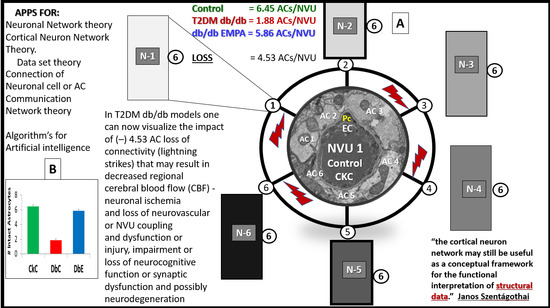
Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing—Six Degrees of Separation
Abstract
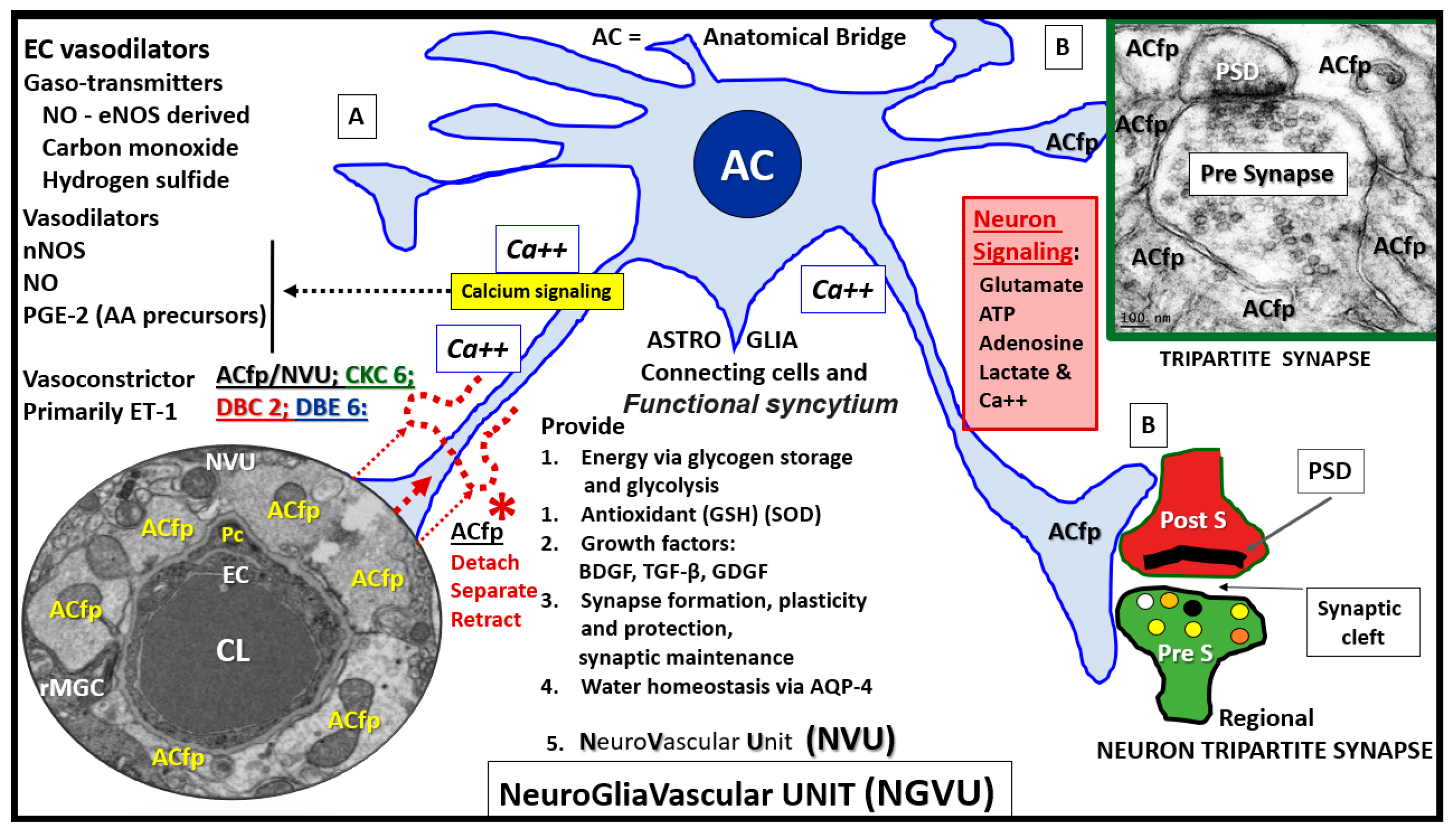
1. Introduction
2. Methods
Image Acquisition and Counting of Astrocyte Foot Processes in Each Model
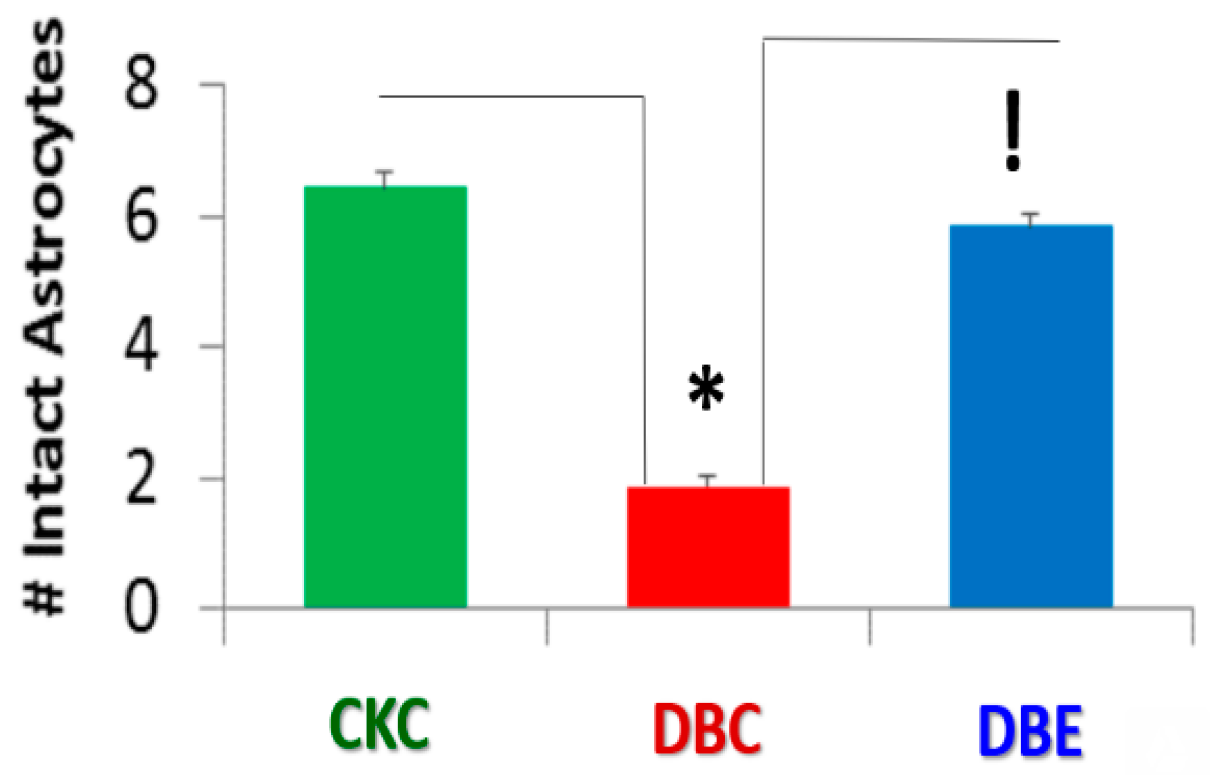
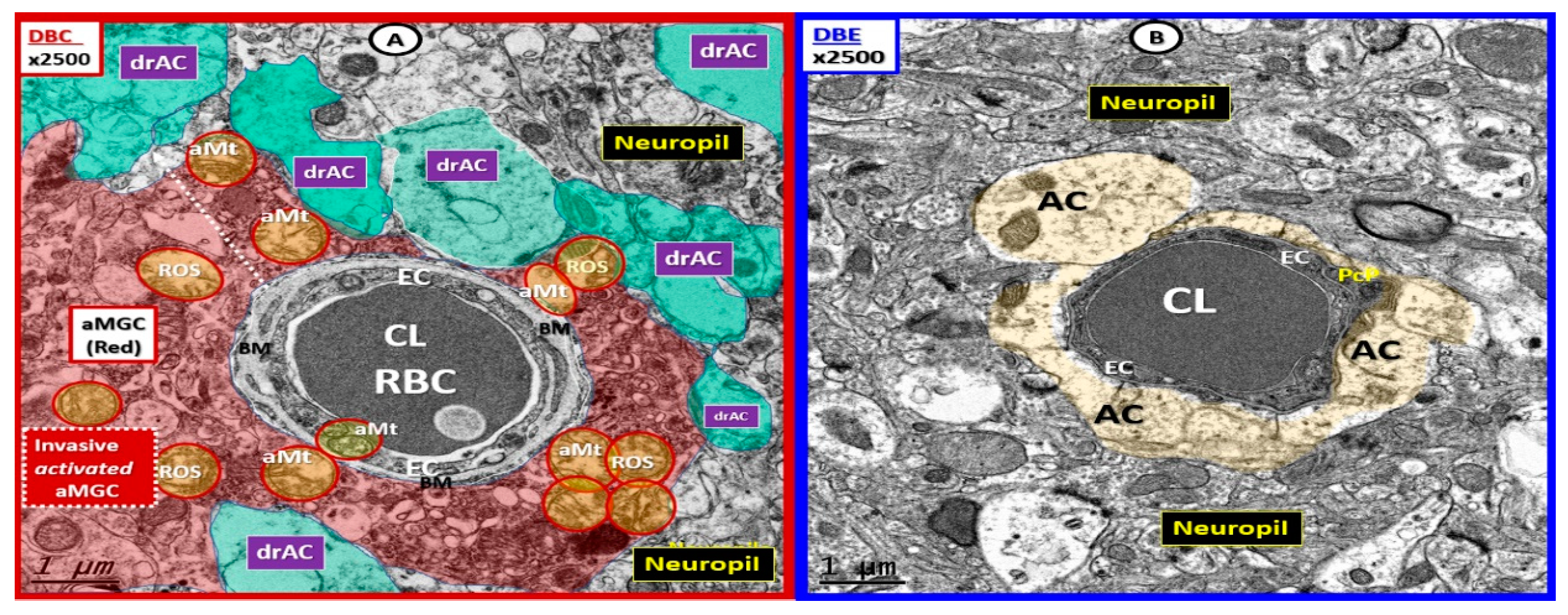
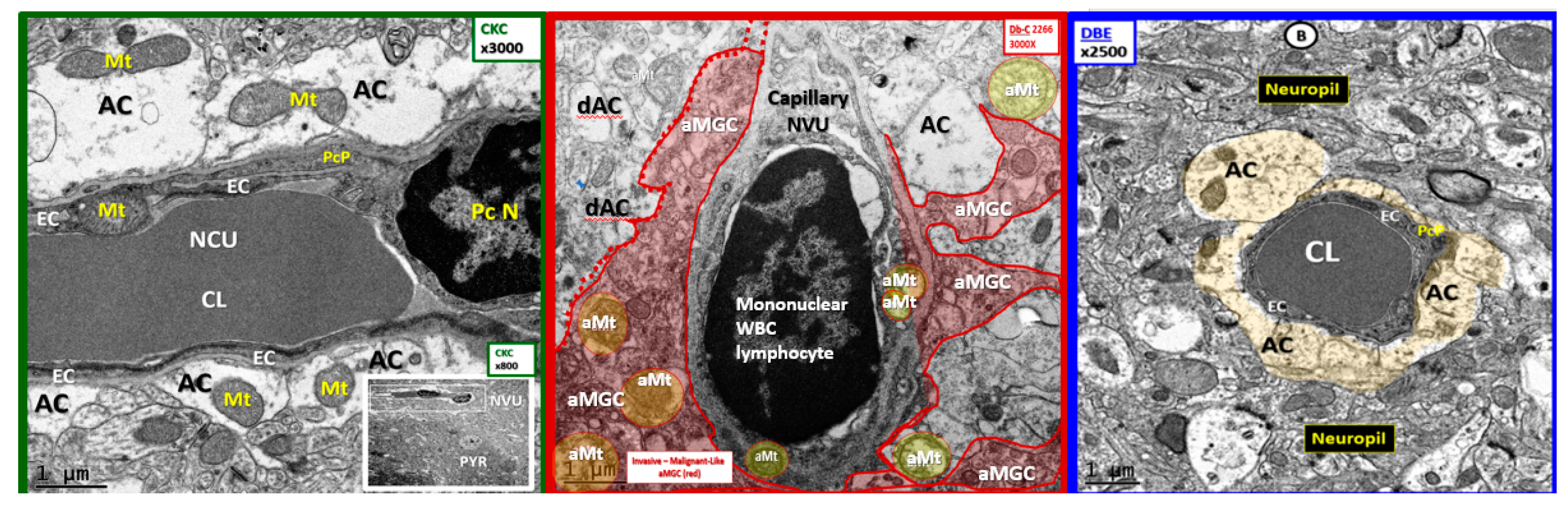
3. Results
4. Discussion
4.1. Astrocytes and Astrocyte Foot Processes May Be Essential to Modulate Informational Processing in the Brain
4.2. Hypothesis: Astrocyte Foot Process Loss from Six to Two per Neurovascular Unit in Diabetic db/db Models could Result in Impaired Modulation of Neuronal Information Processing and Impaired Cognition
4.3. Limitations
5. Conclusions
6. Future Directions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | astrocyte |
| ACfp | astrocyte foot processes |
| BBB | blood-brain barrier |
| BM | basement membrane |
| CBF | cerebral blood flow |
| CKC | control: lean non-diabetic female heterozygous +/-C57B/6Jbackground |
| CL | capillary lumen |
| CNS | central nervous system |
| DBC | db/db homozygous +/+ obese -insulin resistant -female diabetic model |
| DBE | db/db models treated with empagliflozin |
| EC | endothelial cell |
| FIB/SEM | focused ion beam/scanning electron microscopy |
| LOAD | late onset Alzheimer’s disease |
| NVU | neurovascular unit |
| Pc | pericyte |
| PYR | pyramidal neuron |
| T2DM | type 2 diabetes mellitus |
| TEM | transmission electron microscopy |
| TJ/AJ | tight junction/adherens junction |
| RBC | red blood cell |
| SGLT2i | sodium glucose transporter 2 inhibitor |
References
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of The Neurovascular Unit in The Female Diabetic db/db Model—Part I: Astrocyte. Neuroglia 2018, 1, 220–244. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of The Neurovascular Unit in The Female Diabetic db/db Model—Part II: Microglia and Mitochondria. Neuroglia 2018, 1, 311–326. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; DeMarco, V.G. Ultrastructural Remodeling of The Neurovascular Unit in The Female Diabetic db/db Model—Part III: Oligodendrocyte and Myelin. Neuroglia 2018, 1, 351–364. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Empagliflozin (a SGLT2 inhibitor) ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zheng, Y.; Zhao, L.; Chen, M.; Bai, G.; Hu, Y.; Hu, W.; Yan, Z.; Gao, H. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Verkhrasky, A.; Bush, N.O.; Nedergaard, M.; Butt, A. The special case of human astrocytes. Neuroglia 2018, 1, 21–29. [Google Scholar] [CrossRef]
- Szentágothai, J. The ‘module-concept’ in cerebral cortex architecture. Brain Res. 1975, 95, 475–496. [Google Scholar] [CrossRef]
- Drachman, D.A. Do we have brain to spare? Neurology 2005, 64, 2004–2005. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Mai, L.; Zhang, J.Z.; Wang, J.G.; Chang, Y.; Dong, J.D.; Guo, F.Y.; Li, P.A. Diabetes inhibits cerebral ischemia-induced astrocyte activation—an observation in the cingulate cortex. Int. J. Biol. Sci. 2013, 9, 980–988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramos-Rodriguez, J.J.; Ortiz, O.; Jimenez-Palomares, M.; Kay, K.R.; Berrocoso, E.; Murillo-Carretero, M.I.; Perdomo, G.; Spires-Jones, T.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 2013, 38, 2462–2475. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayden, M.R. Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing—Six Degrees of Separation. Brain Sci. 2019, 9, 83. https://doi.org/10.3390/brainsci9040083
Hayden MR. Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing—Six Degrees of Separation. Brain Sciences. 2019; 9(4):83. https://doi.org/10.3390/brainsci9040083
Chicago/Turabian StyleHayden, Melvin R. 2019. "Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing—Six Degrees of Separation" Brain Sciences 9, no. 4: 83. https://doi.org/10.3390/brainsci9040083
APA StyleHayden, M. R. (2019). Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing—Six Degrees of Separation. Brain Sciences, 9(4), 83. https://doi.org/10.3390/brainsci9040083