Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
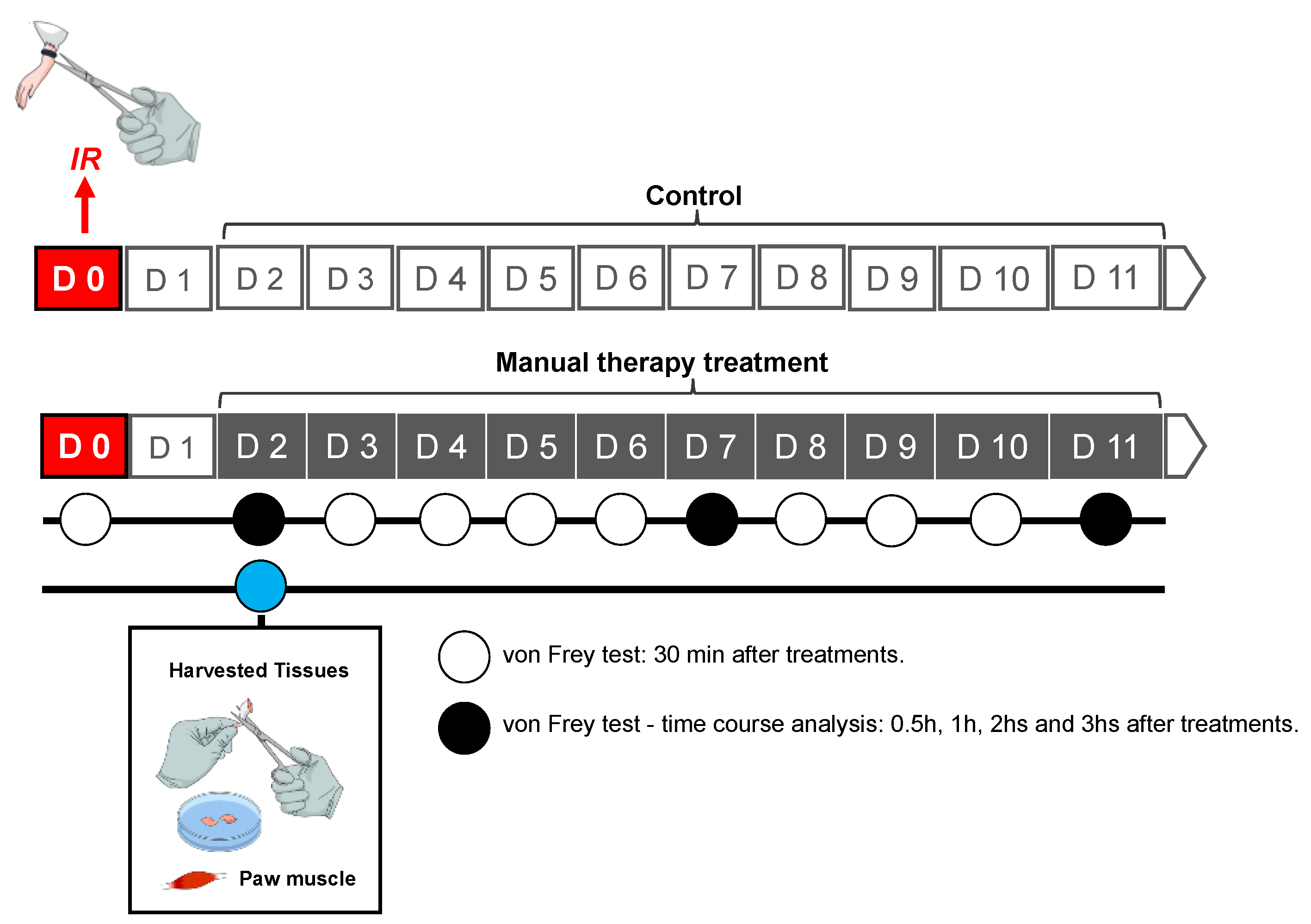
2.2. Animal Model CRPS-I
2.3. MT Treatment
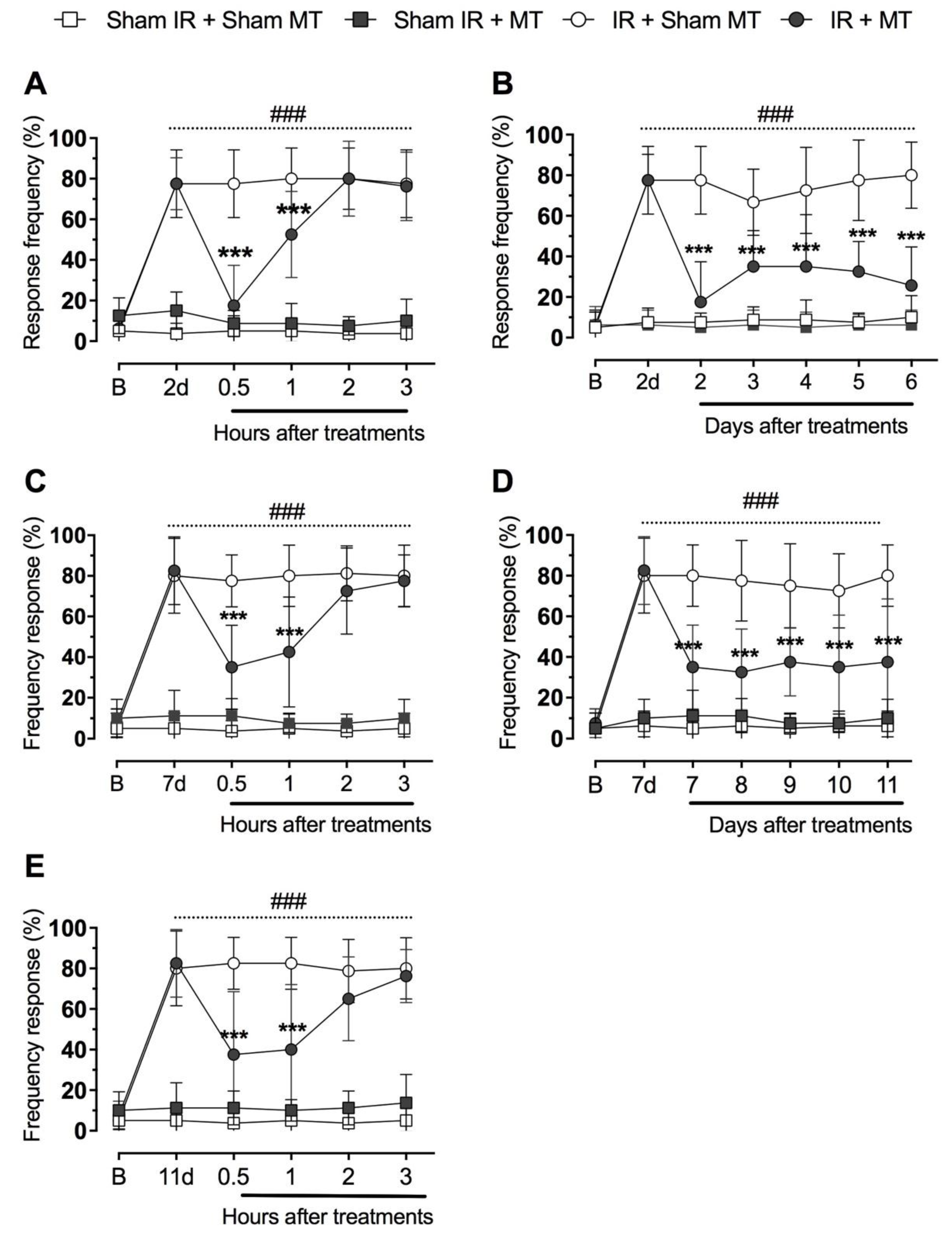
2.4. Mechanical Hyperalgesia
2.5. Sample Collection for Biochemical Analyses
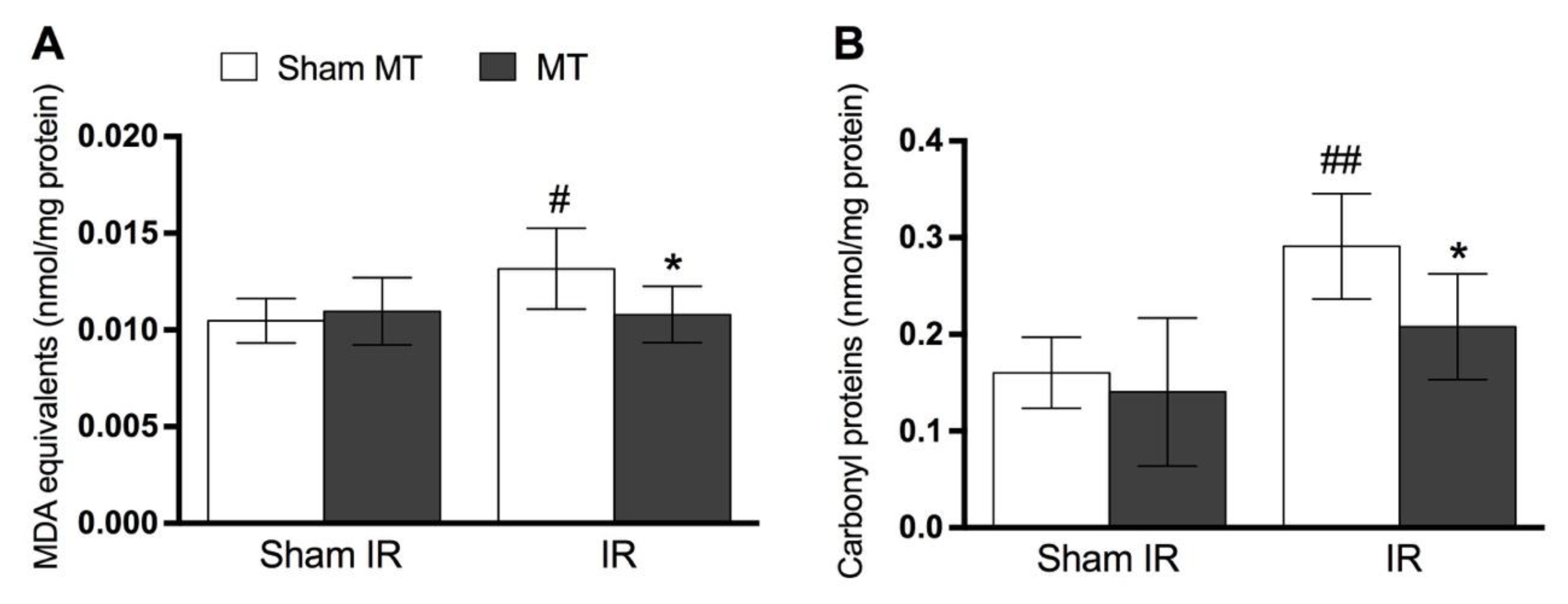
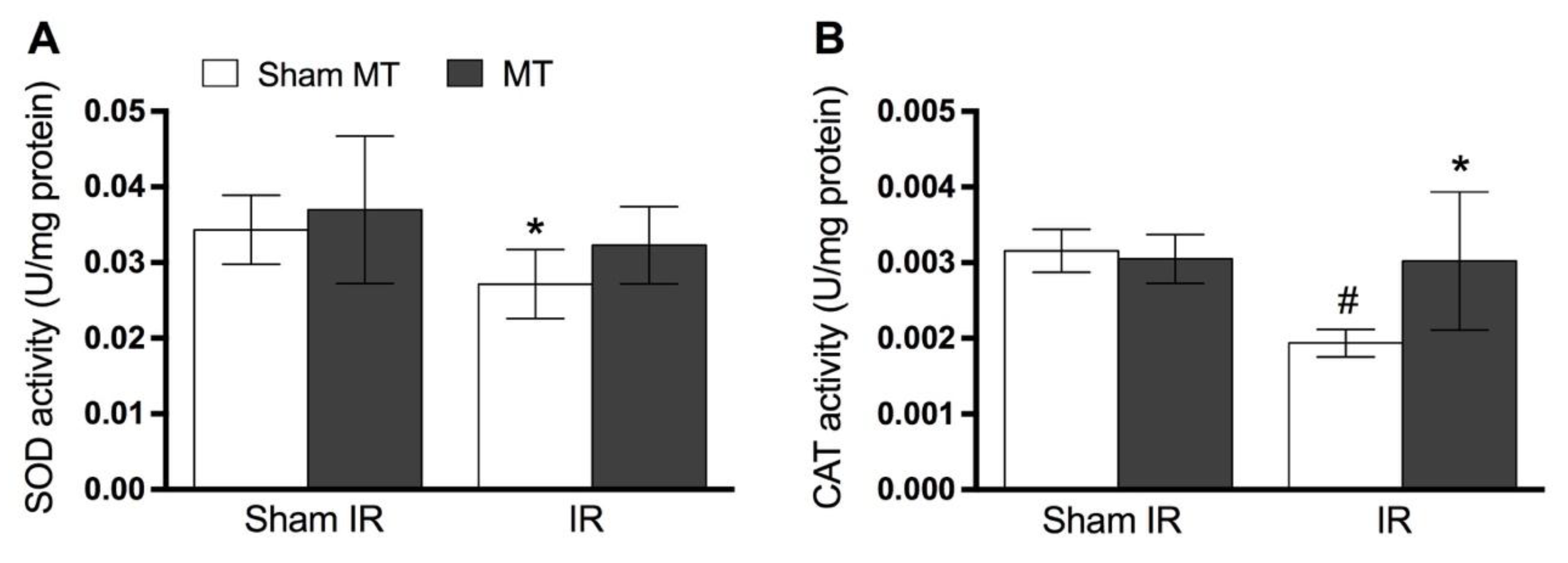
2.6. Determination of Oxidative Stress and Antioxidant Enzymes Levels
2.7. Mitochondrial Function Analyses
2.8. Statistical Analysis
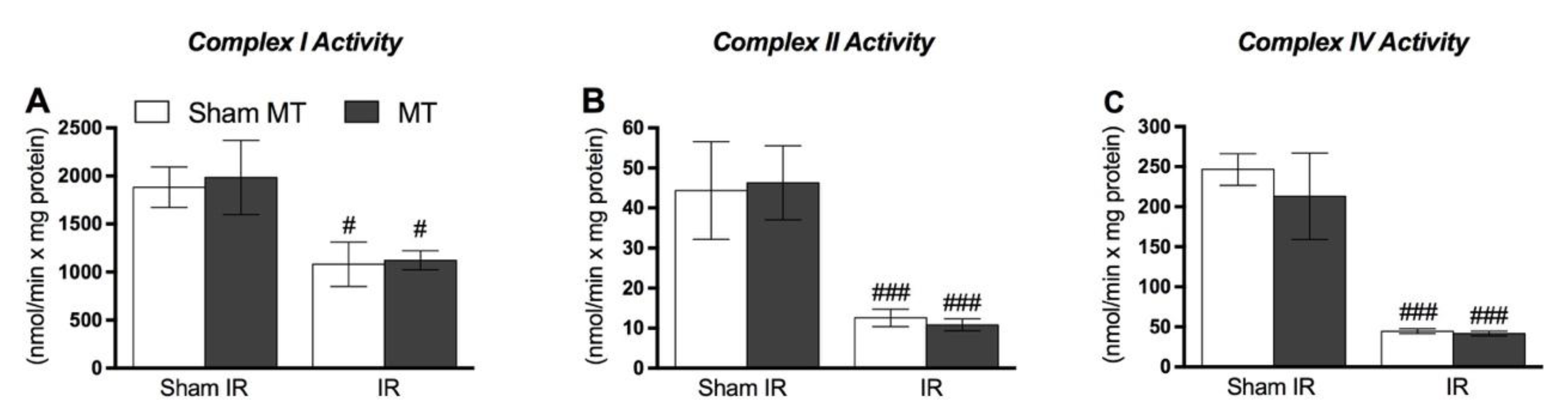
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coderre, T.J.; Xanthos, D.N.; Francis, L.; Bennett, G.J. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 2004, 112, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.F.; Mazzardo-Martins, L.; Cidral-Filho, F.J.; Stramosk, J.; Santos, A.R.S. Ankle joint mobilization affects postoperative pain through peripheral and central adenosine A1 receptors. Phys. Ther. 2013, 93, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Lawand, N.B.; McNearney, T.; Westlund, K.N. Amino acid release into the knee joint: Key role in nociception and inflammation. Pain 2000, 86, 69–74. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, Y.; Choi, J.; Jeon, S.; Kim, D.; Jeong, H.; Lee, Y.; Park, H. Antiallodynic Effects of Bee Venom in an Animal Model of Complex Regional Pain Syndrome Type 1 (CRPS-I). Toxins 2017, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Birklein, F.; Schmelz, M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci. Lett. 2008, 437, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Kingery, W.S. Role of neuropeptide, cytokine, and growth factor signaling in complex regional pain syndrome. Pain Med. 2010, 11, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Maihöfner, C.; Seifert, F.; Markovic, K. Complex regional pain syndromes: New pathophysiological concepts and therapies. Eur. J. Neurol. 2010, 17, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Enax-Krumova, E.K.; Lenz, M.; Frettlöh, J.; Höffken, O.; Reinersmann, A.; Schwarzer, A.; Westermann, A.; Tegenthoff, M.; Maier, C. Changes of the sensory abnormalities and cortical excitability in patients with complex regional pain syndrome of the upper extremity after 6 months of multimodal treatment. Pain Med. 2017, 18, 95–106. [Google Scholar] [CrossRef][Green Version]
- Goris, R.J.; Dongen, L.M.; Winters, H.A. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic. Res. Commun. 1987, 3, 13–18. [Google Scholar] [CrossRef]
- Eisenberg, E.; Shtahl, S.; Geller, R.; Reznick, A.Z.; Sharf, O.; Ravbinovich, M.; Erenreich, A.; Nagler, R.M. Serum and salivary oxidative analysis in Complex Regional Pain Syndrome. Pain 2008, 138, 226–232. [Google Scholar] [CrossRef]
- Millecamps, M.; Laferrire, A.; Ragavendran, J.V.; Stone, L.S.; Coderre, T.J. Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I). Pain 2010, 151, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.M.; D’sa, A.B.; Parks, T.G.; McCaigue, M.D.; Leggett, P.; Halliday, M.I.; Rowlands, B.J. Lower limb ischaemia-reperfusion injury alters gastrointestinal structure and function. Br. J. Surg. 1997, 84, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Besse, J.L.; Gadeyne, S.; Galand-Desmé, S.; Lerat, J.L.; Moyen, B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009, 15, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, P.E.; Tuinebreijer, W.E.; Breederveld, R.S.; Kreis, R.W. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J. Bone Jt. Surg. Am. 2007, 89, 1424–1431. [Google Scholar] [CrossRef]
- Oerlemans, H.M.; Goris, J.; De Boo, T.; Oostendorp, R. Do physical therapy and occupational therapy reduce the impairment percentage in reflex sympathetic dystrophy? Am. J. Phys. Med. Rehab. 1999, 78, 533–539. [Google Scholar] [CrossRef]
- Smith, T.O. How effective is physiotherapy in the treatment of complex regional pain syndrome type I? A review of the literature. Musculoskelet. Care 2005, 3, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Van de Meent, H.; Oerlemans, M.; Bruggeman, A.; Klomp, F.; Van Dongen, R.; Frolke, J.P. Safety of “pain exposure” physical therapy in patients with complex regional pain syndrome type 1. Pain 2011, 152, 1431–1438. [Google Scholar] [CrossRef]
- Sherry, D.D.; Wallace, C.A.; Kelley, C.; Kidder, M.; Sapp, L. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin. J. Pain 1999, 15, 218–223. [Google Scholar] [CrossRef]
- Moss, P.; Sluka, K.; Wright, A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man. Ther. 2007, 12, 109–118. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man. Ther. 2010, 14, 531–538. [Google Scholar] [CrossRef]
- Camarinos, J.; Marinko, L. Effectiveness of manual physical therapy for painful shoulder conditions: A systematic review. J. Man. Manip. Ther. 2009, 17, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Walston, Z.; Hernandez, L.; Yake, D. Utilization of manual therapy to the lumbar spine in conjunction with traditional conservative care for individuals with bilateral lower extremity complex regional pain syndrome: A case series. Physiother. Theory Pract. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Kolberg, C.; Horst, A.; Kolberg, A.; Belló-Klein, A.; Partata, W.A. Effects of High-Velocity, Low-Amplitude Manipulation on Catalase Activity in Men with Neck Pain. J. Manip. Physiol. Ther. 2010, 33, 300–307. [Google Scholar] [CrossRef]
- Martins, D.F.; Bobinski, F.; Mazzardo-Martins, L.; Cidral-filho, F.J.; Nascimento, F.P.; Gadotti, V.M. Ankle Joint Mobilization Decreases Hypersensitivity by Activation of Peripheral Opioid Receptors in a Mouse Model of Postoperative Pain. Pain Med. 2012, 13, 1049–1058. [Google Scholar] [CrossRef]
- Martins, D.F.; Mazzardo-Martins, L.; Cidral-Filho, F.J.; Gadotti, V.M.; Santos, A.R.S. peripheral and spinal activation of cannabinoid receptors by joint mobilization alleviates postoperative pain in mice. Neurosc. 2013, 255, 110–121. [Google Scholar] [CrossRef]
- Solin, A.V.; Liashev, I.D. Opioid peptides effect on lipid peroxidation in long-term stress. Ross. Fiziol. Zhurnal Sechenova 2012, 98, 1016–1020. [Google Scholar]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Raborn, E.S.; Marciano-Cabral, F.; Buckley, N.E.; Martin, B.R.; Cabral, G.A. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: Linkage to the CB2 receptor. J. NeuroImmune Pharmacol. 2008, 3, 117–129. [Google Scholar] [CrossRef]
- Sufianova, G.Z.; Sufianova, A.A.; Shapkin, A.G. Effect of cyclopentyladenosine on lipid peroxidation during focal cerebral ischemia. Bull. Exp. Biol. Med. 2014, 157, 228–230. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Martins, D.F.; Mazzardo-Martins, L.; Soldi, F.; Stramosk, J.; Piovezan, A.P.; Santos, A.R.S. High-intensity swimming exercise reduces neuropathic pain in an animal model of complex regional pain syndrome type I: Evidence for a role of the adenosinergic system. Neuroscience 2013, 234, 69–76. [Google Scholar] [CrossRef]
- Nucci, C.; Mazzardo-Martins, L.; Stramosk, J.; Brethanha, L.C.; Pizzolatti, M.G.; Santos, A.R.; Martins, D.F. Oleaginous extract from the fruits Pterodon pubescens Benth induces antinociception in animal models of acute and chronic pain. J. Ethnopharmacol. 2012, 143, 170–178. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bannister, W.H.; Bannister, J.V. Factor Analysis of the Activities of Superoxide Dismutase, Catalase and Glutathione Peroxidase in Normal Tissues and Neoplastic Cell Lines. Free Radic. Res. Commun. 1987, 4, 1–13. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Cassina, A.; Radi, R. Differential Inhibitory Action of Nitric Oxide and Peroxynitrite on Mitochondrial Electron Transport. Arch. Biochem. Biophys. 1996, 328, 309–316. [Google Scholar] [CrossRef]
- Fischer, J.C.; Ruitenbeek, W.; Berden, J.A.; Trijbels, J.F.; Veerkamp, J.H.; Stadhouders, A.M.; Sengers, R.C.; Janssen, A.J. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta 1985, 153, 23–36. [Google Scholar] [CrossRef]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gerard, B.; Rötig, A.; Saudubray, J.M.; Munnich, A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef]
- Martins, D.F.; Mazzardo-Martins, L.; Gadotti, V.M.; Nascimento, F.P.; Lima, D.A.; Speckhann, B.; Favretto, G.A.; Bobinski, F.; Cargnin-Ferreira, E.; Bressan, E.; et al. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain 2011, 152, 2653–2661. [Google Scholar] [CrossRef]
- Kellogg, E.W.; Fridovich, I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J. Biol. Chem. 1975, 250, 8812–8817. [Google Scholar]
- McCord, J.M. Oxygen-derived radicals: A link between reperfusion injury and inflammation. Fed. Proc. 1987, 46, 2402–2406. [Google Scholar]
- Inauen, W.; Suzuki, M.; Granger, D.N. Mechanisms of cellular injury: Potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc. Endothel. Lymphat. 1989, 5, 143–155. [Google Scholar]
- Partrick, D.A.; Moore, F.A.; Moore, E.E.; Barnett, C.C.; Silliman, C.C. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996, 4, 194–210. [Google Scholar]
- Millecamps, M.; Coderre, T.J. Rats with chronic post-ischemia pain exhibit an analgesic sensitivity profile similar to human patients with complex regional pain syndrome—Type, I. Eur. J. Pharmacol. 2008, 583, 97–102. [Google Scholar] [CrossRef][Green Version]
- Montecucco, F.; Burger, F. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, 1145–1155. [Google Scholar] [CrossRef]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef]
- Boison, D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008, 8, 2–7. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Boemer, G.; Rial, D.; Cordova, F.M.; Mancini, G.; Walz, R.; De Bem, A.F.; Latini, A.; Leal, R.B.; Pinho, R.A.; et al. High-intensity physical exercise disrupts implicit memory in mice: Involvement of the striatal glutathione antioxidant system and intracellular signaling. Neuroscience 2010, 171, 1216–1227. [Google Scholar] [CrossRef]
- Reichelt, M.E.; Shanu, A.; Willems, L.; Witting, P.K.; Ellis, N.A.; Blackburn, M.R.; Headrick, J.P. Endogenous adenosine selectively modulates oxidant stress via the A1 receptor in ischemic hearts. Antioxid. Redox Signal. 2009, 11, 2641–2650. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, A.S.I.; Stramosk, J.; Ludtke, D.D.; Kuci, A.C.C.; Salm, D.C.; Ceci, L.A.; Petronilho, F.; Florentino, D.; Danielski, L.G.; Gassenferth, A.; et al. Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I. Brain Sci. 2019, 9, 197. https://doi.org/10.3390/brainsci9080197
Salgado ASI, Stramosk J, Ludtke DD, Kuci ACC, Salm DC, Ceci LA, Petronilho F, Florentino D, Danielski LG, Gassenferth A, et al. Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I. Brain Sciences. 2019; 9(8):197. https://doi.org/10.3390/brainsci9080197
Chicago/Turabian StyleSalgado, Afonso S. I., Juliana Stramosk, Daniela D. Ludtke, Ana C. C. Kuci, Daiana C. Salm, Lisandro A. Ceci, Fabricia Petronilho, Drielly Florentino, Lucineia G. Danielski, Aline Gassenferth, and et al. 2019. "Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I" Brain Sciences 9, no. 8: 197. https://doi.org/10.3390/brainsci9080197
APA StyleSalgado, A. S. I., Stramosk, J., Ludtke, D. D., Kuci, A. C. C., Salm, D. C., Ceci, L. A., Petronilho, F., Florentino, D., Danielski, L. G., Gassenferth, A., Souza, L. R., Rezin, G. T., Santos, A. R. S., Mazzardo-Martins, L., Reed, W. R., & Martins, D. F. (2019). Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I. Brain Sciences, 9(8), 197. https://doi.org/10.3390/brainsci9080197




