Activation of Membrane Estrogen Receptors Attenuates NOP-Mediated Tactile Antihypersensitivity in a Rodent Model of Neuropathic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Implantation of Cannulae
2.3. Spared Nerve Injury
2.4. Paw Withdrawal Assay
2.5. Drugs
2.6. Immunoblotting
2.7. Data Analysis
3. Results
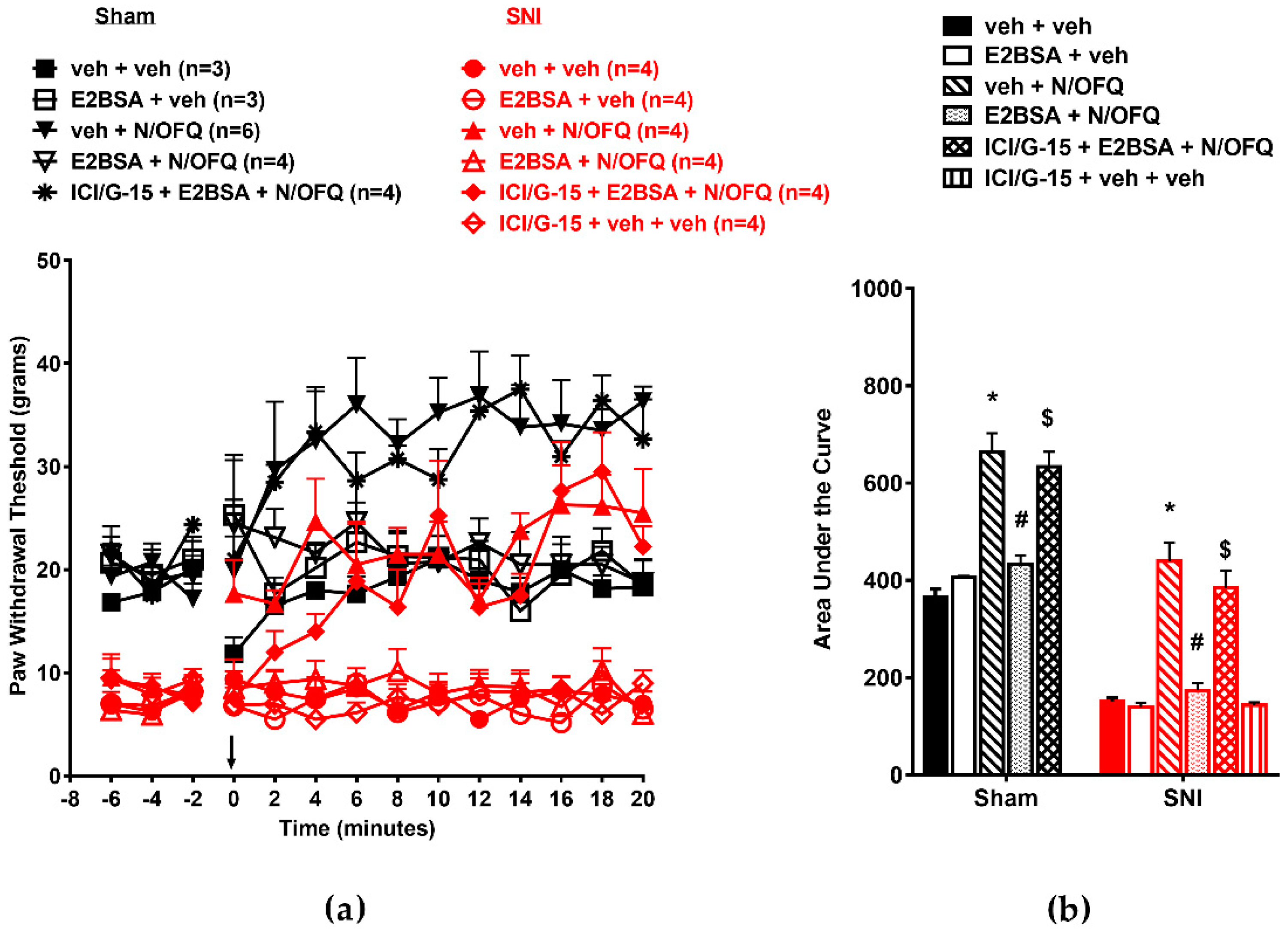
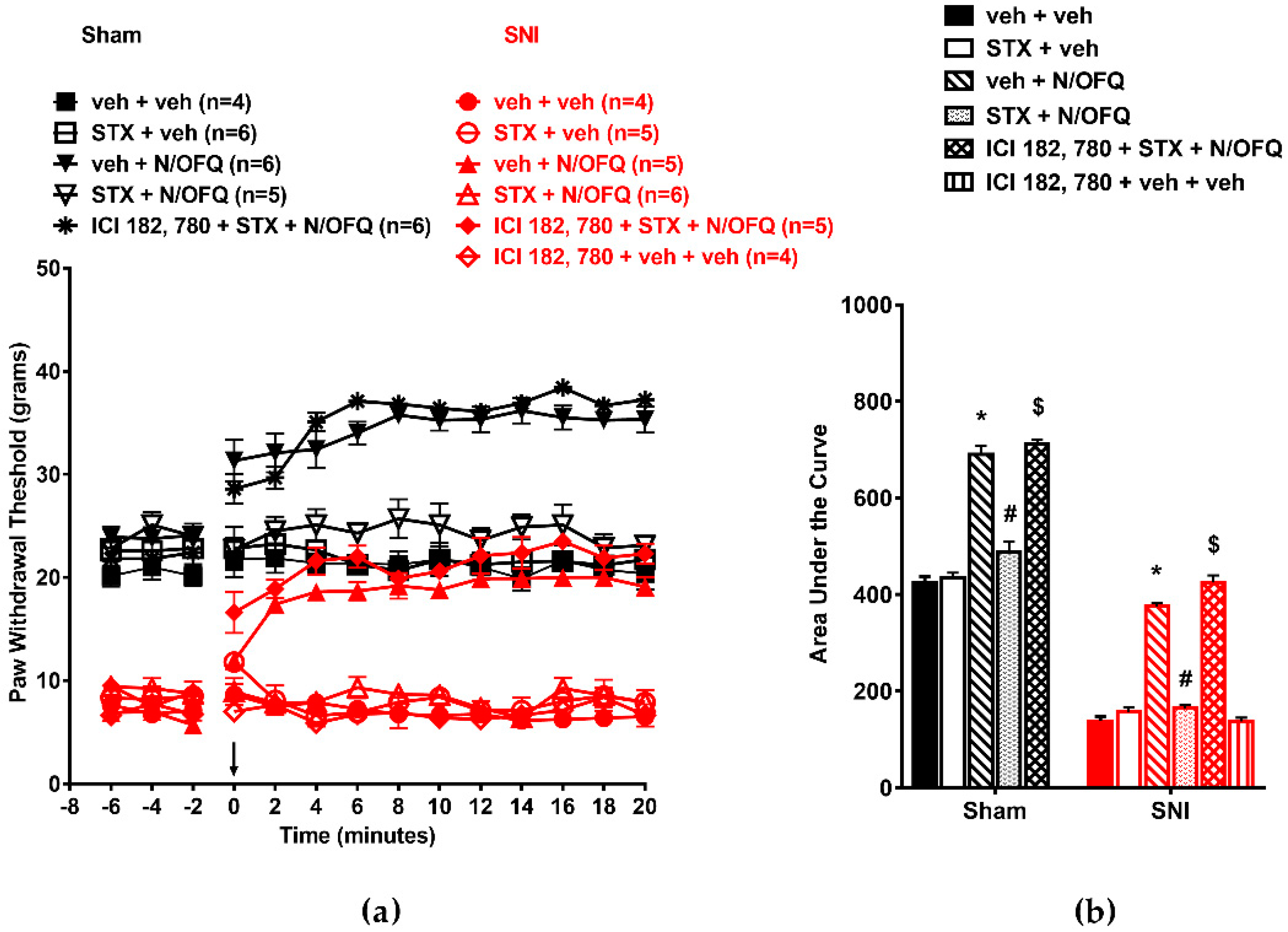
3.1. N/OFQ Reversesd Tactile Hypersensitivity following SNI, and E2-BSA Rapidly Attenuated the Effect of N/OFQ
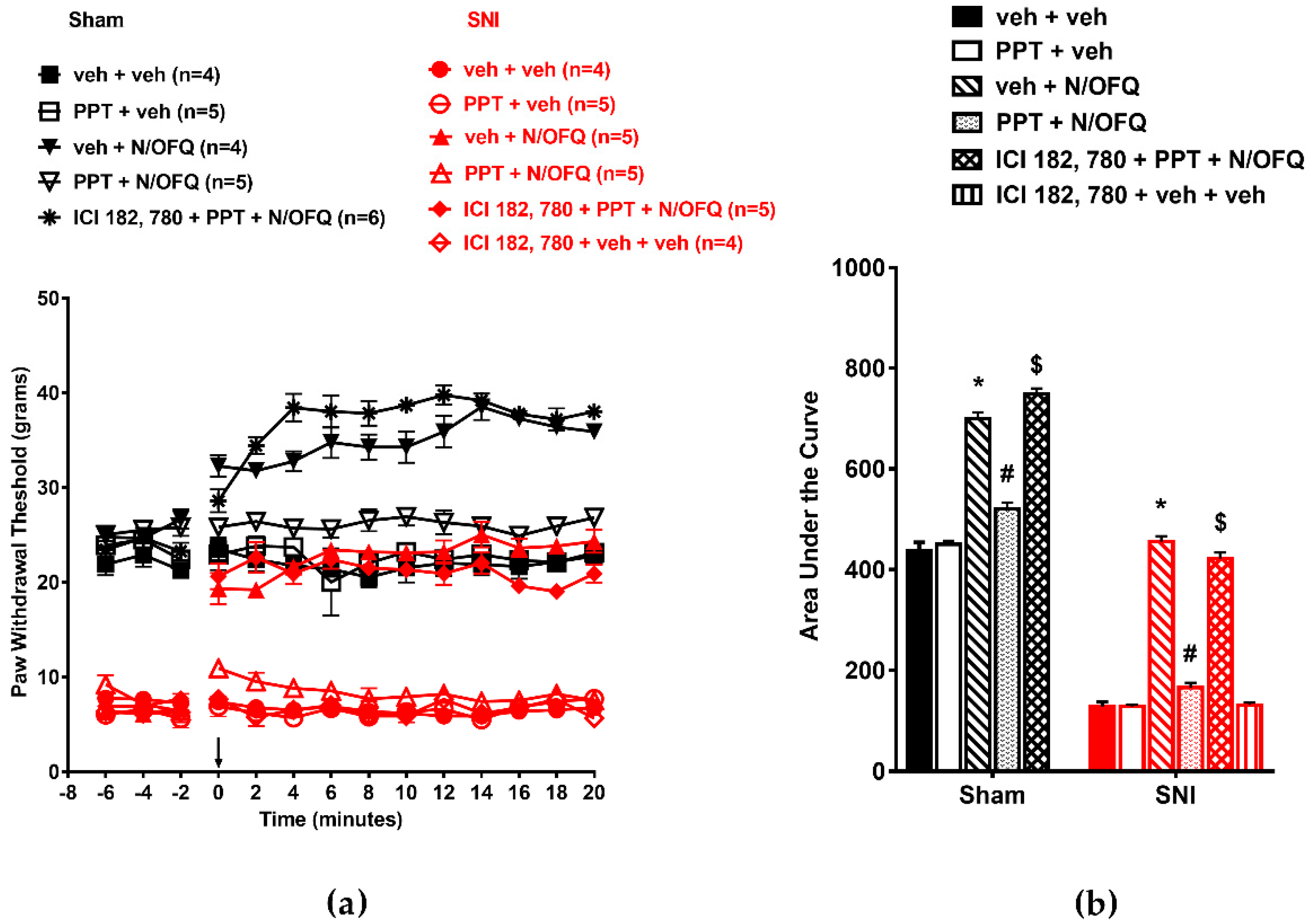
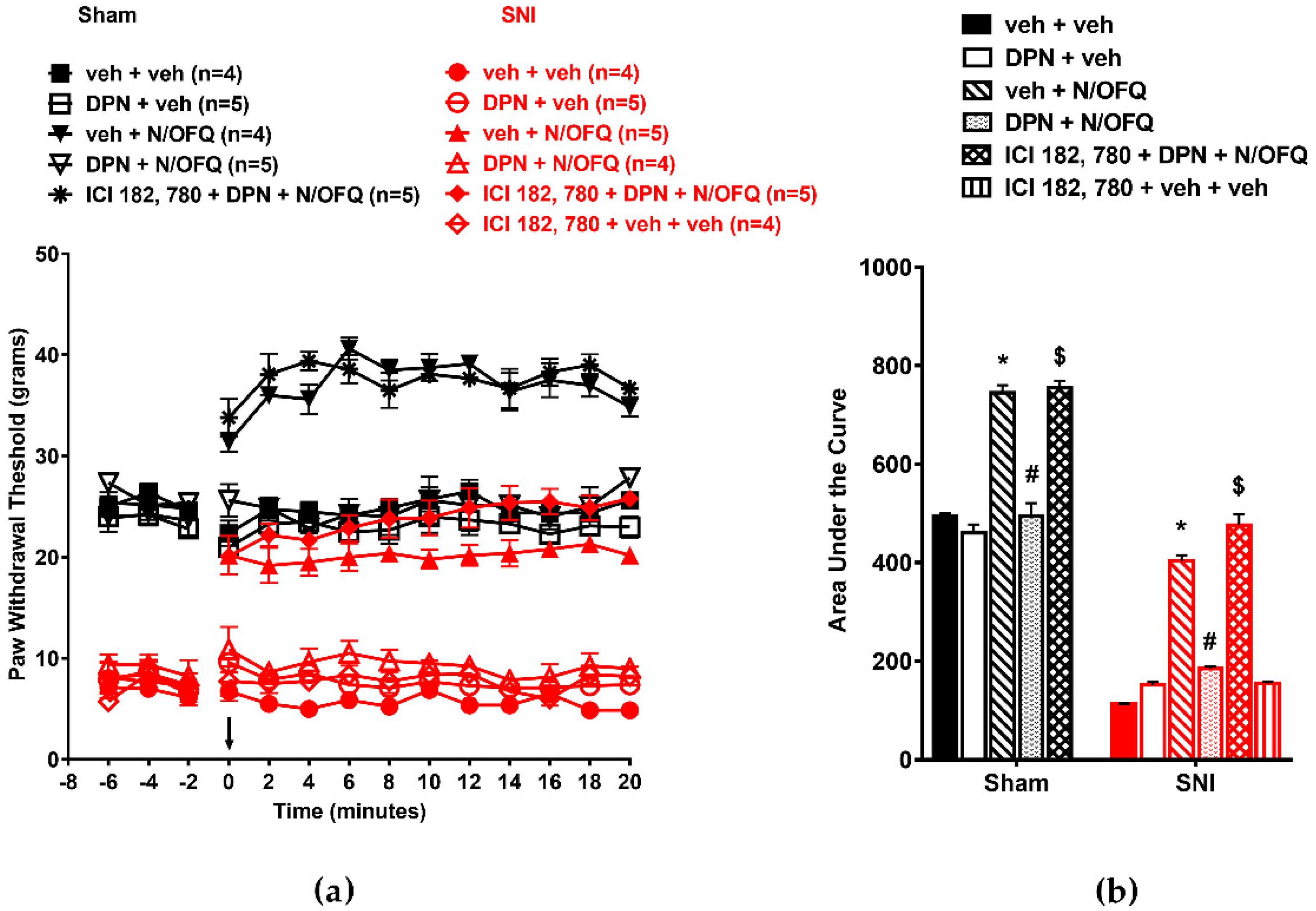
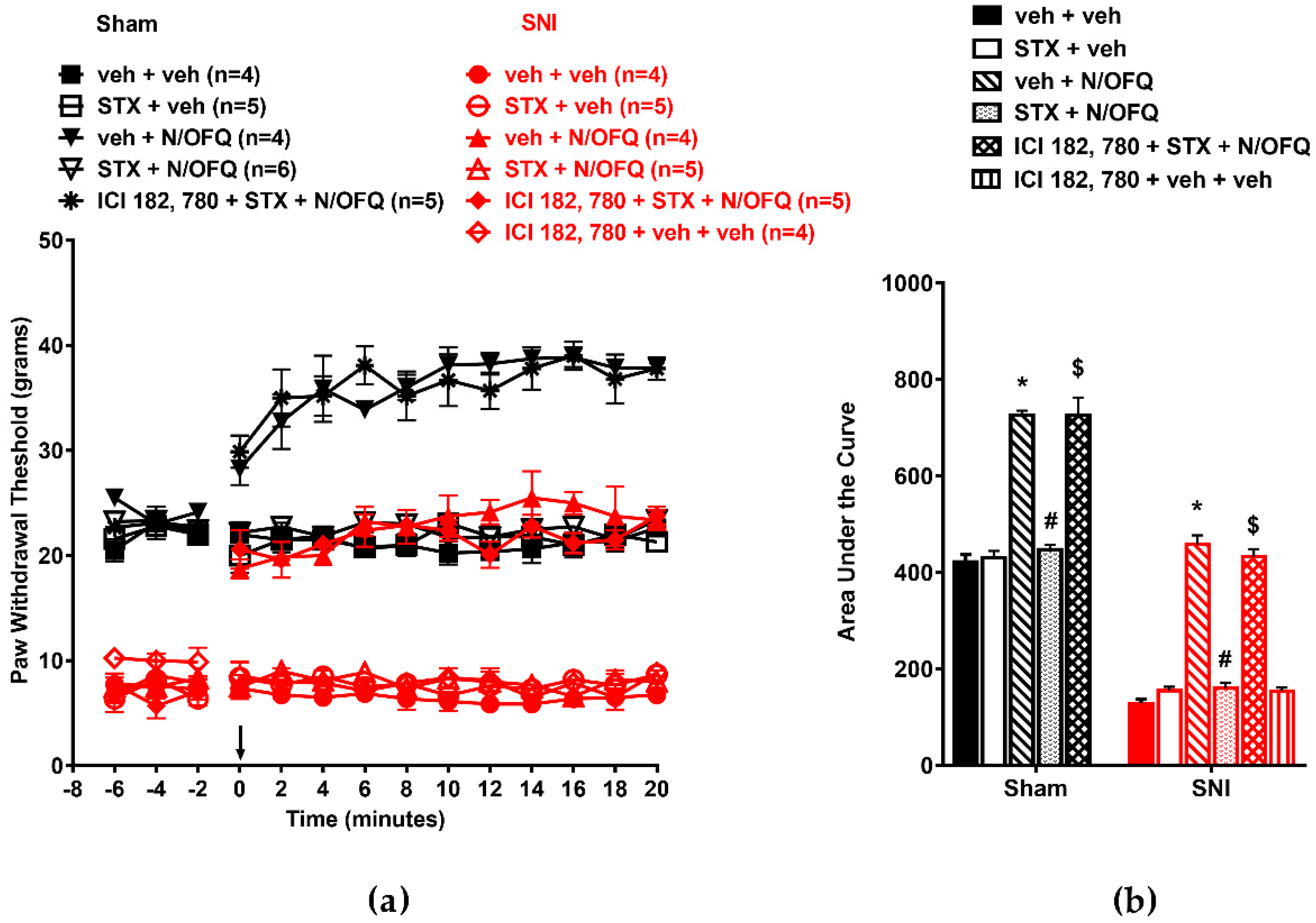
3.2. Selective Activation of ERα Rapidly Attenuated NOP-Mediated Tactile Antihypersensitivity
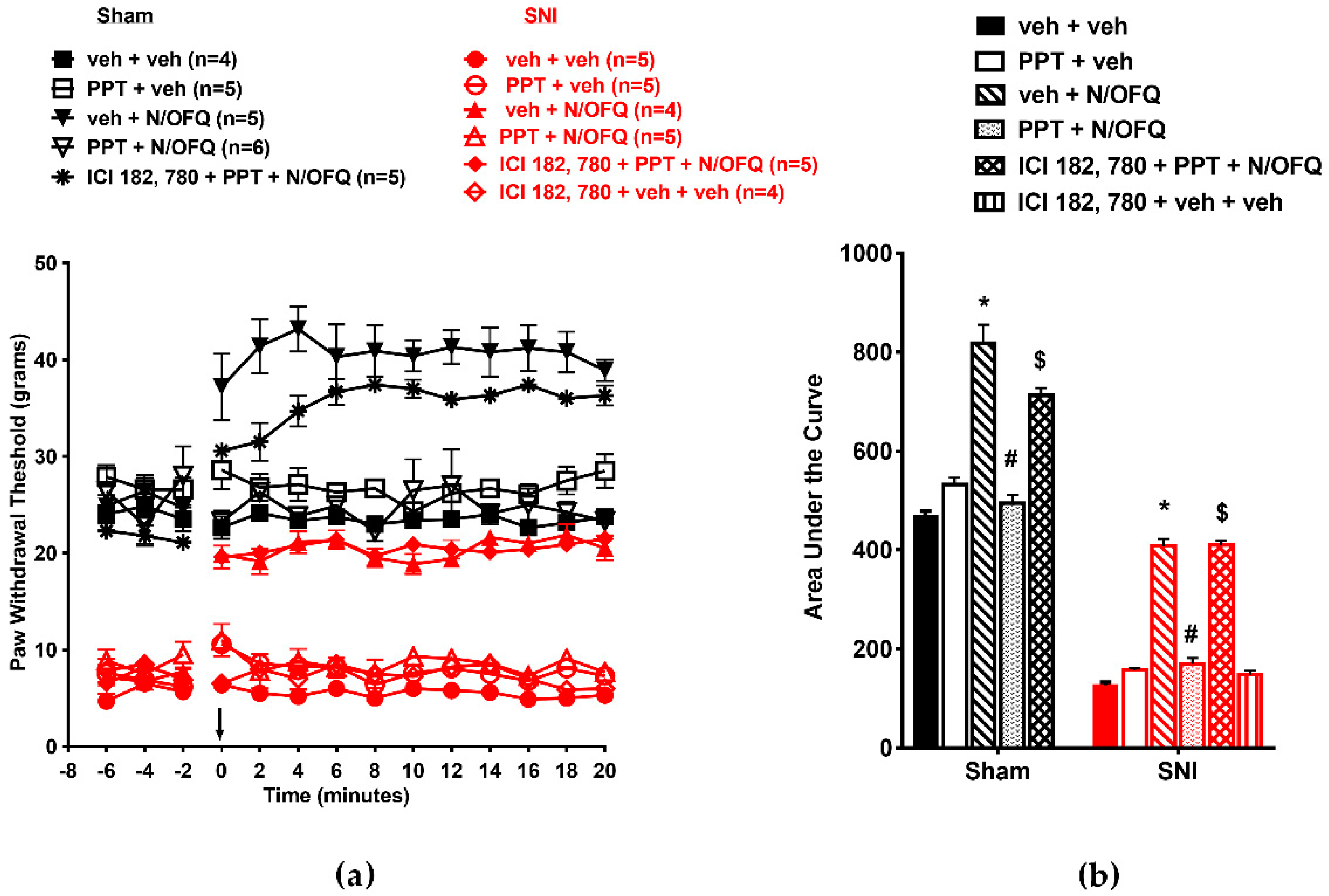
3.3. Selective Activation of ERβ Rapidly Abolished the Effect of N/OFQ
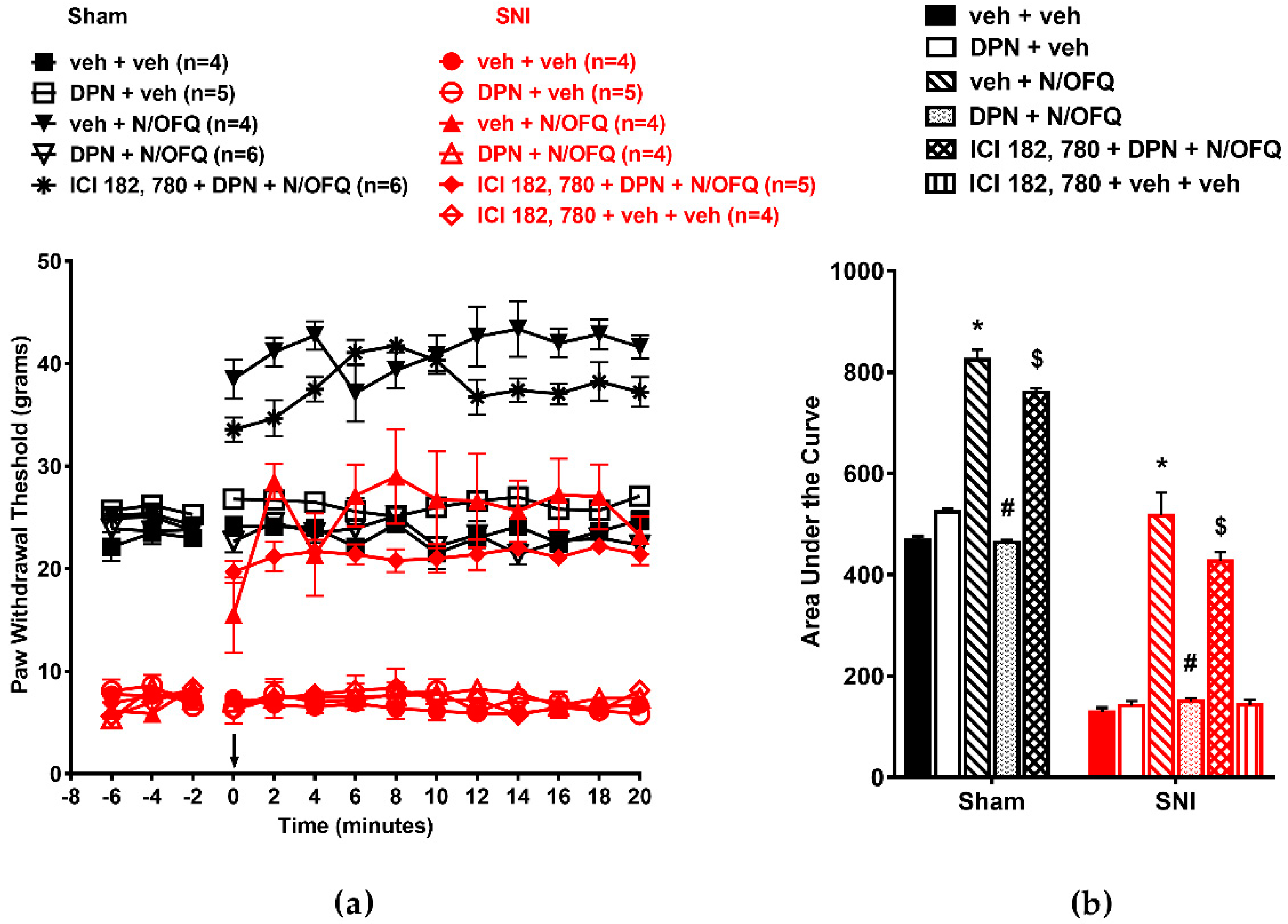
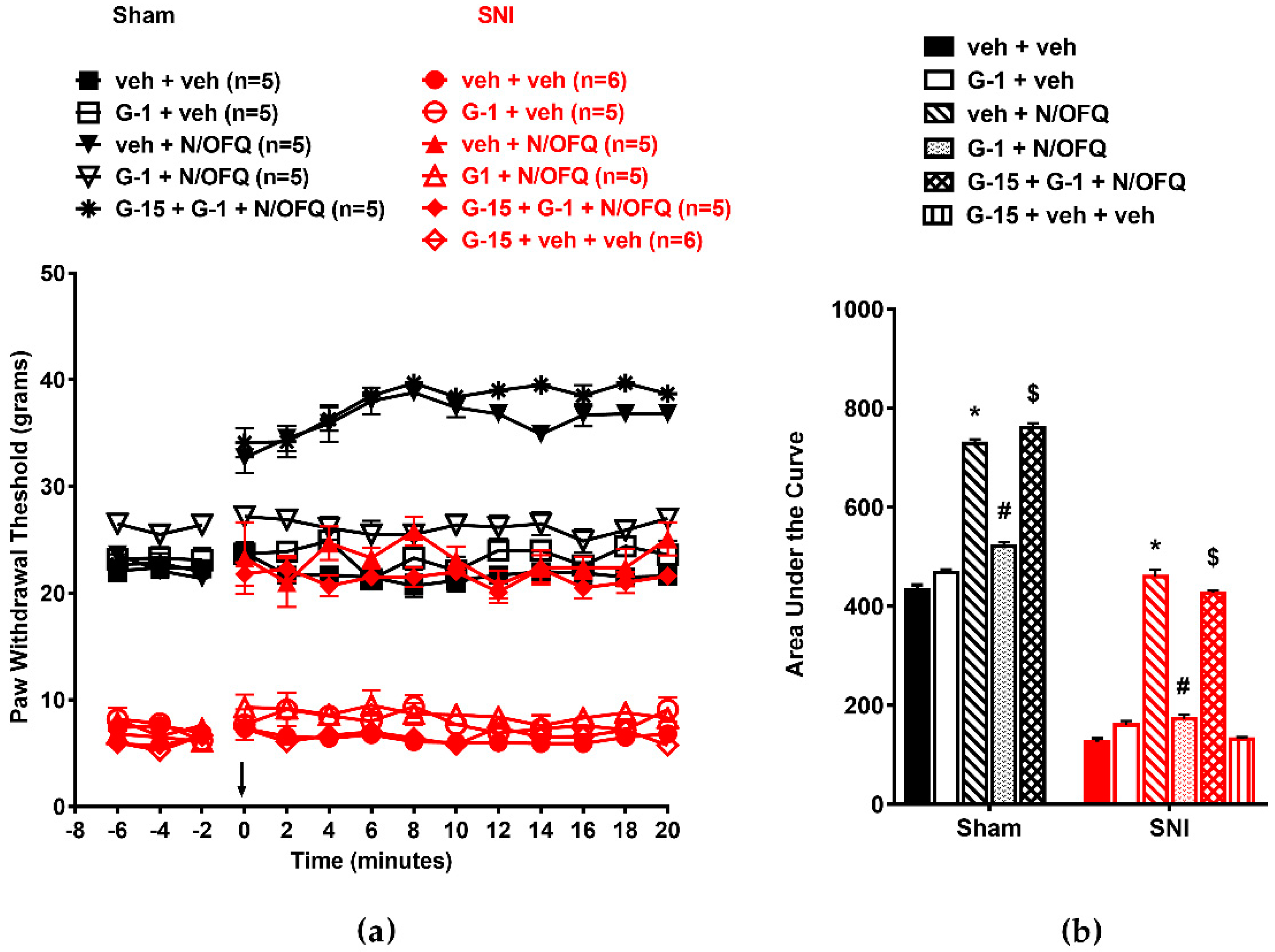
3.4. Selective Activation of GPR30 Rapidly Attenuated the Effect of N/OFQ
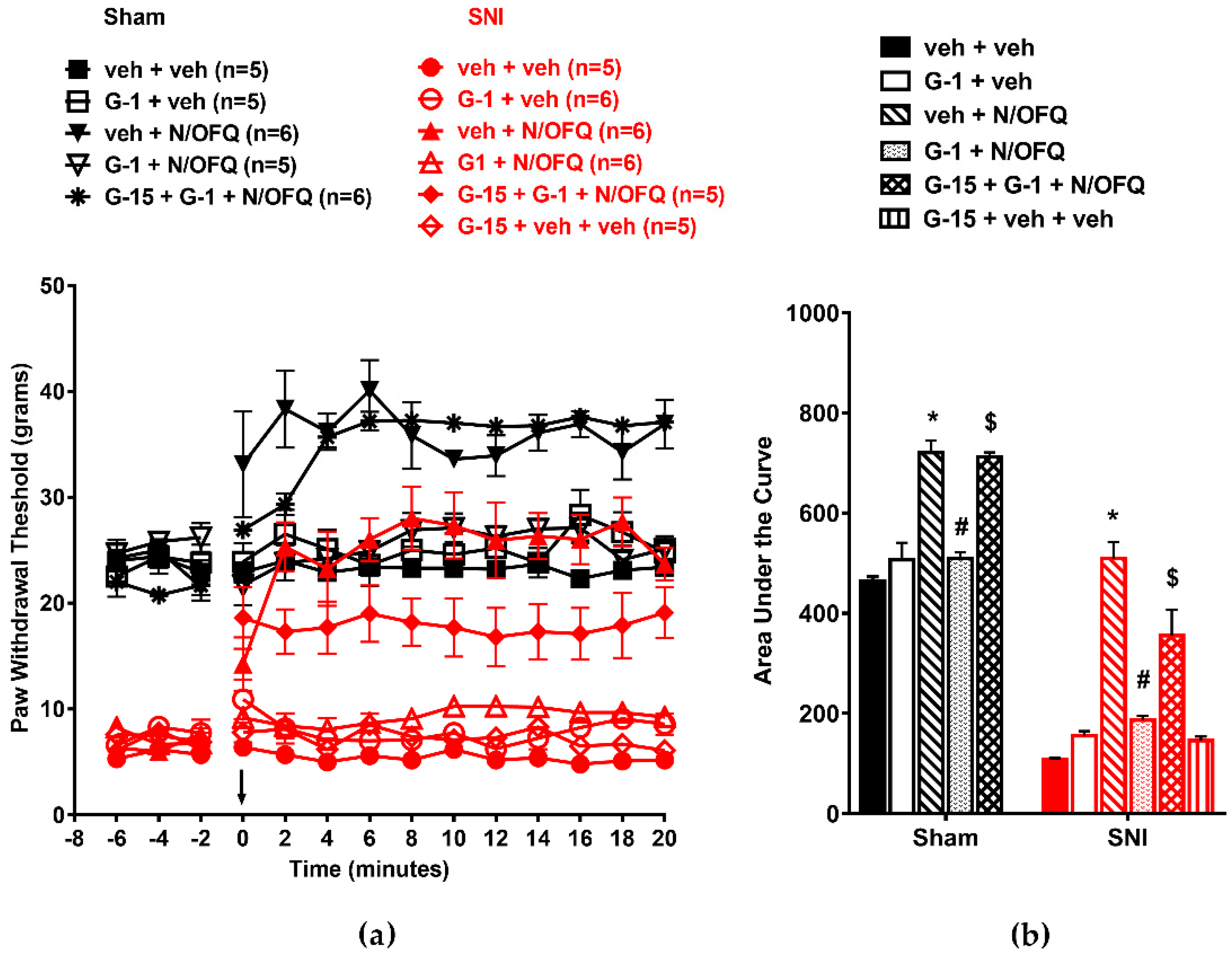
3.5. Selective Activation of Gq-mER Rapidly Abolished the Effects of N/OFQ
3.6. Activation of mERs Attenuated NOP-Mediated Tactile Antihypersensitivity via an ERK-, PKA-, PKC-, and Akt- Independent Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edlund, M.J.; Martin, B.C.; Fan, M.-Y.; Devries, A.; Braden, J.B.; Sullivan, M.D. Risks for Opioid Abuse and Dependence Among Recipients of Chronic Opioid Therapy: Results from the TROUP Study. Drug Alcohol Depend. 2010, 112, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Saez, C.; Mortrud, M.; Bouvier, C.; Williams, J.T.; Low, M.; Grandy, D.K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994, 347, 284–288. [Google Scholar] [CrossRef]
- Mollereau, C.; Parmentier, M.; Mailleux, P.; Butour, J.-L.; Moisand, C.; Chalon, P.; Caput, D.; Vassart, G.; Meunier, J.-C. ORL1, a novel member of the opioid receptor family. FEBS Lett. 1994, 341, 33–38. [Google Scholar] [CrossRef]
- Lutfy, K.; Cowan, A. Buprenorphine: A Unique Drug with Complex Pharmacology. Curr. Neuropharmacol. 2004, 2, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.-C.; Naughton, N.N. Antinociceptive Effects of Nociceptin/Orphanin FQ Administered Intrathecally in Monkeys. J. Pain 2009, 10, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Ko, M.C. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem. Neurosci. 2013, 4, 214–224. [Google Scholar] [CrossRef]
- Houtani, T.; Nishi, M.; Takeshima, H.; Sato, K.; Sakuma, S.; Kakimoto, S.; Ueyama, T.; Noda, T.; Sugimoto, T. Distribution of nociceptin/orphanin FQ precursor protein and receptor in brain and spinal cord: A study using in situ hybridization and X-gal histochemistry in receptor-deficient mice. J. Comp. Neurol. 2000, 424, 489–508. [Google Scholar] [CrossRef]
- Ikeda, K.; Kobayashi, K.; Kobayashi, T.; Ichikawa, T.; Kumanishi, T.; Kishida, H.; Yano, R.; Manabe, T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 1997, 45, 117–126. [Google Scholar] [CrossRef]
- Schroeder, R.A.; Brandes, J.; Buse, D.C.; Calhoun, A.; Eikermann-Haerter, K.; Golden, K.; Halker, R.; Kempner, J.; Maleki, N.; Moriarty, M.; et al. Sex and Gender Differences in Migraine—Evaluating Knowledge Gaps. J. Womens Health (Larchmt) 2018, 27, 965–973. [Google Scholar] [CrossRef]
- Berkley, K.J. Sex differences in pain. Behav. Brain Sci. 1997, 20, 371–380. [Google Scholar] [CrossRef]
- Bi, R.-Y.; Ding, Y.; Gan, Y.-H. A new hypothesis of sex-differences in temporomandibular disorders: Estrogen enhances hyperalgesia of inflamed TMJ through modulating voltage-gated sodium channel 1.7 in trigeminal ganglion? Med. Hypotheses 2015, 84, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995, 38, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, J.; Nag, S.; Mokha, S.S. Activation of Opioid Receptor Like-1 Receptor in the Spinal Cord Produces Sex-Specific Antinociception in the Rat: Estrogen Attenuates Antinociception in the Female, whereas Testosterone Is Required for the Expression of Antinociception in the Male. J. Neurosci. 2006, 26, 13048–13053. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M.; Ulibarri, C.; Leitl, M.D.; Sumner, J.E. Dose- and time-dependent estradiol modulation of morphine antinociception in adult female rats. Eur. J. Pain 2008, 12, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.P.; Nag, S.; Thompson, A.D.; Mokha, S.S. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain 2010, 151, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-J.; Chakrabarti, S.; Schnell, S.; Wessendorf, M.; Gintzler, A.R. Spinal Synthesis of Estrogen and Concomitant Signaling by Membrane Estrogen Receptors Regulate Spinal κ- and μ-Opioid Receptor Heterodimerization and Female-Specific Spinal Morphine Antinociception. J. Neurosci. 2011, 31, 11836–11845. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.M.; Traynor, J.R. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J. Pharmacol. Exp. Ther. 2006, 316, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Small, K.M.; Nag, S.; Mokha, S.S. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an ERK-dependent non-genomic mechanism. Neuroscience 2013, 255, 177–190. [Google Scholar] [CrossRef]
- Kelly, M.J.; Qiu, J.; Ronnekleiv, O.K. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. N. Y. Acad. Sci. 2003, 1007, 6–16. [Google Scholar] [CrossRef]
- Nag, S.; Mokha, S.S. Activation of a Gq-coupled membrane estrogen receptor rapidly attenuates α2-adrenoceptor-induced antinociception via an ERK I/II-dependent, non-genomic mechanism in the female rat. Neuroscience 2014, 267, 122–134. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Saleh, M.C.; Connell, B.J.; Saleh, T.M. Medullary and intrathecal injections of 17beta-estradiol in male rats. Brain Res. 2000, 867, 200–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Zhang, X.M.; Zhao, Z.Q.; Zhang, Y.Q. Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity. J. Biol. Chem. 2012, 287, 33268–33281. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Chen, G.-D.; Tung, K.-C.; Chien, Y.-W.; Lai, C.-Y.; Hsieh, M.-C.; Chiu, C.-H.; Lai, C.-H.; Lee, S.-D.; Lin, T.-B. Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. Am. J. Physiol. Metab. 2009, 297, E416–E426. [Google Scholar] [CrossRef] [PubMed]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; A Parker, M.; E Tkachenko, S.; Savchuck, N.P.; A Sklar, L.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Methods 2006, 2, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Bosch, M.A.; Tobias, S.C.; Grandy, D.K.; Scanlan, T.S.; Rønnekleiv, O.K.; Kelly, M.J. Rapid Signaling of Estrogen in Hypothalamic Neurons Involves a Novel G-Protein-Coupled Estrogen Receptor that Activates Protein Kinase C. J. Neurosci. 2003, 23, 9529–9540. [Google Scholar] [CrossRef]
- Qiu, J.; Bosch, M.A.; Tobias, S.C.; Krust, A.; Graham, S.M.; Murphy, S.J.; Korach, K.S.; Chambon, P.; Scanlan, T.S.; Rønnekleiv, O.K.; et al. A G-Protein-Coupled Estrogen Receptor Is Involved in Hypothalamic Control of Energy Homeostasis. J. Neurosci. 2006, 26, 5649–5655. [Google Scholar] [CrossRef]
- Stevis, P.E.; Deecher, D.C.; Suhadolnik, L.; Mallis, L.M.; Frail, D.E. Differential Effects of Estradiol and Estradiol-BSA Conjugates. Endocrinology 1999, 140, 5455–5458. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Honda, K.; Sawada, H.; Kihara, T.; Urushitani, M.; Nakamizo, T.; Akaike, A.; Shimohama, S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J. Neurosci. Res. 2000, 60, 321–327. [Google Scholar] [CrossRef]
- Kelly, M. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 1999, 64, 64–75. [Google Scholar] [CrossRef]
- Kelly, M.J.; Rønnekleiv, O.K. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol. Cell. Endocrinol. 2009, 308, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Setalo, G.; Guan, X.; Warren, M.; Toran-Allerand, C.D. Estrogen-Induced Activation of Mitogen-Activated Protein Kinase in Cerebral Cortical Explants: Convergence of Estrogen and Neurotrophin Signaling Pathways. J. Neurosci. 1999, 19, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Toran-Allerand, C.D. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann. N. Y. Acad. Sci. 2005, 1052, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.B.; Robinson, S.; Shapiro, R.A.; Dorsa, D.M. Estrogen Receptor (ER)α and ERβ Exhibit Unique Pharmacologic Properties When Coupled to Activation of the Mitogen-Activated Protein Kinase Pathway 1. Endocrinology 2001, 142, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.T. Protein kinase signaling cascades in CNS trauma. IUBMB Life 2005, 57, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, F.-X.; Huang, F.; Lu, Y.-J.; Li, G.-D.; Bao, L.; Xiao, H.-S.; Zhang, X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur. J. Neurosci. 2004, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Q.; Liu, S.; He, D.-D.; Liu, Y.-P.; Song, X.-J. Activation of the cAMP-PKA signaling pathway in rat dorsal root ganglion and spinal cord contributes toward induction and maintenance of bone cancer pain. Behav. Pharmacol. 2014, 25, 267–276. [Google Scholar] [CrossRef]
- Calò, G.; Rizzi, A.; Marzola, G.; Guerrini, R.; Salvadori, S.; Beani, L.; Regoli, D.; Bianchi, C. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br. J. Pharmacol. 1998, 125, 373–378. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Nothacker, H.-P.; Bourson, A.; Ardati, A.; Henningsen, R.A.; Bunzow, J.R.; Grandy, D.K.; Langen, H.; Monsma, F.J.; Civelli, O. Orphanin FQ: A Neuropeptide That Activates an Opioidlike G Protein-Coupled Receptor. Science 1995, 270, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Hao, J.X.; Wiesenfeld-Hallin, Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. NeuroReport 1996, 7, 2092–2094. [Google Scholar] [PubMed]
- Chen, Y.; Sommer, C.; Claudia, S. Activation of the nociceptin opioid system in rat sensory neurons produces antinociceptive effects in inflammatory pain: Involvement of inflammatory mediators. J. Neurosci. Res. 2007, 85, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Courteix, C.; Coudoré-Civiale, M.-A.; Privat, A.-M.; Pelissier, T.; Eschalier, A.; Fialip, J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 2004, 110, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Conde, K.; Meza, C.; Kelly, M.J.; Sinchak, K.; Wagner, E.J.; Conde, K. Estradiol rapidly attenuates ORL-1 receptor-mediated inhibition of proopiomelanocortin neurons via Gq-coupled, membrane-initiated signaling. Neuroendocrinology 2016, 103, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L.; Brailoiu, G.C.; Gao, X.; Brailoiu, E.; Arterburn, J.B.; Prossnitz, E.R.; Oprea, T.I.; Dun, N.J. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J. Neurosci. Res. 2009, 87, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Pappas, T.C.; Gametchu, B.; Watson, C.S. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995, 9, 404–410. [Google Scholar] [CrossRef]
- Razandi, M.; Pedram, A.; Greene, G.L.; Levin, E.R. Cell Membrane and Nuclear Estrogen Receptors (ERs) Originate from a Single Transcript: Studies of ERα and ERβ Expressed in Chinese Hamster Ovary Cells. Mol. Endocrinol. 1999, 13, 307–319. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef]
- Levin, E.R. Membrane oestrogen receptor α signalling to cell functions. J. Physiol. 2009, 587, 5019–5023. [Google Scholar] [CrossRef]
- Raz, L.; Khan, M.M.; Mahesh, V.B.; Vadlamudi, R.K.; Brann, D.W. Rapid Estrogen Signaling in the Brain. Neurosignals 2008, 16, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.A.; Qiu, J.; Bosch, M.A.; Rønnekleiv, O.K.; Kelly, M.J. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signaling in the hypothalamus. J. Neuroendocr. 2009, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; Qiu, J.; Wagner, E.J.; Rønnekleiv, O.K. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS). J. Steroid Biochem. Mol. Boil. 2002, 83, 187–193. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen Signaling through the Transmembrane G Protein–Coupled Receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Shughrue, P.; Petersen, S.; Mokha, S. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience 2003, 118, 769–778. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R.; Bland, K.I. Estrogen Action Via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and cAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Thomas, P. Minireview: G Protein-Coupled Estrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef]
- Revankar, C.M. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Revankar, C.M.; Mitchell, H.D.; Field, A.S.; Burai, R.; Corona, C.; Ramesh, C.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. Synthetic Estrogen Derivatives Demonstrate the Functionality of Intracellular GPR30. ACS Chem. Boil. 2007, 2, 536–544. [Google Scholar] [CrossRef]
- Dickson, R.B.; Clark, C.R. Estrogen Receptors in the Male. Arch. Androl. 1981, 7, 205–217. [Google Scholar] [CrossRef] [PubMed][Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, D.M.; Small, K.M.; Nag, S.; Mokha, S.S. Activation of Membrane Estrogen Receptors Attenuates NOP-Mediated Tactile Antihypersensitivity in a Rodent Model of Neuropathic Pain. Brain Sci. 2019, 9, 147. https://doi.org/10.3390/brainsci9060147
Wright DM, Small KM, Nag S, Mokha SS. Activation of Membrane Estrogen Receptors Attenuates NOP-Mediated Tactile Antihypersensitivity in a Rodent Model of Neuropathic Pain. Brain Sciences. 2019; 9(6):147. https://doi.org/10.3390/brainsci9060147
Chicago/Turabian StyleWright, Danyeal M., Keri M. Small, Subodh Nag, and Sukhbir S. Mokha. 2019. "Activation of Membrane Estrogen Receptors Attenuates NOP-Mediated Tactile Antihypersensitivity in a Rodent Model of Neuropathic Pain" Brain Sciences 9, no. 6: 147. https://doi.org/10.3390/brainsci9060147
APA StyleWright, D. M., Small, K. M., Nag, S., & Mokha, S. S. (2019). Activation of Membrane Estrogen Receptors Attenuates NOP-Mediated Tactile Antihypersensitivity in a Rodent Model of Neuropathic Pain. Brain Sciences, 9(6), 147. https://doi.org/10.3390/brainsci9060147




