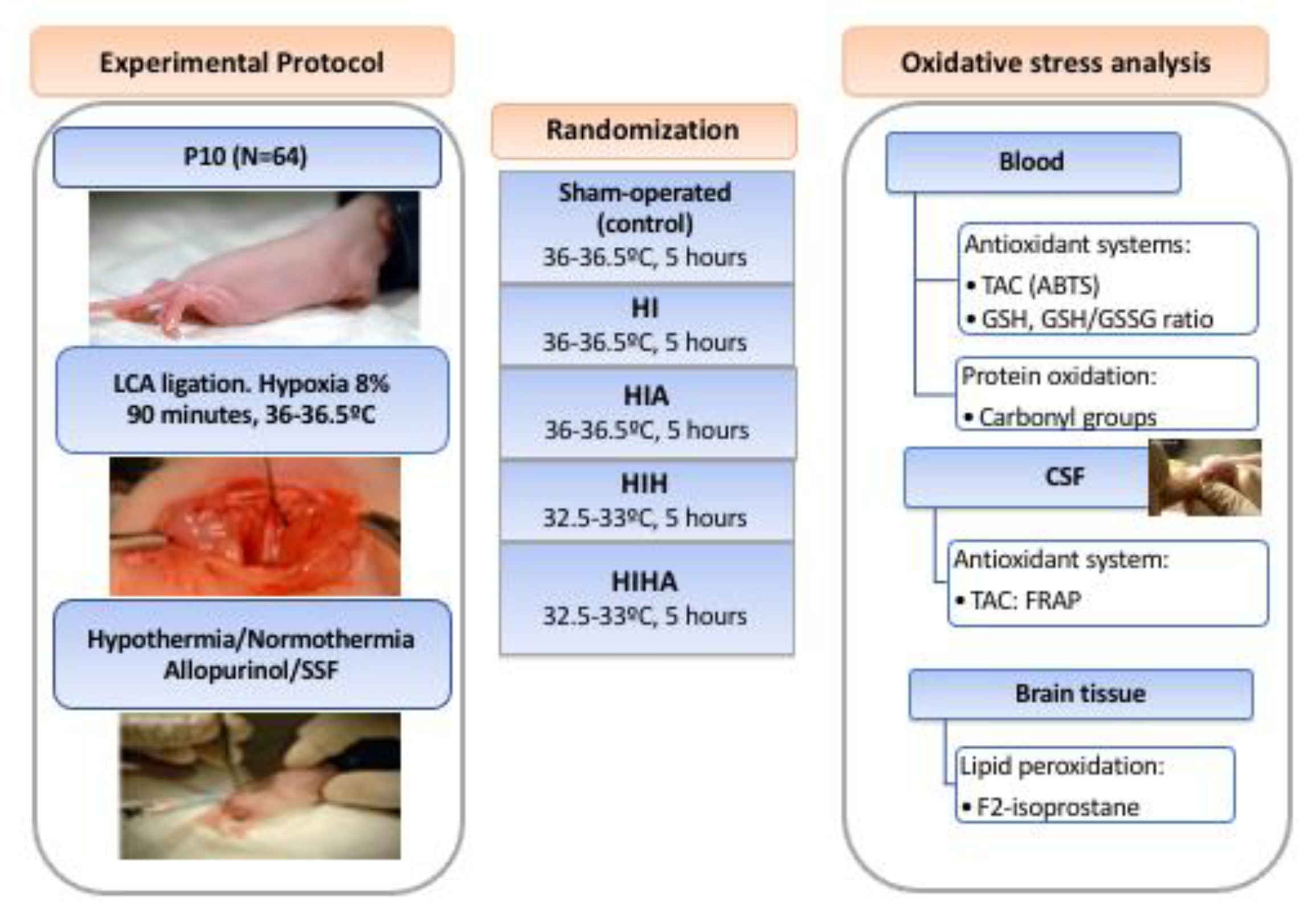
2.1. Experimental Design
2.1.1. Allopurinol
2.1.2. Hypothermia vs. Normothermia
2.2. Samples Obtention and Preparation
2.3. Sample Analysis
2.3.1. Plasma Total Antioxidant Capacity (TAC)
2.3.2. Plasma Glutathione Reduced/Oxidized (GSH/GSSG) Ratio Analysis
2.3.3. Plasma Protein Carbonyl Groups (CG)
2.3.4. Brain 8-Iso-Prostaglandin F2α (8-iso-PGF2α) Quantification
2.4. Statistical Analysis
3. Results
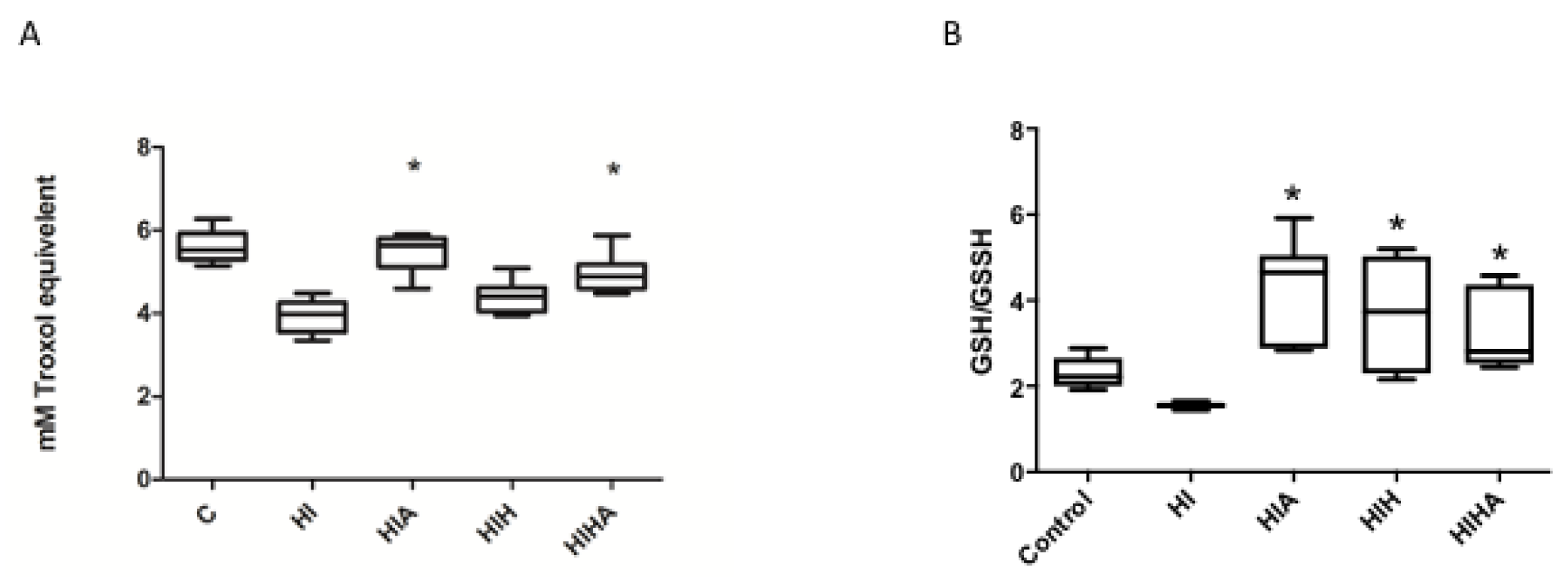
3.1. Allopurinol Administration, Alone or in Combination with Hypothermia, Protects Total Antioxidant Capacity (TAC) after an HI Event
3.2. GSH/GSSG Ratio Was Decreased after an HI Event, and Only Treated Groups Recovered to Normal Values
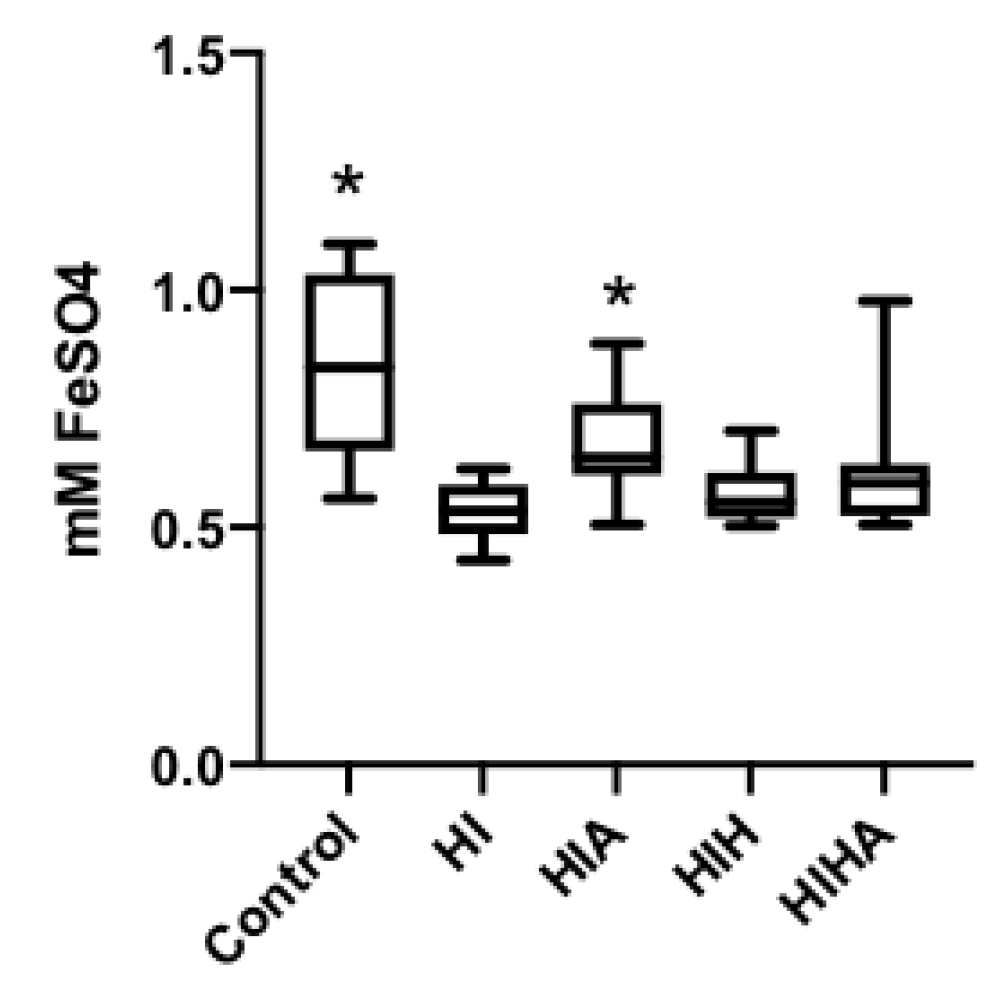
3.3. TAC Levels in CSF Are Preserved When Allopurinol Is Administrated after an HI Event, but Not When Allopurinol Is Administrated in Combination with Hypothermia
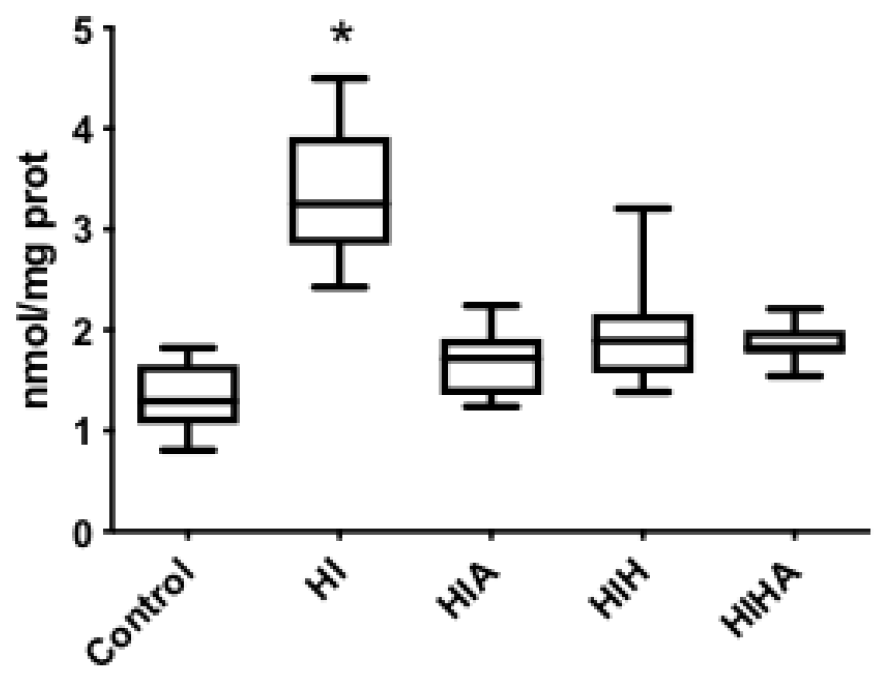
3.4. Protein Oxidation Increase after an HI Event, All Treatments Seem to Prevent Protein Oxidation
3.5. Lipid Peroxidation Increased after an HI Event and Decreased in the Treated Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blencowe, H.; Vos, T.; Lee, A.C.C.; Philips, R.; Lozano, R.; Alvarado, M.; Cousens, S.; Lawn, J.E. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: Introduction, methods overview, and relevant findings from the global burden of disease study. Pediatr. Res. 2013, 74, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.C.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74, 50–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagin, M.A.; Woolcott, C.G.; Vincer, M.J.; Whyte, R.K.; Stinson, D.A. Hypothermia for neonatal hypoxic ischemic encephalopathy. Arch. Pediatr. Adolesc. Med. 2012, 166, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, A.J.; Thoresen, M. Animal studies of neonatal hypothermic neuroprotection have translated well in to practice. Resuscitation 2015, 97, 88–90. [Google Scholar] [CrossRef]
- Perrone, S.; Negro, S.; Tataranno, M.L.; Buonocore, G. Oxidative stress and antioxidant strategies in newborns. J. Matern. Neonatal Med. 2010, 23, 63–65. [Google Scholar] [CrossRef]
- Blomgren, K.; Hagberg, H. Free radicals, mitochondria, and hypoxia–ischemia in the developing brain. Free. Radic. Biol. Med. 2006, 40, 388–397. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.-K. Oxidative stress in hypoxic-ischemic encephalopathy: Molecular mechanisms and therapeutic strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Austin, T.; Aceti, A.; Faldella, G.; Corvaglia, L. Free radicals and neonatal encephalopathy: Mechanisms of injury, biomarkers, and antioxidant treatment perspectives. Pediatr. Res. 2019, 87, 823–833. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, H.; Lu, L.; Ren, P.; Li, L. Neonatal hypoxic–ischemic encephalopathy: Emerging therapeutic strategies based on pathophysiologic phases of the injury. J. Matern. Neonatal Med. 2018, 32, 3685–3692. [Google Scholar] [CrossRef]
- Perrone, S.; Szabó, M.; Bellieni, C.V.; Longini, M.; Bangó, M.; Kelen, D.; Treszl, A.; Negro, S.; Tataranno, M.L.; Buonocore, G. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr. Neurol. 2010, 43, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Drury, P.P.; Gunn, E.R.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Clin. Perinatol. 2014, 41, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Vannucci, R.C.; Towfighi, J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr. Res. 1990, 27, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadid, M.; Moison, R.; Steendijk, P.; Hiltermann, L.; Berger, H.M.; van Bel, F. The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr. Res. 1998, 44, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bel, F.; Shadid, M.; Moison, R.M.W.; Dorrepaal, C.A.; Fontijn, J.; Monteiro, L.; van de Bor, M.; Berger, H.M. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics 1998, 101, 185–193. [Google Scholar] [CrossRef]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Bona, E.; Hagberg, H.; Løberg, E.M.; Bågenholm, R.; Thoresen, M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: Short- and long-term outcome. Pediatr. Res. 1998, 43, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef]
- Rodríguez-Fanjul, J.; Fernández-Feijóo, C.D.; Lopez-Abad, M.; Ramos, M.G.L.; Caballé, R.B.; Alcántara-Horillo, S.; Camprubí, M.C. Neuroprotection with hypothermia and allopurinol in an animal model of hypoxic-ischemic injury: Is it a gender question? PLoS ONE 2017, 12, e0184643. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fanjul, J.; Fernández-Feijóo, C.D.; Camprubí, M.C. A new technique for collection of cerebrospinal fluid in rat pups. J. Exp. Neurosci. 2015, 9, 37–41. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- González-Diez, B.; Cavia, M.; Torres, G.; Abaigar, P.; Muñiz, P. Effect of a hemodiafiltration session with on-line regeneration of the ultrafiltrate on oxidative stress. Blood Purif. 2008, 26, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-García, R.; Gerardi, G.; Rivero-Pérez, M.D.; González-San José, M.L.; García-Lomillo, J.; Muñiz, P. Wine pomace seasoning attenuates hyperglycaemia-induced endothelial dysfunction and oxidative damage in endothelial cells. J. Funct. Foods 2016, 22, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013, 136, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.; Elmahdy, H.; El-Dib, M.; Rowisha, M.; Awny, M.; Elgohary, T.; Elbatch, M.; Hamisa, M.; El-Mashad, A.-R. Melatonin use for neuroprotection in perinatal asphyxia: A randomized controlled pilot study. J. Perinatol. 2015, 35, 186–191. [Google Scholar] [CrossRef]
- Jatana, M.; Singh, I.; Singh, A.K.; Jenkins, D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2006, 59, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Maiwald, C.A.; Annink, K.V.; Rüdiger, M.; Benders, M.J.N.L.; van Bel, F.; Allegaert, K.; Naulaers, G.; Bassler, D.; Klebermaß-Schrehof, K.; Vento, M.; et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): Study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr. 2019, 27, 210. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, R.; Li, H.N.; Yao, W.X.; Qiao, H.Q.; Wang, S.J.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Oxymatrine attenuated hypoxic-ischemic brain damage in neonatal rats via improving antioxidant enzyme activities and inhibiting cell death. Neurochem. Int. 2015, 89, 17–27. [Google Scholar] [CrossRef]
- Wei, W.; Lan, X.-B.; Liu, N.; Yang, J.-M.; Du, J.; Ma, L.; Zhang, W.-J.; Niu, J.-G.; Sun, T.; Yu, J.-Q. Echinacoside alleviates hypoxic-ischemic brain injury in neonatal rat by enhancing antioxidant capacity and inhibiting apoptosis. Neurochem. Res. 2019, 44, 1582–1592. [Google Scholar] [CrossRef]
- Dirnagl, U.; Lindauer, U.; Them, A.; Schreiber, S.; Pfister, H.-W.; Koedel, U.; Reszka, R.; Freyer, D.; Villringer, A. Global cerebral ischemia in the rat: Online monitoring of oxygen free radical production using chemiluminescence in vivo. Br. J. Pharmacol. 1995, 15, 929–940. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Tsuruta, R.; Fujita, M.; Aki, H.S.; Kutsuna, S.; Kawamura, Y.; Wakatsuki, J.; Aoki, T.; Kobayashi, C.; Kasaoka, S.; et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009, 1305, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Palmer, C.; Towfighi, J.; Roberts, R.L.; Heitjan, D.F. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr. Res. 1993, 33, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mittal, R.; Khanna, H.D.; Basu, S. Free radical injury and blood-brain barrier permeability in hypoxic-ischemic encephalopathy. Pediatrics 2008, 122, e722–e727. [Google Scholar] [CrossRef]
- Nagel, S.; Su, Y.; Horstmann, S.; Heiland, S.; Gardner, H.; Koziol, J.; Martinez-Torres, F.J.; Wagner, S. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat—Effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008, 1188, 198–206. [Google Scholar] [CrossRef]
- Kaandorp, J.J.; Benders, M.J.; Schuit, E.; Rademaker, C.M.; Oudijk, M.A.; Porath, M.M.; Oetomo, S.B.; Wouters, M.G.; van Elburg, R.; Franssen, M.T.; et al. Maternal allopurinol administration during suspected fetal hypoxia: A novel neuroprotective intervention? A multicentre randomised placebo controlled trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F216–F223. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Brambrink, A.M.; Price, A.C.; Kaiser, A.; Agnew, D.M.; Ichord, R.N.; Traystman, R.J. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol. Dis. 2000, 7, 169–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, S.G.; Roberts, R.J.; Delemos, R.A.; Lawrence, R.A.; Coalson, J.J.; King, R.J.; Null, J.D.M.; Gerstmann, D.R. Allopurinol-induced effects in premature baboons with respiratory distress syndrome. J. Appl. Physiol. 1991, 70, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Alataş, O.; Sahin, A.; Colak, O.; Inal, M.; Köken, T.; Yaşar, B.; Karahüseyinoglu, E. Beneficial effects of allopurinol on glutathione levels and glutathione peroxidase activity in rat ischaemic acute renal failure. J. Int. Med. Res. 1996, 24, 33–39. [Google Scholar] [CrossRef]
- Okatani, Y.; Wakatsuki, A.; Kaneda, C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.J.; Mei, Y.W.; Sun, S.G.; Tong, E.T. Effects of immediate and delayed mild hypothermia on endogenous antioxidant enzymes and energy metabolites following global cerebral ischemia. Chin. Med. J. 2011, 124, 2764–2766. [Google Scholar] [PubMed]
- Zhao, H.; Chen, Y. Effects of mild hypothermia therapy on the levels of glutathione in rabbit blood and cerebrospinal fluid after cardiopulmonary resuscitation. Iran. J. Basic Med. Sci. 2015, 18, 194–198. [Google Scholar]
- Mueller-Burke, D.; Koehler, R.C.; Martin, L.J. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int. J. Dev. Neurosci. 2008, 26, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Yang, Z.-J.; Carter, E.L.; Martin, L.J.; Koehler, R.C. Striatal neuroprotection from neonatal hypoxia-ischemia in piglets by antioxidant treatment with EUK-134 or edaravone. Dev. Neurosci. 2011, 33, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Negro, S.; Benders, M.J.; Tataranno, M.L.; Coviello, C.; de Vries, L.S.; van Bel, F.; Groenendaal, F.; Longini, M.; Proietti, F.; Belvisi, E.; et al. Early prediction of hypoxic-ischemic brain injury by a new panel of biomarkers in a population of term newborns. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, H.; Pazos, M.R.; Alvarez, A.; Mohammed, N.; Santos, M.; Arizti, M.; Alvarez, F.J.; Martinez-Orgado, J. Effects of cannabidiol and hypothermia on short-term brain damage in new-born piglets after acute hypoxia-ischemia. Front. Neurosci. 2016, 10, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, J.G.; Ravi, R.; Stull, L.B.; Murphy, A.M. Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2005, 289, H1512–H1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorini, C.; Ciccoli, L.; Leoncini, S.; Carloni, S.; Perrone, S.; Comporti, M.; Balduini, W.; Buonocore, G. Free iron, total F2-isoprostanes and total F4-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: Neuroprotective effect of melatonin. J. Pineal Res. 2009, 46, 148–154. [Google Scholar] [CrossRef]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; D’Avila, R.; Morsch, A.L.B.; Stein, F.D.S.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huun, M.U.; Garberg, H.T.; Escobar, J.; Chafer, C.; Vento, M.; Holme, I.M.; Saugstad, O.D.; Solberg, R. DHA reduces oxidative stress following hypoxia-ischemia in newborn piglets: A study of lipid peroxidation products in urine and plasma. J. Perinat. Med. 2017, 46, 209–217. [Google Scholar] [CrossRef]
- Huun, M.U.; Garberg, H.T.; Buonocore, G.; Longini, M.; Belvisi, E.; Bazzini, F.; Proietti, F.; Saugstad, O.D.; Solberg, R. Regional differences of hypothermia on oxidative stress following hypoxia-ischemia: A study of DHA and hypothermia on brain lipid peroxidation in newborn piglets. J. Perinat. Med. 2018, 47, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán Fernández-Feijóo, C.; Rodríguez-Fanjul, J.; Lopez-Abat, M.; Hadley, S.; Cavia-Saiz, M.; Muñiz, P.; Arnaez, J.; Fernández-Lorenzo, J.R.; Camprubí Camprubí, M. Effects of Hypothermia and Allopurinol on Oxidative Status in a Rat Model of Hypoxic Ischemic Encephalopathy. Antioxidants 2021, 10, 1523. https://doi.org/10.3390/antiox10101523
Durán Fernández-Feijóo C, Rodríguez-Fanjul J, Lopez-Abat M, Hadley S, Cavia-Saiz M, Muñiz P, Arnaez J, Fernández-Lorenzo JR, Camprubí Camprubí M. Effects of Hypothermia and Allopurinol on Oxidative Status in a Rat Model of Hypoxic Ischemic Encephalopathy. Antioxidants. 2021; 10(10):1523. https://doi.org/10.3390/antiox10101523
Chicago/Turabian StyleDurán Fernández-Feijóo, Cristina, Javier Rodríguez-Fanjul, Miriam Lopez-Abat, Stephanie Hadley, Mónica Cavia-Saiz, Pilar Muñiz, Juan Arnaez, José Ramón Fernández-Lorenzo, and Marta Camprubí Camprubí. 2021. "Effects of Hypothermia and Allopurinol on Oxidative Status in a Rat Model of Hypoxic Ischemic Encephalopathy" Antioxidants 10, no. 10: 1523. https://doi.org/10.3390/antiox10101523
APA StyleDurán Fernández-Feijóo, C., Rodríguez-Fanjul, J., Lopez-Abat, M., Hadley, S., Cavia-Saiz, M., Muñiz, P., Arnaez, J., Fernández-Lorenzo, J. R., & Camprubí Camprubí, M. (2021). Effects of Hypothermia and Allopurinol on Oxidative Status in a Rat Model of Hypoxic Ischemic Encephalopathy. Antioxidants, 10(10), 1523. https://doi.org/10.3390/antiox10101523





