Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility
Abstract
1. Introduction
2. Qualitative mtDNA Alterations
2.1. Oxidative Stress in Mitochondrial DNA-Dependant Aging
2.2. Oxidative Stress in Ovarian Follicle Mitochondrial DNA-Dependant Aging
2.3. Evidence about mtDNA Oocyte Alterations
2.4. Evidence about mtDNA Granulosa Cell Alterations
2.5. Mitochondrial Unfolded Protein Response (mtUPR)
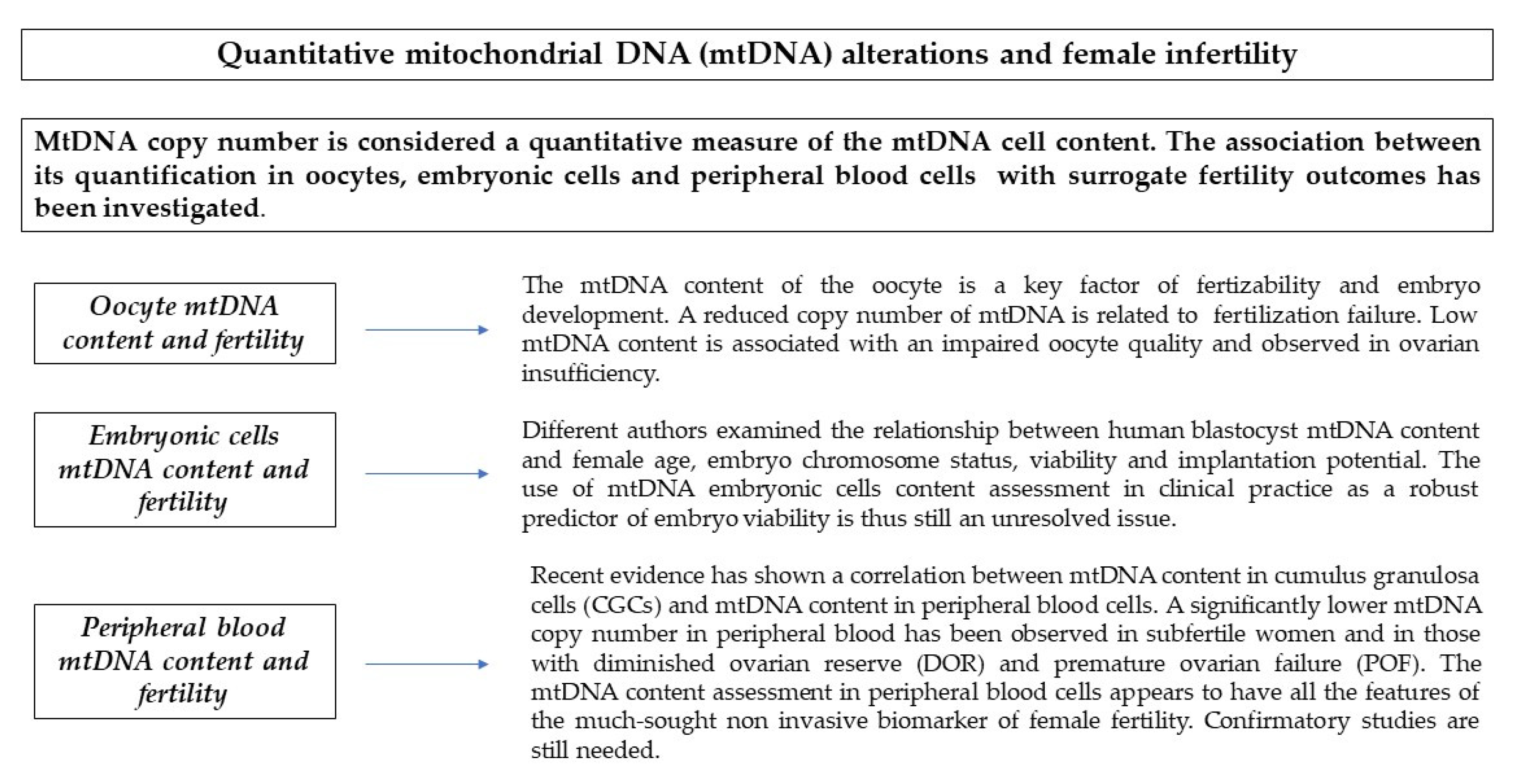
3. Quantitative Alterations
3.1. Oocytes mtDNA Content and Fertility
3.2. Granulosa Cells mtDNA Content and Fertility
3.3. Embryonic Cells mtDNA Content and Fertility
3.4. Peripheral Blood Cells mtDNA Content and Fertility
4. Proposed Interventions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kasapoğlu, I.; Seli, E. Mitochondrial Dysfunction and Ovarian Aging. Endocrinology 2020, 161, bqaa001. [Google Scholar]
- May-Panloup, P.; Boucret, L.; De La Barca, J.-M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Updat. 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.-W.; Cao, J.; E Smith, A.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.; Karri, S.; Gunn, D.; Hurd, W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 101168. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Chrétien, M.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef]
- Kim, J.; Seli, E. Mitochondria as a Biomarker for IVF Outcome. Reproduction 2019, 157, R235–R242. [Google Scholar] [CrossRef]
- Reynier, P.; May-Panloup, P.; Chretien, M.-F.; Morgan, C.; Jean, M.; Savagner, F.; Barriere, P.; Malthiery, Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Fragouli, E.; Spath, K.; Alfarawati, S.; Kaper, F.; Craig, A.; Michel, C.E.; Kokocinski, F.; Cohen, J.; Munne, S.; Wells, D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015, 11, e1005241. [Google Scholar]
- Cozzolino, M.; Marin, D.; Sisti, G. New Frontiers in IVF: mtDNA and autologous germline mitochondrial energy transfer. Reprod. Biol. Endocrinol. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Chappel, S. The Role of Mitochondria from Mature Oocyte to Viable Blastocyst. Obstet. Gynecol. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-R.; Zhang, M.; Jiang, Z.; Seli, E. Mitochondrial dysfunction and ovarian aging. Am. J. Reprod. Immunol. 2017, 77, e12651. [Google Scholar] [CrossRef] [PubMed]
- Zsurka, G.; Peeva, V.; Kotlyar, A.B.; Kunz, W.S. Is There Still Any Role for Oxidative Stress in Mitochondrial DNA-Dependent Aging? Genes 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.R.; Salk, J.J.; Schmitt, M.W.; Loeb, L.A. Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage. PLoS Genet. 2013, 9, e1003794. [Google Scholar] [CrossRef]
- Hoekstra, J.G.; Bs, M.J.H.; Montine, T.J.; Kennedy, S.R. Mitochondrial DNA mutations increase in early stage Alzheimer disease and are inconsistent with oxidative damage. Ann. Neurol. 2016, 80, 301–306. [Google Scholar] [CrossRef]
- Szczepanowska, K.; Trifunovic, A. Origins of mtDNA mutations in ageing. Essays Biochem. 2017, 61, 325–337. [Google Scholar] [CrossRef]
- Kauppila, T.E.; Kauppila, J.H.; Larsson, N.-G. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef]
- DeBalsi, K.L.; Hoff, K.E.; Copeland, W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017, 33, 89–104. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J. Mitochondrial Nucleoid: Shield and Switch of the Mitochondrial Genome. Oxidative Med. Cell. Longev. 2017, 2017, 8060949. [Google Scholar] [CrossRef]
- Trifunovic, A.; Hansson, A.; Wredenberg, A.; Rovio, A.T.; Dufour, E.; Khvorostov, I.; Spelbrink, J.N.; Wibom, R.; Jacobs, H.T.; Larsson, N. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2005, 102, 17993–17998. [Google Scholar] [CrossRef] [PubMed]
- Buccione, R.; Schroeder, A.C.; Eppig, J.J. Interactions between Somatic Cells and Germ Cells Throughout Mammalian Oogenesis1. Biol. Reprod. 1990, 43, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Updat. 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, M.; Li, X.; Wang, H.; Ma, M.; Zhang, S.; Guo, Y.; Wang, S.; Wang, Y.; Duan, N.; et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum. Reprod. 2017, 32, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Luoma, P.; Melberg, A.; Rinne, J.O.; Kaukonen, J.A.; Nupponen, N.N.; Chalmers, R.M.; Oldfors, A.; Rautakorpi, I.; Peltonen, L.; Majamaa, K.; et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: Clinical and molecular genetic study. Lancet 2004, 364, 875–882. [Google Scholar] [CrossRef]
- Chan, C.; Liu, V.; Lau, E.; Yeung, W.; Ng, E.H.Y.; Ho, P. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol. Hum. Reprod. 2005, 11, 843–846. [Google Scholar] [CrossRef] [PubMed]
- A Barritt, J.; Cohen, J.; A Brenner, C. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod. Biomed. Online 2000, 1, 96–100. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F. The aging ovary—The poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Seifer, D.B.; DeJesus, V.; Hubbard, K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil. Steril. 2002, 78, 1046–1048. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef]
- Boucret, L.; De La Barca, J.M.C.; Moriniere, C.; Desquiret, V.; Ferre-L’Hotellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Zhang, G.; Wan, Y.; Zhang, Y.; Lan, S.; Jia, R.; Wang, Z.; Fan, Y.; Wang, F. Expression of Mitochondria-Associated Genes (PPARGC1A,NRF-1,BCL-2andBAX) in Follicular Development and Atresia of Goat Ovaries. Reprod. Domest. Anim. 2015, 50, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Casper, R.F.; Diez-Juan, A.; Simon, C.; Domar, A.D.; Frydman, R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil. Steril. 2016, 105, 548–559. [Google Scholar] [CrossRef]
- Miquel, J.; Economos, A.; Fleming, J.; Johnson, J. Mitochondrial role in cell aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Burhans, W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2013, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Lin, L.T.; Li, C.J.; Kao, P.G.; Tsai, H.W.; Chen, S.N.; Wen, Z.H.; Wang, P.H.; Tsui, K.H. Combining Bioinformatics and Experiments to Identify CREB1 as a Key Regulator in Senescent Granulosa Cells. Diagnostics 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Martinus, R.D.; Garth, G.P.; Webster, T.L.; Cartwright, P.; Naylor, D.J.; Høj, P.B.; Hoogenraad, N.J. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur. J. Biochem. 1996, 240, 98–103. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Levichkin, I.V.; Stasinopoulos, S.; Ryan, M.T.; Hoogenraad, N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002, 21, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.M.; Haynes, C.M. UPR(mt)-mediated cytoprotection and organismal aging. Biochim. Biophys. Acta. 2015, 1847, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, C.; Haynes, C.M.; Yang, Y.; Harding, H.P.; Ron, D. Ubiquitin-Like Protein 5 Positively Regulates Chaperone Gene Expression in the Mitochondrial Unfolded Protein Response. Genetics 2006, 174, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Wang, T.; Horvath, T.L. Mitochondrial unfolded protein response: A stress response with implications for fertility and reproductive aging. Fertil. Steril. 2019, 111, 197–204. [Google Scholar] [CrossRef]
- Santos, T.A.; El Shourbagy, S.; John, J.C.S. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef]
- Konstantinidis, M.; Alfarawati, S.; Hurd, D.; Paolucci, M.; Shovelton, J.; Fragouli, E.; Wells, D. Simultaneous assessment of aneuploidy, polymorphisms, and mitochondrial DNA content in human polar bodies and embryos with the use of a novel microarray platform. Fertil. Steril. 2014, 102, 1385–1392. [Google Scholar] [CrossRef]
- Cecchino, G.N.; Seli, E.; Da Motta, E.L.A.; García-Velasco, J.A. The role of mitochondrial activity in female fertility and assisted reproductive technologies: Overview and current insights. Reprod. Biomed. Online 2018, 36, 686–697. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Clément, A.; Seegers, V.; Boucret, L.; Ferré-L’ Hotellier, V.; Bouet, P.; Descamps, P.; Procaccio, V.; Reynier, P.; May-Panloup, P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum. Reprod. 2017, 32, 607–614. [Google Scholar] [CrossRef]
- Taugourdeau, A.; Desquiret-Dumas, V.; Hamel, J.F.; Chupin, S.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; et al. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J. Assist. Reprod. Genet. 2018, 36, 223–228. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, S.; Gong, F.; Lu, C.; Lin, G.; Hu, L. The mitochondrial DNA copy number of cumulus granulosa cells may be related to the maturity of oocyte cytoplasm. Hum. Reprod. 2020, 35, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Cecchino, G.N.; García-Velasco, J.A. Mitochondrial DNA copy number as a predictor of embryo viability. Fertil. Steril. 2019, 111, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Diez-Juan, A.; Rubio, C.; Marin, C.; Martinez, S.; Al-Asmar, N.; Riboldi, M.; Díaz-Gimeno, P.; Valbuena, D.; Simón, C. Mitochondrial DNA content as a viability score in human euploid embryos: Less is better. Fertil. Steril. 2015, 104, 534–541.e1. [Google Scholar] [CrossRef] [PubMed]
- Spinella, F.; Cotroneo, E.; Bono, S.; Biricik, A.; Greco, E.; Minasi, M.G.; Ruberti, A.; Fiorentino, F. Quantification of mitochondrial DNA in preimplantation embryos: A tool to predict implantation potential of chromosomally normal embryos. Hum. Reprod. 2016, 31, i26. [Google Scholar]
- Ravichandran, K.; McCaffrey, C.; Grifo, J.; Morales, A.; Perloe, M.; Munne, S.; Wells, D.; Fragouli, E. Mitochondrial DNA quantification as a tool for embryo viability assessment: Retrospective analysis of data from single euploid blastocyst transfers. Hum. Reprod. 2017, 32, 1282–1292. [Google Scholar] [CrossRef]
- Victor, A.R.; Brake, A.J.; Tyndall, J.C.; Griffin, D.K.; Zouves, C.G.; Barnes, F.L.; Viotti, M. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil. Steril. 2017, 107, 34–42.e3. [Google Scholar] [CrossRef]
- Fragouli, E.; McCaffrey, C.; Ravichandran, K.; Spath, K.; Grifo, J.A.; Munné, S.; Wells, D. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: A blinded prospective non-selection study. Hum. Reprod. 2017, 32, 2340–2347. [Google Scholar] [CrossRef]
- Treff, N.R.; Zhan, Y.; Tao, X.; Olcha, M.; Han, M.; Rajchel, J.; Morrison, L.; Morin, S.J.; Scott, R.T. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum. Reprod. 2017, 32, 1–9. [Google Scholar] [CrossRef]
- de Los Santos, M.J.; Diez Juan, A.; Mifsud, A.; Mercader, A.; Meseguer, M.; Rubio, C.; Pellicer, A. Variables associated with mitochondrial copy number in human blastocysts: What can we learn from trophectoderm biopsies? Fertil. Steril. 2018, 109, 110–117. [Google Scholar] [CrossRef]
- Lledo, B.; A Ortiz, J.; Morales, R.; García-Hernández, E.; Ten, J.; Bernabeu, A.; Llácer, J. Comprehensive mitochondrial DNA analysis and IVF outcome. Hum. Reprod. Open 2018, 2018, hoy023. [Google Scholar] [CrossRef]
- Klimczak, A.M.; Pacheco, L.E.; Lewis, K.E.; Massahi, N.; Richards, J.P.; Kearns, W.G.; Saad, A.F.; Crochet, J.R. Embryonal mitochondrial DNA: Relationship to embryo quality and transfer outcomes. J. Assist. Reprod. Genet. 2018, 35, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-X.; Chen, C.-H.; Lin, S.-Y.; Lin, Y.-H.; Tzeng, C.-R. Adjusted mitochondrial DNA quantification in human embryos may not be applicable as a biomarker of implantation potential. J. Assist. Reprod. Genet. 2019, 36, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.T.; Sun, L.; Zhan, Y.; Marin, D.; Tao, X.; Seli, E. Mitochondrial DNA content is not predictive of reproductive competence in euploid blastocysts. Reprod. Biomed. Online 2020, 41, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.; Ravichandran, K.; McCaffrey, C.; Grifo, J.; Morales, A.; Perloe, M.; Munne, S.; Fragouli, E. Reply: Mitochondrial DNA Quantification—the devil in the detail. Hum. Reprod. 2017, 32, 2150–2151. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.; Somigliana, E.; Cacciatore, C.; Busnelli, M.; Rossetti, R.; Bonetti, S.; Paffoni, A.; Mari, D.; Ragni, G.; Persani, L. Italian Network for the study of Ovarian Dysfunctions. Blood cell mitochondrial DNA content and premature ovarian aging. PLoS ONE 2012, 7, e42423. [Google Scholar] [CrossRef]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef]
- Busnelli, A.; Lattuada, D.; Rossetti, R.; Paffoni, A.; Persani, L.; Fedele, L.; Somigliana, E. Mitochondrial DNA copy number in peripheral blood: A potential non-invasive biomarker for female subfertility. J. Assist. Reprod. Genet. 2018, 35, 1987–1994. [Google Scholar] [CrossRef]
- Knez, J.; Marrachelli, V.G.; Cauwenberghs, N.; Winckelmans, E.; Zhang, Z.; Thijs, L.; Brguljan-Hitij, J.; Plusquin, M.; Delles, C.; Monleón, D.; et al. Peripheral blood mitochondrial DNA content in relation to circulating metabolites and inflammatory markers: A population study. PLoS ONE 2017, 12, e0181036. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Ashar, F.N.; Zhang, Y.; Longchamps, R.J.; Lane, J.; Moes, A.; Grove, M.L.; Mychaleckyj, J.C.; Taylor, K.D.; Coresh, J.; Rotter, J.I.; et al. Association of Mitochondrial DNA Copy Number with Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1247–1255. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.R.; Park, I.H.; Huh, J.H.; Son, J.W.; Kim, K.W.; Park, K.-S.; Cha, S.K.; Sohn, J.H.; Jung, D.-H.; et al. A prospective study of leucocyte mitochondrial DNA content and deletion in association with the metabolic syndrome. Diabetes Metab. 2017, 43, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Verhoeven, J.E.; Picard, M.; Lin, J.; Sidney, S.; Epel, E.S.; Penninx, B.W.J.H.; Puterman, E. Associations Between Cellular Aging Markers and Metabolic Syndrome: Findings from the CARDIA Study. J. Clin. Endocrinol. Metab. 2017, 103, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Abdulhasan, M.K.; Li, Q.; Dai, J.; Abu-Soud, H.M.; Puscheck, E.E.; Rappolee, D. CoQ10 increases mitochondrial mass and polarization, ATP and Oct4 potency levels, and bovine oocyte MII during IVM while decreasing AMPK activity and oocyte death. J. Assist. Reprod. Genet. 2017, 34, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Boots, C.; Boudoures, A.; Zhang, W.; Drury, A.; Moley, K.H. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum. Reprod. 2016, 31, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Hannam, T.; Jurisicova, A.; Esfandiari, N.; Casper, R.F. Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clin. Med. Insights: Reprod. Heal. 2014, 8, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Sun, A.-G.; Zhao, S.-G.; Liu, H.; Ma, S.-Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H.-B. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 8, CD007807. [Google Scholar]


| Study | Design | Biopsy Site | Nr. of Embryos/Blastocysts Analyzed | mtDNA Quantification Method | Impact of mtDNA Copy Number on Implantation | Association between mtDNA Copy Number and Female Age | Impact of mtDNA Copy Number on Euploidy | Impact of mtDNA Copy Number on Embryo Morphology |
|---|---|---|---|---|---|---|---|---|
| Fragouli et al., 2015 [9] | Prospective study | Trophectoderm | 379 embryos | Relative quantification | Euploid blastocysts that successfully implanted were shown to contain a lower mtDNA quantity than those failing to implant | Significantly higher quantity of mtDNA in embryos from older women | mtDNA levels were elevated in aneuploid embryos | NR |
| Diez-Juan et al., 2015 [53] | Retrospective study | Trophectoderm | 310 euploid embryos | Relative quantification | The relative mtDNA content of day-3 and euploid embryos that successfully implanted resulted significantly lower than that of those that failed to implant | The quantity of mtDNA was significantly higher in embryos from older women | NR | Trend toward an increased mtDNA content in poorer quality embryos |
| Spinella et al., 2016 [54] | NR | Trophectoderm | 96 blastocysts | Relative quantification | Blastocysts that implanted and resulted in baby born, were shown to contain lower mtDNA quantities compared with those that failed to implant | NR | The relative quantity of mtDNA was significantly lower in euploid embryos | Fully expanded (Grade 5 or 6) euploid blastocysts had an mtDNA average value 1.6-fold lower than euploid blastocysts with expansion grade 3 |
| Ravichandran et al., 2017 [55] | Retrospective study | Trophectoderm | 1505 euploid blastocysts | Relative quantification | Of embryos containing ‘elevated’ amounts of mtDNA, none implanted | Female patients in the youngest age bracket had average mtDNA levels that were significantly lower than those in the oldest age bracket | NR | None |
| Victor et al., 2017 [56] | Retrospective study | Trophectoderm | 1396 embryos | Relative quantification | Absence of an association | No differences in mtDNA scores between age groups | Absence of an association | NR |
| Fragouli et al., 2017 [57] | Prospective study | Trophectoderm | 199 euploid blastocysts | Relative quantification | Elevated mtDNA was accompanied by implantation failure | The mtDNA quantity appeared to increase with advancing female age, although the difference was not statistically significant | NR | NR |
| Treff et al., 2017 [58] | Retrospective study | Trophectoderm | 374 euploid blastocysts | Relative quantification | None | mtDNA copy number was negatively correlated with oocyte age | NR | NR |
| de Los Santos et al., 2018 [59] | Retrospective study | Trophectoderm | 1641 biopsied blastocysts | Relative quantification | NR | Neither age nor AMH levels were found to be associated with mtDNA quantity | Lower amount of mtDNA in euploid blastocyst | Blastocysts with poor quality TE had more chances of carrying higher mtDNA values |
| Lledo et al., 2018 [60] | Prospective study | Trophectoderm | 159 blastocysts | Relative quantification | Reduction in ongoing pregnancy rate associated with elevated mtDNA copy number | mtDNA copy number was negatively correlated with female age | NR | NR |
| Klimczak et al., 2018 [61] | Retrospective study | Trophectoderm | 1510 blastocysts | Relative quantification | None | No correlation between mtDNA content and the patients’ age | NR | Embryos with higher mtDNA content were found to be of poorer quality (grade 3) relative to grades 1 and 2 |
| Lee et al., 2019 [62] | Double-blind, observational, prospective | Blastomere or Trophoectoderm | 1617 embryos | Realative quantification and adjusted calculation | Absence of an association | Significant but weak correlation | Adjusted mtDNA quantification significantly lower in euploid blastocyst | NR |
| Scott et al., 2020 [63] | Prospective study | Trophectoderm | 615 euploid blastocysts | Relative quantification | Absence of an association | No correlation was observed between maternal age and relative mtDNA copy number | NA | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busnelli, A.; Navarra, A.; Levi-Setti, P.E. Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility. Antioxidants 2021, 10, 55. https://doi.org/10.3390/antiox10010055
Busnelli A, Navarra A, Levi-Setti PE. Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility. Antioxidants. 2021; 10(1):55. https://doi.org/10.3390/antiox10010055
Chicago/Turabian StyleBusnelli, Andrea, Annalisa Navarra, and Paolo Emanuele Levi-Setti. 2021. "Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility" Antioxidants 10, no. 1: 55. https://doi.org/10.3390/antiox10010055
APA StyleBusnelli, A., Navarra, A., & Levi-Setti, P. E. (2021). Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility. Antioxidants, 10(1), 55. https://doi.org/10.3390/antiox10010055




