Diffusion Tensor Magnetic Resonance Imaging for Differentiating Multiple System Atrophy Cerebellar Type and Spinocerebellar Ataxia Type 3
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. DTI Data Collection
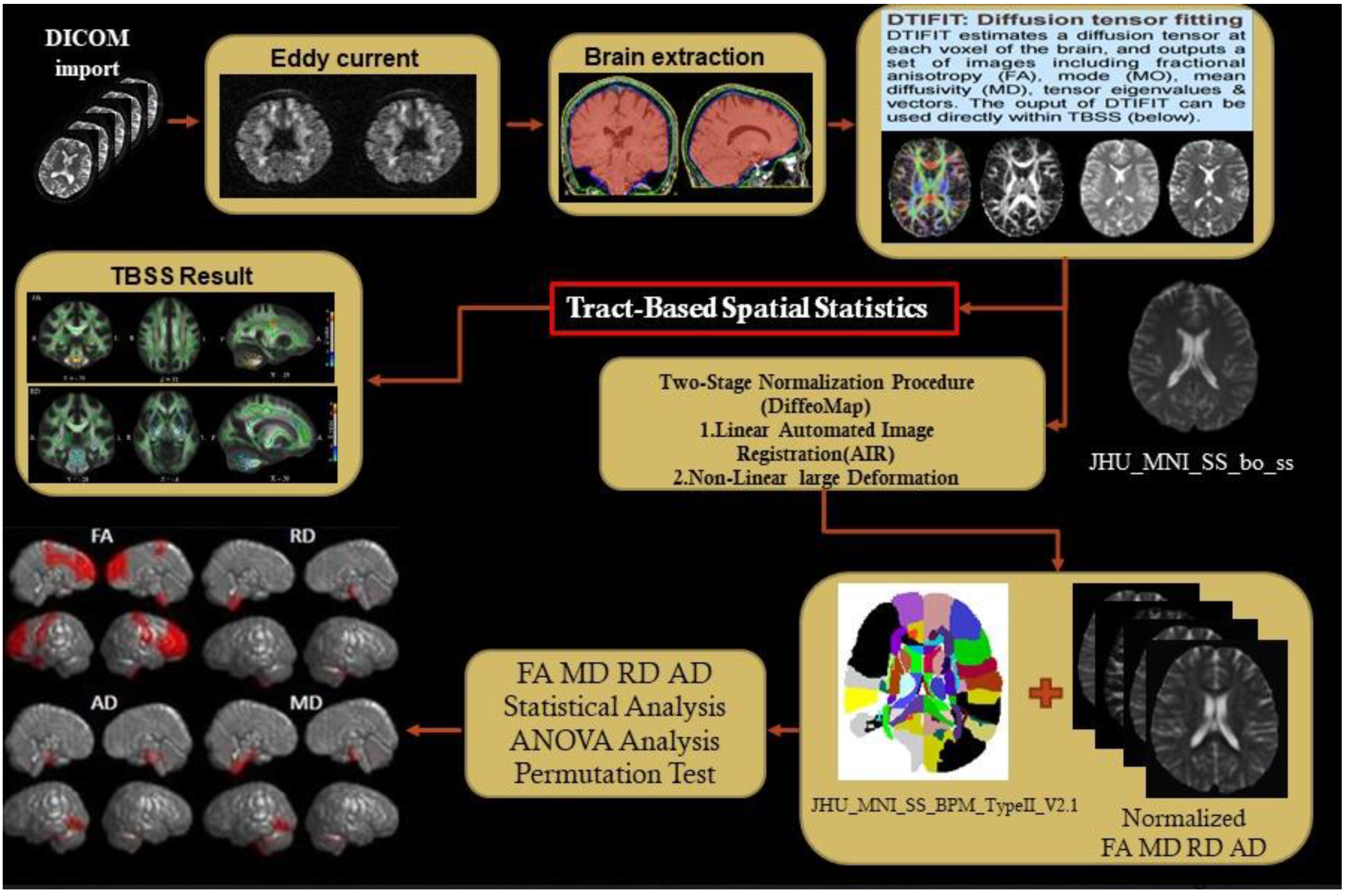
2.3. Diffusion Tensor Images Preprocessing
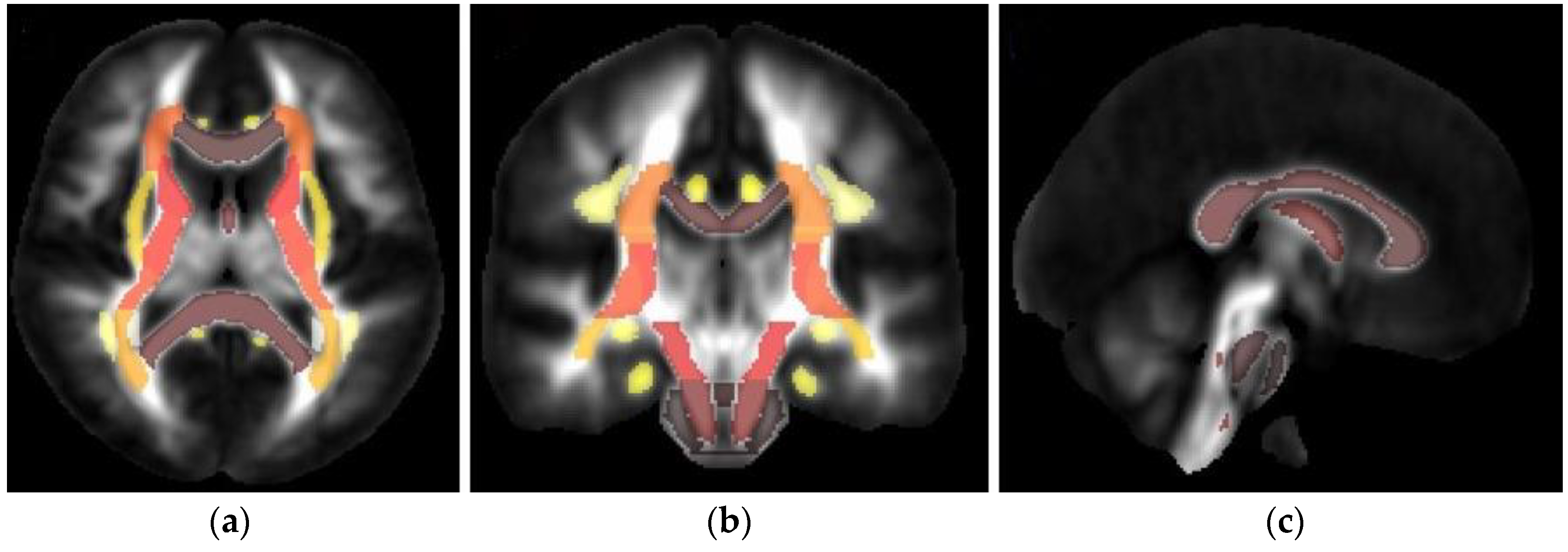
2.4. TBSS and Structural Parcellation
2.5. Statistical Analysis
2.6. K-Means Clustering
3. Results
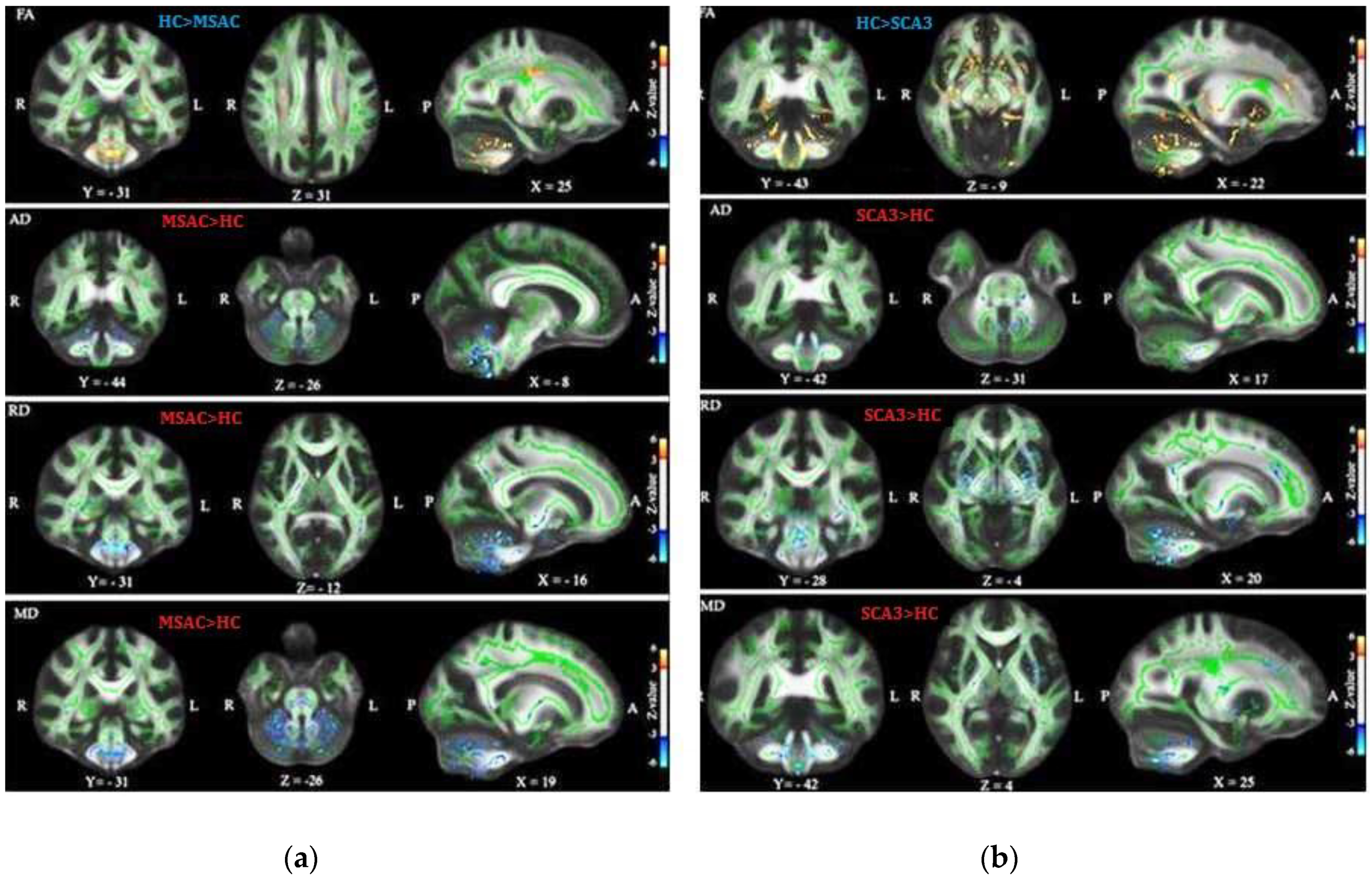
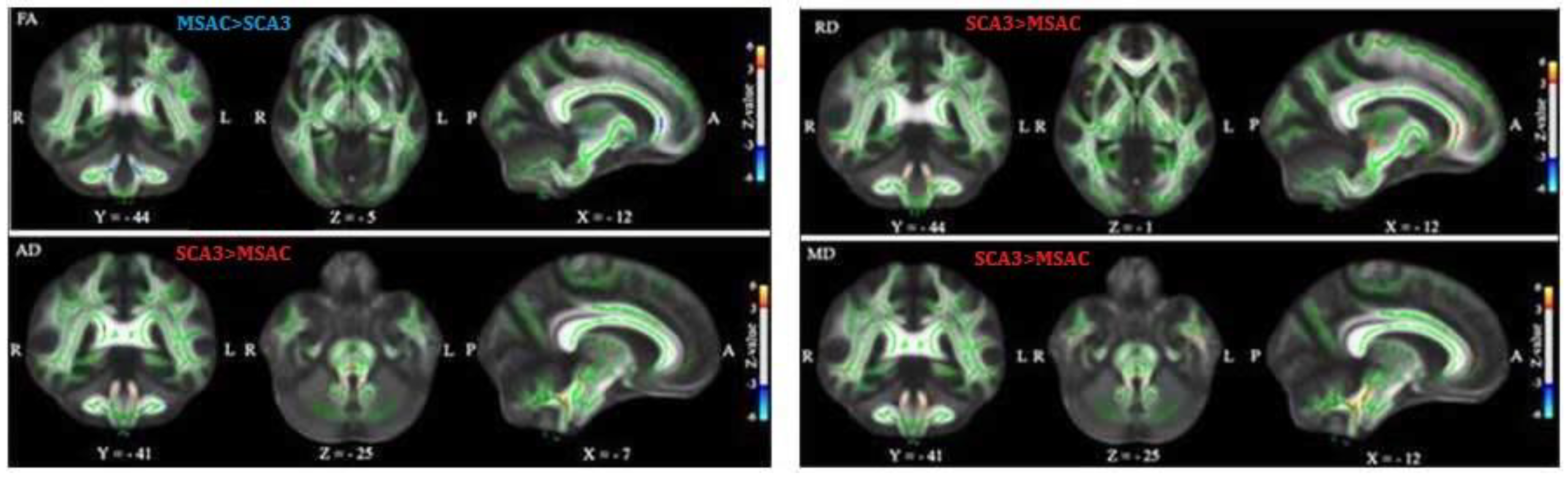
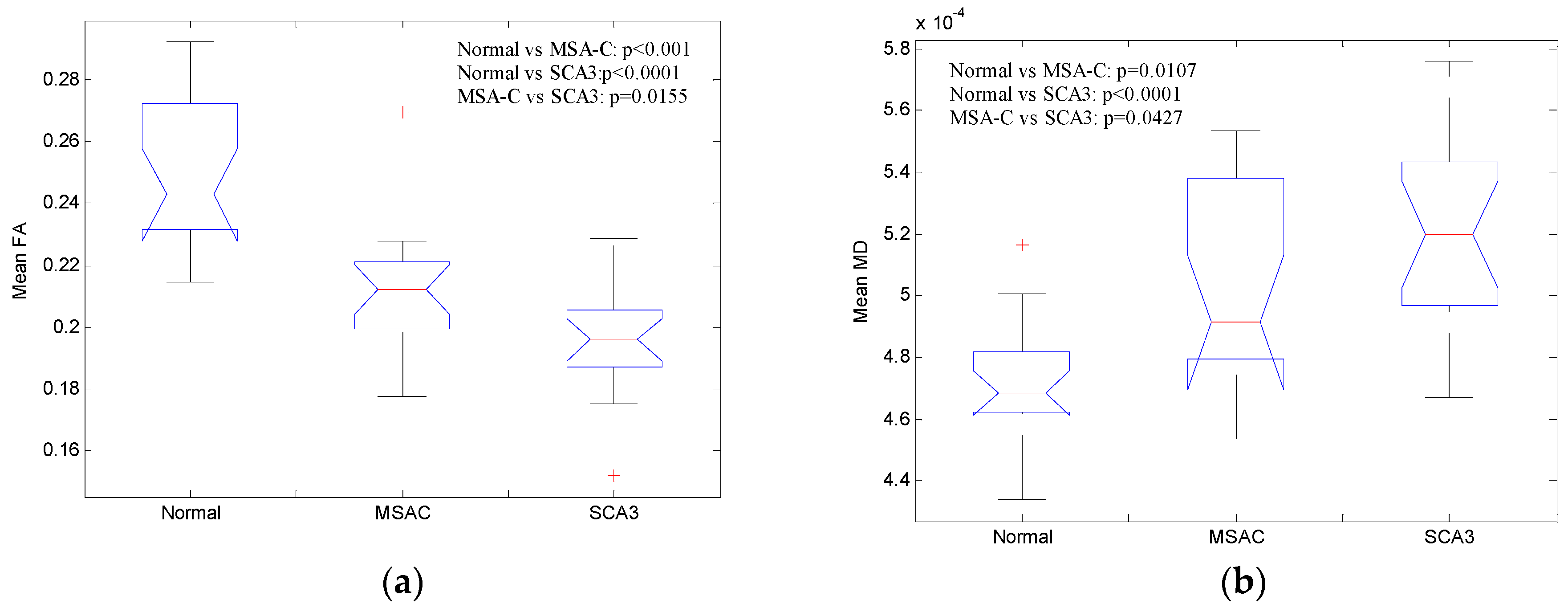
3.1. Patients with SCA3 or MSA-C Demonstrated Significant Decrease in FA and Increase in AD, RD, and MD
3.2. Patients with SCA3 Demonstrated Significant Decrease in FA and Increases in RD, AD, and MD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paulson, H. Machado–Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012, 103, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.R.; Wu, Y.T.; Jao, C.W.; Soong, B.W.; Lirng, J.F.; Wu, H.M.; Wang, P.S. CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3. Neuroimage Clin. 2017, 13, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Xia, G. Ataxia. Continuum 2016, 22, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Scherzed, W.; Brunt, E.R.; Heinsen, H.; De Vos, R.; Seidel, K.; Bürk, K.; Schöls, L.; Auburger, G.; Del Turco, D.; Deller, T.; et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3). Cerebellum 2012, 11, 749–760. [Google Scholar] [CrossRef]
- Koeppen, A.H. The pathogenesis of spinocerebellar ataxia. Cerebellum 2005, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Konagaya, Y.; Kimura, B.; Ishida, M.; Fujii, T. Purification and properties of a histidine decarboxylase from Tetragenococcus muriaticus, a halophilic lactic acid bacterium. J. Appl. Microbiol. 2002, 92, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Shyu, K.K.; Jao, C.W.; Liao, Y.L.; Wang, T.Y.; Wu, H.M.; Wang, P.S.; Soong, B.W. Quantifying cerebellar atrophy in multiple system atrophy of the cerebellar type (MSA-C) using three-dimensional gyrification index analysis. NeuroImage 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.T.; Shyu, K.K.; Jao, C.W.; Wang, Z.Y.; Soong, B.W.; Wu, H.M.; Wang, P.S. Fractal dimension analysis for quantifying cerebellar morphological change of multiple system atrophy of the cerebellar type (MSA-C). NeuroImage 2012, 49, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Pemde, H.K.; Bakhshi, S.; Kalra, V. Olivopontocerebellar atrophy: A case report. Brain Dev. 1995, 17, 130–132. [Google Scholar] [CrossRef]
- Konagaya, M.; Konagaya, Y.; Sakai, M.; Matsuoka, Y.; Hashizume, Y. Progressive cerebral atrophy in multiple system atrophy. J. Neurol. Sci. 2002, 195, 123–127. [Google Scholar] [CrossRef]
- Lee, E.A.; Cho, H.I.; Kim, S.S.; Lee, W.Y. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat. Disord. 2004, 10, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.H.; Simpson, K.; Durazzo, T.C.; Gazdzinski, S.; Meyerhoff, D.J. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: Abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009, 173, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Subramony, S.H. Degenerative Ataxias: Challenges in clinical research. Ann. Clin. Transl. Neurol. 2017, 4, 53–60. [Google Scholar] [CrossRef]
- Kang, J.S.; Klein, J.C.; Baudrexel, S.; Deichmann, R.; Nolte, D.; Hilker, R. White matter damage is related to ataxia severity in SCA3. J. Neurol. 2014, 261, 291–299. [Google Scholar] [CrossRef]
- Guimarães, R.P.; D’Abreu, A.; Yasuda, C.L.; Franca, M.C., Jr.; Silva, B.H.; Cappabianco, F.A.; Bergo, F.P.; Lopes-Cendes, I.T.; Cendes, F. A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3. Mov. Disord. 2013, 28, 1125–1132. [Google Scholar] [CrossRef]
- Konagaya, M.; Sakai, M.; Matsuoka, Y.; Konagaya, Y.; Hashizume, Y. Multiple system atrophy with remarkable frontal lobe atrophy. Acta Neuropathol. 1999, 97, 423–428. [Google Scholar] [CrossRef]
- Ji, L.; Zhu, D.; Xiao, C.; Shi, J. Tract based spatial statistics in multiple system atrophy: A comparison between clinical subtypes. Parkinsonism Relat. Disord. 2014, 20, 1050–1055. [Google Scholar] [CrossRef]
- Brenneis, C.; Boesch, S.M.; Egger, K.E.; Seppi, K.; Scherfler, C.; Schocke, M.; Wenning, G.K.; Poewe, W. Cortical atrophy in the cerebellar variant of multiple system atrophy: A voxel-based morphometry study. Mov. Disord. 2006, 21, 159–165. [Google Scholar] [CrossRef]
- Goel, G.; Pal, P.K.; Ravishankar, S.; Venkatasubramanian, G.; Jayakumar, P.N.; Krishna, N.; Purushottam, M.; Saini, J.; Faruq, M.; Mukherji, M.; et al. Gray matter volume deficits in spinocerebellar ataxia: An optimized voxel based morphometric study. Parkinsonism Relat. Disord. 2011, 17, 521–527. [Google Scholar] [CrossRef]
- Lukas, C.; Schöls, L.; Bellenberg, B.; Rüb, U.; Przuntek, H.; Schmid, G.; Köster, O.; Suchan, B. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: A voxel-based morphometry study. Neurosci. Lett. 2006, 408, 230–235. [Google Scholar] [CrossRef]
- Lopes, T.M.; Anelyssa, D.; Junior, M.C.; Yasuda, C.L.; Betting, L.E.; Samara, A.B.; Castellano, G.; Somazz, J.C.; Balthazar, M.L.; Lopes-Cendes, I.; et al. Widespread neuronal damage and cognitive dysfunction in spinocerebellar ataxia type 3. J. Neurol. 2013, 260, 2370–2379. [Google Scholar] [CrossRef]
- Reetz, K.; Costa, A.S.; Mirzazade, S.; Lehmann, A.; Juzek, A.; Rakowicz, M.; Boguslawska, R.; Schöls, L.; Linnemann, C.; Mariotti, C.; et al. Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain 2013, 136, 905–917. [Google Scholar] [CrossRef]
- Lirng, J.F.; Wang, P.S.; Chen, H.C.; Soong, B.W.; Guo, W.Y.; Wu, H.M.; Chang, C.Y. Differences between Spinocerebellar Ataxias and Multiple System Atrophy-Cerebellar Type on Proton Magnetic Resonance Spectroscopy. PLoS ONE 2012, 7, e47925. [Google Scholar] [CrossRef]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef]
- Wang, P.S.; Wu, H.M.; Lin, C.P.; Soong, B.W. Use of diffusion tensor imaging to identify similarities and differences between cerebellar and Parkinsonism forms of multiple system atrophy. Neuroradiology 2011, 53, 471–481. [Google Scholar] [CrossRef]
- Bang, O.Y.; Huh, K.; Lee, P.H.; Kim, H.J. Clinical and neuroradiological features of patients with spinocerebellar ataxias from Korean kindreds. Arch. Neurol. 2003, 60, 1566–1574. [Google Scholar] [CrossRef]
- Soong, B.W.; Lu, Y.C.; Choo, K.B.; Lee, H.Y. Frequency analysis of autosomal dominant cerebellar ataxias in Taiwanese patients and clinical and molecular characterization of spinocerebellar ataxia type 6. Arch. Neurol. 2001, 58, 1105–1109. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 2011, 213, 560–570. [Google Scholar] [CrossRef]
- O’Donnell, L.J.; Westin, C.F. An introduction to diffusion tensor image analysis. Neurosurg. Clin. 2011, 22, 185–196. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Østby, Y.; Fjell, A.M.; Westlye, L.T.; Due-Tønnessen, P.; Walhovd, K.B. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex 2010, 20, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.M.; Yeatman, J.D.; Lee, E.S.; Barde, L.H.; Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric researchers and clinicians. JDBP 2010, 31, 346. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Hurley, S.A.; Samsonov, A.A.; Adluru, N.; Hosseinbor, A.P.; Mossahebi, P.; Tromp, D.P.; Zakszewski, E.; Field, A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011, 1, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 15, 1487–1505. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wen, G.; Deng, Z.; Liu, J.; Fan, Y. Accurate non-maximum suppression for object detection in high-resolution remote sensing images. Remote Sens. Lett. 2018, 9, 237–246. [Google Scholar] [CrossRef]
- Winkler, A.M.; .Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef]
- Kucuk, U.; Eyuboglu, M.; Kucuk, H.O.; Degirmencioglu, G. Importance of using proper post hoc test with ANOVA. Int. J. Cardiol. 2016, 209, 346. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Suckling, J.; Overmeyer, S.; Rabe-Hesketh, S.; Taylor, E.; Brammer, M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging 1999, 18, 32–42. [Google Scholar] [CrossRef]
- MacQueen, J.B. Some Methods for classification and Analysis of Multivariate Observations. In Proceedings of the 5th Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, 7 January 1966; University of California Press: Berkeley, CA, USA, 1967; Volume 1, pp. 281–297. [Google Scholar]
- Alashwal, H.; El Halaby, M.; Crouse, J.J.; Abdalla, A.; Moustafa, A.A. The Application of Unsupervised Clustering Methods to Alzheimer’s Disease. Front. Comput. Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef]
- Rulseh, A.M.; Keller, J.; Rusz, J.; Syka, M.; Brozova, H.; Rusina, R.; Havrankova, P.; Zarubova, K.; Malikova, H.; Jech, R.; et al. Diffusion tensor imaging in the characterization of multiple system atrophy. Neuropsych. Dis. Treat. 2016, 12, 2181–2187. [Google Scholar] [CrossRef]
- Blatter, D.D.; Bigler, E.D.; Gale, S.D.; Johnson, S.C.; Anderson, C.V.; Burnett, B.M.; Parker, N.; Kurth, S.; Horn, S.D. Quantitative volumetric analysis of brain MR: Normative database spanning 5 decades of life. Am. J. Neuroradiol. 1995, 16, 241–251. [Google Scholar] [PubMed]
- Krogsrud, S.K.; Fjell, A.M.; Tamnes, C.K.; Grydeland, H.; Mor, L.; Due-Tønnesse, P.; Bjørnerud, A.; Sampaio-Baptist, C.; Andersson, J.; Johansen-Berg, H.; et al. Changes in white matter microstructure in the developing brain—A longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage 2016, 124, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The biological basis of diffusion anisotropy. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. In Diffusion MRI; Academic Press: Cambridge, MA, USA, 2009; pp. 105–126. [Google Scholar]
- Kawai, Y.; Takeda, A.; Abe, Y.; Washimi, Y.; Tanaka, F.; Sobue, G. Cognitive impairments in Machado-Joseph disease. Arch. Neurol. 2004, 61, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Braga-Neto, P.; Dutra, L.A.; Pedroso, J.L.; Felício, A.C.; Alessi, H.; Santos-Galduroz, R.F.; Bertolucci, P.H.; Castiglioni, M.L.; Bressan, R.A.; de Garrido, G.E.; et al. Cognitive deficits in Machado–Joseph disease correlate with hypoperfusion of visual system areas. Cerebellum 2012, 11, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain 2006, 129, 290–292. [Google Scholar] [CrossRef]
- De Rezende, T.J.; D’abreu, A.; Guimarães, R.P.; Lopes, T.M.; Lopes-Cendes, I.; Cendes, F.; Castellano, G.; França, M.C., Jr. Cerebral cortex involvement in Machado–Joseph disease. Eur. J. Neurol. 2015, 22, 277-e24. [Google Scholar] [CrossRef]
- D’Abreu, A.; França, M.C., Jr.; Yasuda, C.L.; Campos, B.A.; Lopes-Cendes, I.; Cendes, F. Neocortical Atrophy in Machado-Joseph Disease: A Longitudinal Neuroimaging Study. J. Neuroimaging 2012, 22, 285–291. [Google Scholar] [CrossRef]
- Honea, R.; Crow, T.J.; Passingham, D.; Mackay, C.E. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 2005, 162, 2233–2245. [Google Scholar] [CrossRef]
- Wolf, U.; Rapoport, M.J.; Schweizer, T.A. Evaluating the affective component of the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 245–253. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J. Comp. Neurol. 1989, 289, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.S.; Hayashi, S.; Hasegawa, M.; Wakabayashi, K.; Yamada, M.; Yoshimoto, M.; Ishikawa, A.; Iwatsubo, T.; Takahashi, H. Co-localization of a-synuclein and phosphorylated tau in neuronal and glial cytoplasmic inclusions in a patient with multiple system atrophy of long duration. Acta Neuropathol. 2001, 101, 285–293. [Google Scholar] [PubMed]
- Shibuya, K.; Nagatomo, H.; Iwabuchi, K.; Inoue, M.; Yagishita, S.; Itoh, Y. Asymmetrical temporal lobe atrophy with massive neuronal inclusions in multiple system atrophy. J. Neurol. Sci. 2000, 179, 50–58. [Google Scholar] [CrossRef]
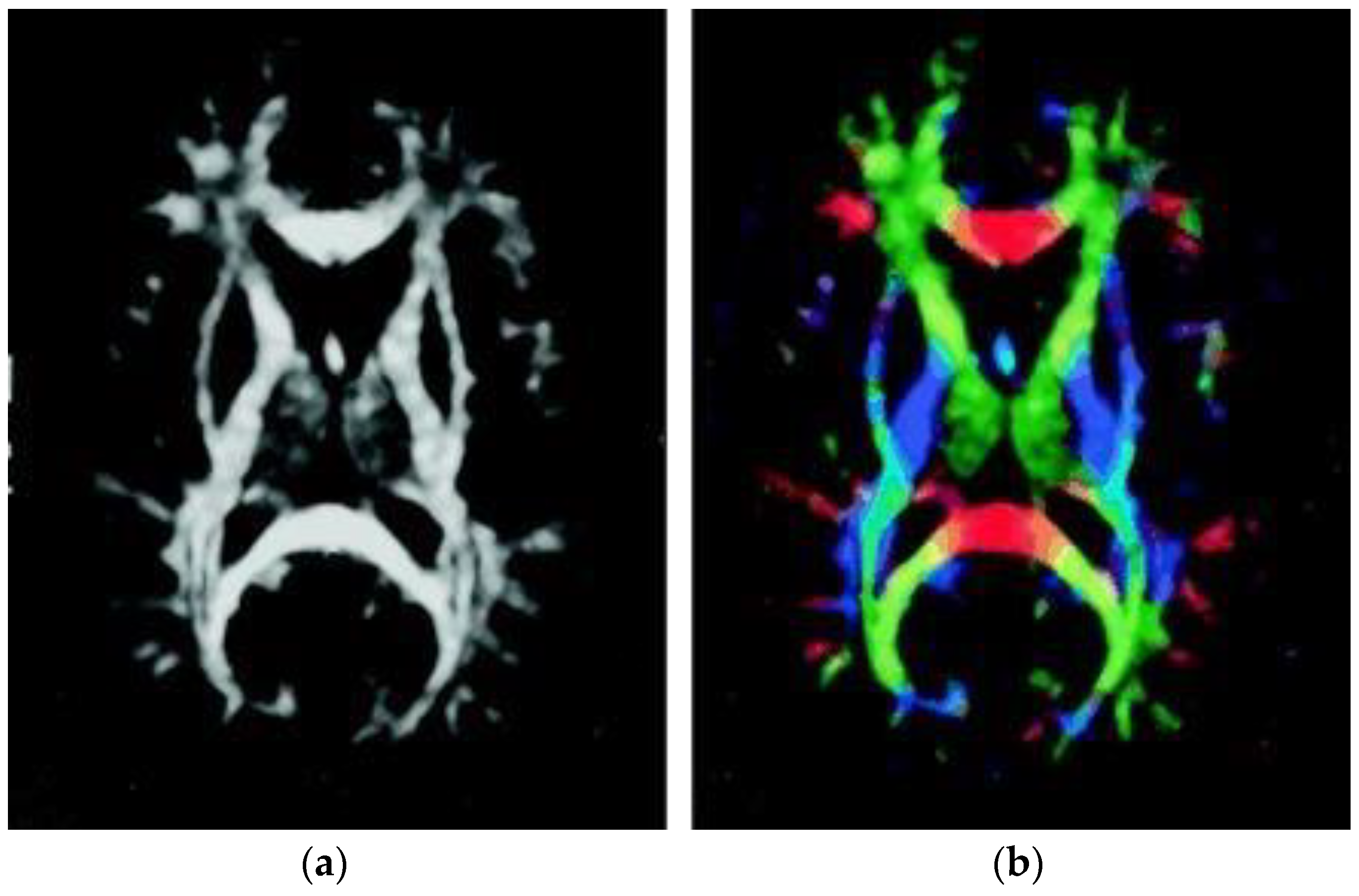

| Group | Subjects | Sex (M/F) | Age |
|---|---|---|---|
| Control | 30 | 15/15 | 46.57 ± 16.2 |
| SCA3 | 15 | 7/8 | 43.8 ± 14.8 |
| MAS-C | 15 | 7/8 | 56.2 ± 6.62 |
| Measurement | Formula |
|---|---|
| Fractional Anisotropy (FA) | where = ( + + )/3 |
| Mean Diffusivity (MD) | (λ1 + λ2 + λ3 )/3 |
| Axial Diffusivity (AD) | λ1 |
| Radial Diffusivity (RD) | (λ2 + λ3 )/2 |
| a. ANOVA analysis pass FDR between HC and SCA3 (α value = 0.05) | |||||
| Tract name | HC v.s. SCA3 | FA p-value | RD p-value | MD p-value | |
| ATRL | 3.00E−08 | 0.000578 | 0.003286 | ||
| ATRR | 1.27E−08 | 1.01E−05 | 9.19E−05 | ||
| CCF | 1.88E−08 | 0.000136 | 0.001167 | ||
| CgLL | 3.12E−06 | 1.40E−07 | 6.29E−07 | ||
| CgLR | 1.72E−06 | 4.22E−09 | 1.48E−08 | ||
| CgUL | 1.37E−08 | 1.58E−06 | 1.72E−05 | ||
| CgUR | 3.75E−08 | 1.47E−05 | 0.00016 | ||
| CSTL | FA  RD,MD RD,MD  | 1.10E-05 | 0.000329 | 0.001618 | |
| CSTR | 5.45E-05 | 5.33E−05 | 0.000192 | ||
| IFOL | 3.34E−07 | 0.000712 | 0.005848 | ||
| IFOR | 4.00E−07 | 0.000138 | 0.001238 | ||
| ILFL | 1.57E−05 | 0.001387 | 0.006487 | ||
| ILFR | 3.42E−06 | 0.000251 | 0.00152 | ||
| SLFBL | 3.66E−06 | 3.21E−05 | 0.000186 | ||
| SLFBR | 2.98E−06 | 4.22E−05 | 0.000215 | ||
| UNCL | 1.03E−09 | 0.000147 | 0.00245 | ||
| UNCR | 5.67E−09 | 4.27E−05 | 0.000682 | ||
| b. ANOVA analysis pass FDR between NC and MSAC (α value = 0.05) | |||||
| Tract name | HC v.s. MSAC | FA p-value | RD p-value | MD p-value | |
| ATRL | 5.24E−05 | 0.000236 | 0.000688 | ||
| ATRR | 0.000164 | 1.94E−05 | 7.84E−05 | ||
| CCF | 0.01287 | 0.014828 | 0.032523 | ||
| CgLL | 7.03E−09 | 2.12E−09 | 1.88E−08 | ||
| CgLR | 1.32E−09 | 2.00E−11 | 2.67E−10 | ||
| CgUL | 7.05E−06 | 4.26E−07 | 2.77E−06 | ||
| CgUR | 3.49E−05 | 9.77E−05 | 0.00042 | ||
| CSTL | FA  RD,MD RD,MD  | 0.010202 | 0.006932 | 0.011976 | |
| CSTR | 0.008366 | 0.004881 | 0.009157 | ||
| IFOL | 0.002727 | 0.003176 | 0.008229 | ||
| IFOR | 0.003212 | 0.005515 | 0.013653 | ||
| ILFL | 0.000557 | 0.000261 | 0.000811 | ||
| ILFR | 8.61E−05 | 0.000509 | 0.001974 | ||
| SLFBL | 0.000247 | 0.000689 | 0.001565 | ||
| SLFBR | 0.00264 | 0.006788 | 0.012182 | ||
| UNCL | 0.003825 | 0.001234 | 0.003551 | ||
| UNCR | 0.008912 | 0.00186 | 0.00538 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, C.-W.; Soong, B.-W.; Huang, C.-W.; Duan, C.-A.; Wu, C.-C.; Wu, Y.-T.; Wang, P.-S. Diffusion Tensor Magnetic Resonance Imaging for Differentiating Multiple System Atrophy Cerebellar Type and Spinocerebellar Ataxia Type 3. Brain Sci. 2019, 9, 354. https://doi.org/10.3390/brainsci9120354
Jao C-W, Soong B-W, Huang C-W, Duan C-A, Wu C-C, Wu Y-T, Wang P-S. Diffusion Tensor Magnetic Resonance Imaging for Differentiating Multiple System Atrophy Cerebellar Type and Spinocerebellar Ataxia Type 3. Brain Sciences. 2019; 9(12):354. https://doi.org/10.3390/brainsci9120354
Chicago/Turabian StyleJao, Chi-Wen, Bing-Wen Soong, Chao-Wen Huang, Chien-An Duan, Chih-Chun Wu, Yu-Te Wu, and Po-Shan Wang. 2019. "Diffusion Tensor Magnetic Resonance Imaging for Differentiating Multiple System Atrophy Cerebellar Type and Spinocerebellar Ataxia Type 3" Brain Sciences 9, no. 12: 354. https://doi.org/10.3390/brainsci9120354
APA StyleJao, C.-W., Soong, B.-W., Huang, C.-W., Duan, C.-A., Wu, C.-C., Wu, Y.-T., & Wang, P.-S. (2019). Diffusion Tensor Magnetic Resonance Imaging for Differentiating Multiple System Atrophy Cerebellar Type and Spinocerebellar Ataxia Type 3. Brain Sciences, 9(12), 354. https://doi.org/10.3390/brainsci9120354




