Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Apparatus
2.3. Procedure
2.4. Assessment of Behavior
2.5. GAD65/67 Immunostaining
2.6. Quantification
2.7. Gene Expression Analyses
2.8. Statistics
3. Results
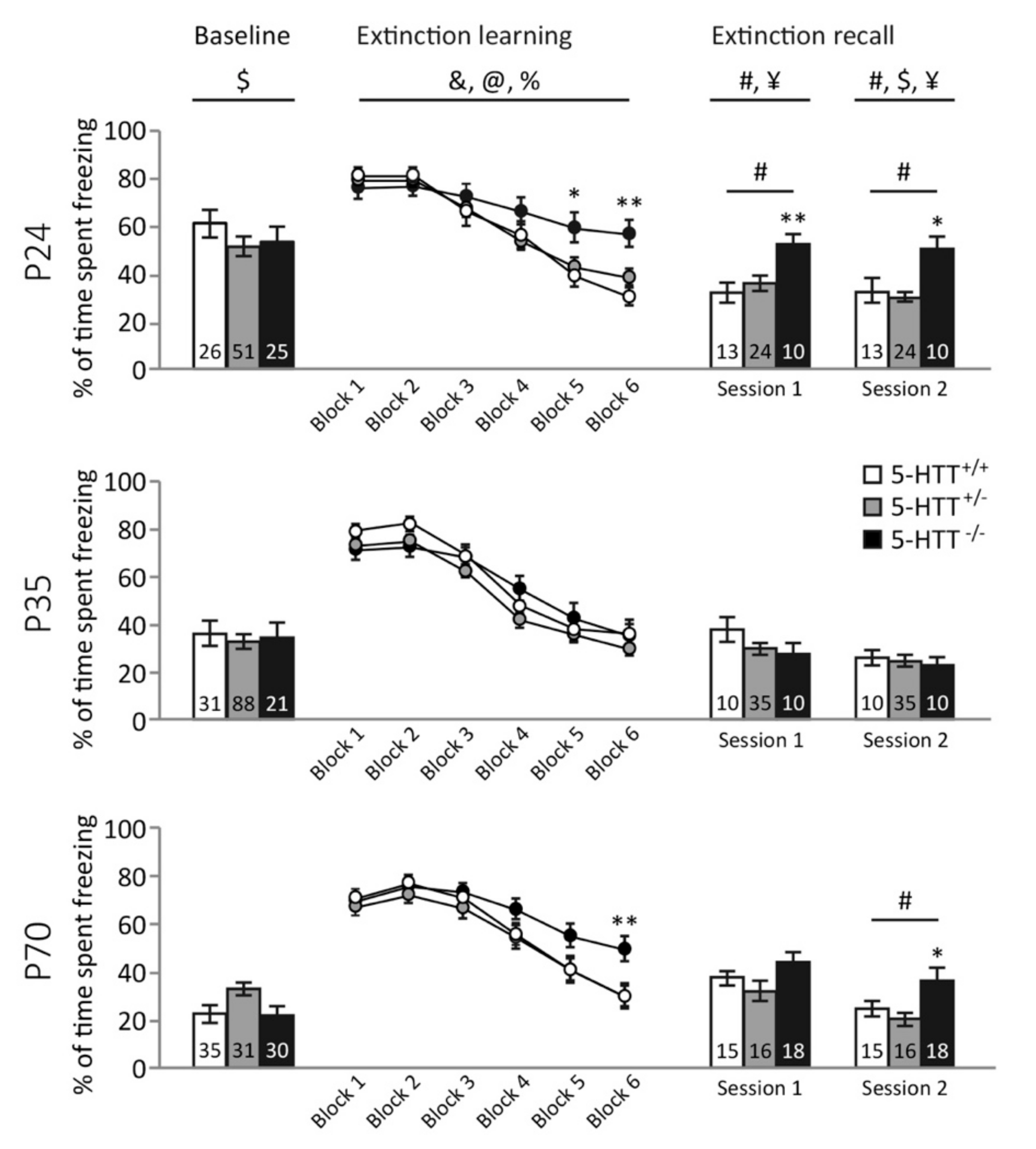
3.1. Freezing Behavior
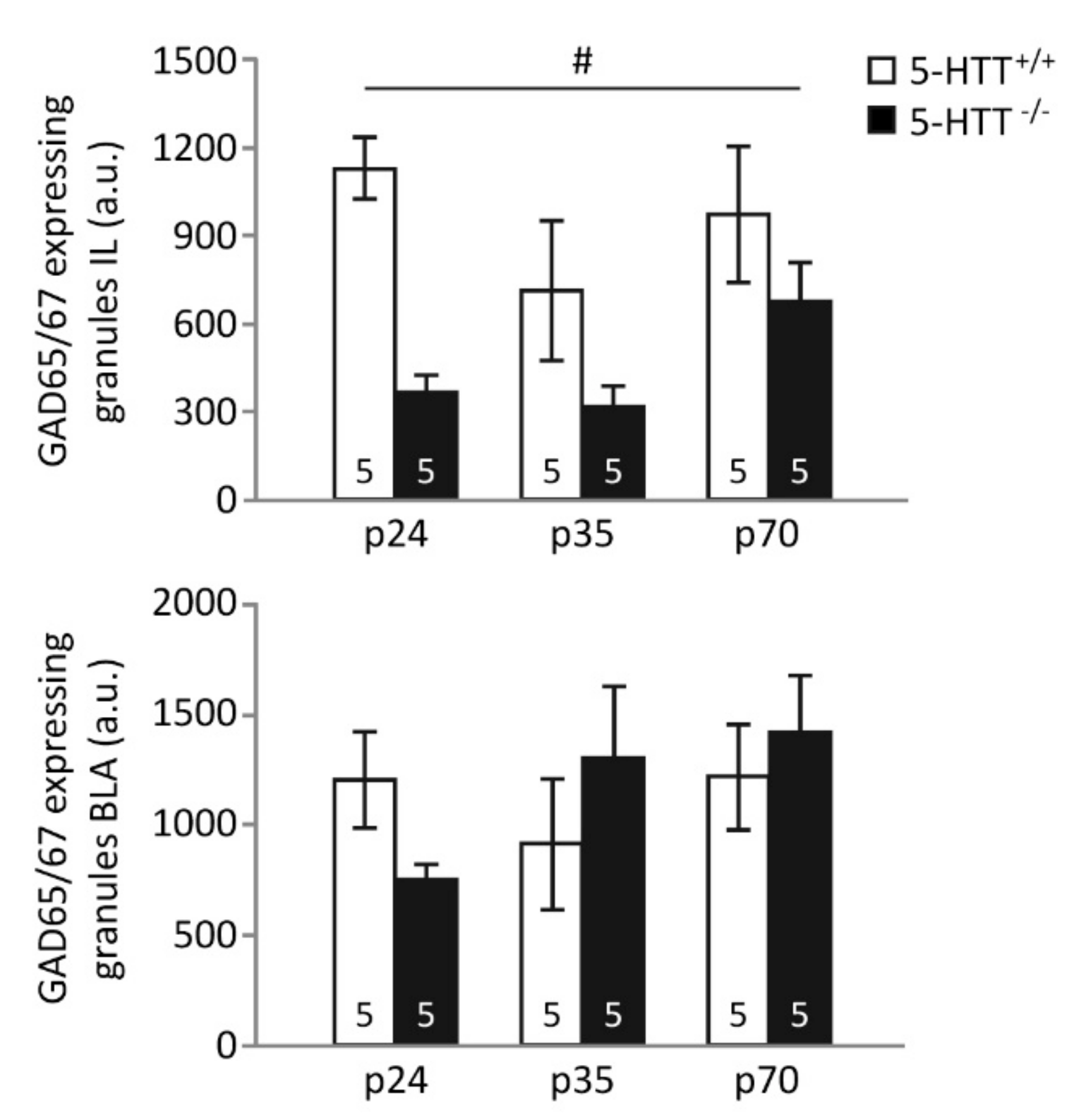
3.2. GAD65/67 Immunoreactivity
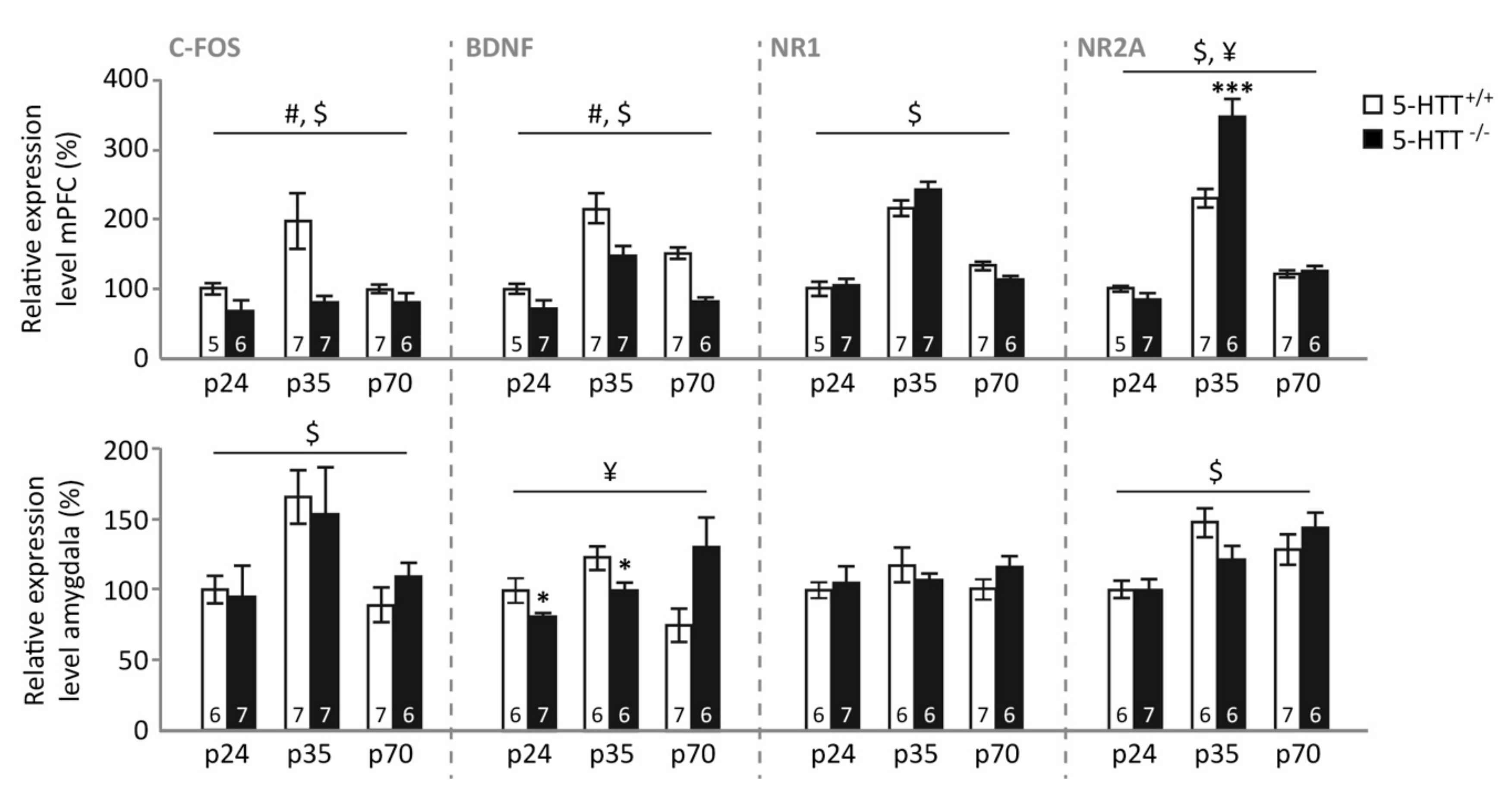
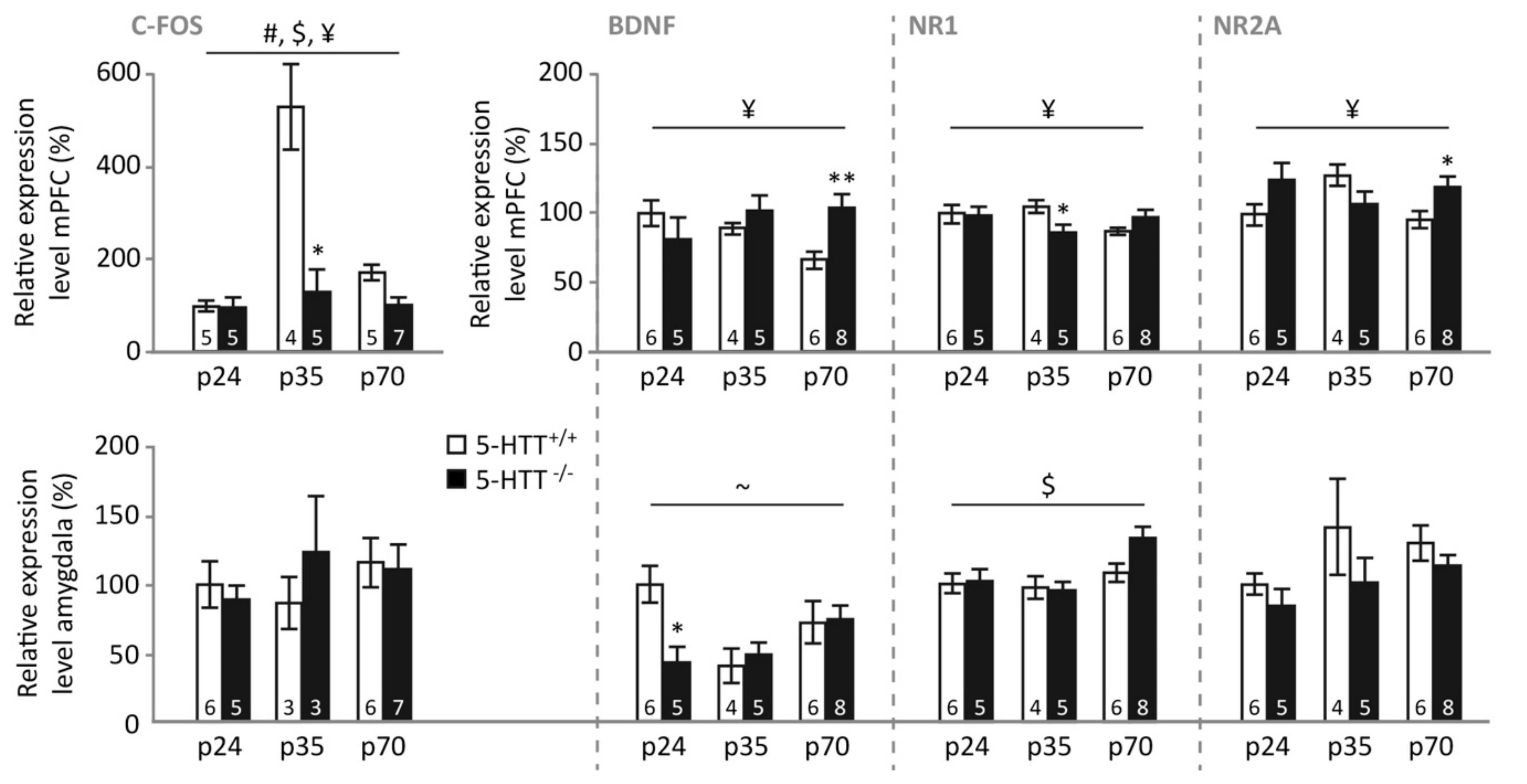
3.3. Gene Expression Levels Neuronal Plasticity and Activity Genes
3.4. Gene Expression following Fear Extinction Learning
3.5. Gene Expression following Fear Extinction Recall
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somerville, L.H.; Kelley, W.M.; Heatherton, T.F. Self-esteem Modulates Medial Prefrontal Cortical Responses to Evaluative Social Feedback. Cereb. Cortex 2010, 20, 3005–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pine, D.S.; Coplan, J.D.; Papp, L.A.; Klein, R.G.; Martinez, J.M.; Kovalenko, P.; Tancer, N.; Moreau, D.; Dummit, E.S.; Shaffer, D.; et al. Ventilatory Physiology of Children and Adolescents With Anxiety Disorders. Arch. Gen. Psychiatry 1998, 55, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Somerville, L.H.; Casey, B. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010, 20, 236–241. [Google Scholar] [Green Version]
- Somerville, L.H.; Jones, R.M.; Casey, B.J. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010, 72, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Dahl, R.E. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 2004, 1021, 1–22. [Google Scholar] [CrossRef]
- Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef]
- Britton, J.C.; Lissek, S.; Grillon, C.; Norcross, M.A.; Pine, D.S. Development of anxiety: The role of threat appraisal and fear learning. Depress. Anxiety 2011, 28, 5–17. [Google Scholar] [CrossRef]
- Heller, A.S.; Cohen, A.O.; Dreyfuss, M.F.W.; Casey, B.J. Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc. Cogn. Affect. Neurosci. 2016, 11, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Hare, T.A.; Tottenham, N.; Galvan, A.; Voss, H.U.; Glover, G.H.; Casey, B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry 2008, 63, 927–934. [Google Scholar] [CrossRef]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef]
- Kim-Cohen, J.; Caspi, A.; Moffitt, T.E.; Harrington, H.; Milne, B.J.; Poulton, R. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Arch. Gen. Psychiatry 2003, 60, 709–717. [Google Scholar] [CrossRef]
- Pattwell, S.S.; Duhoux, S.; Hartley, C.A.; Johnson, D.C.; Jing, D.; Elliott, M.D.; Ruberry, E.J.; Powers, A.; Mehta, N.; Yang, R.R.; et al. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. USA 2012, 109, 16318–16323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallum, J.; Kim, J.H.; Richardson, R. Impaired Extinction Retention in Adolescent Rats: Effects of D-Cycloserine. Neuropsychopharmacology 2010, 35, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.D.; Bisby, M.A.; Richardson, R. Impaired fear extinction in adolescent rodents: Behavioural and neural analyses. Neurosci. Biobehav. Rev. 2016, 70, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Pattwell, S.S.; Bath, K.G.; Casey, B.J.; Ninan, I.; Leea, F.S. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. USA 2011, 108, 1182–1187. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, J.; Cicchetti, P.; Xagoraris, A.; Romanski, L. The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. J. Neurosci. 1990, 10, 1062–1069. [Google Scholar] [CrossRef]
- Saffari, R.; Teng, Z.; Zhang, M.; Kravchenko, M.; Hohoff, C.; Ambrée, O.; Zhang, W. NPY+−, but not PV+− GABAergic neurons mediated long-range inhibition from infra- to prelimbic cortex. Transl. Psychiatry 2016, 6, e736. [Google Scholar] [CrossRef]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala Inhibitory Circuits and the Control of Fear Memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Krettek, J.E.; Price, J.L. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J. Comp. Neurol. 1977, 172, 687–722. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Carter, A.G. Synaptic Mechanisms Underlying Strong Reciprocal Connectivity between the Medial Prefrontal Cortex and Basolateral Amygdala. J. Neurosci. 2013, 33, 15333–15342. [Google Scholar] [CrossRef] [Green Version]
- Little, J.P.; Carter, A.G. Subcellular Synaptic Connectivity of Layer 2 Pyramidal Neurons in the Medial Prefrontal Cortex. J. Neurosci. 2012, 32, 12808–12819. [Google Scholar] [Green Version]
- Floresco, S.B.; Tse, M.T. Dopaminergic Regulation of Inhibitory and Excitatory Transmission in the Basolateral Amygdala-Prefrontal Cortical Pathway. J. Neurosci. 2007, 27, 2045–2057. [Google Scholar] [Green Version]
- Dilgen, J.; Tejeda, H.A.; O’Donnell, P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J. Neurophysiol. 2013, 110, 221–229. [Google Scholar] [Green Version]
- McGarry, L.M.; Carter, A.G. Inhibitory Gating of Basolateral Amygdala Inputs to the Prefrontal Cortex. J. Neurosci. 2016, 36, 9391–9406. [Google Scholar]
- Sotres-Bayon, F.; Sierra-Mercado, D.; Pardilla-Delgado, E.; Quirk, G.J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 2012, 76, 804–812. [Google Scholar] [CrossRef]
- Bang, S.J.; Commons, K.G. Forebrain GABAergic Projections From the Dorsal Raphe Nucleus Identified by Using GAD67–GFP Knock-In Mice. J. Comp. Neurol. 2012, 520, 4157–4167. [Google Scholar] [CrossRef]
- Henny, P.; Jones, B.E. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur. J. Neurosci. 2008, 27, 654–670. [Google Scholar] [Green Version]
- Michels, L.; Schulte-Vels, T.; Schick, M.; O’Gorman, R.L.; Zeffiro, T.; Hasler, G.; Mueller-Pfeiffer, C. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res. Neuroimaging 2014, 224, 288–295. [Google Scholar] [CrossRef]
- Baker, K.D.; Richardson, R. Pharmacological evidence that a failure to recruit NMDA receptors contributes to impaired fear extinction retention in adolescent rats. Neurobiol. Learn Mem. 2017, 143, 18–26. [Google Scholar] [CrossRef]
- Dincheva, I.; Lynch, N.B.; Lee, F.S. The Role of BDNF in the development of fear learning. Depress. Anxiety 2016, 33, 907–916. [Google Scholar] [CrossRef]
- Garpenstrand, H.; Annas, P.; Ekblom, J.; Oreland, L.; Fredrikson, M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav. Neurosci. 2001, 115, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Klucken, T.; Alexander, N.; Schweckendiek, J.; Merz, C.J.; Kagerer, S.; Osinsky, R.; Walter, B.; Vaitl, D.; Hennig, J.; Stark, R. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Soc. Cogn. Affect. Neurosci. 2013, 8, 318–325. [Google Scholar] [CrossRef]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef]
- Pezawas, L.; Meyer-Lindenberg, A.; Drabant, E.M.; A Verchinski, B.; E Munoz, K.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat. Neurosci. 2005, 8, 828–834. [Google Scholar] [CrossRef]
- Pacheco, J.; Beevers, C.G.; Benavides, C.; McGeary, J.; Stice, E.; Schnyer, D.M. Frontal-Limbic White Matter Pathway Associations with the Serotonin Transporter Gene Promoter Region (5-HTTLPR) Polymorphism. J. Neurosci. 2009, 29, 6229–6233. [Google Scholar] [CrossRef] [Green Version]
- Witteveen, J.S.; Middelman, A.; Van Hulten, J.A.; Martens, G.J.M.; Homberg, J.R.; Kolk, S.M.; Martens, G.J.M. Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front. Cell. Neurosci. 2013, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef]
- Homberg, J.R.; Schubert, D.; Gaspar, P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol. Sci. 2010, 31, 60–65. [Google Scholar] [CrossRef]
- Homberg, J.R.; Lesch, K.-P. Looking on the Bright Side of Serotonin Transporter Gene Variation. Biol. Psychiatry 2011, 69, 513–519. [Google Scholar] [CrossRef]
- Narayanan, V.; Heiming, R.S.; Jansen, F.; Lesting, J.; Sachser, N.; Pape, H.-C.; Seidenbecher, T. Social Defeat: Impact on Fear Extinction and Amygdala-Prefrontal Cortical Theta Synchrony in 5-HTT Deficient Mice. PLoS ONE 2011, 6, e22600. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.D.; Wang, Z.; Klosinski, L.P.; Guo, Y.; Herman, D.H.; Celikel, T.; Dong, H.W.; Holschneider, D.P. Mapping Functional Brain Activation Using [14C]-Iodoantipyrine in Male Serotonin Transporter Knockout Mice. PLoS ONE 2011, 6, e23869. [Google Scholar] [CrossRef]
- Hartley, C.A.; McKenna, M.C.; Salman, R.; Holmes, A.; Casey, B.J.; Phelps, E.A.; Glatt, C.E. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proc. Natl. Acad. Sci. USA 2012, 109, 5493–5498. [Google Scholar] [CrossRef] [Green Version]
- Wellman, C.L.; Izquierdo, A.; Garrett, J.E.; Martin, K.P.; Carroll, J.; Millstein, R.; Lesch, K.-P.; Murphy, D.L.; Holmes, A. Impaired Stress-Coping and Fear Extinction and Abnormal Corticolimbic Morphology in Serotonin Transporter Knock-Out Mice. J. Neurosci. 2007, 27, 684–691. [Google Scholar] [CrossRef]
- Shan, L.; Schipper, P.; Nonkes, L.J.P.; Homberg, J.R. Impaired Fear Extinction as Displayed by Serotonin Transporter Knockout Rats Housed in Open Cages Is Disrupted by IVC Cage Housing. PLoS ONE 2014, 9, e91472. [Google Scholar] [CrossRef]
- Nonkes, L.J.; De Pooter, M.; Homberg, J.R. Behavioural therapy based on distraction alleviates impaired fear extinction in male serotonin transporter knockout rats. J. Psychiatry Neurosci. 2012, 37, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Guo, H.-Y.; Heuvel, C.N.A.M.V.D.; Van Heerikhuize, J.; Homberg, J.R. Impaired fear extinction in serotonin transporter knockout rats is associated with increased 5-hydroxymethylcytosine in the amygdala. CNS Neurosci. Ther. 2018, 24, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Miceli, S.; Kasri, N.N.; Joosten, J.; Huang, C.; Kepser, L.; Proville, R.; Selten, M.M.; Van Eijs, F.; Azarfar, A.; Homberg, J.R.; et al. Reduced Inhibition within Layer IV of Sert Knockout Rat Barrel Cortex is Associated with Faster Sensory Integration. Cereb. Cortex 2017, 27, 933–949. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, F.; Guidotti, G.; Middelman, A.; Racagni, G.; Homberg, J.; Riva, M.A. Lack of Serotonin Transporter Alters BDNF Expression in the Rat Brain During Early Postnatal Development. Mol. Neurobiol. 2013, 48, 244–256. [Google Scholar] [CrossRef]
- Karel, P.; Calabrese, F.; Riva, M.; Brivio, P.; van der Veen, B.; Reneman, L.; Verheij, M.; Homberg, J. d-Cycloserine enhanced extinction of cocaine-induced conditioned place preference is attenuated in serotonin transporter knockout rats. Addict. Biol. 2018, 23, 120–129. [Google Scholar] [CrossRef]
- Smits, B.M.G.; Mudde, J.B.; Van De Belt, J.; Verheul, M.; Olivier, J.; Homberg, J.; Guryev, V.; Cools, A.R.; A Ellenbroek, B.; A Plasterk, R.H.; et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenetics Genom. 2006, 16, 159–169. [Google Scholar]
- Olivier, J.; Van Der Hart, M.; Van Swelm, R.; Dederen, P.; Homberg, J.; Cremers, T.; Deen, P.; Cuppen, E.; Cools, A.; Ellenbroek, B. A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 2008, 152, 573–584. [Google Scholar] [CrossRef]
- Nonkes, L.J.; Tomson, K.; Mærtin, A.; Dederen, J.; Maes, J.R.; Homberg, J. Orbitofrontal cortex and amygdalar over-activity is associated with an inability to use the value of expected outcomes to guide behaviour in serotonin transporter knockout rats. Neurobiol. Learn. Mem. 2010, 94, 65–72. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schipper, P.; Kiliaan, A.J.; Homberg, J.R. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav. Pharmacol. 2011, 22, 324–334. [Google Scholar] [CrossRef]
- Schipper, P.; Nonkes, L.J.; Karel, P.; Kiliaan, A.J.; Homberg, J.R. Serotonin transporter genotype x construction stress interaction in rats. Behav. Brain 2011, 223, 169–175. [Google Scholar] [CrossRef]
- Kroeze, Y.; Dirven, B.; Janssen, S.; Kröhnke, M.; Barte, R.M.; Middelman, A.; Van Bokhoven, H.; Zhou, H.; Homberg, J.R. Perinatal reduction of functional serotonin transporters results in developmental delay. Neuropharmacology 2016, 109, 96–111. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Kasahara, Y.; Hall, F.S.; Lesch, K.-P.; Murphy, D.L.; Uhl, G.R.; Sora, I. Developmental alterations in anxiety and cognitive behavior in serotonin transporter mutant mice. Psychopharmacology 2014, 231, 4119–4133. [Google Scholar] [CrossRef]
- Hefner, K.; Holmes, A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav. Brain Res. 2007, 176, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Maren, S.; Phan, K.L.; Liberzon, I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Vollmer, L.L.; Schmeltzer, S.; Schurdak, J.; Ahlbrand, R.; Rush, J.; Dolgas, C.M.; Baccei, M.L.; Sah, R. Neuropeptide Y Impairs Retrieval of Extinguished Fear and Modulates Excitability of Neurons in the Infralimbic Prefrontal Cortex. J. Neurosci. 2016, 36, 1306–1315. [Google Scholar] [CrossRef]
- Henson, M.A.; Tucker, C.J.; Zhao, M.; Dudek, S.M. Long-term depression-associated signaling is required for an in vitro model of NMDA receptor-dependent synapse pruning. Neurobiol. Learn Mem. 2017, 138, 39–53. [Google Scholar] [CrossRef]
- Molteni, R.; Cattaneo, A.; Calabrese, F.; Macchi, F.; Olivier, J.D.; Racagni, G.; Ellenbroek, B.A.; Gennarelli, M.; Riva, M.A. Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as in humans. Neurobiol. Dis. 2010, 37, 747–755. [Google Scholar] [CrossRef]
- Guidotti, G.; Calabrese, F.; Auletta, F.; Olivier, J.; Racagni, G.; Homberg, J.; Riva, M.A. Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: Modulation by antidepressant treatment. Neuropsychopharmacology 2012, 37, 746–758. [Google Scholar] [CrossRef]
- Vieira, P.A.; Corches, A.; Lovelace, J.W.; Westbrook, K.B.; Mendoza, M.; Korzus, E. Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiol. Learn. Mem. 2015, 119, 52–62. [Google Scholar] [CrossRef]
- Endres, T.; Lessmann, V. Age-dependent deficits in fear learning in heterozygous BDNF knock-out mice. Learn. Mem. 2012, 19, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.S. The effects of social housing on extinction of fear conditioning in rapid eye movement sleep-deprived rats. Exp. Brain 2014, 232, 1459–1467. [Google Scholar] [CrossRef]
- Crone, E.A.; Dahl, R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| BDNF tot | AAGTCTGCATTACATTCCTCGA | GTTTTCTGAAAGAGGGACAGTTTAT | TGTGGTTTGTTGCCGTTGCCAAG |
| NR1 | TCATCTCTAGCCAGGTCTACG | CAGAGTAGATGGACATTCGGG | TGGGAGTGAAGTGGTCGTTGGG |
| NR2A | GCACCAGTACATGACCAGATTC | ACCAGTTTACAGCCTTCATCC | CGTCCAACTTCCCGGTTTTCAAGC |
| c-Fos | TCCTTACGGACTCCCCAC | CTCCGTTTCTCTTCCTCTTCAG | TGCTCTACTTTGCCCCTTCTGCC |
| β-actin | CACTTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG | TCTGGGTCATCTTTTCACGGTTGGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipper, P.; Brivio, P.; de Leest, D.; Madder, L.; Asrar, B.; Rebuglio, F.; Verheij, M.M.M.; Kozicz, T.; Riva, M.A.; Calabrese, F.; et al. Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence. Brain Sci. 2019, 9, 118. https://doi.org/10.3390/brainsci9050118
Schipper P, Brivio P, de Leest D, Madder L, Asrar B, Rebuglio F, Verheij MMM, Kozicz T, Riva MA, Calabrese F, et al. Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence. Brain Sciences. 2019; 9(5):118. https://doi.org/10.3390/brainsci9050118
Chicago/Turabian StyleSchipper, Pieter, Paola Brivio, David de Leest, Leonie Madder, Beenish Asrar, Federica Rebuglio, Michel M. M. Verheij, Tamas Kozicz, Marco A. Riva, Francesca Calabrese, and et al. 2019. "Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence" Brain Sciences 9, no. 5: 118. https://doi.org/10.3390/brainsci9050118
APA StyleSchipper, P., Brivio, P., de Leest, D., Madder, L., Asrar, B., Rebuglio, F., Verheij, M. M. M., Kozicz, T., Riva, M. A., Calabrese, F., Henckens, M. J. A. G., & Homberg, J. R. (2019). Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence. Brain Sciences, 9(5), 118. https://doi.org/10.3390/brainsci9050118








