Astrocytic Response to Acutely- and Chronically-Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microelectrode Arrays and Implant Surgery
2.3. Immunohistochemistry
2.4. Image and Statistical Analysis
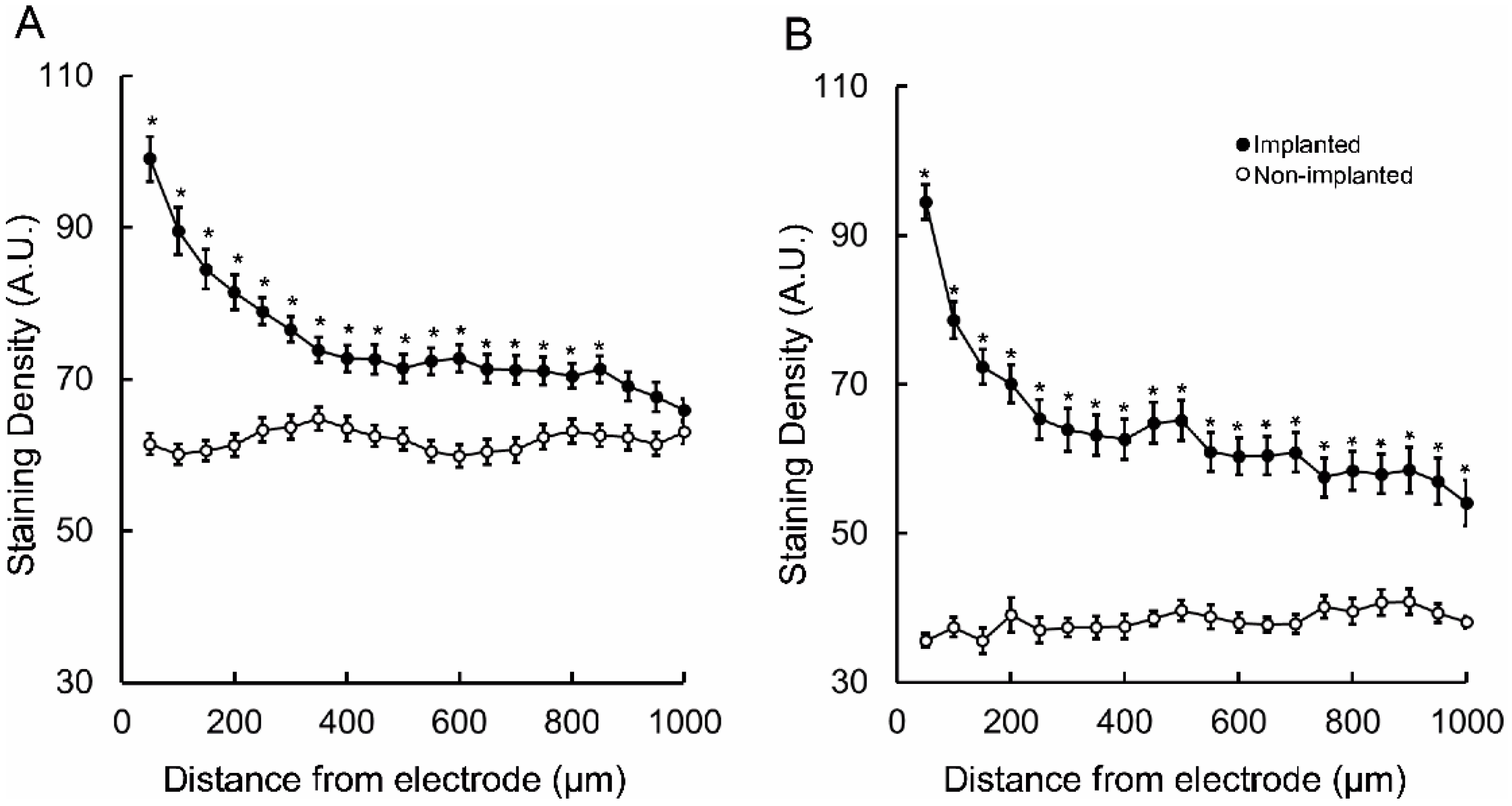
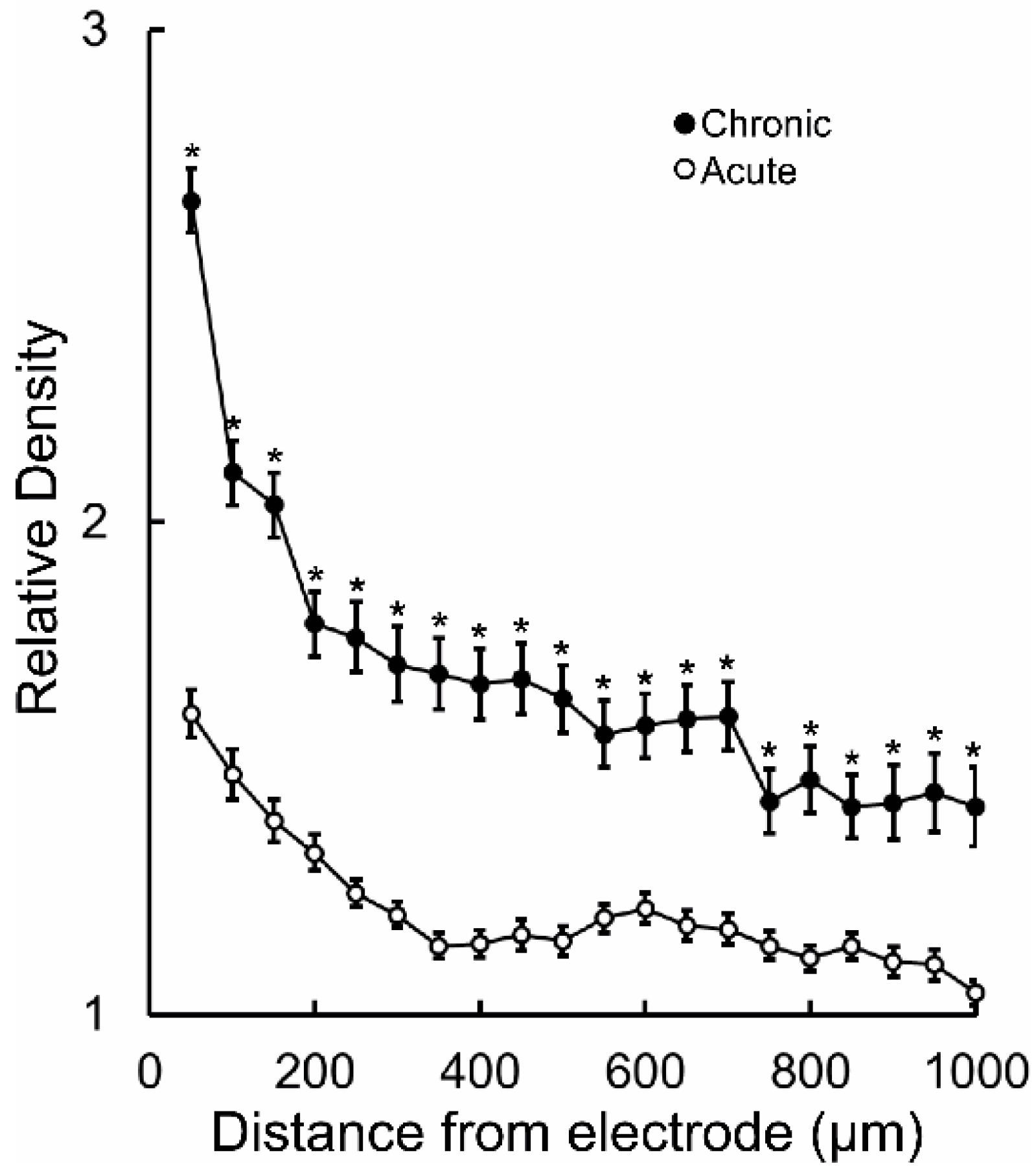
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lebedev, M.A.; Nicolelis, M.A.L. Brain–machine interfaces: Past, present and future. Trends Neurosci. 2006, 29, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.A.M.; Morya, E.; Faber, J.; Santos, J.R.; Guimaraes, J.S.; Lemos, N.A.M.; Sameshima, K.; Pereira, A.; Ribeiro, S.; Nicolelis, M.A.L. Comprehensive Analysis of Tissue Preservation and Recording Quality from Chronic Multielectrode Implants. PLoS ONE 2011, 6, 1–15. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.-S.; Dieme, R.; Sankar, V.; Mayrand, R.C.; Nishida, T.; Streit, W.J.; Sanchez, J.C. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.; Orazem, M.E.; Sanchez, J.C.; Nishida, T. Corrosion of tungsten microelectrodes used in neural recording applications. J. Neurosci. Methods 2011, 198, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Griffith, R.; Sutin, J. Reactive astrocyte formation in vivo is regulated by noradrenergic axons. J. Comp. Neurol. 1996, 371, 362–375. [Google Scholar] [CrossRef]
- Griffith, R.; Soria, J.; Wood, J.G. Regulation of Microglial Tyrosine Phosphorylation in Response to Neuronal Injury. Exp. Neurol. 2000, 161, 297–305. [Google Scholar] [CrossRef]
- Streit, W.J.; Graeber, M.B.; Kreutzberg, G.W. Functional plasticity of microglia: A review. Glia 1988, 1, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Stice, P.; Muthuswamy, J. Assessment of gliosis around moveable implants in the brain. J. Neural Eng. 2009, 6, 046004. [Google Scholar] [CrossRef] [Green Version]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Xue, Q.-S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef]
- Chaplin, T.A.; Yu, H.-H.; Soares, J.G.M.; Gattass, R.; Rosa, M.G.P. A Conserved Pattern of Differential Expansion of Cortical Areas in Simian Primates. J. Neurosci. 2013, 33, 15120–15125. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.F.; Leopold, D.A. The marmoset monkey as a model for visual neuroscience. Neurosci. Res. 2015, 93, 20–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brok, H.P.M.; Bauer, J.; Jonker, M.; Blezer, E.; Amor, S.; Bontrop, R.E.; Laman, J.D.; ‘t Hart, B.A. Non-human primate models of multiple sclerosis: Non-human primate models of MS. Immunol. Rev. 2001, 183, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Hikishima, K.; Iriki, A.; Sasaki, E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal Neonatal Med. 2012, 17, 336–340. [Google Scholar] [CrossRef]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Capadona, J.R. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J. Neural Eng. 2012, 9, 046020. [Google Scholar] [CrossRef]
- Potter-Baker, K.A.; Ravikumar, M.; Burke, A.A.; Meador, W.D.; Householder, K.T.; Buck, A.C.; Sunil, S.; Stewart, W.G.; Anna, J.P.; Tomaszewski, W.H.; et al. A comparison of neuroinflammation to implanted microelectrodes in rat and mouse models. Biomaterials 2014, 35, 5637–5646. [Google Scholar] [CrossRef] [Green Version]
- Stephan, H.; Baron, G.; Schwerdtfeger, W.K. The Brain of the Common Marmoset (Callithrix jacchus): A Stereotaxic Atlas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Perez-Mendes, P.; Blanco, M.M.; Calcagnotto, M.E.; Cinini, S.M.; Bachiega, J.; Papoti, D.; Covolan, L.; Tannus, A.; Mello, L.E. Modeling epileptogenesis and temporal lobe epilepsy in a non-human primate. Epilepsy Res. 2011, 96, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Griffith, R.W.; Humphrey, D.R. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci. Lett. 2006, 406, 81–86. [Google Scholar] [CrossRef]
- Ribeiro, M.; Santana, M.B.; Araujo, M. Neuronal signal description after chronic stainless steel microelectrode array implants in marmosets. In Proceedings of the XXIV Congresso Brasileiro de Engenharia Biomédica—CBEB, Uberlândia, Brazil, 13–17 October 2014; Andrade, A.O., Rocha, A.F., Eds.; pp. 2620–2623. [Google Scholar]
- Porada, I.; Bondar, I.; Spatz, W.; Krüger, J. Rabbit and monkey visual cortex: More than a year of recording with up to 64 microelectrodes. J. Neurosci. Methods 2000, 95, 13–28. [Google Scholar] [CrossRef]
- Bérces, Z.; Tóth, K.; Márton, G.; Pál, I.; Kováts-Megyesi, B.; Fekete, Z.; Ulbert, I.; Pongrácz, A. Neurobiochemical changes in the vicinity of a nanostructured neural implant. Sci. Rep. 2016, 6, 35944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronenbuerger, M.; Nolte, K.W.; Coenen, V.A.; Burgunder, J.M.; Krauss, J.K.; Weis, J. Brain Alterations With Deep Brain Stimulation: New Insight from a Neuropathological Case Series. Mov. Disord. 2015, 30, 1125–1130. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budoff, S.A.; Yano, K.M.; de Mesquita, F.C.; Doerl, J.G.; de Santana, M.B.; Nascimento, M.S.L.; Kunicki, A.C.B.; de Araújo, M.F.P. Astrocytic Response to Acutely- and Chronically-Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain. Brain Sci. 2019, 9, 19. https://doi.org/10.3390/brainsci9020019
Budoff SA, Yano KM, de Mesquita FC, Doerl JG, de Santana MB, Nascimento MSL, Kunicki ACB, de Araújo MFP. Astrocytic Response to Acutely- and Chronically-Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain. Brain Sciences. 2019; 9(2):19. https://doi.org/10.3390/brainsci9020019
Chicago/Turabian StyleBudoff, Samuel A., Kim M. Yano, Fernanda C. de Mesquita, Jhulimar G. Doerl, Maxwell B. de Santana, Manuela S. L. Nascimento, Ana Carolina B. Kunicki, and Mariana F. P. de Araújo. 2019. "Astrocytic Response to Acutely- and Chronically-Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain" Brain Sciences 9, no. 2: 19. https://doi.org/10.3390/brainsci9020019
APA StyleBudoff, S. A., Yano, K. M., de Mesquita, F. C., Doerl, J. G., de Santana, M. B., Nascimento, M. S. L., Kunicki, A. C. B., & de Araújo, M. F. P. (2019). Astrocytic Response to Acutely- and Chronically-Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain. Brain Sciences, 9(2), 19. https://doi.org/10.3390/brainsci9020019




