1. Introduction
Population ageing is a global phenomenon that has attracted international attention in the twenty-first century. Statistics indicate that older adults (aged >60 years) accounted for 11% of the global population in 2000, and estimates suggest that this population will double and thus account for 22% of the world population by 2050 [
1]. Alzheimer’s disease (AD) is a chronic neurodegenerative disease that severely impairs a person’s cognitive function, thus hindering their self-care ability and inducing disordered psychological behaviour [
2]. According to the WHO, approximately 35.6 million people were affected by dementia in 2012, representing an annual increase of 4.6 million cases. The number of AD patients is expected to double within decades if timely and effective preventive measures and interventions are not provided [
3].
Mild cognitive impairment (MCI) is a transitional stage between normal brain ageing and early-stage dementia (particularly AD). Although people with MCI are affected by symptoms of memory impairment at an earlier than normal stage, their memory functions remain satisfactory and do not severely affect their daily lives or activities [
4]. Older adults commonly experience decreases in cognitive function with ageing, and this trend is particularly evident with respect to memory function [
5]. In the initial stage, AD is frequently associated with MCI and the gradual development of cognitive impairment and functional disorders within a few years [
6]. Therefore, MCI accelerates the risk of AD development, and in particular, memory impairment is a major symptom in patients with MCI. In the absence of timely treatment, 50% of patients with MCI will develop AD within 5 years [
7]. Current studies of AD prevention have shifted from the clinical phase to the preclinical phase, given the particular importance of MCI prevention and reduction in older adults (aged ≥65 years). In other words, early MCI diagnosis and intervention are critical to preventing the development of AD.
Psychomotor speed (PS) is a sensitive index used to measure psychomotor function [
8]. PS tests are used to measure central brain functions, such as the reaction speed and accuracy, during the performance of simple or complicated actions after receiving stimulation. Early studies of PS measurement included only the simple reaction time and choice reaction time [
9,
10,
11]. (Pate and Margolin, 1994; Baddeley et al., 2001; Levinoff et al., 2005). However, these parameters comprise only a fraction of PS behaviours. According to Sobin and Sackeim [
12], who conducted an observation of the psychomotor characteristics of patients with depression, four levels of PS can be observed and measured: gross motor activity, body movements, speech and motor response time. A slow PS manifests in multiple forms, and the external signs mainly involve slowness in basic movements (e.g., whole body activity or fine motor activities), slowness and incoherence in language, and reduced calculation abilities. People with a severely slowed PS may experience a decline in their performance of daily activities (e.g., bathing, getting out of bed, dressing and answering phone calls) and may be incapable of making prompt decisions in response to unexpected incidents [
13]. (MBF, 2008). Patients with MCI exhibit evident deficiencies in their abilities to perform dual tasks [
14]. Moreover, a slow PS may be particularly evident in patients with mental illnesses, such as depression [
15,
16] or schizophrenia [
17]. Studies have indicated that depression is a risk factor for the development of dementia and MCI, and that the cognitive function symptoms exhibited by patients with depression and MCI are highly consistent with the symptoms associated with a slow PS [
18,
19]. Therefore, this study used an improvement in PS as the basis for establishing an intervention strategy intended to delay and prevent continuous impairments in cognitive function in patients with MCI.
Recent studies have explored the critical ability of physical exercise to improve cognitive abilities and brain function. Numerous studies have verified the protective effect of physical exercise on cognitive function in later life [
20,
21], as well as the different effects of variation in the form, intensity and frequency of exercise [
22,
23]. The effects of an exercise intervention on the cognitive functions of young and older adults have been investigated by several studies. Levin, Leon, Karssemeijer and their teams [
24,
25,
26] utilizes systematic review and meta-analysis to analyse the combined cognitive and physical exercises on cognitive function in older adults with MCI or dementia. The results of their studies reported positive cognitive benefits for older people with MCI or dementia.
Opportune exercise training has critical effects on the cognitive functions of older adults. In older adults, physical condition is related closely to the degeneration of hippocampal brain tissue. Exercise can induce fibroblast growth factor expression in the hippocampus and increase the hippocampal volume in older adults. Consequently, these effects alter the brain structure, enhance neuronal vitality and delay the degeneration of cognitive function in this population. Therefore, exercise is clearly a valuable component of MCI prevention [
27].
Currently, no treatments prevent or reverse the course of AD, and only a few options can be used to ease and improve symptoms temporarily [
28]. MCI intervention strategies comprise both drug and non-drug interventions, such as physical training, cognitive interventions and psychological interventions. Most studies have recommended that MCI treatments focus primarily on non-drug interventions to prevent and reduce the side effects of drugs [
29,
30]. There are several studies that used psychomotor speed, processing speed and reaction time to measure the effects of physical training for the improvement of participants [
31]. Pluncevic-Gligoroska [
16] and Poon’ studies aimed to examine the possible relationship between psychomotor speed expressed by the Trial Making Test (TMT), the results suggest that regular physical activity have a positive impact on cognitive processes. Poon’s studies also conclude that the psychomotor speed of fit young and older adults is faster than the speeds of their less fit age-mates, and that physical training programs results in the improvement of participants’ response speeds.
Although several studies have investigated the beneficial effects of different types of physical exercises (e.g., Tai Chi, bowling/croquet, cycling, resistance training, waling, psychomotor exercises and aerobic exercises) on psychomotor and cognitive functions in old age [
24,
25,
32,
33], previous studies have rarely reported using a structured therapeutic psychomotor limbs-exercise programme as a treatment on the effects of older adults with MCI who reside in urban communities and nursing homes. In addition, singular exercise training methods have been used in previous studies, and the conventional content designs of these methods have frequently neglected mind and body related exercises and PS, a sensitive indicator of cognitive function. This study had the following goals:
To determine whether PS is correlated with cognition level in patients with MCI and whether it can be regarded as an indicator to measure the cognitive functions of patients with MCI;
to assess a potential structured limbs exercise programme to improve the cognitive functions of MCI patients according to their PS and cognition level; and
to evaluate a limb exercise project intended to improve the PS of patients with MCI to improve cognitive functions such as cognition level and electroencephalography (EEG) results.
2. Materials and Methods
2.1. Participants
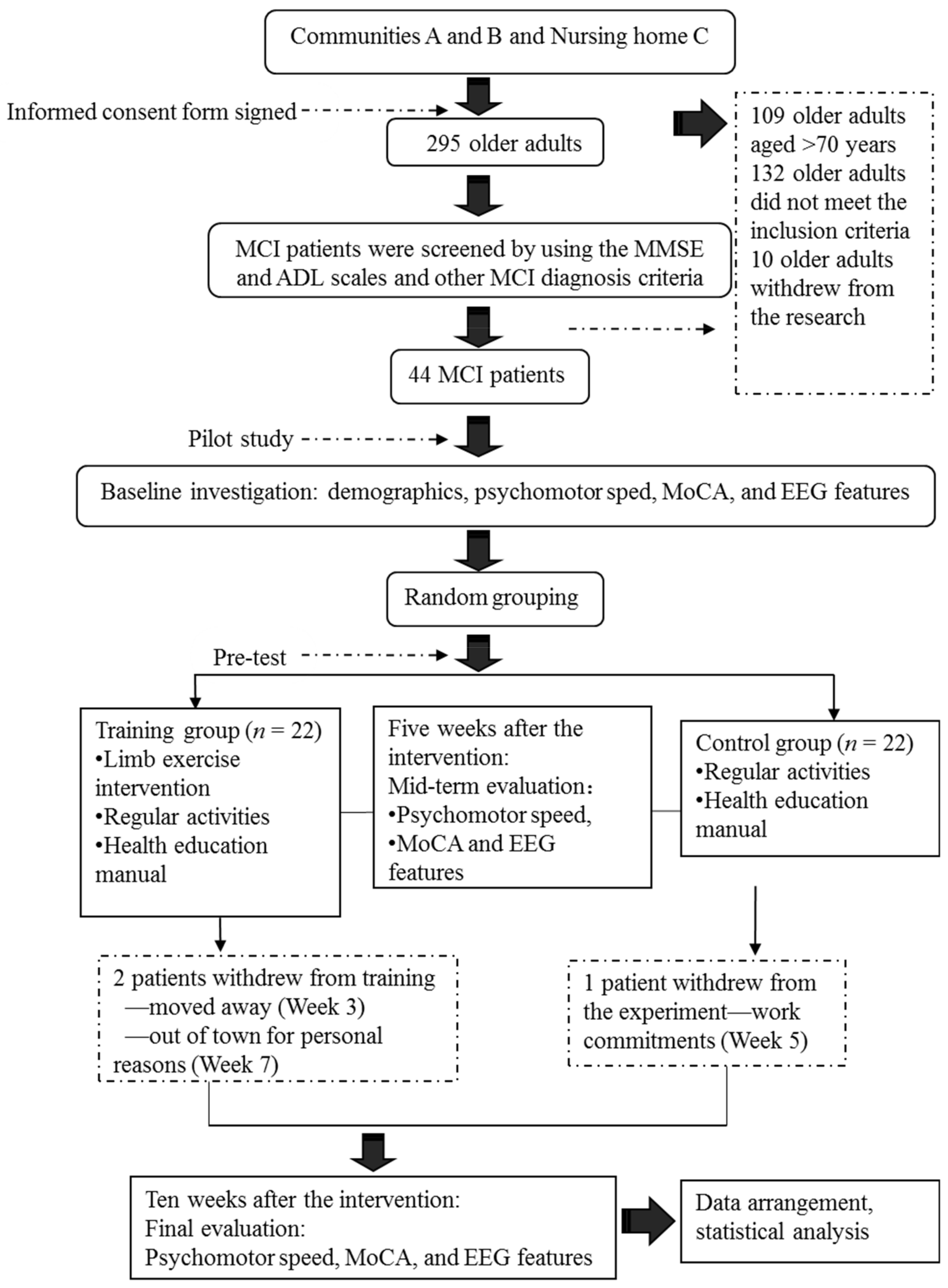
Our screening of older adults residing in Communities A and B and Nursing Home C yielded 44 elderly patients with MCI. We divided the subjects randomly into a limb exercise training intervention group and a control group. The criteria for participant recruitment are shown in
Table 1.
Sample Size Calculation: Prior to the data collection, power analysis was performed to determine the sample size. Based on our pilot study of the experimental design, we determined a large effect (Cohen’s
f = 0.502) of the intervention on the cognition level (measured using the Montreal Cognitive Assessment (MoCA)). At an α value of 0.05 and β (power) value of 0.95, our power analysis indicated that 19 participants per group would be required to detect a large effect (
N = 38). The decision was made to oversample (
N = 44) to accommodate an expected attrition rate of 15%. The 44 subjects resided in 15 residential quarters of two urban communities. Equal numbers of patients were included in the control and experimental groups because classrooms were assigned randomly to the two groups (
Table 2). A power analysis indicated that the sample size was adequate for the proposed study.
2.2. Instruments
We randomly selected 44 elderly participants aged 60–70 years. The Mini Mental State Examination (MMSE) and Activities of Daily Living (ADL) Scale were used to screen subjects for the study. The following test instruments were used to collect data at the baseline, interim and post-intervention time points and to verify the results of the intervention: for PS, the Finger Tapping Test (FTT) and Purdue Pegboard Test (PPT); and for cognitive level, MoCA and EEG (Emotive EPOC, Emotive Company, SF, USA.
2.3. Structured Limbs-Exercises
The limb exercise at this stage aims to develop the task execution capacity, attentiveness, fine motor skills, hand–eye coordination, reaction time, balance, and upper and lower body coordination of MCI patients (
Table 3).
2.4. Experimental Design
This study adopted a quasi-experimental design that included both a randomised control trial and a questionnaire. Under this quasi-experimental design, the study applied grouped comparisons among the baseline, interim and post-test time points, and used a repeated measures analysis of variance (ANOVA) for the data analysis. Subjects were allocated randomly to the experimental and control groups. Details of the test arrangements are shown in
Table 4.
2.5. Intervention and Procedures
Five patients with MCI who satisfied the recruitment criteria and passed a screening protocol based on the MMSE and ADL scales were included in a pilot study. This pilot study was conducted to improve the preliminary procedures at an early experimental stage, derive relevant assumptions and increase the rates of success of subsequent experiments in the official programme. The formal research intervention was commenced upon completing the pilot study.
During the formal experiment, the subjects in the control group participated in regular activities at a community senior activity centre (e.g., reading, chess and card games, singing and dancing). In addition to these regular daily activities, the subjects in the experimental group participated in a limb exercise intervention. They were divided into several subgroups (n = 7 or 8 per group). The duration of each limb exercise session was 60 min, and the sessions were held three times per week. During the first week, the researcher taught the limb exercise to the subjects. Beginning in the second week, subgroups of subjects were asked to participate in the limb exercise intervention. The subjects were also required to keep a record of their training conditions. The researcher and group heads monitored and instructed the subjects in each group.
The research intervention procedures are described in
Figure 1.
2.6. Statistics and Data Analysis
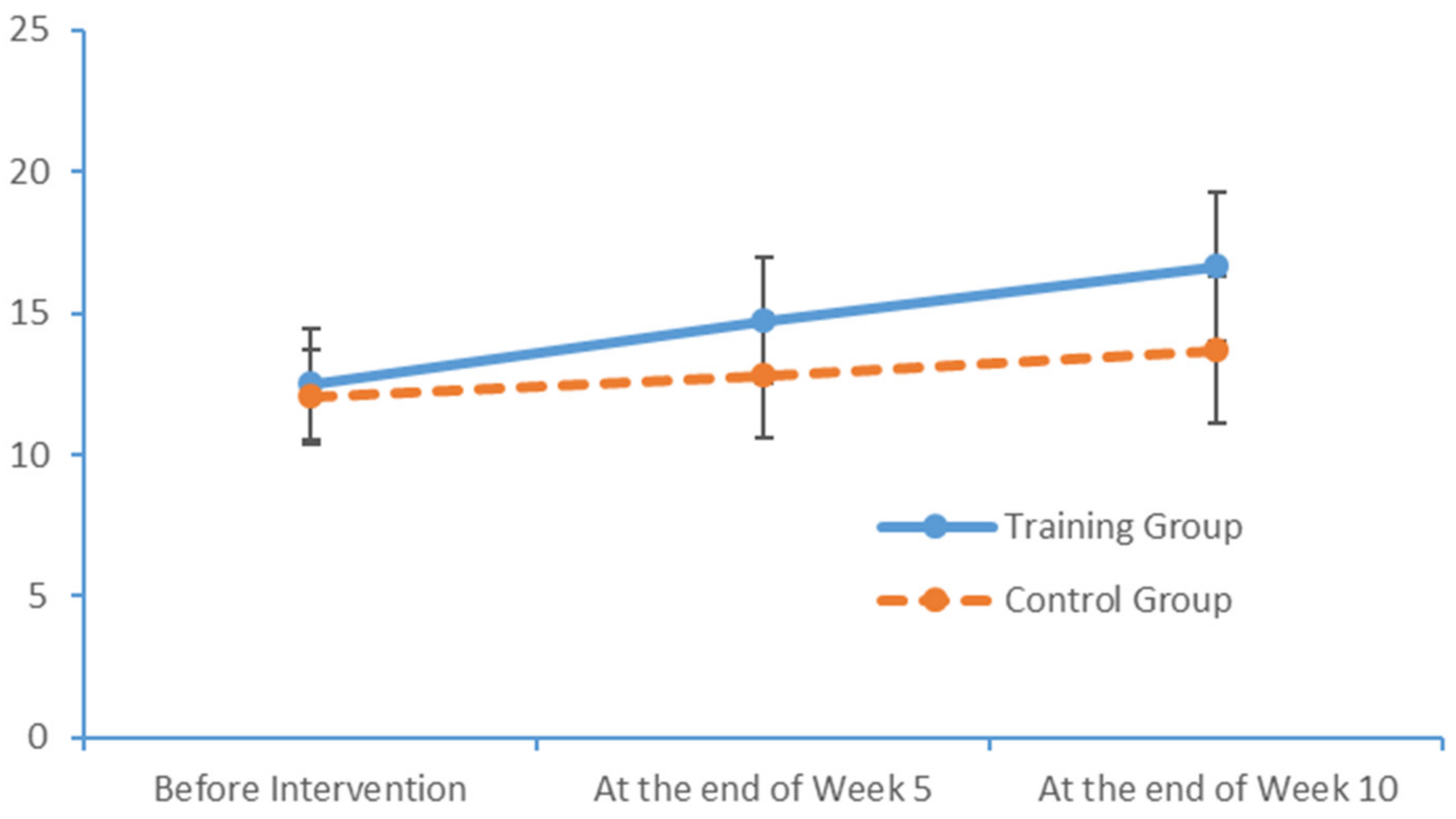
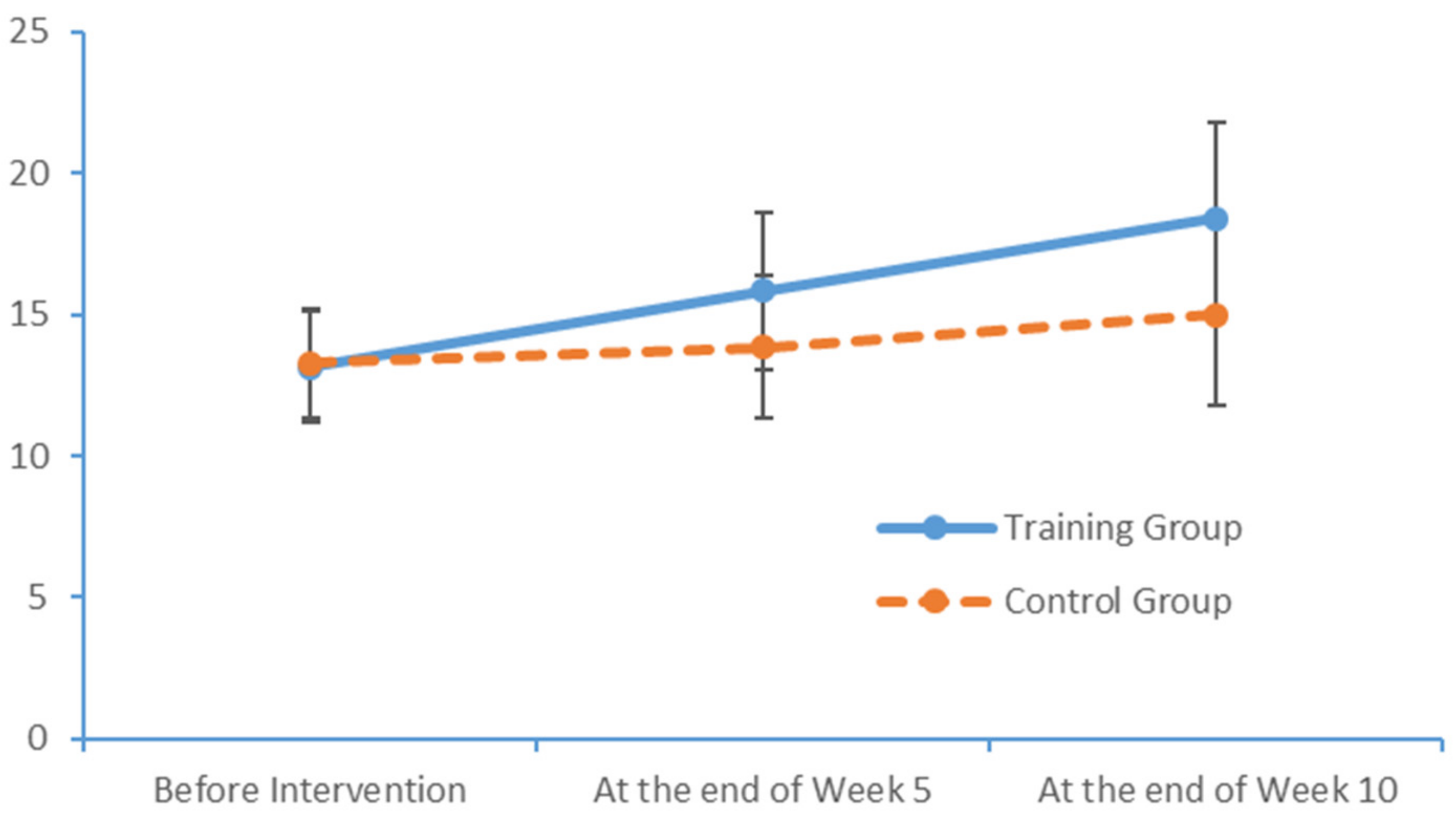
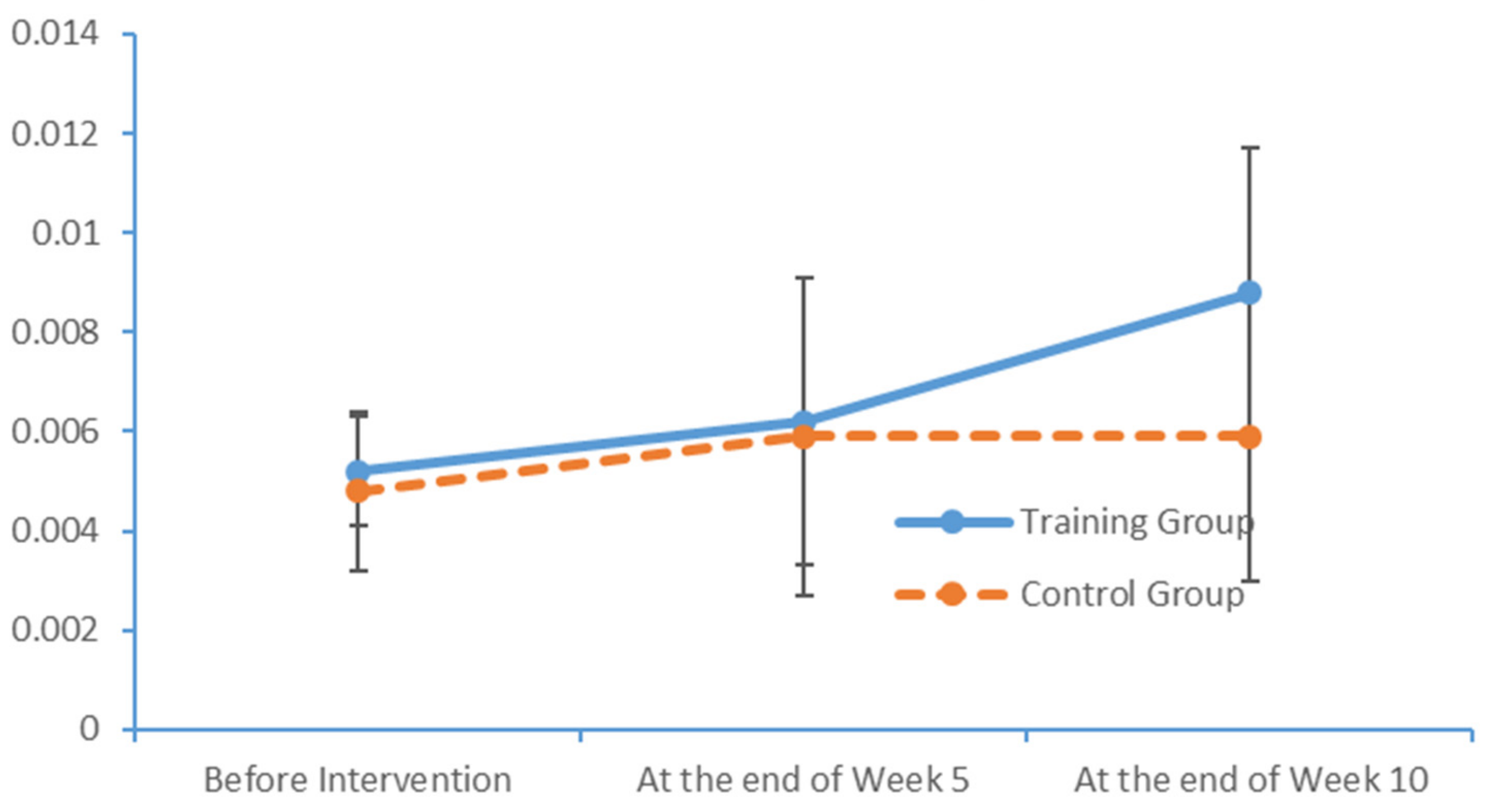
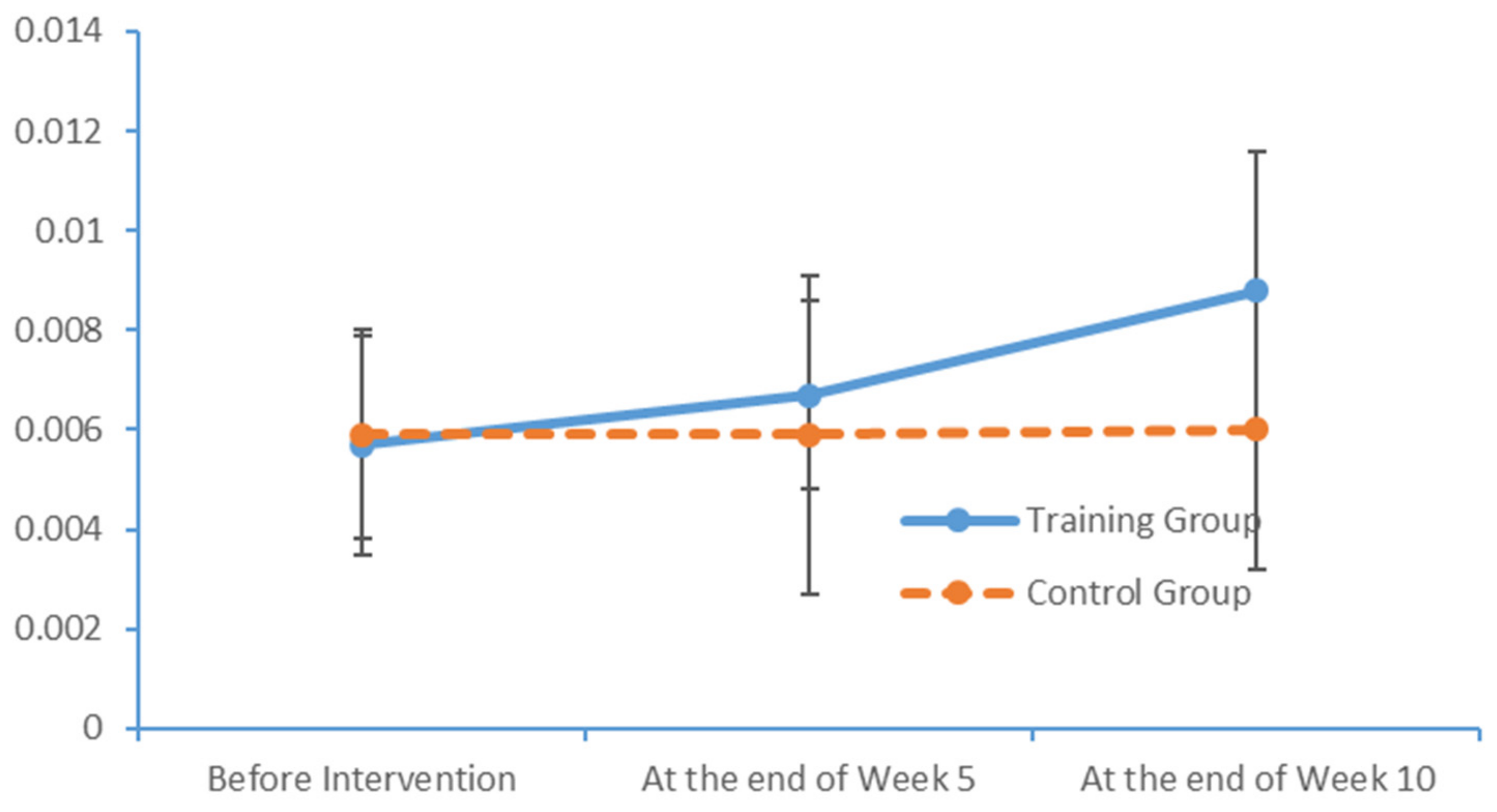
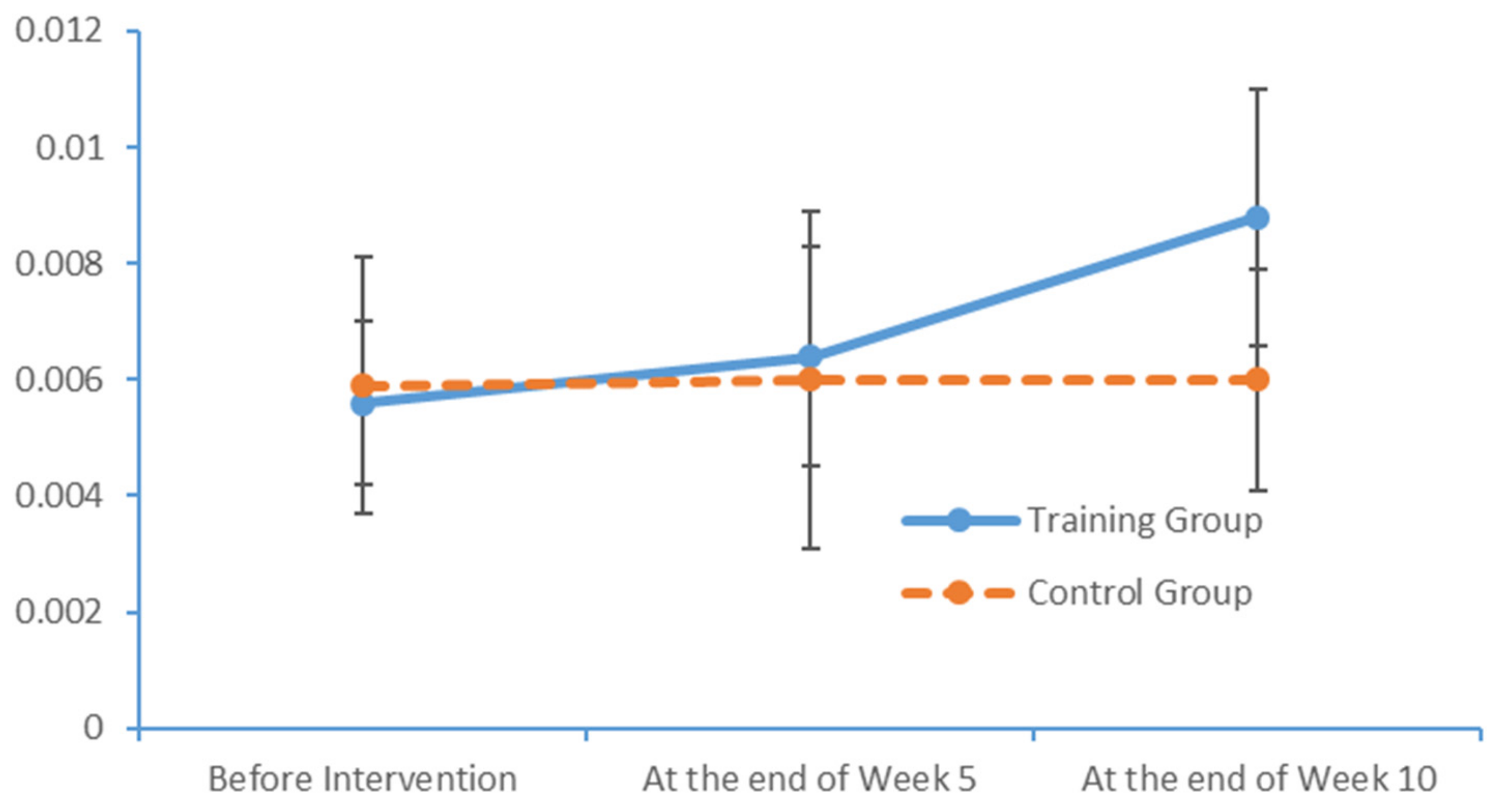
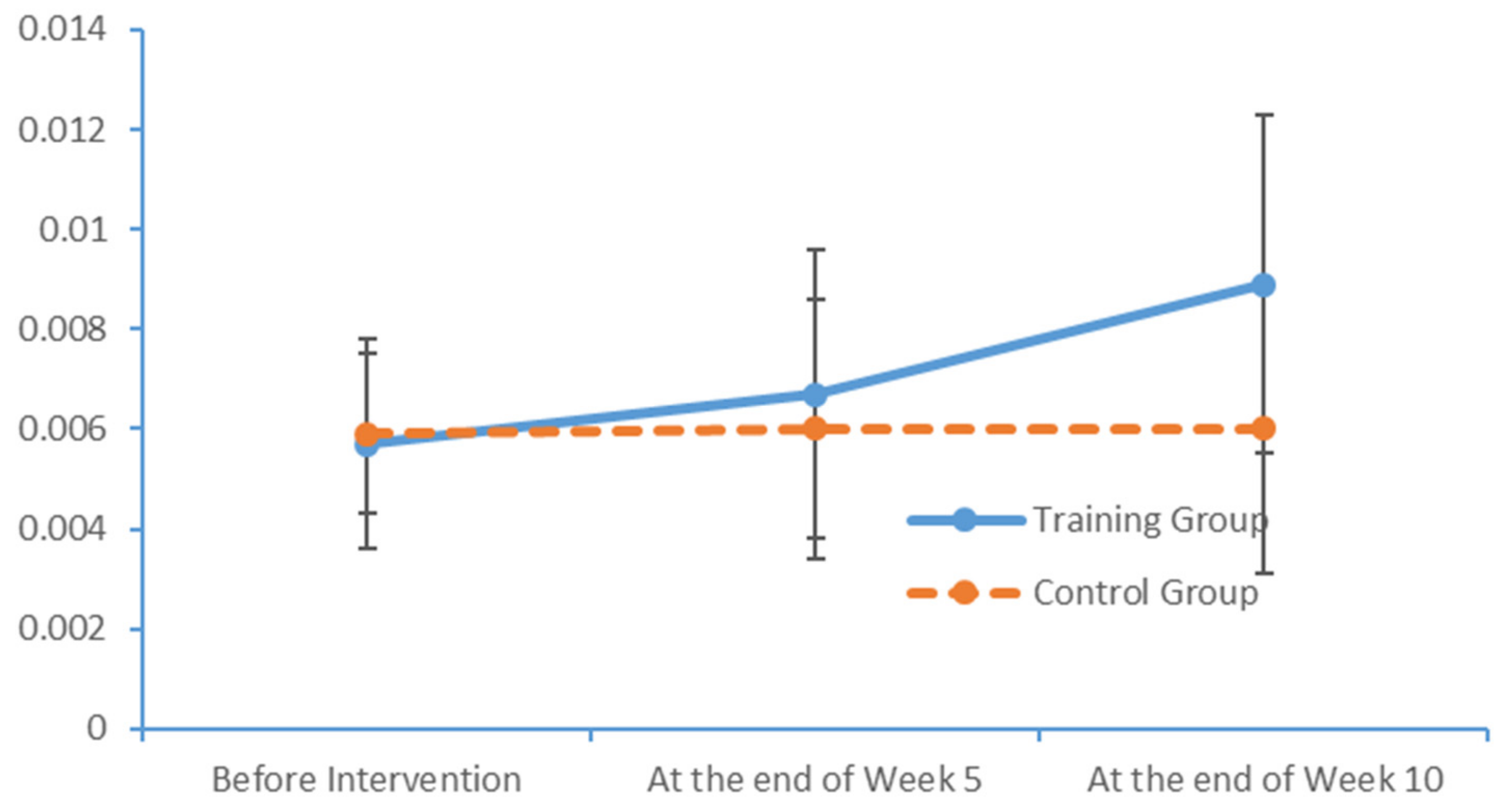
SPSS Version 18 was used to organise and analyse the data. The χ2 test and an independent sample t-test were used to compare the baseline levels of all variables between the two groups. To evaluate the measurements and ranked data with non-normal distributions, the Mann–Whitney U rank-sum test was used to balance the distributions of the related variables between the groups before the intervention. Spearman’s rank correlation coefficient was used to investigate the relationship between the subjects’ PS and cognition levels.
Significant differences in the subjects’ PS, MoCA scores and EEG features were analysed to evaluate the influence of the limb exercise intervention on cognitive functions.
4. Discussion
In this study, we evaluated the forms, content and intensity of limb exercises designed to foster an increase in PS. The objectives of this study were to investigate the ability of these exercises to maintain the cognitive functions of patients with MCI, and to expose the effects of the exercises on cognitive functions in this patient population.
4.1. Correlation between the PSs and Cognitive Functions of Patients with MCI
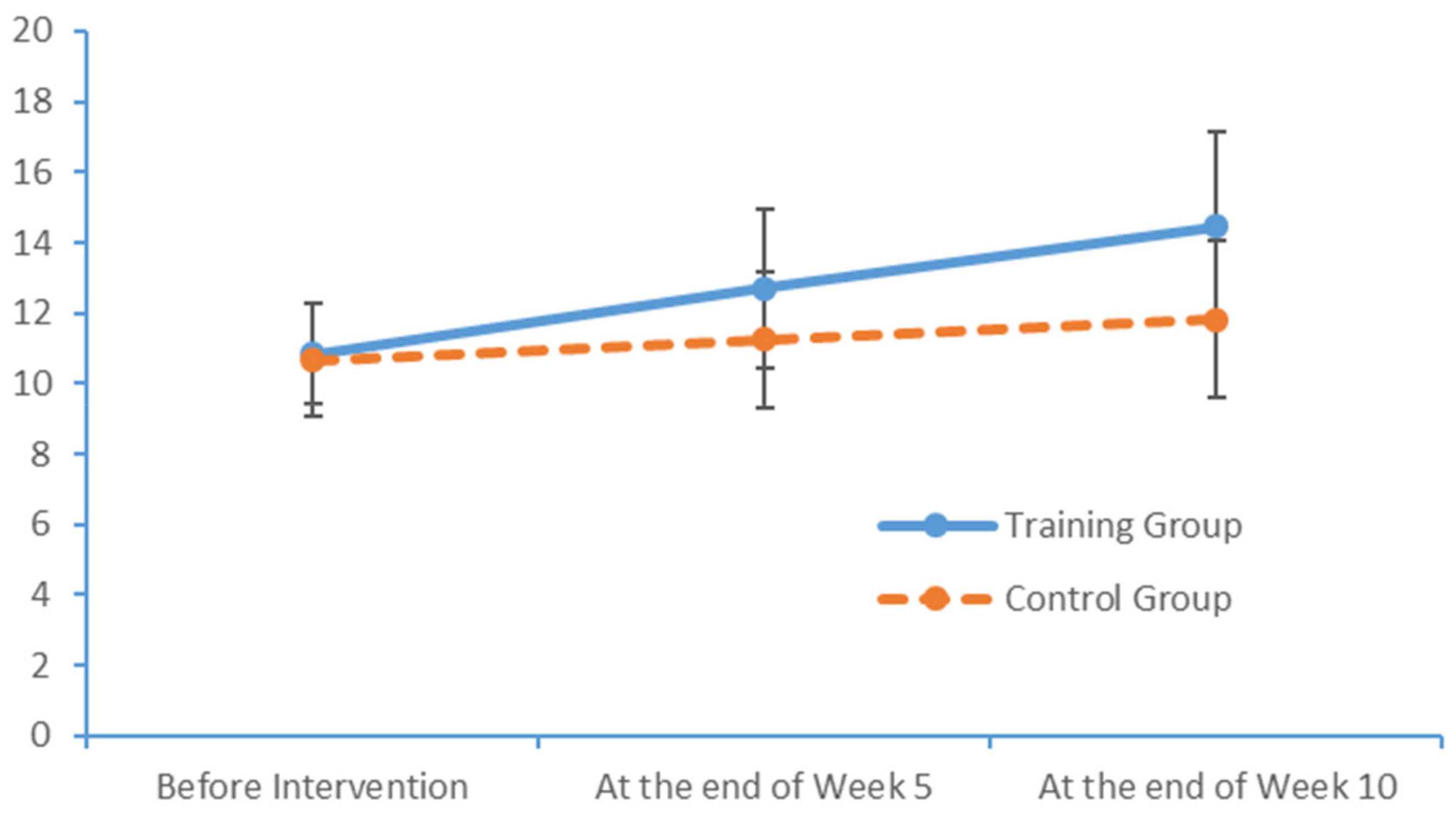
In our study, the patients with MCI had mean finger tap frequencies of 45.00 for the left hand and 48.39 for the right hand, compared with an average of 37 per 10 seconds measured among healthy Chinese adults aged >45 years [
31,
32,
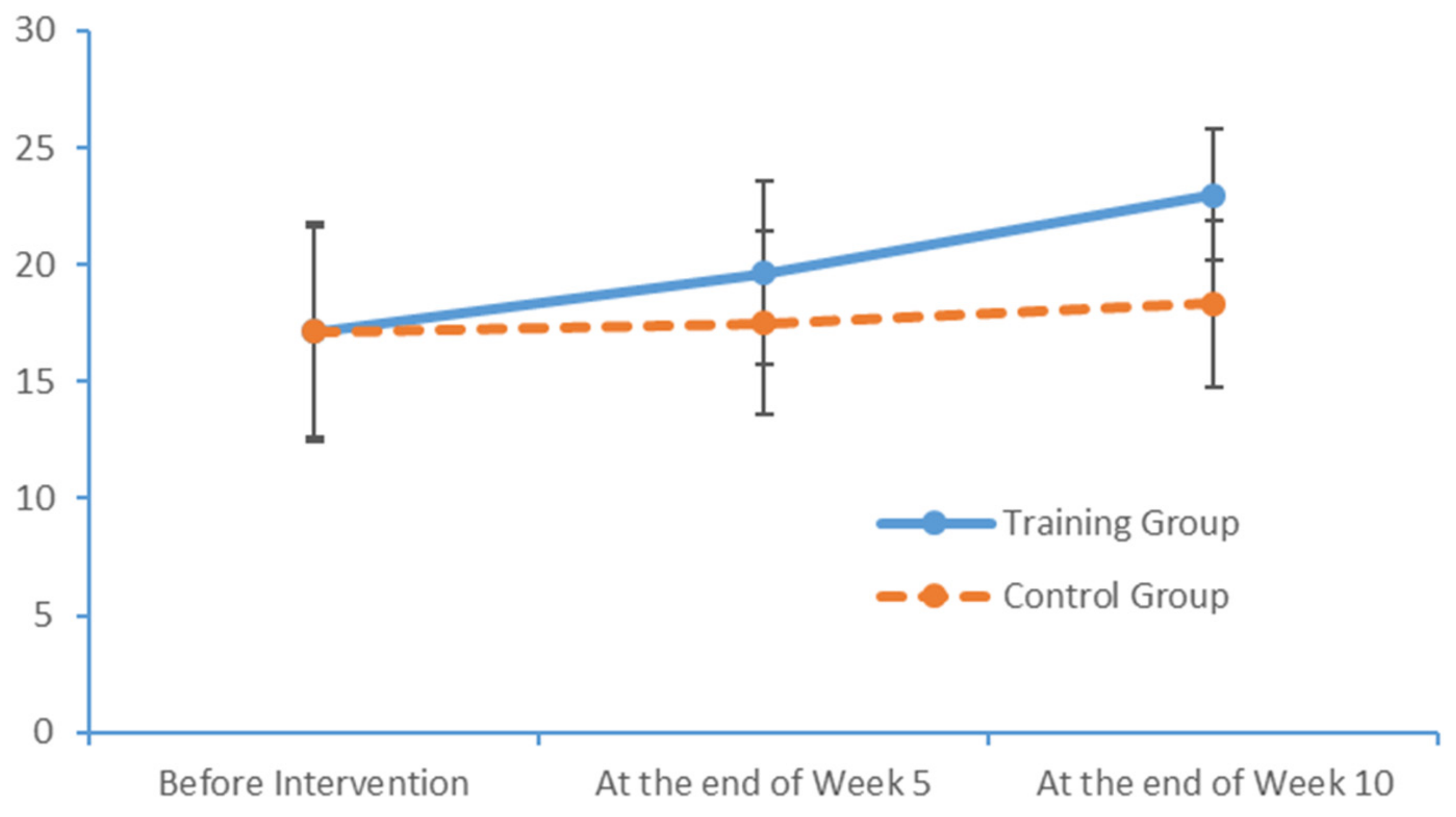
33]. In other words, PS was far lower in the patients with MCI than in healthy adults. In the PPT, the subjects in our study achieved results similar to those of healthy older adults, as reported by Sun and Desrosiers [
34,
35]. That earlier study, however, did not test the cognitive functions of healthy elderly adults. Consequently, the PPT results of subjects in our study cannot yet be compared with those of healthy elderly adults with a normal level of cognitive function.
In our study, the MoCA scale scores revealed a relatively obvious decline in visuospatial and executive functions, consistent with the results of previously reported studies [
36]. Most of the patients with MCI exhibited normal levels of ability with respect to place orientation but were unsure of the day of the week. This type of confusion might be due to the retired status of elderly adults and an associated lack of concern regarding the day of the week. In summary, our findings suggest that patients with MCI have accumulated damage in multiple cognitive domains, although the levels of damage vary between these domains. The most severe and prevalent damage was observed in the domains of delayed recall and visuospatial and executive function. An understanding of the characteristics of cognitive impairment in elderly adults with MCI could aid in the identification and diagnosis of community-dwelling patients, who could then be referred for an early-stage intervention. Moreover, our study results revealed significant correlations between the scores obtained in all dimensions of the FTT and PPT and all dimensions of the MoCA total score. Furthermore, the scores for all dimensions of the FTT were significantly correlated with those for all dimensions of the PPT. These results confirm that PS is a valid indicator of cognitive function and can be used for the preliminary screening of patients with MCI.
The results may also support an analysis of the predictive value of the PS for the diagnosis of MCI. Therefore, an understanding of the characteristics of PS in elderly adults with MCI would assist in the early identification of affected patients, as well as early disease interventions.
4.2. Influences of Limb Exercise Training on the PS of Patients with MCI
During the limb exercise training project, the PPT and ball-holding exercises mainly targeted the fine motor control abilities of the hands, which indicate a person’s capacity to complete a specific task mainly via movements of the minor muscles in the hands and fingers in coordination with psychological activity (e.g., perception and attention). Previous studies [
37] have investigated the influence of hand activities, such as clapping, forming and releasing a fist, and consecutive finger reversal with both hands, on cerebral cortex activation. Notably, clapping exerts the maximum effect on functions throughout the brain. Specifically, clapping induces maximum changes in the motion system and premotor areas, whereas consecutive finger reversal with both hands induces a maximum change in the prefrontal area. Activity in these brain areas is closely related to cognitive function. In this study, the fine motor abilities of patients with MCI were identified, and structured exercises were then designed to target fine motor training specifically. Statistical feedback from the FTT was examined and used to train the subjects with respect to the fine motor control abilities of the hands. This intervention profoundly prevented and mitigated the decline in cognitive function and fine motor ability in the hands observed in patients with MCI.
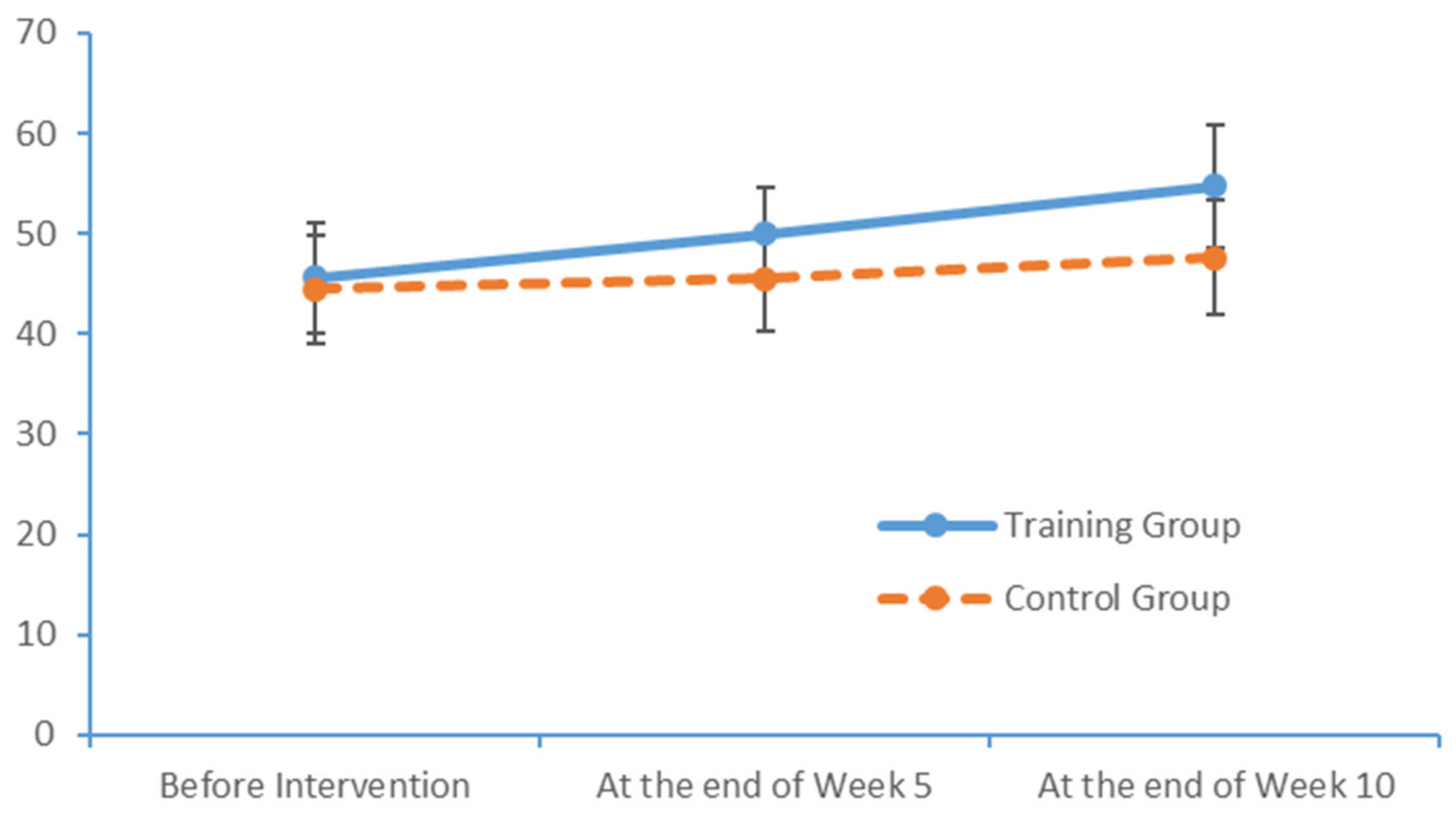
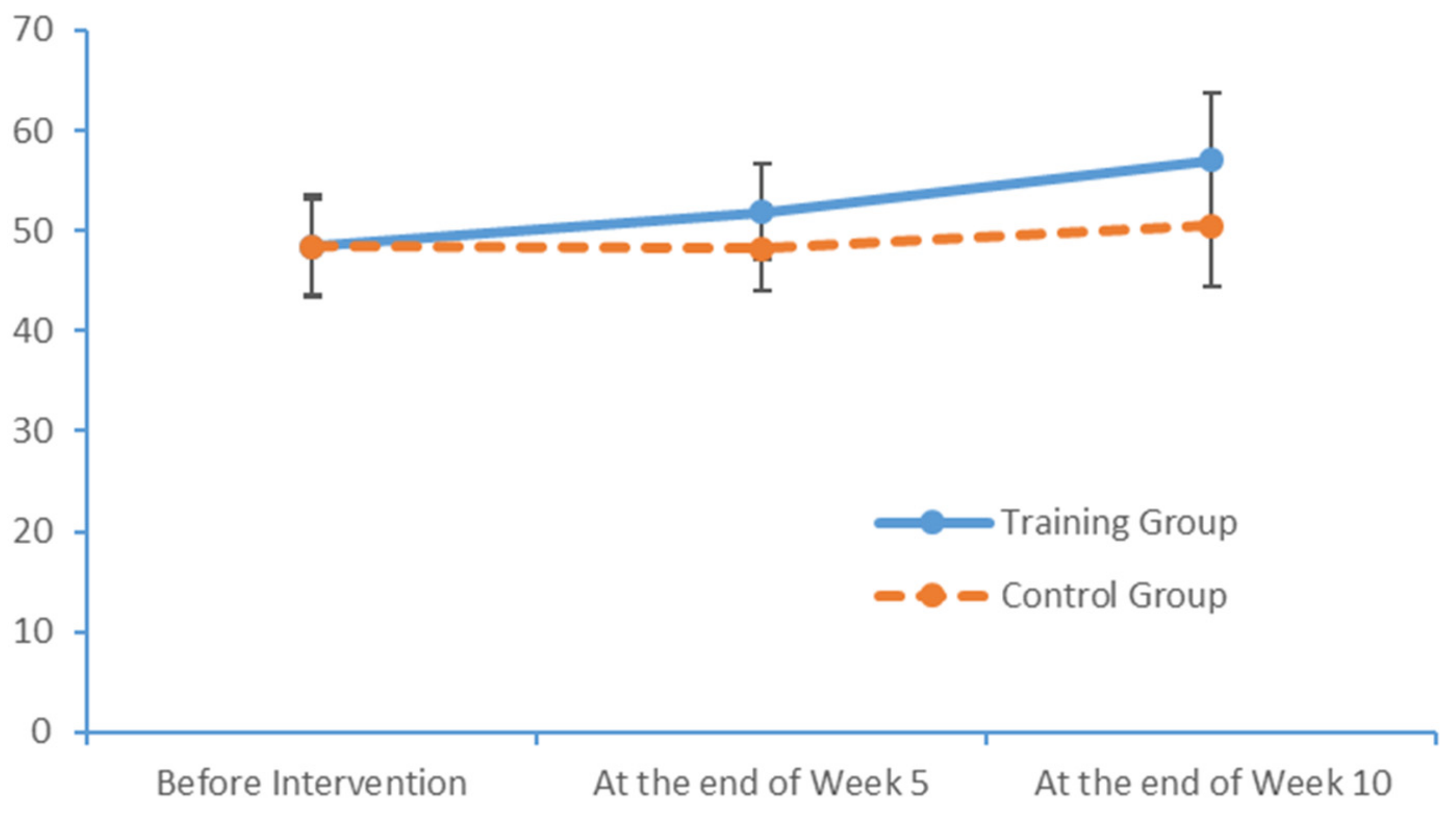
4.3. Influence of the Limb Exercise Training Intervention on the Cognition Levels of Patients with MCI
After a 10-week limb exercise intervention, patients with MCI in our training intervention group exhibited an improvement of 5.85 points in the total MoCA score. Moreover, this improvement was statistically significant relative to the outcome of the control group. This study referenced the structured limb exercise interventions proposed by foreign scholars, Chinese cultural customs and the conditions of Chinese community- and nursing home-dwelling patients with MCI when designing the intervention. The results confirm that this limb exercise training intervention improved the cognitive functions of patients with MCI, consistent with the findings of several previous studies [
38,
39].
4.4. Effect of Limb Exercise Training on the EEG Activities of Patients with MCI
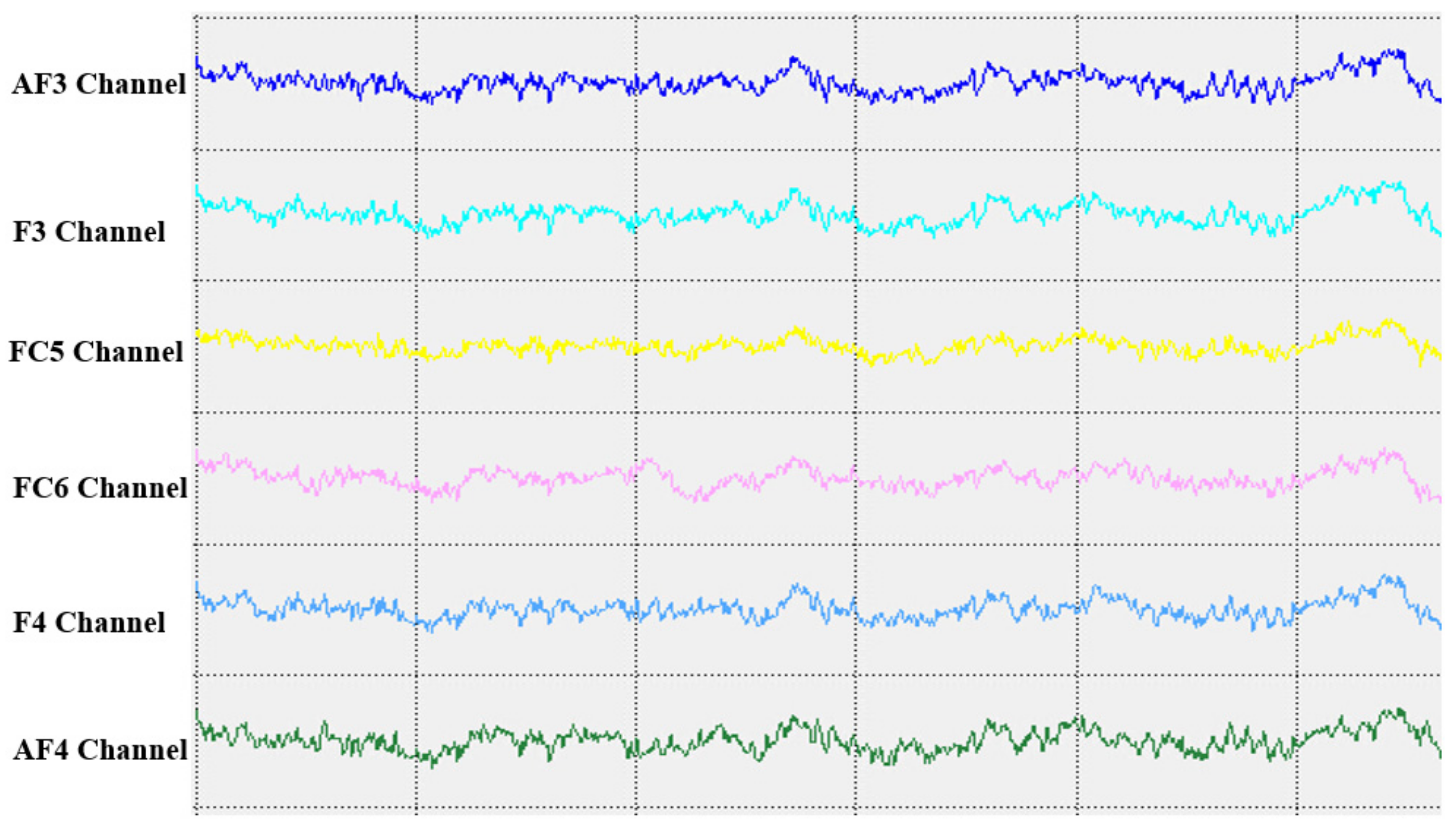
In the cerebral hemispheres, bioelectrical activities are triggered by cerebral cortical pyramidal cells and postsynaptic potentials and are regulated by nonspecific thalamic nuclei. The rhythms of EEG activity are affected by stimuli and feedback among the thalamus, brainstem reticular formation and cerebral cortex. Berger discovered that cerebral bioelectrical activities could be recorded via the scalp (
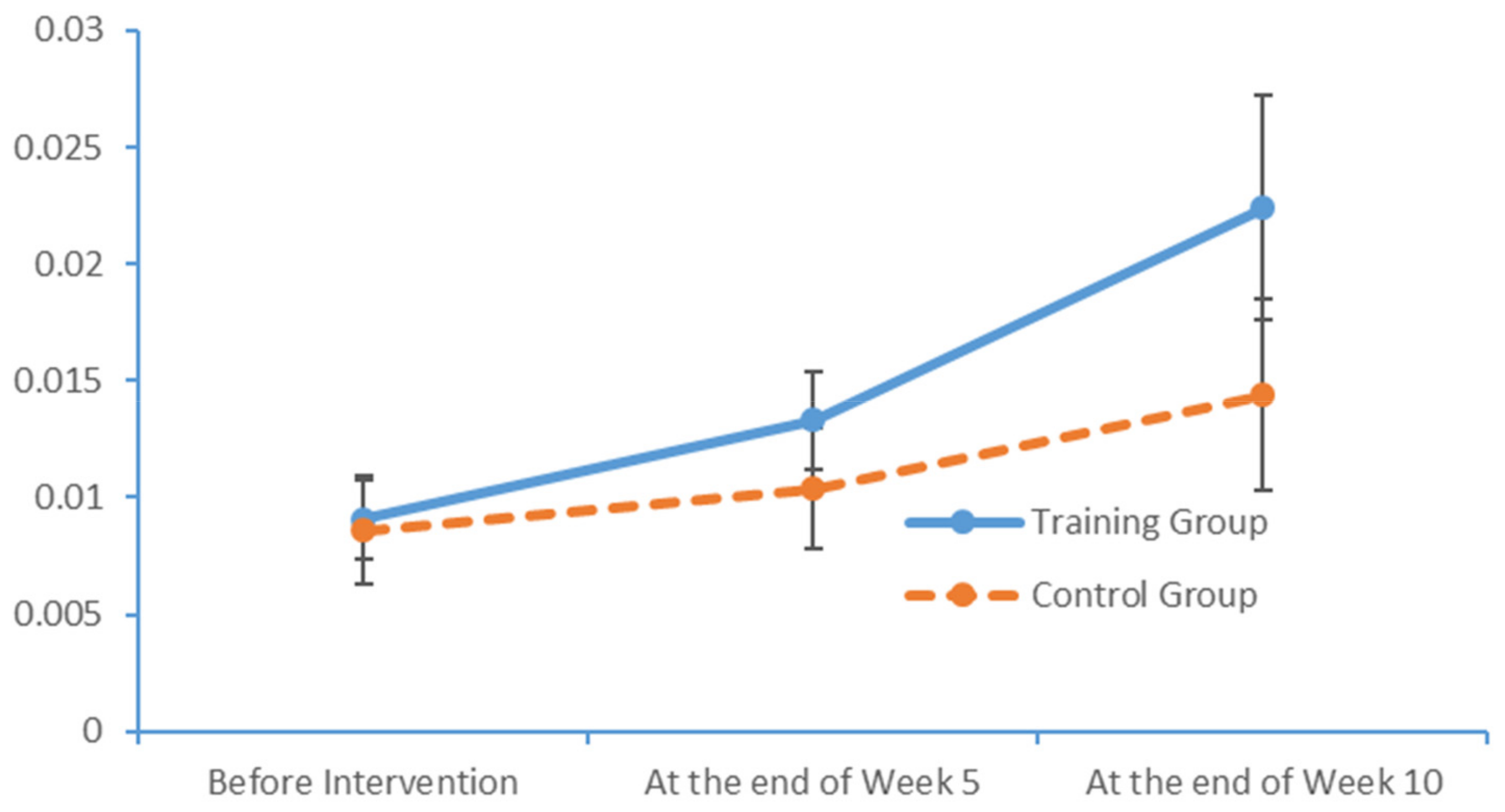
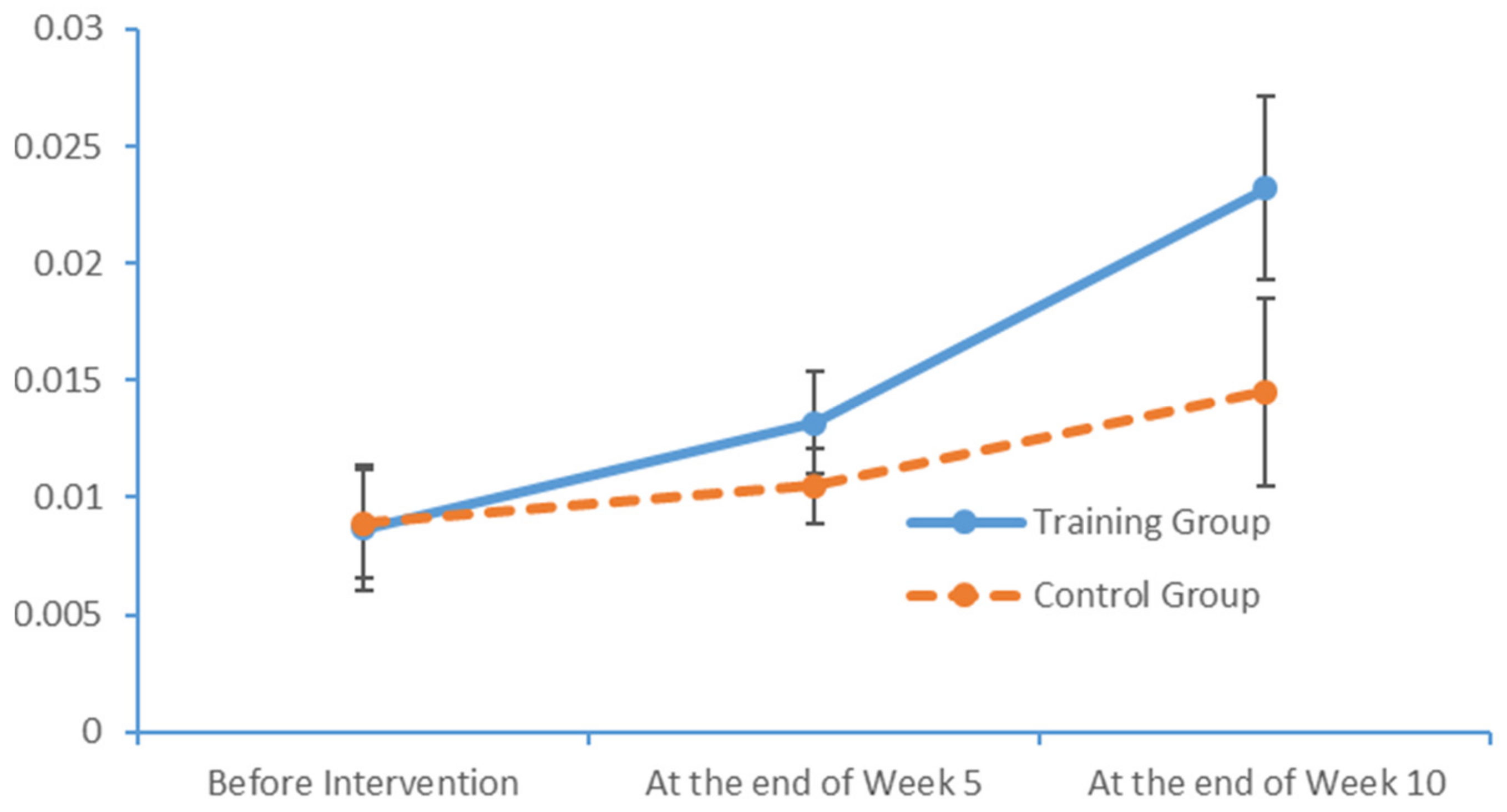
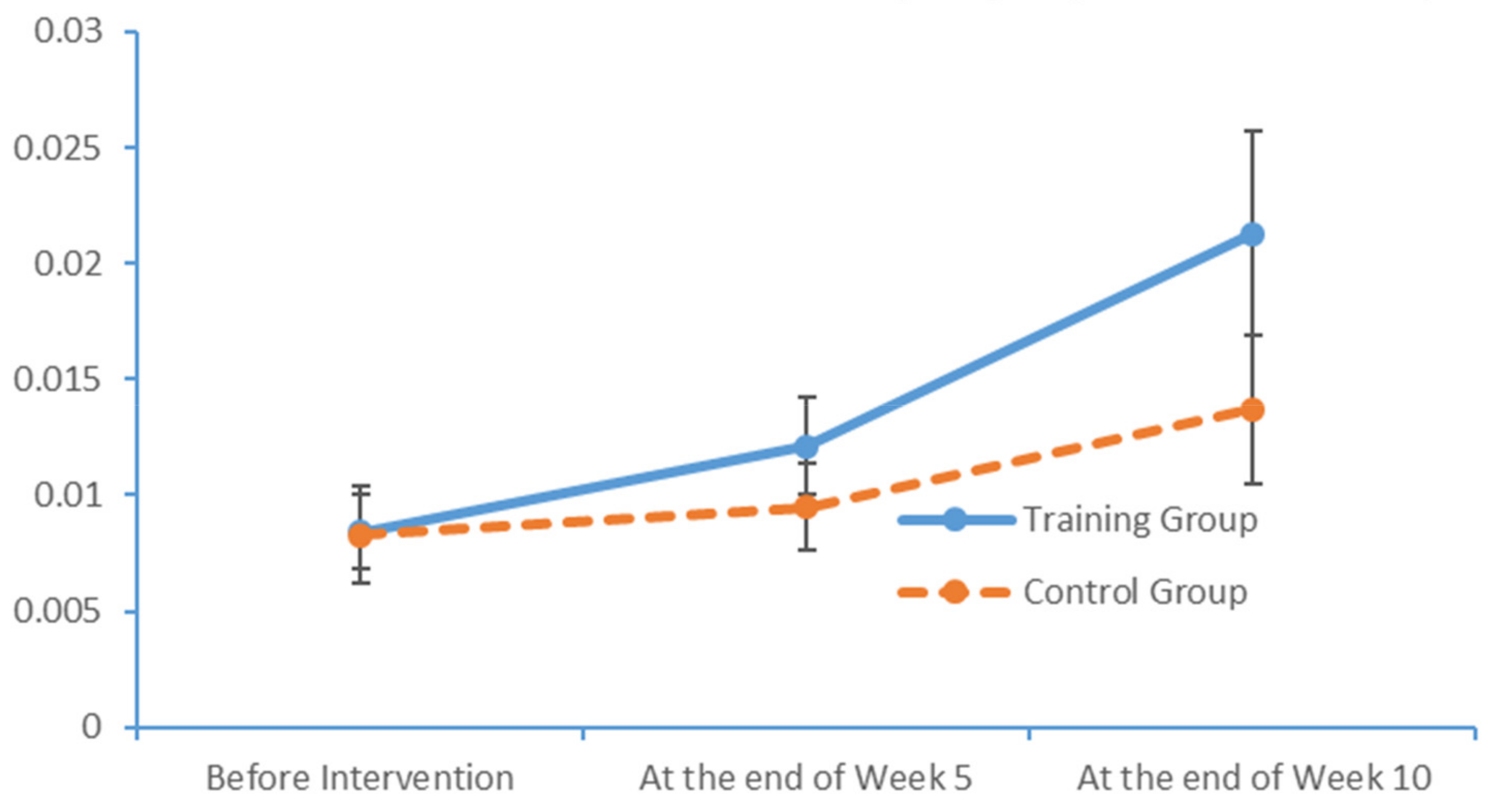
www.epub.org). In a healthy adult, the EEG mainly comprises alpha and beta waves, of which the former comprises the majority. The two brainwaves are distributed in different areas of the brain. Alpha waves are distributed mainly in the occipital and parietal lobes, whereas beta waves are located mainly in the frontal and temporal lobes. The activities of alpha and beta waves are stable and change in response to pathological alterations in the brain. The run charts generated in this study explicitly revealed changes in the alpha wave power values in all brain regions over time, as well as prominent variations in the data collected from the training group over time. As patients with MCI face a high risk of AD, an understanding of the EEG characteristics in this population is highly valuable for MCI prevention.
4.5. Design of Limb Exercise Training Interventions Intended to Enhance the Health of Older Adults
Physical activity is critical to physical development in older adults. This type of activity can alter the social status and behaviour of this population and yield improvements in the quality of life. This study explored the physical and psychological development of older adults and used various theories and skills from physical training studies to provide education, consultation and training related to physical health knowledge and skills. Consequently, the intervention developed in this study helped the participants to cultivate regular exercise habits and improve their physical capacities and cognitive functions, while ensuring harmonious physical and psychological development.
For older adults, a combination of limb exercises and health-related activities enables the development of a comprehensive education system that further enhances conventional nursing skills and yields improvements in physical status, cognitive functioning and overall health quality. This type of intervention enables the development of training programmes from diverse perspectives (e.g., physical skills, cognition, behaviour and emotions) and the incorporation of numerous scientific subjects, including physical education, psychology, physiology and nutrition.
Limb exercise training is a scientific approach in which nursing personnel are provided with pre-service training in the aspects of caring for older adults and integrating physical activities with health education. Nursing personnel who participate in this pre-service training must then apply the newly acquired training methods. Moreover, the inclusion of whole-body physical activities has increased the flexibility and creativity of these training methods. Although previous studies have not proposed alternative limb exercise training with the intent to improve the MCI conditions of older adults, our results suggest that nursing homes should continue to incorporate these limb exercises experimentally in combination with other physical activities intended to improve the PS of patients with MCI.