Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Effects of Drugs/Combinations on the Inflammatory Pain Model
2.1.1. Animals
2.1.2. Induction of Inflammatory Pain Model
2.1.3. Assessment of Mechanical Allodynia
2.1.4. Data Analysis
2.2. Assay of Intracranial Self-Stimulation
2.2.1. Animals
2.2.2. Surgery
2.2.3. Apparatus
2.2.4. Behavioral Procedure
2.2.5. Data Analysis
3. Results
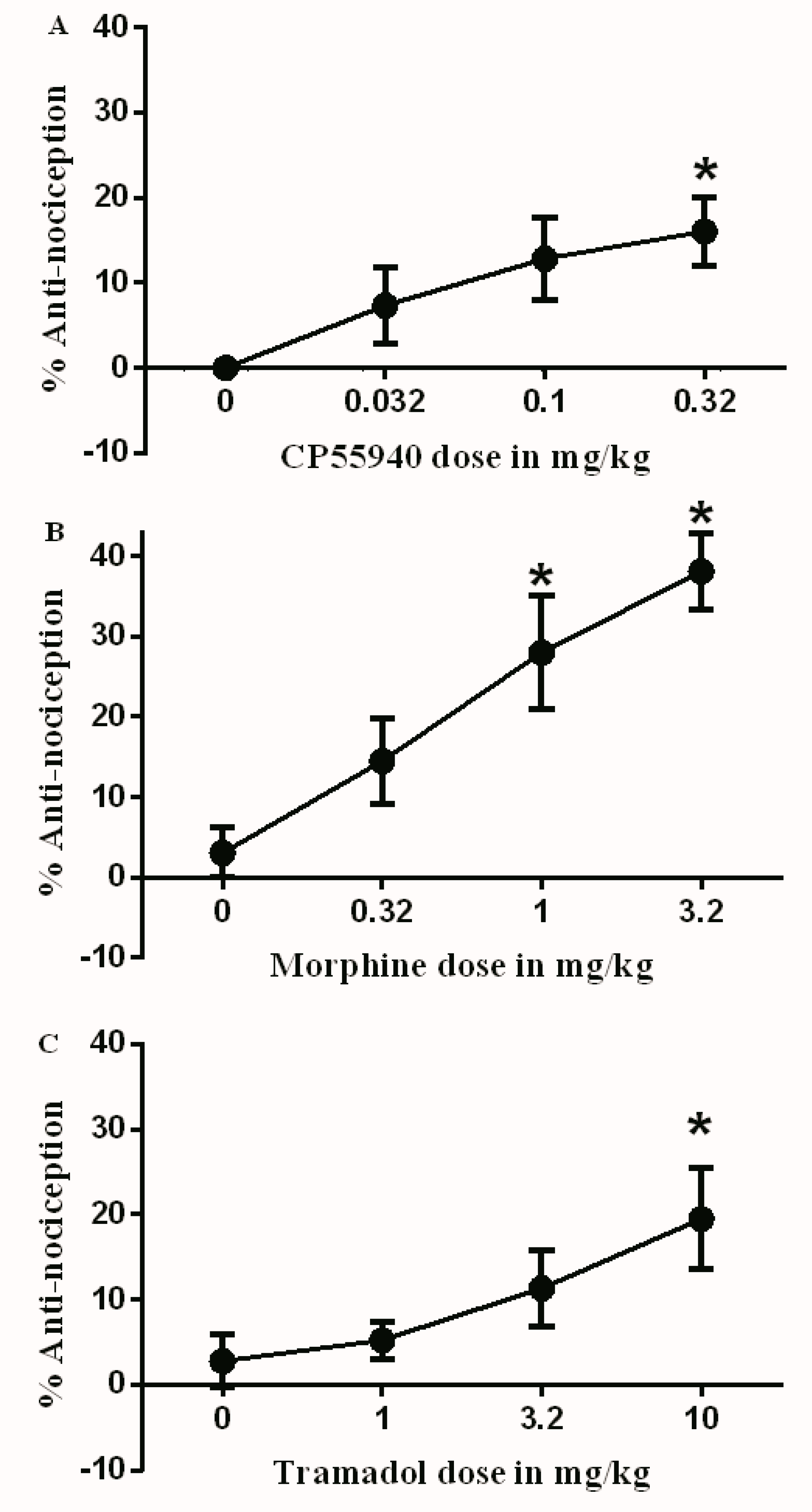
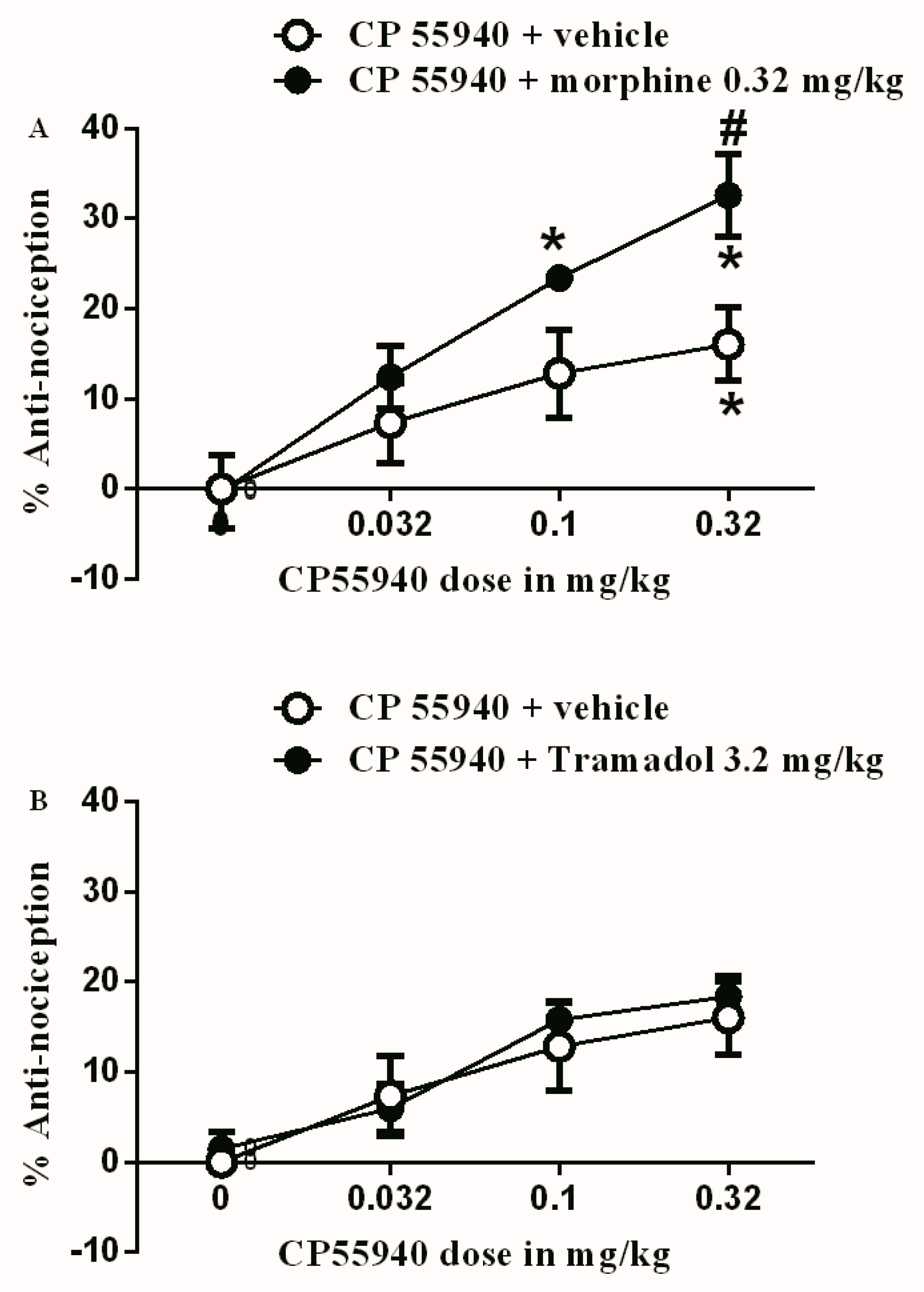
3.1. Effects of Test Drugs on Inflammatory Pain Model
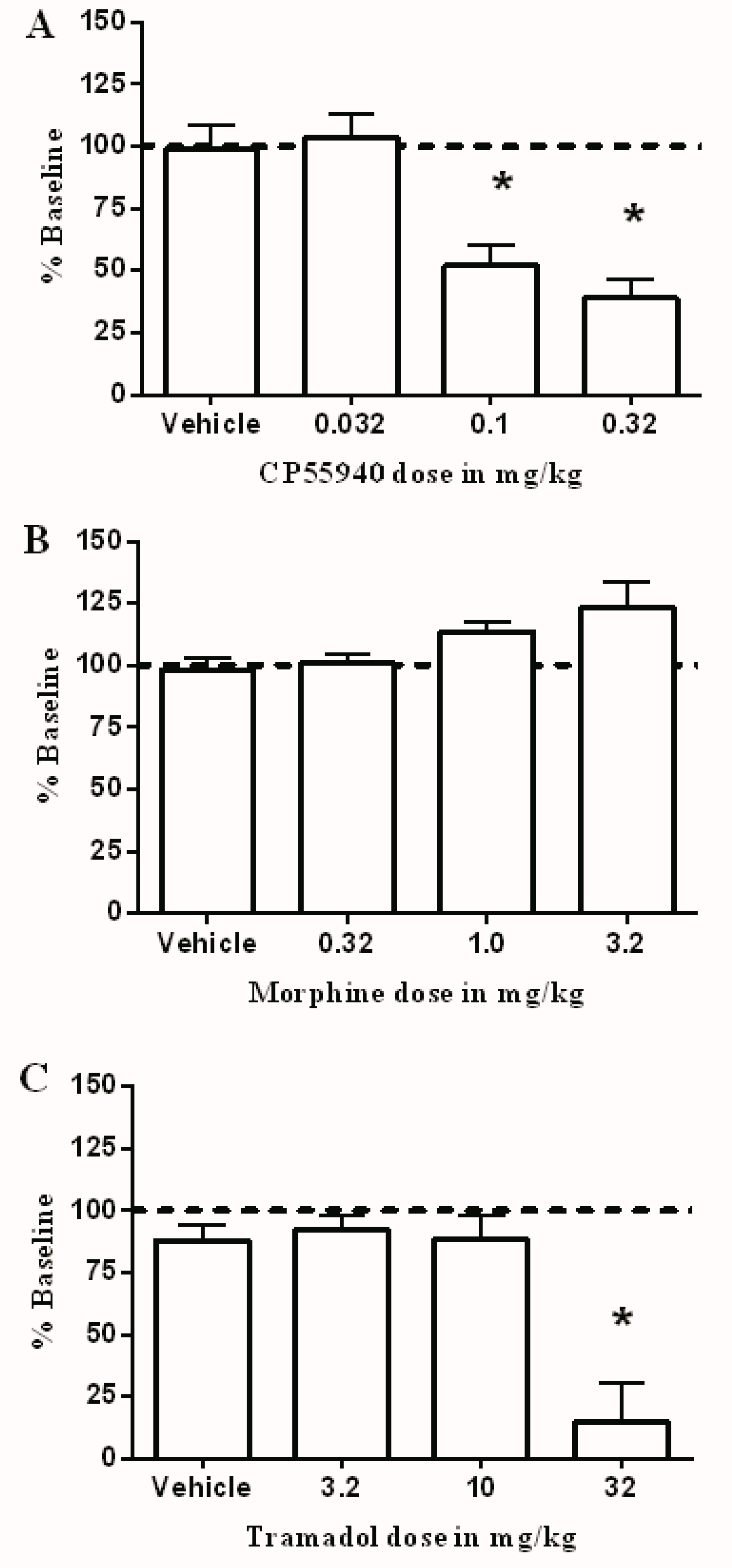
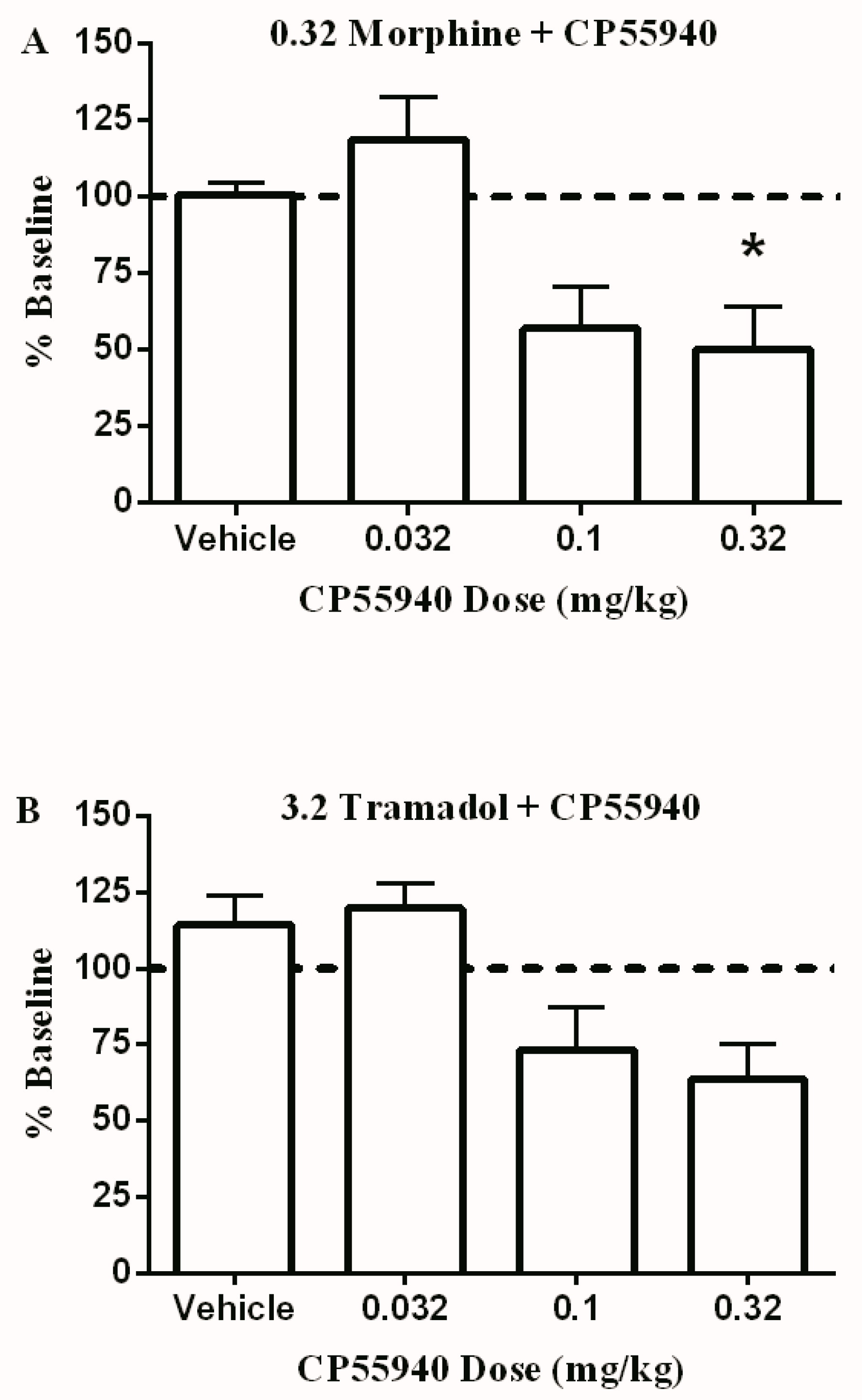
3.2. Effects of Test Drugs on Intracranial Self-Stimulation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hestehave, S.; Abelson, K.S.P.; Bronnum Pedersen, T.; Munro, G. The analgesic efficacy of morphine varies with rat strain and experimental pain model: Implications for target validation efforts in pain drug discovery. Eur. J. Pain 2019, 23, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Altarifi, A.A.; Miller, L.L.; Negus, S.S. Role of micro-opioid receptor reserve and micro-agonist efficacy as determinants of the effects of micro-agonists on intracranial self-stimulation in rats. Behav. Pharmacol. 2012, 23, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.L.; Ineck, J.R. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann. Pharmacother. 2006, 40, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B. Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology 2012, 89, 237–246. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef]
- Minervini, V.; France, C.P. Effects of morphine/CP55940 mixtures on an impulsive choice task in rhesus monkeys. Behav. Pharmacol. 2018, 29, 60–70. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Karst, M.; Wippermann, S.; Ahrens, J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 2010, 70, 2409–2438. [Google Scholar] [CrossRef]
- Strangman, N.M.; Patrick, S.L.; Hohmann, A.G.; Tsou, K.; Walker, J.M. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Res. 1998, 813, 323–328. [Google Scholar] [CrossRef]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Whiteside, G.; Fowler, C.J.; Hohmann, A.G. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res. Rev. 2009, 60, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Hohmann, A.G. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005, 168, 509–554. [Google Scholar]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Selley, D.E.; Sim-Selley, L.J.; Welch, S.P. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur. J. Pharmacol. 2007, 571, 129–137. [Google Scholar] [CrossRef]
- Maguire, D.R.; Yang, W.; France, C.P. Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: Antinociception, drug discrimination, and drug self-administration. J. Pharmacol. Exp. Ther. 2013, 345, 354–362. [Google Scholar] [CrossRef]
- Miller, L.L.; Picker, M.J.; Umberger, M.D.; Schmidt, K.T.; Dykstra, L.A. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. J. Pharmacol. Exp. Ther. 2012, 342, 177–187. [Google Scholar] [CrossRef]
- Manzanares, J.; Corchero, J.; Romero, J.; Fernandez-Ruiz, J.J.; Ramos, J.A.; Fuentes, J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999, 20, 287–294. [Google Scholar] [CrossRef]
- Parolaro, D.; Massi, P. Cannabinoids as potential new therapy for the treatment of gliomas. Expert Rev. Neurother. 2008, 8, 37–49. [Google Scholar] [CrossRef]
- Nadal, X.; La Porta, C.; Andreea Bura, S.; Maldonado, R. Involvement of the opioid and cannabinoid systems in pain control: New insights from knockout studies. Eur. J. Pharmacol. 2013, 716, 142–157. [Google Scholar] [CrossRef]
- Tham, S.M.; Angus, J.A.; Tudor, E.M.; Wright, C.E. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br. J. Pharmacol. 2005, 144, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, D.L.; McCarthy, E.A. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J. Pharmacol. Exp. Ther. 2003, 304, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Gennings, C.; Shih, M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur. J. Pharmacol. 2006, 530, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Bushlin, I.; Rozenfeld, R.; Devi, L.A. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol. 2010, 10, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Couey, P.; Shade, S.B.; Kelly, M.E.; Benowitz, N.L. Cannabinoid-opioid interaction in chronic pain. Clin. Pharmacol. Ther. 2011, 90, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; McMahon, L.R.; Gerak, L.R.; Becker, G.L.; France, C.P. Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: Discrimination and antinociception. Psychopharmacology 2008, 199, 199–208. [Google Scholar] [CrossRef]
- Driessen, B.; Reimann, W. Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro. Br. J. Pharmacol. 1992, 105, 147–151. [Google Scholar] [CrossRef]
- Driessen, B.; Reimann, W.; Giertz, H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br. J. Pharmacol. 1993, 108, 806–811. [Google Scholar] [CrossRef]
- Aldossary, S.A.; Alsalem, M.; Kalbouneh, H.; Haddad, M.; Azab, B.; Al-shboul, O. The role of transient receptor potential vanilloid receptor 1 and peroxisome proliferator-activated receptors-α in mediating the antinociceptive effects of palmitoylethanolamine in rats. NeuroReport 2019, 30, 32–37. [Google Scholar] [CrossRef]
- Alsalem, M.; Altarifi, A.; Kalbouneh, H.; Al-Zer, H.; Azab, B.; El-Salem, K. Role of PPARα and PPARγ in Mediating the Analgesic Properties of Ibuprofen in vivo and the Effects of Dual PPARα/γ Activation in Inflammatory Pain Model in the Rat. Int. J. Pharmacol. 2016, 12, 812–820. [Google Scholar] [CrossRef]
- Kwilasz, A.J.; Negus, S.S. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J. Pharmacol. Exp. Ther. 2012, 343, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bijjem, K.R.; Sharma, P.L. Anti-inflammatory and antihyperalgesic effects of the combination of ibuprofen and hemin in adjuvant-induced arthritis in the Wistar rat. Inflammopharmacology 2011, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Leitl, M.D.; Potter, D.N.; Cheng, K.; Rice, K.C.; Carlezon, W.A., Jr.; Negus, S.S. Sustained pain-related depression of behavior: Effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol. Pain 2014, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Jun, I.G.; Kim, S.H.; Park, J.Y. Interaction of morphine and selective serotonin receptor inhibitors in rats experiencing inflammatory pain. J. Korean Med. Sci. 2012, 27, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Okada, M.; Kohara, A.; Kiso, T.; Toya, T.; Iwai, A. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: Time course of progression and efficacy of analgesics. J. Pharmacol. Exp. Ther. 2003, 306, 490–497. [Google Scholar] [CrossRef]
- Bianchi, M.; Martucci, C.; Ferrario, P.; Franchi, S.; Sacerdote, P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: The effects of analgesic drugs. Anesth. Analg. 2007, 104, 949–954. [Google Scholar] [CrossRef]
- Gould, S.A.; Doods, H.; Lamla, T.; Pekcec, A. Pharmacological characterization of intraplantar Complete Freund’s Adjuvant-induced burrowing deficits. Behav. Brain Res. 2016, 301, 142–151. [Google Scholar] [CrossRef]
- Choong, K.C.; Su, X.; Urban, M.O. Effect of CP55,940 on mechanosensory spinal neurons following chronic inflammation. Neurosci. Lett. 2007, 414, 105–109. [Google Scholar] [CrossRef]
- Martin, W.J.; Loo, C.M.; Basbaum, A.I. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain 1999, 82, 199–205. [Google Scholar] [CrossRef]
- Craft, R.M.; Kandasamy, R.; Davis, S.M. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Delta(9)-tetrahydrocannabinol in the rat. Pain 2013, 154, 1709–1717. [Google Scholar] [CrossRef]
- Sain, N.M.; Liang, A.; Kane, S.A.; Urban, M.O. Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1(-/-) and not CB2(-/-) mice in models of acute and persistent pain. Neuropharmacology 2009, 57, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Massi, P.; Panerai, A.E.; Parolaro, D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000, 109, 155–163. [Google Scholar] [CrossRef]
- Maguire, D.R.; France, C.P. Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur. J. Pharmacol. 2018, 819, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Grim, T.W.; Wiebelhaus, J.M.; Morales, A.J.; Negus, S.S.; Lichtman, A.H. Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depend. 2015, 150, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Moerke, M.J.; Negus, S.S. Role of agonist efficacy in exposure-induced enhancement of mu opioid reward in rats. Neuropharmacology 2019, 151, 180–188. [Google Scholar] [CrossRef]
- Vlachou, S.; Nomikos, G.G.; Panagis, G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology 2005, 179, 498–508. [Google Scholar] [CrossRef]
- Altarifi, A.A.; Negus, S.S. Some determinants of morphine effects on intracranial self-stimulation in rats: Dose, pretreatment time, repeated treatment, and rate dependence. Behav. Pharmacol. 2011, 22, 663–673. [Google Scholar] [CrossRef]
- Negus, S.S.; Moerke, M.J. Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 2019, 112, 23–31. [Google Scholar] [CrossRef]
- Rutten, K.; De Vry, J.; Robens, A.; Tzschentke, T.M.; van der Kam, E.L. Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. Eur. J. Pain 2011, 15, 299–305. [Google Scholar] [CrossRef]
- Cha, H.J.; Song, M.J.; Lee, K.W.; Kim, E.J.; Kim, Y.H.; Lee, Y. Dependence potential of tramadol: Behavioral pharmacology in rodents. Biomol. Ther. 2014, 22, 558–562. [Google Scholar] [CrossRef]
- Cannon, C.Z.; Kissling, G.E.; Hoenerhoff, M.J.; King-Herbert, A.P.; Blankenship-Paris, T. Evaluation of dosages and routes of administration of tramadol analgesia in rats using hot-plate and tail-flick tests. Lab Anim. 2010, 39, 342–351. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalem, M.; Altarifi, A.; Haddad, M.; Aldossary, S.A.; Kalbouneh, H.; Aldaoud, N.; Saleh, T.; El-Salem, K. Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats. Brain Sci. 2019, 9, 328. https://doi.org/10.3390/brainsci9110328
Alsalem M, Altarifi A, Haddad M, Aldossary SA, Kalbouneh H, Aldaoud N, Saleh T, El-Salem K. Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats. Brain Sciences. 2019; 9(11):328. https://doi.org/10.3390/brainsci9110328
Chicago/Turabian StyleAlsalem, Mohammad, Ahmad Altarifi, Mansour Haddad, Sara A. Aldossary, Heba Kalbouneh, Nour Aldaoud, Tareq Saleh, and Khalid El-Salem. 2019. "Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats" Brain Sciences 9, no. 11: 328. https://doi.org/10.3390/brainsci9110328
APA StyleAlsalem, M., Altarifi, A., Haddad, M., Aldossary, S. A., Kalbouneh, H., Aldaoud, N., Saleh, T., & El-Salem, K. (2019). Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats. Brain Sciences, 9(11), 328. https://doi.org/10.3390/brainsci9110328




