Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival
Abstract
:1. Introduction
2. Experimental Section
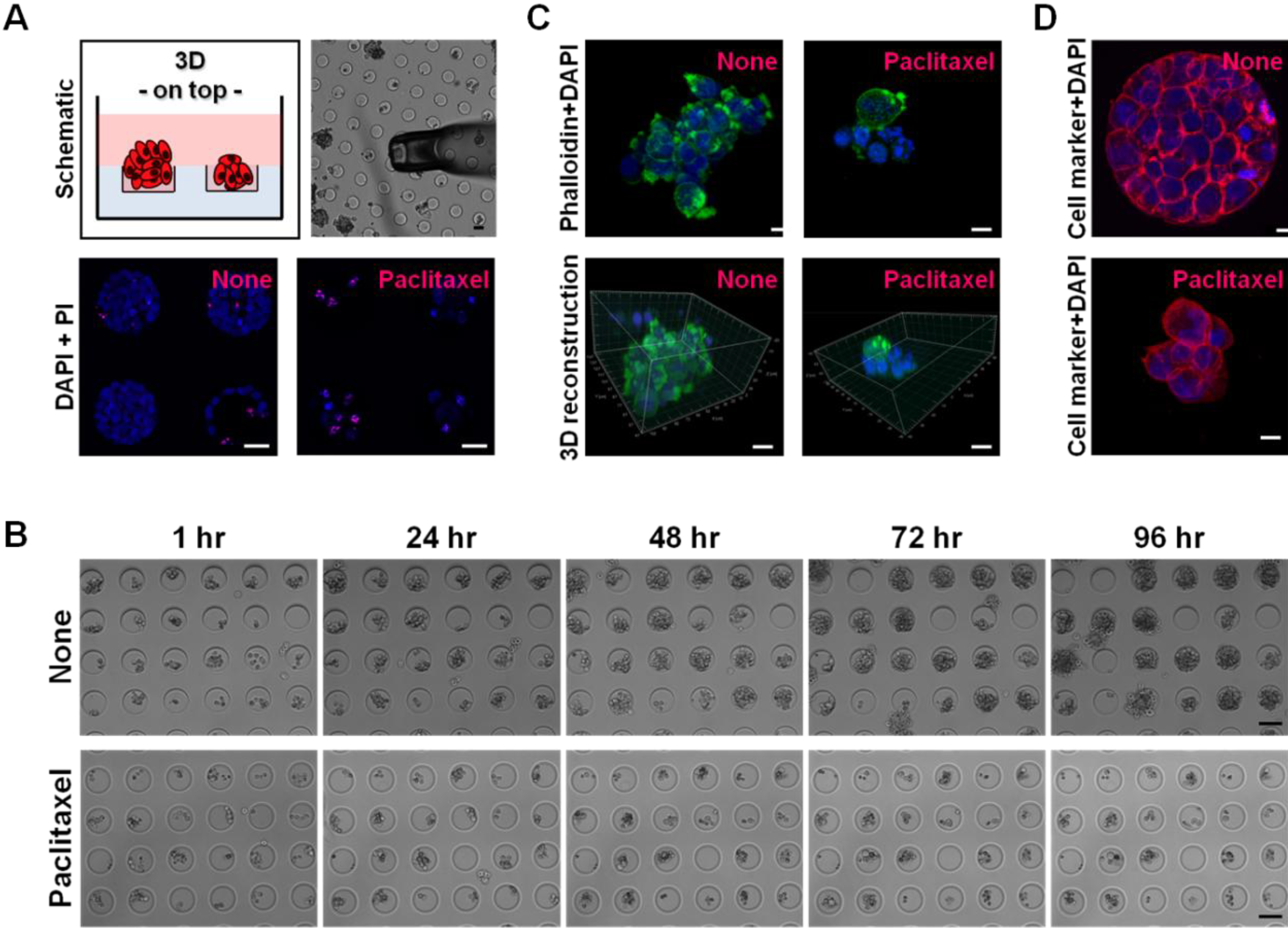
3. Results and Discussion
3.1. Hydrogel Microwell Arrays Allow the Aggregation of Ovarian Cancer Cells
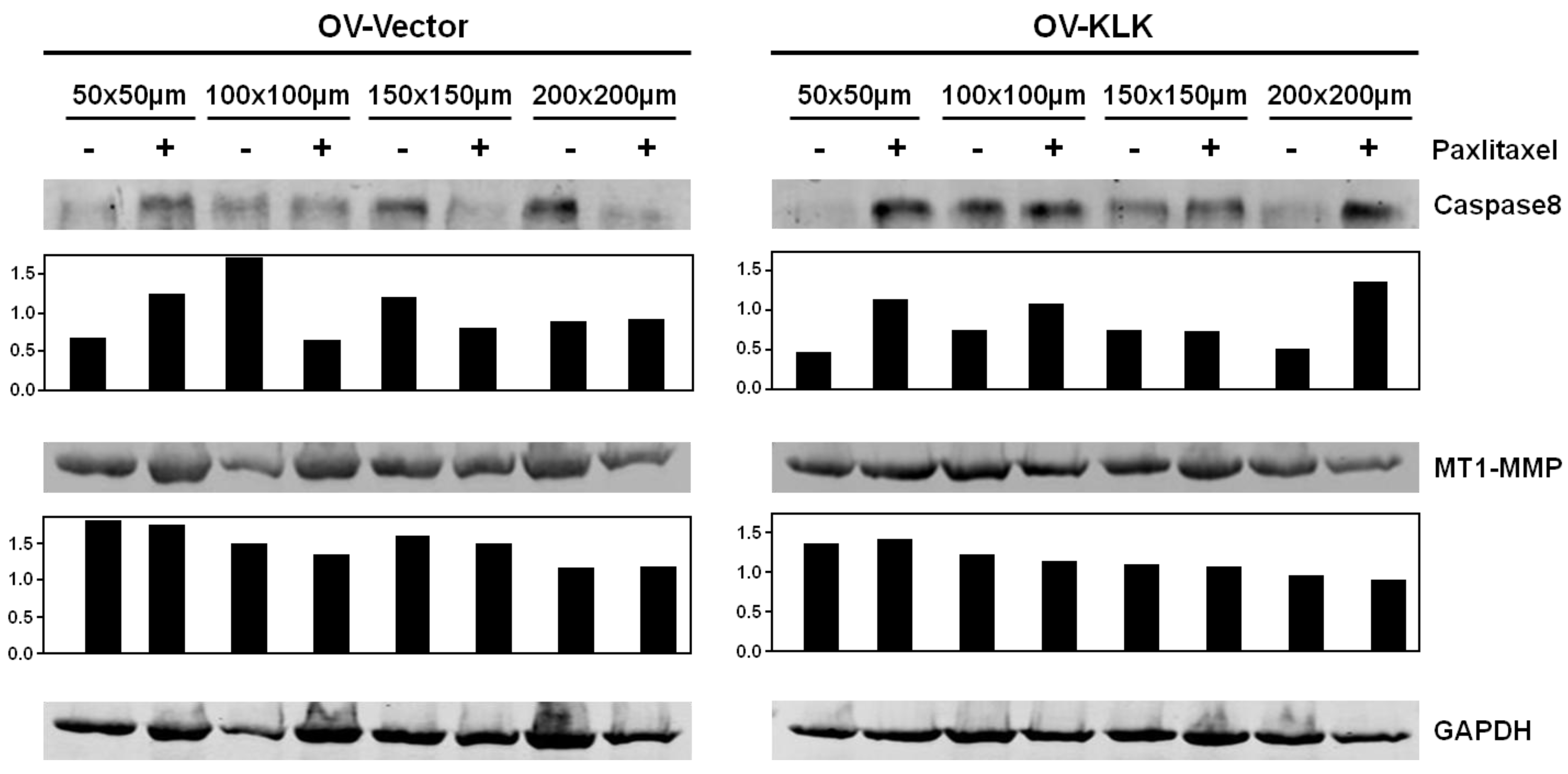
3.2. KLK-Expressing Cells Increase Aggregation and Viability upon Paclitaxel Treatment
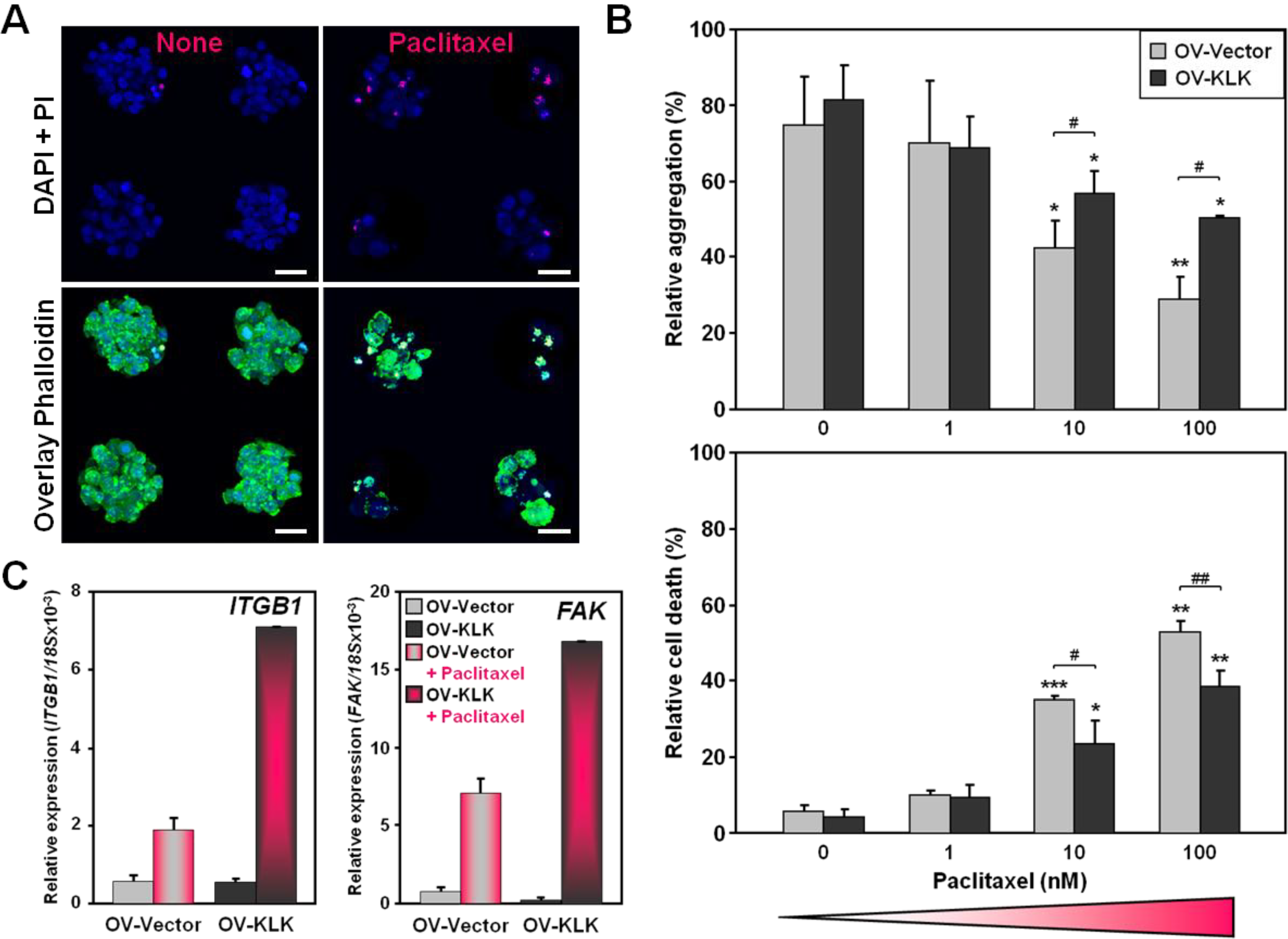
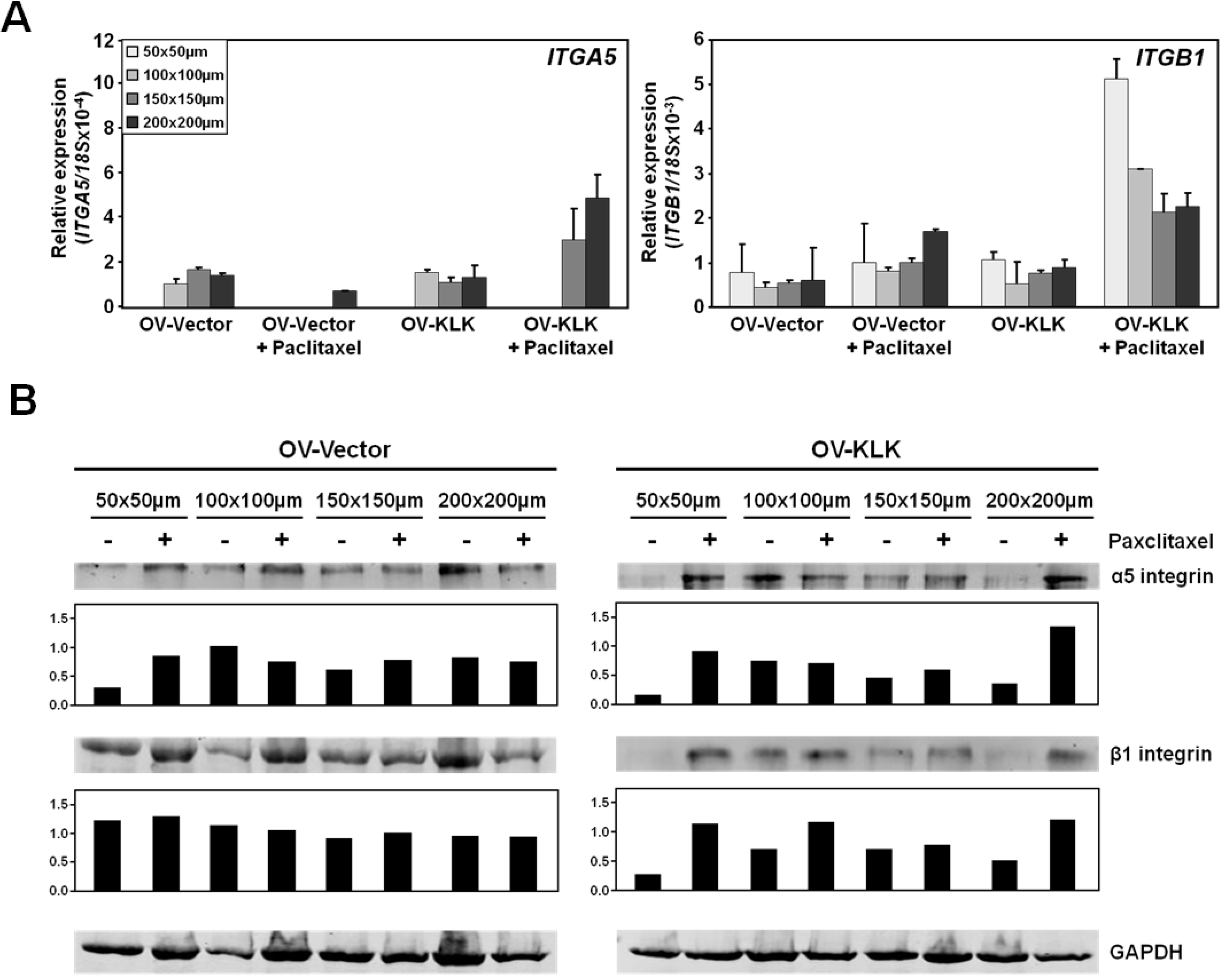
3.3. Paclitaxel Treatment Alters Integrin Expression of Tailor-Made KLK-Expressing Cell Aggregates
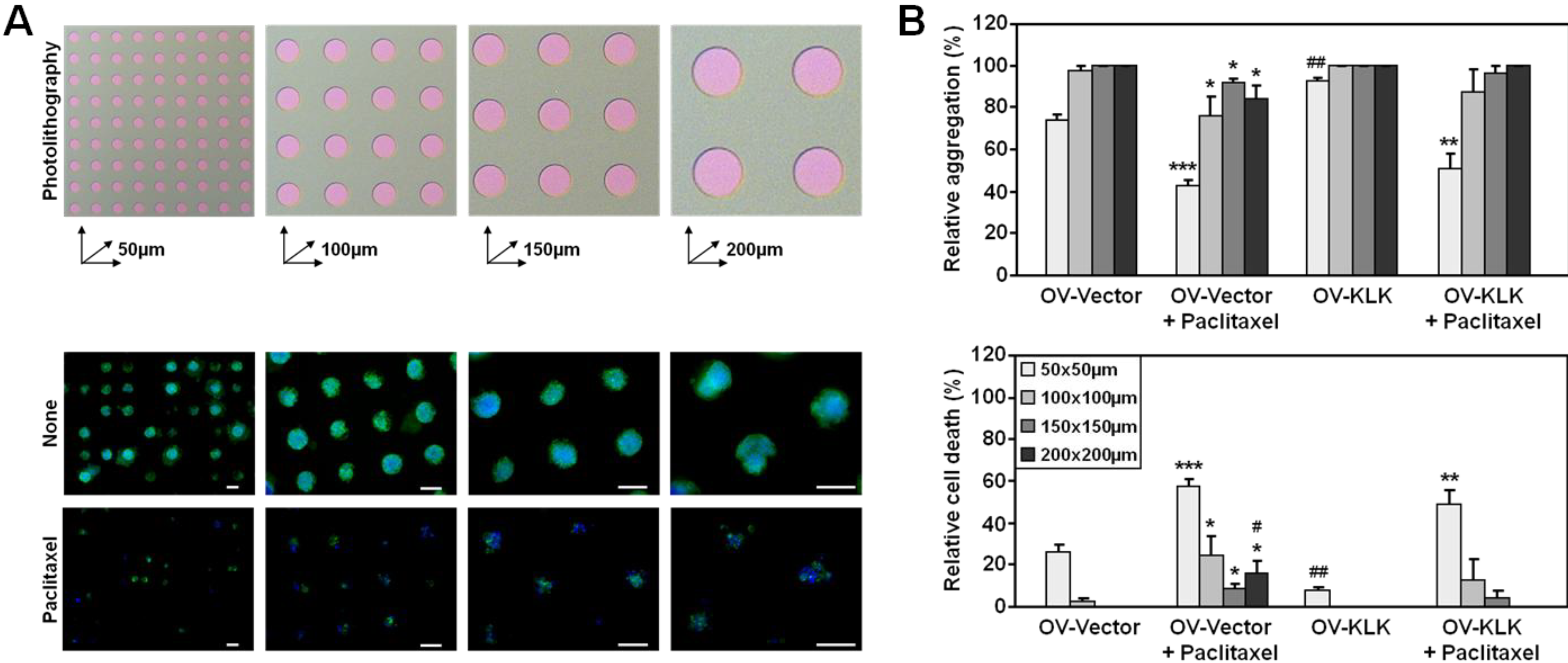
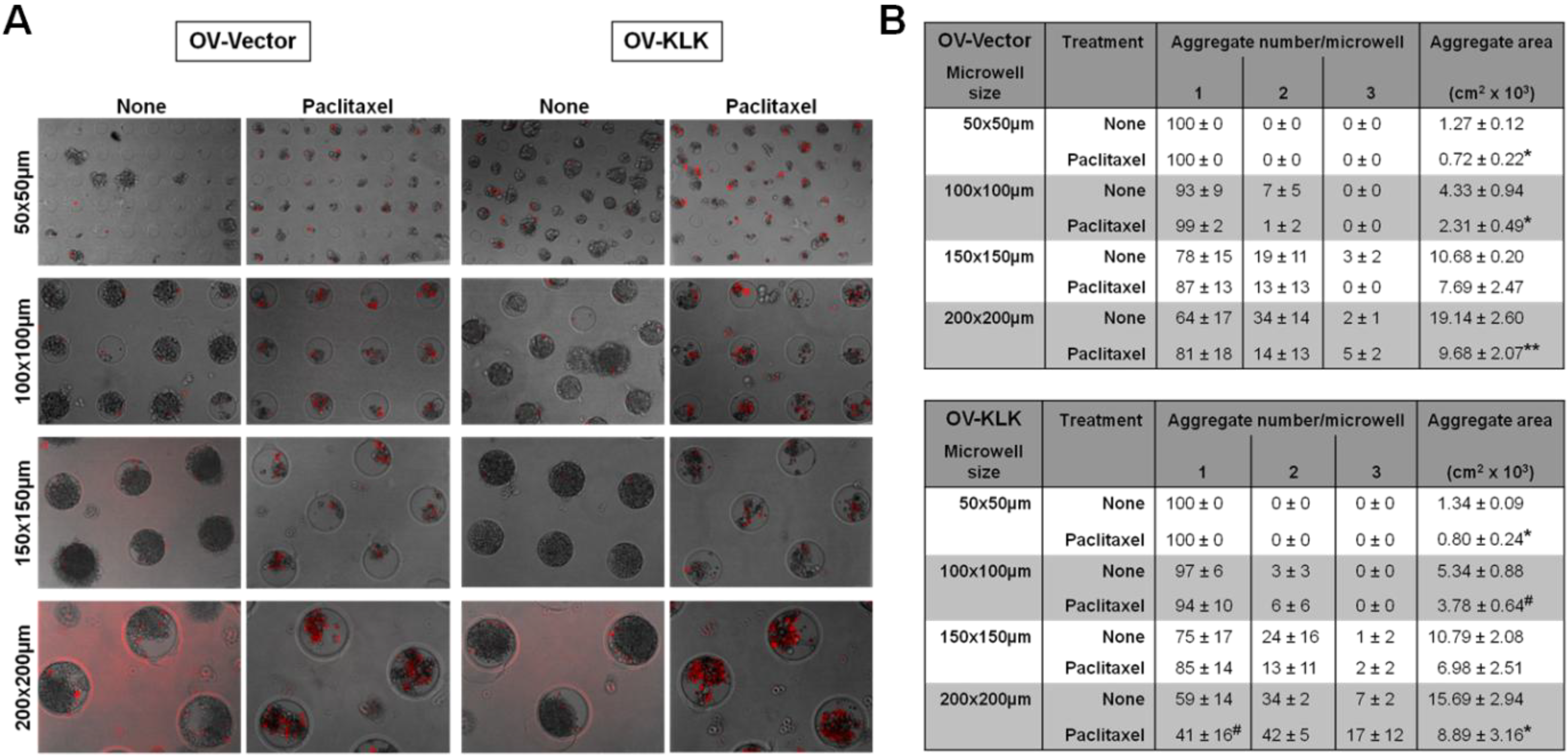
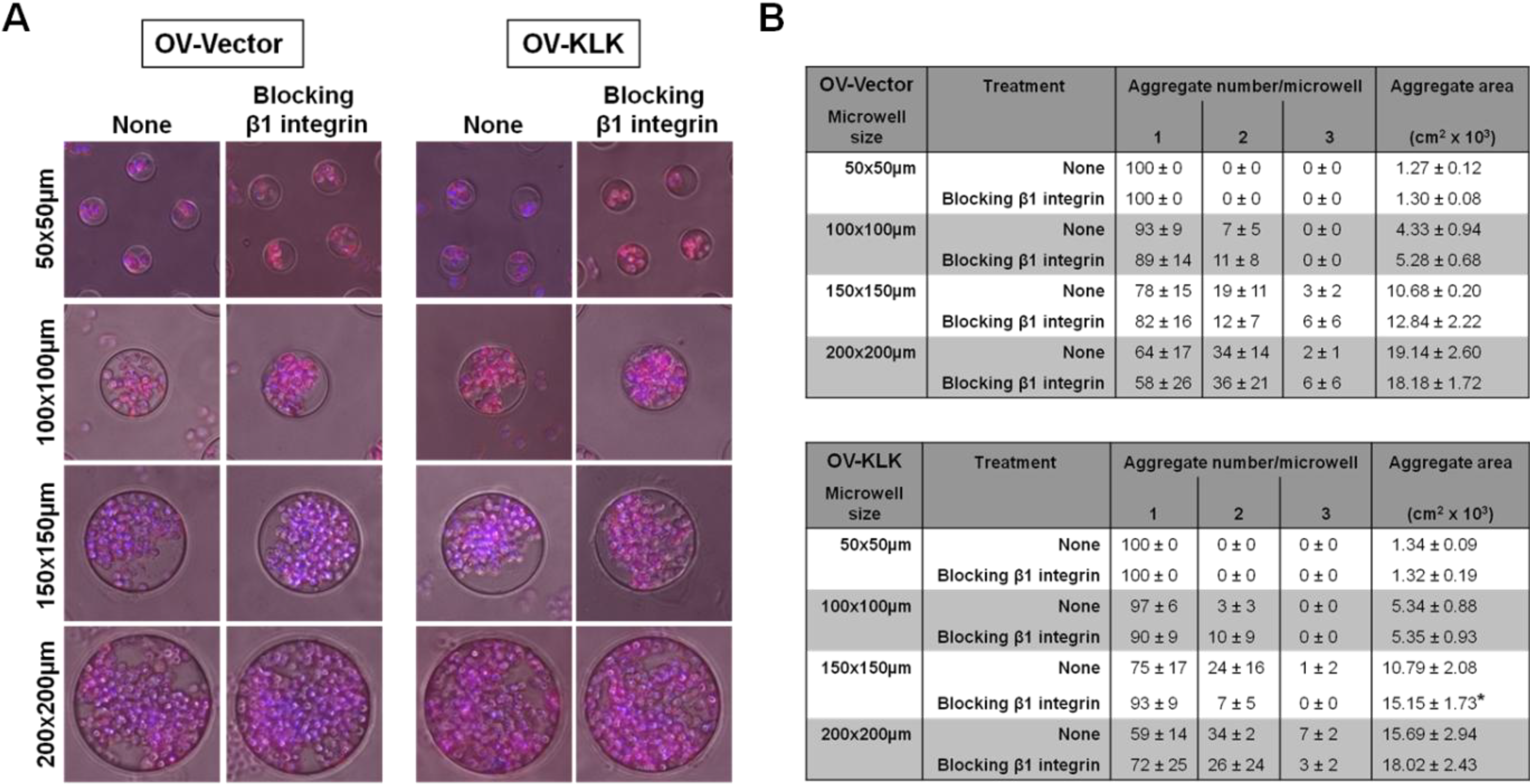
3.4. Blocking of Integrin Function Does Not Affect Cell Aggregation
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Inman, J.L.; Bissell, M.J. Apical polarity in three-dimensional culture systems: Where to now? J. Biol. 2010, 9, 2. [Google Scholar] [CrossRef]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Debnath, J.; Brugge, J.S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 2005, 5, 675–688. [Google Scholar] [CrossRef]
- Hebner, C.; Weaver, V.M.; Debnath, J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu. Rev. Pathol. 2008, 3, 313–339. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell. Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol. Oncol. 2009, 113, 143–148. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef]
- Borgono, C.A.; Diamandis, E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 2004, 4, 876–890. [Google Scholar] [CrossRef]
- Ramani, V.C.; Haun, R.S. The extracellular matrix protein fibronectin is a substrate for kallikrein 7. Biochem. Biophys. Res. Commun. 2008, 369, 1169–1173. [Google Scholar] [CrossRef]
- Obiezu, C.V.; Michael, I.P.; Levesque, M.A.; Diamandis, E.P. Human kallikrein 4: Enzymatic activity, inhibition, and degradation of extracellular matrix proteins. Biol. Chem. 2006, 387, 749–759. [Google Scholar]
- Michael, I.P.; Sotiropoulou, G.; Pampalakis, G.; Magklara, A.; Ghosh, M.; Wasney, G.; Diamandis, E.P. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 2005, 280, 14628–14635. [Google Scholar] [CrossRef]
- Ghosh, M.C.; Grass, L.; Soosaipillai, A.; Sotiropoulou, G.; Diamandis, E.P. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumor cells. Tumor Biol. 2004, 25, 193–199. [Google Scholar] [CrossRef]
- Witz, C.A.; Montoya-Rodriguez, I.A.; Cho, S.; Centonze, V.E.; Bonewald, L.F.; Schenken, R.S. Composition of the extracellular matrix of the peritoneum. J. Soc. Gynecol. Investig. 2001, 8, 299–304. [Google Scholar] [CrossRef]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Invest. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Yousef, G.M.; Diamandis, E.P. The human kallikrein gene family: New biomarkers for ovarian cancer. Cancer Treat. Res. 2009, 149, 165–187. [Google Scholar] [CrossRef]
- Clements, J.A.; Willemsen, N.M.; Myers, S.A.; Dong, Y. The tissue kallikrein family of serine proteases: Functional roles in human disease and potential as clinical biomarkers. Crit. Rev. Clin. Lab. Sci. 2004, 41, 265–312. [Google Scholar] [CrossRef]
- Obiezu, C.V.; Diamandis, E.P. Human tissue kallikrein gene family: Applications in cancer. Cancer Lett. 2005, 224, 1–22. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Li, L.; Zheng, Y.; Simon, I.; Wolfert, R.L.; Valik, D.; Nekulova, M.; Simickova, M.; Frgala, T.; Diamandis, E.P. Prediction of ovarian cancer prognosis and response to chemotherapy by a serum-based multiparametric biomarker panel. Brit. J. Cancer 2008, 99, 1103–1113. [Google Scholar] [CrossRef]
- Dong, Y.; Stephens, C.; Walpole, C.; Swedberg, J.E.; Boyle, G.M.; Parsons, P.G.; McGuckin, M.A.; Harris, J.M.; Clements, J.A. Paclitaxel resistance and multicellular spheroid formation are induced by kallikrein-related peptidase 4 in serous ovarian cancer cells in an ascites mimicking microenvironment. PLoS One 2013, 8, e57056. [Google Scholar] [CrossRef]
- Dong, Y.; Tan, O.L.; Loessner, D.; Stephens, C.; Walpole, C.; Boyle, G.M.; Parsons, P.G.; Clements, J.A. Kallikrein-related peptidase 7 promotes multicellular aggregation via the α5β1 integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res. 2010, 70, 2624–2633. [Google Scholar]
- Xi, Z.; Kaern, J.; Davidson, B.; Klokk, T.I.; Risberg, B.; Trope, C.; Saatcioglu, F. Kallikrein 4 is associated with paclitaxel resistance in ovarian cancer. Gynecol. Oncol. 2004, 94, 80–85. [Google Scholar] [CrossRef]
- Loessner, D.; Quent, V.M.; Kraemer, J.; Weber, E.C.; Hutmacher, D.W.; Magdolen, V.; Clements, J.A. Combined expression of KLK4, KLK5, KLK6, and KLK7 by ovarian cancer cells leads to decreased adhesion and paclitaxel-induced chemoresistance. Gynecol. Oncol. 2012, 127, 569–578. [Google Scholar] [CrossRef]
- Prezas, P.; Arlt, M.J.; Viktorov, P.; Soosaipillai, A.; Holzscheiter, L.; Schmitt, M.; Talieri, M.; Diamandis, E.P.; Kruger, A.; Magdolen, V. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7 increases the malignant phenotype of ovarian cancer cells. Biol. Chem. 2006, 387, 807–811. [Google Scholar]
- Zutter, M.M. Integrin-mediated adhesion: Tipping the balance between chemosensitivity and chemoresistance. Adv. Exp. Med. Biol. 2007, 608, 87–100. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Cabodi, S.; del Pilar Camacho-Leal, M.; Di Stefano, P.; Defilippi, P. Integrin signalling adaptors: Not only figurants in the cancer story. Nat. Rev. Cancer 2010, 10, 858–870. [Google Scholar] [CrossRef]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A.; Peter, M.E.; Ramakrishnan, V.; Yamada, S.D.; Lengyel, E. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008, 68, 2329–2339. [Google Scholar] [CrossRef]
- Shield, K.; Riley, C.; Quinn, M.A.; Rice, G.E.; Ackland, M.L.; Ahmed, N. α2β1 integrin affects metastatic potential of ovarian carcinoma spheroids by supporting disaggregation and proteolysis. J. Carcinog. 2007, 6, 11. [Google Scholar] [CrossRef]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Ruff, L.E.; Skubitz, A.P. β1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Cordey, M.; Limacher, M.; Kobel, S.; Taylor, V.; Lutolf, M.P. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells 2008, 26, 2586–2594. [Google Scholar] [CrossRef]
- Roccio, M.; Gobaa, S.; Lutolf, M.P. High-throughput clonal analysis of neural stem cells in microarrayed artificial niches. Integr. Biol. 2012, 4, 391–400. [Google Scholar] [CrossRef]
- Gobaa, S.; Hoehnel, S.; Roccio, M.; Negro, A.; Kobel, S.; Lutolf, M.P. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 2011, 8, 949–955. [Google Scholar] [CrossRef]
- Mobus, V.; Gerharz, C.D.; Press, U.; Moll, R.; Beck, T.; Mellin, W.; Pollow, K.; Knapstein, P.G.; Kreienberg, R. Morphological, immunohistochemical and biochemical characterization of 6 newly established human ovarian carcinoma cell lines. Int. J. Cancer 1992, 52, 76–84. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- ImageJ. Available online: http://rsb.info.nih.gov/ij/ (accessed on 2 July 2013).
- Imaris. Available online: http://www.bitplane.com (accessed on 2 July 2013).
- Moss, N.M.; Barbolina, M.V.; Liu, Y.; Sun, L.; Munshi, H.G.; Stack, M.S. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: A potential role in I. p. metastatic dissemination. Cancer Res. 2009, 69, 7121–7129. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Loessner, D.; Rizzi, S.; Kaplan, D.L.; Mooney, D.J.; Clements, J.A. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010, 28, 125–133. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar]
- Zietarska, M.; Maugard, C.M.; Filali-Mouhim, A.; Alam-Fahmy, M.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.M. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol. Carcinog. 2007, 46, 872–885. [Google Scholar] [CrossRef]
- Helleman, J.; Jansen, M.P.; Burger, C.; van der Burg, M.E.; Berns, E.M. Integrated genomics of chemotherapy resistant ovarian cancer: A role for extracellular matrix, TGFbeta and regulating microRNAs. Int. J. Biochem. Cell Biol. 2010, 42, 25–30. [Google Scholar]
- Frankel, A.; Buckman, R.; Kerbel, R.S. Abrogation of taxol-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res. 1997, 57, 2388–2393. [Google Scholar]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumors. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Hehlgans, S.; Haase, M.; Cordes, N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim. Biophys. Acta 2007, 1775, 163–180. [Google Scholar]
- Sood, A.K.; Coffin, J.E.; Schneider, G.B.; Fletcher, M.S.; DeYoung, B.R.; Gruman, L.M.; Gershenson, D.M.; Schaller, M.D.; Hendrix, M.J. Biological significance of focal adhesion kinase in ovarian cancer: Role in migration and invasion. Am. J. Pathol. 2004, 165, 1087–1095. [Google Scholar] [CrossRef]
- Judson, P.L.; He, X.; Cance, W.G.; van Le, L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 1999, 86, 1551–1556. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Eng, C.H.; Schlaepfer, D.D.; Marcantonio, E.E.; Gundersen, G.G. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 2004, 303, 836–839. [Google Scholar] [CrossRef]
- Halder, J.; Landen, C.N., Jr.; Lutgendorf, S.K.; Li, Y.; Jennings, N.B.; Fan, D.; Nelkin, G.M.; Schmandt, R.; Schaller, M.D.; Sood, A.K. Focal adhesion kinase silencing augments docetaxel-mediated apoptosis in ovarian cancer cells. Clin. Cancer Res. 2005, 11, 8829–8836. [Google Scholar] [CrossRef]
- Burleson, K.M.; Casey, R.C.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Skubitz, A.P. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol. Oncol. 2004, 93, 170–181. [Google Scholar] [CrossRef]
- Burleson, K.M.; Boente, M.P.; Pambuccian, S.E.; Skubitz, A.P. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J. Transl. Med. 2006, 4, 6. [Google Scholar] [CrossRef]
- Kellouche, S.; Fernandes, J.; Leroy-Dudal, J.; Gallet, O.; Dutoit, S.; Poulain, L.; Carreiras, F. Initial formation of IGROV1 ovarian cancer multicellular aggregates involves vitronectin. Tumor Biol. 2010, 31, 129–139. [Google Scholar] [CrossRef]
- Burleson, K.M.; Hansen, L.K.; Skubitz, A.P. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin. Exp. Metastasis 2004, 21, 685–697. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- Karp, J.M.; Yeh, J.; Eng, G.; Fukuda, J.; Blumling, J.; Suh, K.Y.; Cheng, J.; Mahdavi, A.; Borenstein, J.; Langer, R.; Khademhosseini, A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 2007, 7, 786–794. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Barbolina, M.V.; Stack, M.S. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin. Cell Dev. Biol. 2008, 19, 24–33. [Google Scholar] [CrossRef]
Appendix
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loessner, D.; Kobel, S.; Clements, J.A.; Lutolf, M.P.; Hutmacher, D.W. Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival. Microarrays 2013, 2, 208-227. https://doi.org/10.3390/microarrays2030208
Loessner D, Kobel S, Clements JA, Lutolf MP, Hutmacher DW. Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival. Microarrays. 2013; 2(3):208-227. https://doi.org/10.3390/microarrays2030208
Chicago/Turabian StyleLoessner, Daniela, Stefan Kobel, Judith A. Clements, Matthias P. Lutolf, and Dietmar W. Hutmacher. 2013. "Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival" Microarrays 2, no. 3: 208-227. https://doi.org/10.3390/microarrays2030208
APA StyleLoessner, D., Kobel, S., Clements, J. A., Lutolf, M. P., & Hutmacher, D. W. (2013). Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival. Microarrays, 2(3), 208-227. https://doi.org/10.3390/microarrays2030208





