Effects of Acute Normobaric Hypoxia on Memory Interference
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
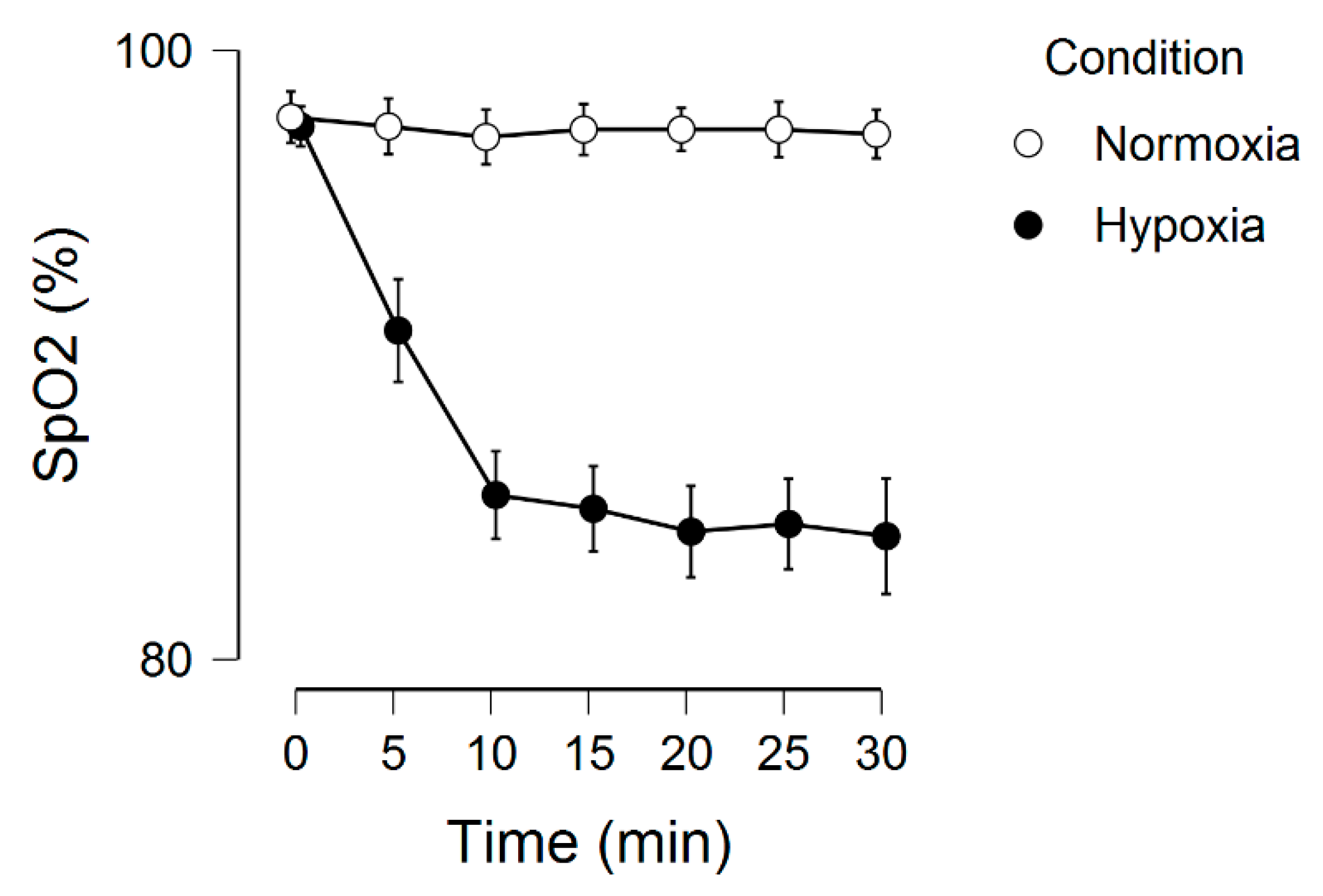
2.3. Normoxia and Hypoxia
2.4. Memory Function
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, G.E.; Peterson, C. Decreases in the release of acetylcholine in vitro with low oxygen. Biochem. Pharmacol. 1982, 31, 111–115. [Google Scholar] [CrossRef]
- Gibson, G.E.; Pulsinelli, W.; Blass, J.P.; Duffy, T.E. Brain dysfunction in mild to moderate hypoxia. Am. J. Med. 1981, 70, 1247–1254. [Google Scholar] [CrossRef]
- Virues-Ortega, J.; Buela-Casal, G.; Garrido, E.; Alcazar, B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 2004, 14, 197–224. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Aquino-Lemos, V.; Santos, R.V.; Antunes, H.K.; Lira, F.S.; Luz Bittar, I.G.; Caris, A.V. Acute physical exercise under hypoxia improves sleep, mood and reaction time. Physiol. Behav. 2016, 154, 90–99. [Google Scholar] [CrossRef]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef]
- Sun, S.; Loprinzi, P.D.; Guan, H.; Zou, L.; Kong, Z.; Hu, Y. The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina 2019, 55, 43. [Google Scholar] [CrossRef]
- Lei, O.K.; Kong, Z.; Loprinzi, P.D.; Shi, Q.; Sun, S.; Zou, L. Severe Hypoxia Does Not Offset the Benefits of Exercise on Cognitive Function in Sedentary Young Women. Int. J. Environ. Res. Public Health 2019, 16, 1003. [Google Scholar] [CrossRef]
- Nakata, H.; Miyamoto, T.; Ogoh, S.; Kakigi, R.; Shibasaki, M. Effects of acute hypoxia on human cognitive processing: A study using ERPs and SEPs. J. Appl. Physiol. 2017, 123, 1246–1255. [Google Scholar] [CrossRef]
- Burton, R.L.; Lek, I.; Caplan, J.B. Associative independence revisited: Competition between conflicting associations can be resolved or even reversed in one trial. Q. J. Exp. Psychol. (Hove) 2017, 70, 832–857. [Google Scholar] [CrossRef]
- Yoon, J.; Seo, Y.; Kim, J.; Lee, I. Hippocampus is required for paired associate memory with neither delay nor trial uniqueness. Learn. Mem. 2012, 19, 1–8. [Google Scholar] [CrossRef][Green Version]
- Hossmann, K.A. The hypoxic brain. Insights from ischemia research. Adv. Exp. Med. Biol. 1999, 474, 155–169. [Google Scholar]
- Koolschijn, R.S.; Emir, U.E.; Pantelides, A.C.; Nili, H.; Behrens, T.E.J.; Barron, H.C. The Hippocampus and Neocortical Inhibitory Engrams Protect against Memory Interference. Neuron 2019, 101, 528–541. [Google Scholar] [CrossRef]
- Fowler, B.; Prlic, H.; Brabant, M. Acute hypoxia fails to influence two aspects of short-term memory: Implications for the source of cognitive deficits. Aviat. Space Environ. Med. 1994, 65, 641–645. [Google Scholar]
- Aquino Lemos, V.; Antunes, H.K.; Santos, R.V.; Lira, F.S.; Tufik, S.; Mello, M.T. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012, 49, 1298–1306. [Google Scholar] [CrossRef]
- Asmaro, D.; Mayall, J.; Ferguson, S. Cognition at altitude: Impairment in executive and memory processes under hypoxic conditions. Aviat. Space Environ. Med. 2013, 84, 1159–1165. [Google Scholar] [CrossRef]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bartsch, P.; Luks, A.M.; MacInnis, M.J. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef]
- Blough, J.; Loprinzi, P.D. Experimental manipulation of psychological control scenarios: Implications for exercise and memory research. Psych 2019, 1, 279–289. [Google Scholar] [CrossRef]
- Crawford, L.; Loprinzi, P.D. Effects of intensity-specific acute exercise on paired-associative memory and memory interference. Psych 2019, 1, 290–305. [Google Scholar] [CrossRef]
- Dale, E.A.; Ben Mabrouk, F.; Mitchell, G.S. Unexpected benefits of intermittent hypoxia: Enhanced respiratory and nonrespiratory motor function. Physiology 2014, 29, 9–48. [Google Scholar] [CrossRef]
- Taylor, L.; Watkins, S.L.; Marshall, H.; Dascombe, B.J.; Foster, J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front. Physiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Cai, Z.; Manalo, D.J.; Wei, G.; Rodriguez, E.R.; Fox-Talbot, K.; Lu, H. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 2003, 108, 79–85. [Google Scholar] [CrossRef]
- Costa, D.C.; Alva, N.; Trigueros, L.; Gamez, A.; Carbonell, T.; Rama, R. Intermittent hypobaric hypoxia induces neuroprotection in kainate-induced oxidative stress in rats. J. Mol. Neurosci. 2013, 50, 402–410. [Google Scholar] [CrossRef]
- Cummings, K.J.; Wilson, R.J. Time-dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1571–R1580. [Google Scholar] [CrossRef]
- Neubauer, J.A. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J. Appl. Physiol. 2001, 90, 1593–1599. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2019, 39, 9–14. [Google Scholar] [CrossRef]
- Martens, L.K.; Kirschner, K.M.; Warnecke, C.; Scholz, H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J. Biol. Chem. 2007, 282, 14379–14388. [Google Scholar] [CrossRef]
- Clarke, R.J.; Johnson, J.W. Voltage-dependent gating of NR1/2B NMDA receptors. J. Physiol. 2008, 586, 5727–5741. [Google Scholar] [CrossRef]
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.F.; Carvalho, A.L.; Duarte, C.B. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol. Cell. Neurosci. 2007, 35, 208–219. [Google Scholar] [CrossRef]
- Kim, J.H.; Roberts, D.S.; Hu, Y.; Lau, G.C.; Brooks-Kayal, A.R.; Farb, D.H. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J. Neurochem. 2012, 120, 210–219. [Google Scholar] [CrossRef]
- Nakai, T.; Nagai, T.; Tanaka, M.; Itoh, N.; Asai, N.; Enomoto, A. Girdin phosphorylation is crucial for synaptic plasticity and memory: A potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J. Neurosci. 2014, 34, 14995–15008. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Kent, B.A.; Oomen, C.A.; Clemenson, G.D.; Gage, F.H.; Saksida, L.M. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013, 5, 759–768. [Google Scholar] [CrossRef]
- Fulco, C.S.; Beidleman, B.A.; Muza, S.R. Effectiveness of preacclimatization strategies for high-altitude exposure. Exerc. Sport. Sci. Rev. 2013, 41, 55–63. [Google Scholar] [CrossRef]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem. Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef]


| Normoxia (PIO2 = 150 mmHG, FIO2 = 0.21) † | Normobaric Hypoxia (PIO2 = 87 mmHG, FIO2 = 0.12, simulated altitude of 4000 m) ‡ | ||||||
|---|---|---|---|---|---|---|---|
| 30-min exposure † | Memory Task | 20-min rest * | Delayed Memory Recall | 30-min exposure ‡ | Memory Task | 20-min rest * | Delayed Memory Recall |
| Study Set 1: AB, DE | Cued Recall 1: A__, D__ | Study Set 2: AC, FG | Cued Recall 2: A__, F__ | MMFR: A__ __ D__ __ F__ __ |
|---|---|---|---|---|
| BABY HUNTER | SPIDER ________ | FOREST CITY | ARROW _______ | BABY ____ ____ |
| SUPPER SHERIFF | FOREST ________ | ARROW THEATER | FOREST ______ | CHERRY ____ ____ |
| SHOE MOVIE | BABY ________ | BABY SALAD | TIGER ______ | ARROW ____ ____ |
| SPIDER CANDLE | CHERRY ________ | TIGER HOTEL | SUPPER _______ | SUPPER ____ ____ |
| MONKEY GARDEN | MONKEY ________ | MONKEY ENGINE | LADY _______ | TIGER ____ ____ |
| FOREST BATTLE | SUPPER _______ | LADY BUTTER | CANNON _______ | SHOE ___ ___ |
| CHERRY MONEY | APPLE ________ | CANNON HAMMER | BABY _______ | MONKEY ____ ____ |
| APPLE DIAMOND | WEDDING ______ | SUPPER JACKET | MONKEY ______ | LADY ____ _____ |
| SPIDER ____ ____ | ||||
| FOREST ____ ____ | ||||
| APPLE ____ ____ | ||||
| CANNON ____ ____ |
| Variable | Point Estimate | SD |
|---|---|---|
| Age, mean years | 21.0 | 2.1 |
| % Female | 52.4 | |
| Race-Ethnicity, % | ||
| Mexican American | 4.5 | |
| Other Hispanic | 4.5 | |
| Non-Hispanic white | 50.0 | |
| Non-Hispanic black | 9.1 | |
| Multi-race/other | 31.8 | |
| BMI, mean kg/m2 | 26.8 | 5.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loprinzi, P.D.; Matalgah, A.; Crawford, L.; Yu, J.J.; Kong, Z.; Wang, B.; Liu, S.; Zou, L. Effects of Acute Normobaric Hypoxia on Memory Interference. Brain Sci. 2019, 9, 323. https://doi.org/10.3390/brainsci9110323
Loprinzi PD, Matalgah A, Crawford L, Yu JJ, Kong Z, Wang B, Liu S, Zou L. Effects of Acute Normobaric Hypoxia on Memory Interference. Brain Sciences. 2019; 9(11):323. https://doi.org/10.3390/brainsci9110323
Chicago/Turabian StyleLoprinzi, Paul D., Aala’a Matalgah, Lindsay Crawford, Jane J. Yu, Zhaowei Kong, Bo Wang, Shijie Liu, and Liye Zou. 2019. "Effects of Acute Normobaric Hypoxia on Memory Interference" Brain Sciences 9, no. 11: 323. https://doi.org/10.3390/brainsci9110323
APA StyleLoprinzi, P. D., Matalgah, A., Crawford, L., Yu, J. J., Kong, Z., Wang, B., Liu, S., & Zou, L. (2019). Effects of Acute Normobaric Hypoxia on Memory Interference. Brain Sciences, 9(11), 323. https://doi.org/10.3390/brainsci9110323






