Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation
Abstract
1. Introduction
2. Materials and Methods
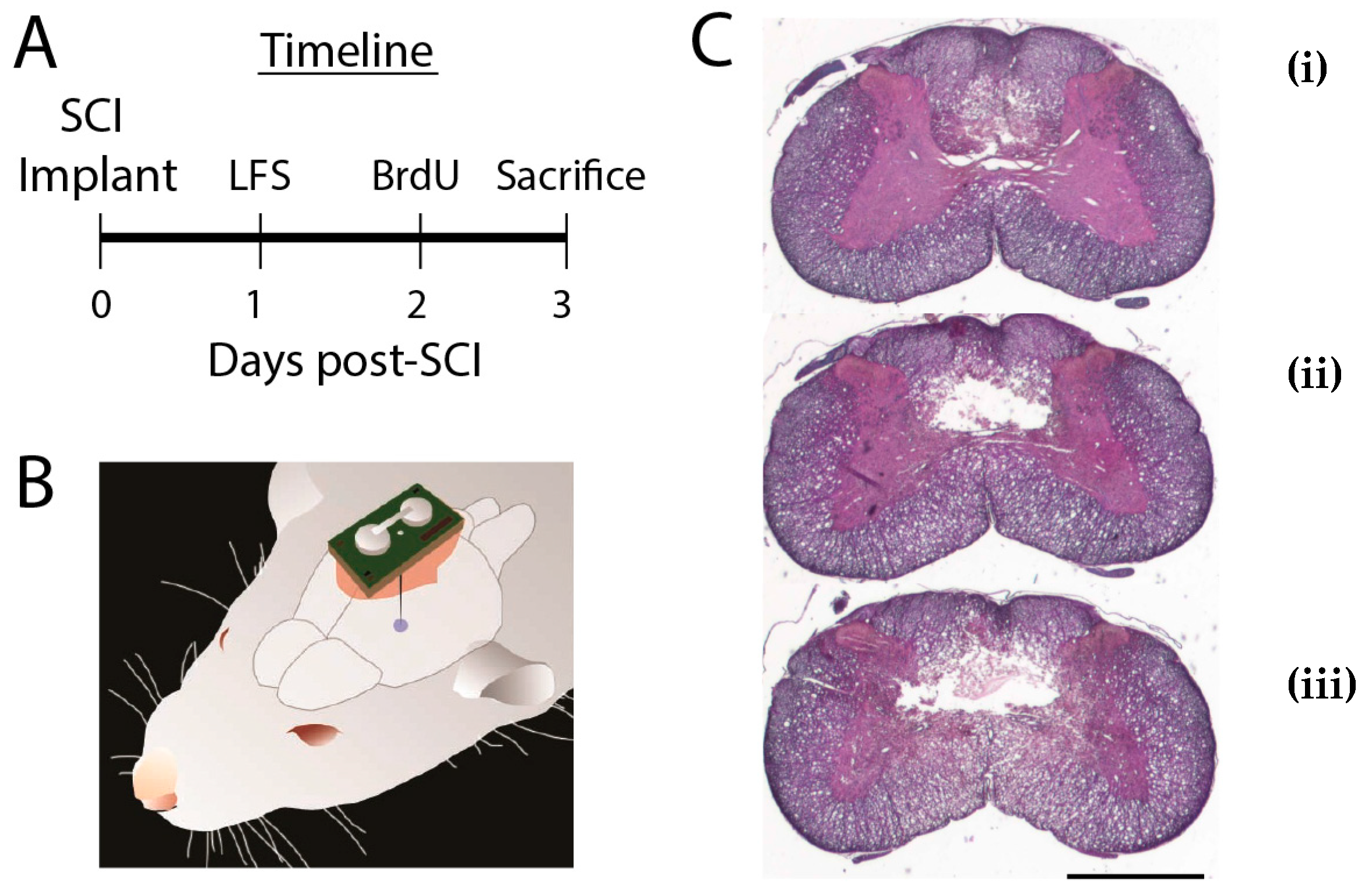
2.1. Animal Procedures
2.2. Stimulators
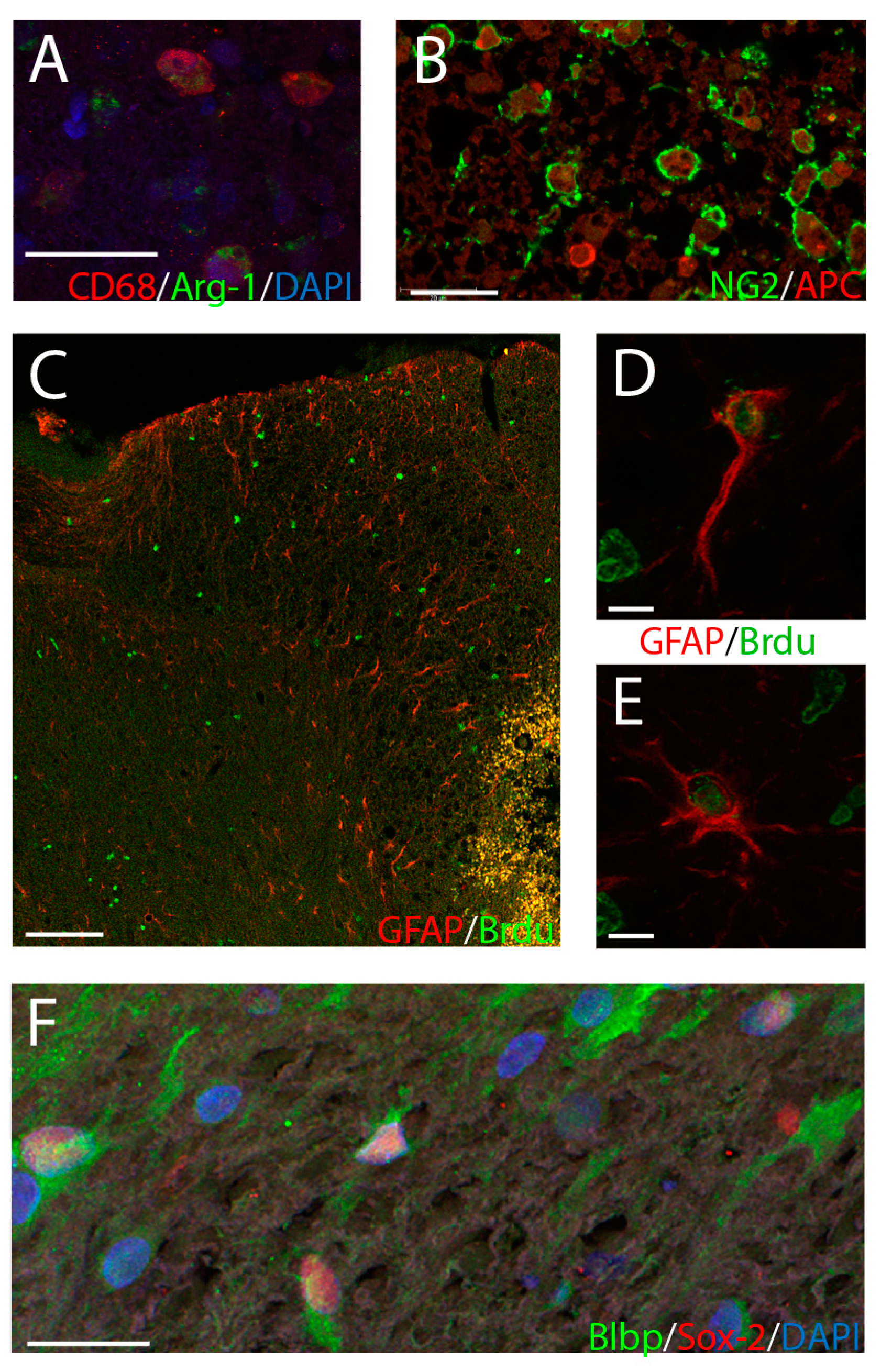
2.3. Histological Analysis
2.4. Statistical Analyses
3. Results
3.1. Did SCI Lesions Differ Between LFS and Control Groups?
3.2. Were Cellular Effects of LFS Specific to the Injury Site?
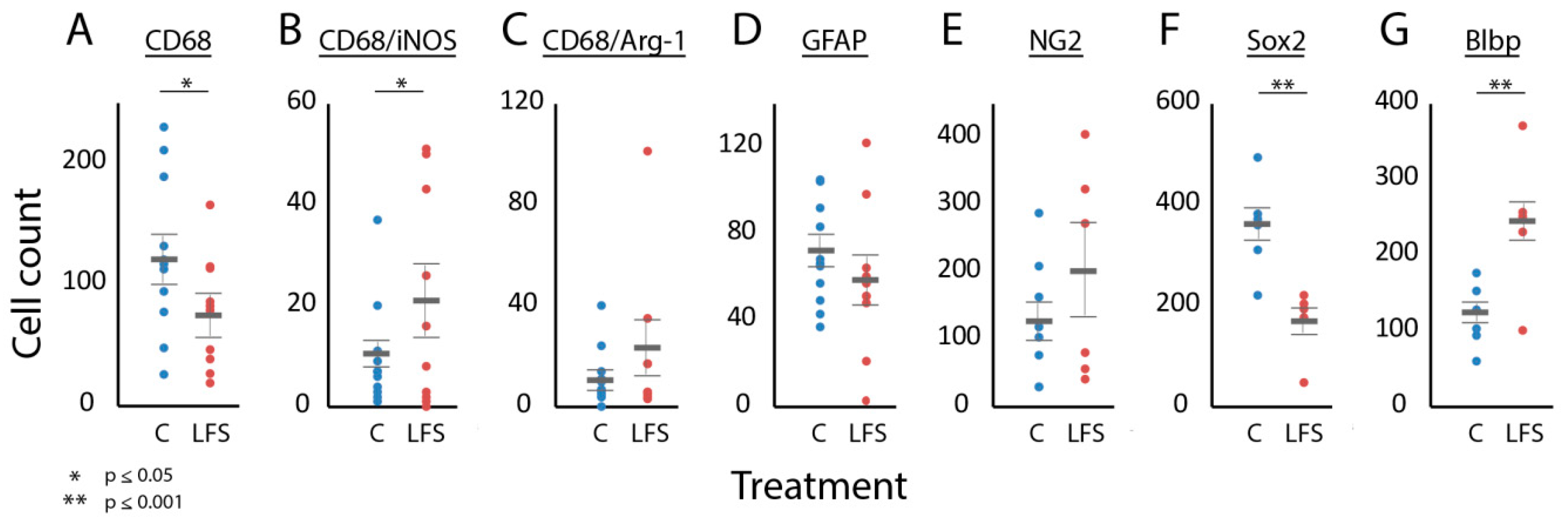
3.3. Was Immune and Neural Progenitor Cell Expression at the Injury Site Influenced by LFS?
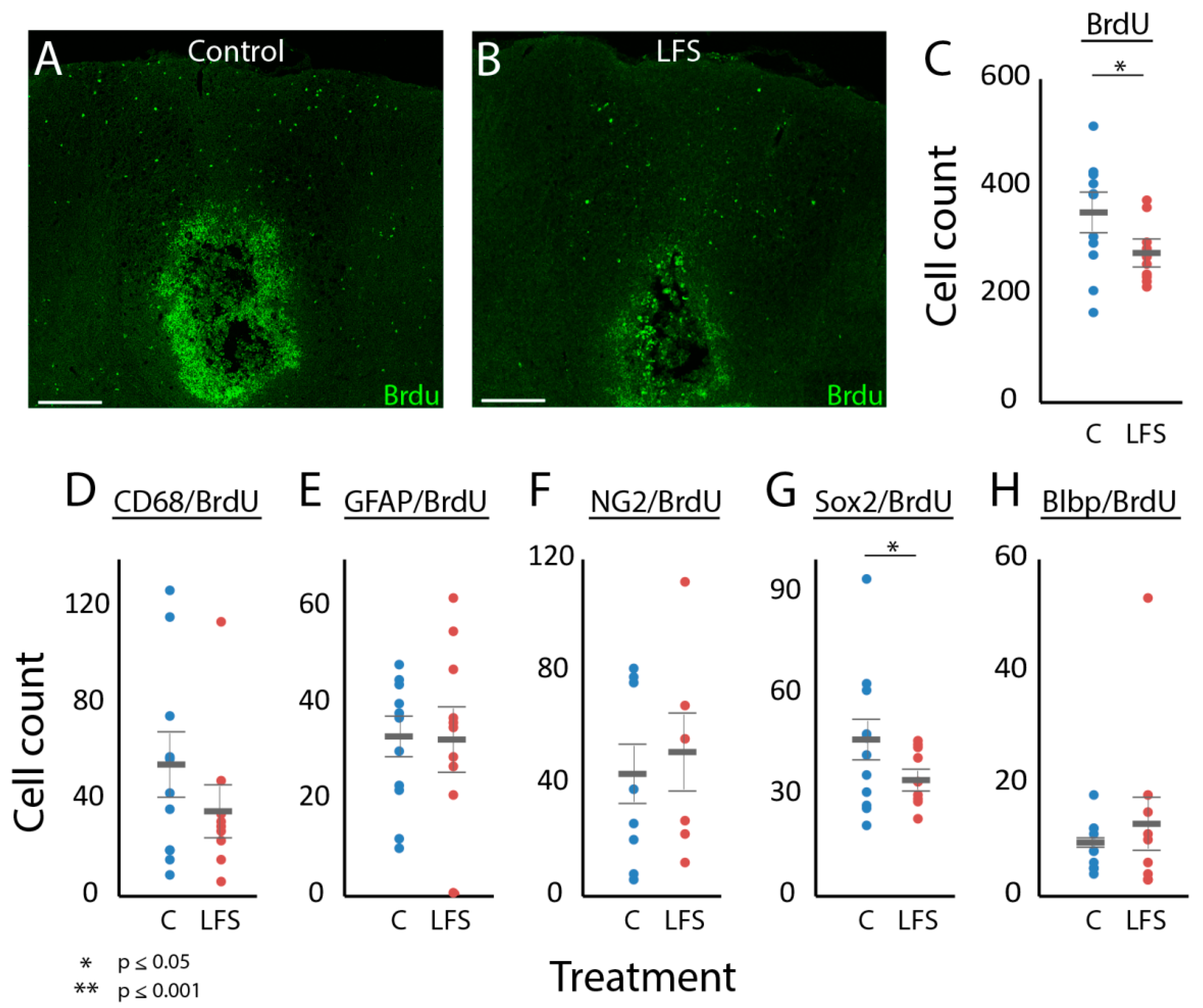
3.4. Was LFS Associated with Changes in Cellular Proliferation?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.M.; Mishra, A.; Yang, P.F.; Wang, F.; Gore, J.C. Injury alters intrinsic functional connectivity within the primate spinal cord. Proc. Natl. Acad. Sci. USA 2015, 112, 5991–5996. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Fermanian, C.; Fermanian, J.; Lanteri-Minet, M.; Alchaar, H.; Bouhassira, D. Neuropathic pain: Are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 2008, 138, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and health status among adults with back and neck problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef]
- Hentall, I.D.; Gonzalez, M.M. Promotion of recovery from thoracic spinal cord contusion in rats by stimulation of medullary raphe or its midbrain input. Neurorehabil. Neural Repair 2012, 26, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Mathews, K.; Gorrie, C.A. Temporal Response of Endogenous Neural Progenitor Cells Following Injury to the Adult Rat Spinal Cord. Front. Cell Neurosci. 2016, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Jermakowicz, W.J.; Hentall, I.D.; Jagid, J.R.; Luca, C.C.; Adcock, J.; Martinez-Arizala, A.; Widerstrom-Noga, E. Deep Brain Stimulation Improves the Symptoms and Sensory Signs of Persistent Central Neuropathic Pain from Spinal Cord Injury: A Case Report. Front. Hum. Neurosci. 2017, 11, 177. [Google Scholar] [CrossRef]
- Keay, K.A.; Bandler, R. Periaqueductal Gray. In The Rat Nervous System, 4th ed.; Elsevier: Waltham, MA, USA, 2015. [Google Scholar]
- Pereira, E.A.; Aziz, T.Z. Neuropathic pain and deep brain stimulation. Neurotherapeutics 2014, 11, 496–507. [Google Scholar] [CrossRef]
- Vasudeva, V.S.; Abd-El-Barr, M.; Chi, J. Lumbosacral spinal cord epidural stimulation enables recovery of voluntary movement after complete motor spinal cord injury. Neurosurgery 2014, 75, N14–N15. [Google Scholar] [CrossRef]
- McPherson, J.G.; Miller, R.R.; Perlmutter, S.I. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc. Natl. Acad. Sci. USA 2015, 112, 12193–12198. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Brus-Ramer, M.; Martin, J.H.; McDonald, J.W. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 2010, 479, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Carballosa Gonzalez, M.M.; Blaya, M.O.; Alonso, O.F.; Bramlett, H.M.; Hentall, I.D. Midbrain raphe stimulation improves behavioral and anatomical recovery from fluid-percussion brain injury. J. Neurotrauma 2013, 30, 119–130. [Google Scholar] [CrossRef]
- Hentall, I.D.; Burns, S.B. Restorative effects of stimulating medullary raphe after spinal cord injury. J. Rehabil. Res. Dev. 2009, 46, 109–122. [Google Scholar] [CrossRef]
- Vitores, A.A.; Sloley, S.S.; Martinez, C.; Carballosa-Gautam, M.M.; Hentall, I.D. Some Autonomic Deficits of Acute or Chronic Cervical Spinal Contusion Reversed by Interim Brainstem Stimulation. J. Neurotrauma 2018, 35, 560–572. [Google Scholar] [CrossRef]
- Madsen, P.M.; Sloley, S.S.; Vitores, A.A.; Carballosa-Gautam, M.M.; Brambilla, R.; Hentall, I.D. Prolonged stimulation of a brainstem raphe region attenuates experimental autoimmune encephalomyelitis. Neuroscience 2017, 346, 395–402. [Google Scholar] [CrossRef][Green Version]
- Chang, Y.W.; Goff, L.A.; Li, H.; Kane-Goldsmith, N.; Tzatzalos, E.; Hart, R.P.; Young, W.; Grumet, M. Rapid induction of genes associated with tissue protection and neural development in contused adult spinal cord after radial glial cell transplantation. J. Neurotrauma 2009, 26, 979–993. [Google Scholar] [CrossRef]
- Carballosa-Gonzalez, M.M.; Vitores, A.; Hentall, I.D. Hindbrain raphe stimulation boosts cyclic adenosine monophosphate and signaling proteins in the injured spinal cord. Brain Res. 2014, 1543, 165–172. [Google Scholar] [CrossRef]
- Hentall, I.D. A long-lasting wireless stimulator for small mammals. Front. Neuroengin. 2013, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Machado, A.G.; Cooperrider, J.; Truong-Furmaga, H.; Johnson, M.; Krishna, V.; Chen, Z.; Gale, J.T. Semi-automated method for estimating lesion volumes. J. Neurosci. Methods 2013, 213, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dong, W.; Xie, P.; Cheng, P.; Bai, S.; Ren, Y.; Wang, G.; Chen, X.; Cui, C.; Zhuang, Y.; et al. The Effect of Pre-Condition Cerebella Fastigial Nucleus Electrical Stimulation within and beyond the Time Window of Thrombolytic on Ischemic Stroke in the Rats. PLoS ONE 2015, 10, e0128447. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Pfeiffer-Jensen, M.; Drewes, A.M.; Farmer, A.D.; Deleuran, B.W.; Stengaard-Pedersen, K.; Brock, B.; Brock, C. Vagal influences in rheumatoid arthritis. Scand. J. Rheumatol. 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Abe, C.; Inoue, T.; Inglis, M.A.; Viar, K.E.; Huang, L.; Ye, H.; Rosin, D.L.; Stornetta, R.L.; Okusa, M.D.; Guyenet, P.G. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 2017, 20, 700–707. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, D.; Saika, F.; Matsuzaki, S.; Kishioka, S. Pharmacological Regulation of Neuropathic Pain Driven by Inflammatory Macrophages. Int. J. Mol. Sci. 2017, 18, 2296. [Google Scholar] [CrossRef]
- Baganz, N.L.; Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Luo, T.; Li, Q. Impaired hypothalamic-pituitary-adrenal axis and its feedback regulation in serotonin transporter knockout mice. Psychoneuroendocrinology 2009, 34, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, G.; Matyash, V.; Pannasch, U.; Mamer, L.; Boddeke, H.W.; Kettenmann, H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav. Immun. 2012, 26, 419–428. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Frisca, F.; Pinto, A.R.; Pebay, A.; Tang, J.K.; Siegel, A.L.; Kaslin, J.; Currie, P.D. Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 2014, 4, 187–200. [Google Scholar] [CrossRef]
- Gomez-Lopez, S.; Wiskow, O.; Favaro, R.; Nicolis, S.K.; Price, D.J.; Pollard, S.M.; Smith, A. Sox2 and Pax6 maintain the proliferative and developmental potential of gliogenic neural stem cells In vitro. Glia 2011, 59, 1588–1599. [Google Scholar] [CrossRef]
- Li, T.; Peng, M.; Yang, Z.; Zhou, X.; Deng, Y.; Jiang, C.; Xiao, M.; Wang, J. 3D-printed IFN-gamma-loading calcium silicate-beta-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater 2018, 71, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Calizo, L.H.; Akanwa, A.; Ma, X.; Pan, Y.Z.; Lemos, J.C.; Craige, C.; Heemstra, L.A.; Beck, S.G. Raphe serotonin neurons are not homogenous: Electrophysiological, morphological and neurochemical evidence. Neuropharmacology 2011, 61, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Son, H. Effects of serotonin on erythropoietin expression in mouse hippocampus. Exp. Neurobiol. 2013, 22, 45–50. [Google Scholar] [CrossRef]
- Arnold, S.A.; Hagg, T. Serotonin 1A receptor agonist increases species- and region-selective adult CNS proliferation, but not through CNTF. Neuropharmacology 2012, 63, 1238–1247. [Google Scholar] [CrossRef]
- Ievins, A.; Moritz, C.T. Therapeutic Stimulation for Restoration of Function After Spinal Cord Injury. Physiology (Bethesda) 2017, 32, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.A.; Hagg, T. Anti-inflammatory treatments during the chronic phase of spinal cord injury improve locomotor function in adult mice. J. Neurotrauma 2011, 28, 1995–2002. [Google Scholar] [CrossRef]
| Marker | Spinal Cord Location | |||||
|---|---|---|---|---|---|---|
| Rostral | Lesion | Caudal | ||||
| Control | LFS | Control | LFS | Control | LFS | |
| BrdU | 135 ± 14 | 147 ± 17 | 587 ± 50 | 519 ± 49 | 237 ± 24 | 209 ± 21 |
| CD68 | 33 ± 8 | 34 ± 7 | 300 ± 39 * | 224 ± 28 * | 90 ± 21 | 74 ± 11 |
| GFAP | 175 ± 19 | 206 ± 31 | 187 ± 29 | 185 ± 29 | 114 ± 20 | 101 ± 23 |
| DWM | GM | VWM | ||||
| Control | LFS | Control | LFS | Control | LFS | |
| BrdU | 105 ± 9 | 88 ± 11 | 239 ± 24 * | 187 ± 16 * | 243 ± 27 | 234 ± 22 |
| CD68 | 61 ± 13 * | 39 ± 8 * | 114 ± 15 * | 71 ± 8 * | 125 ± 11 | 114 ± 12 |
| CD68/iNOS | 4 ± 2 * | 11 ± 4 * | 6 ± 2 | 10 ± 3 | 6 ± 2 | 9 ± 3 |
| CD68/Arg-1 | 5 ± 1 | 9 ± 3 | 8 ± 3 | 13 ± 3 | 8 ± 2 | 14 ± 5 |
| GFAP | 28 ± 6 | 28 ± 7 | 73 ± 11 | 64 ± 10 | 86 ± 12 | 93 ± 12 |
| NG2 | 81 ± 22 * | 149 ± 39 * | 46 ± 9 | 48 ± 10 | 54 ± 10 | 55 ± 15 |
| Sox-2 | 101 ± 16 | 91 ± 13 | 155 ± 22 * | 78 ± 12 * | 128 ± 23 * | 81 ± 17 * |
| Blbp | 68 ± 15 | 102 ± 21 | 51 ± 8 * | 140 ± 25 * | 62 ± 15 | 107 ± 21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermakowicz, W.J.; Sloley, S.S.; Dan, L.; Vitores, A.; Carballosa-Gautam, M.M.; Hentall, I.D. Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation. Brain Sci. 2019, 9, 124. https://doi.org/10.3390/brainsci9060124
Jermakowicz WJ, Sloley SS, Dan L, Vitores A, Carballosa-Gautam MM, Hentall ID. Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation. Brain Sciences. 2019; 9(6):124. https://doi.org/10.3390/brainsci9060124
Chicago/Turabian StyleJermakowicz, Walter J., Stephanie S. Sloley, Lia Dan, Alberto Vitores, Melissa M. Carballosa-Gautam, and Ian D. Hentall. 2019. "Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation" Brain Sciences 9, no. 6: 124. https://doi.org/10.3390/brainsci9060124
APA StyleJermakowicz, W. J., Sloley, S. S., Dan, L., Vitores, A., Carballosa-Gautam, M. M., & Hentall, I. D. (2019). Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation. Brain Sciences, 9(6), 124. https://doi.org/10.3390/brainsci9060124





