Mid-Frontal Theta Modulates Response Inhibition and Decision Making Processes in Emotional Contexts
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
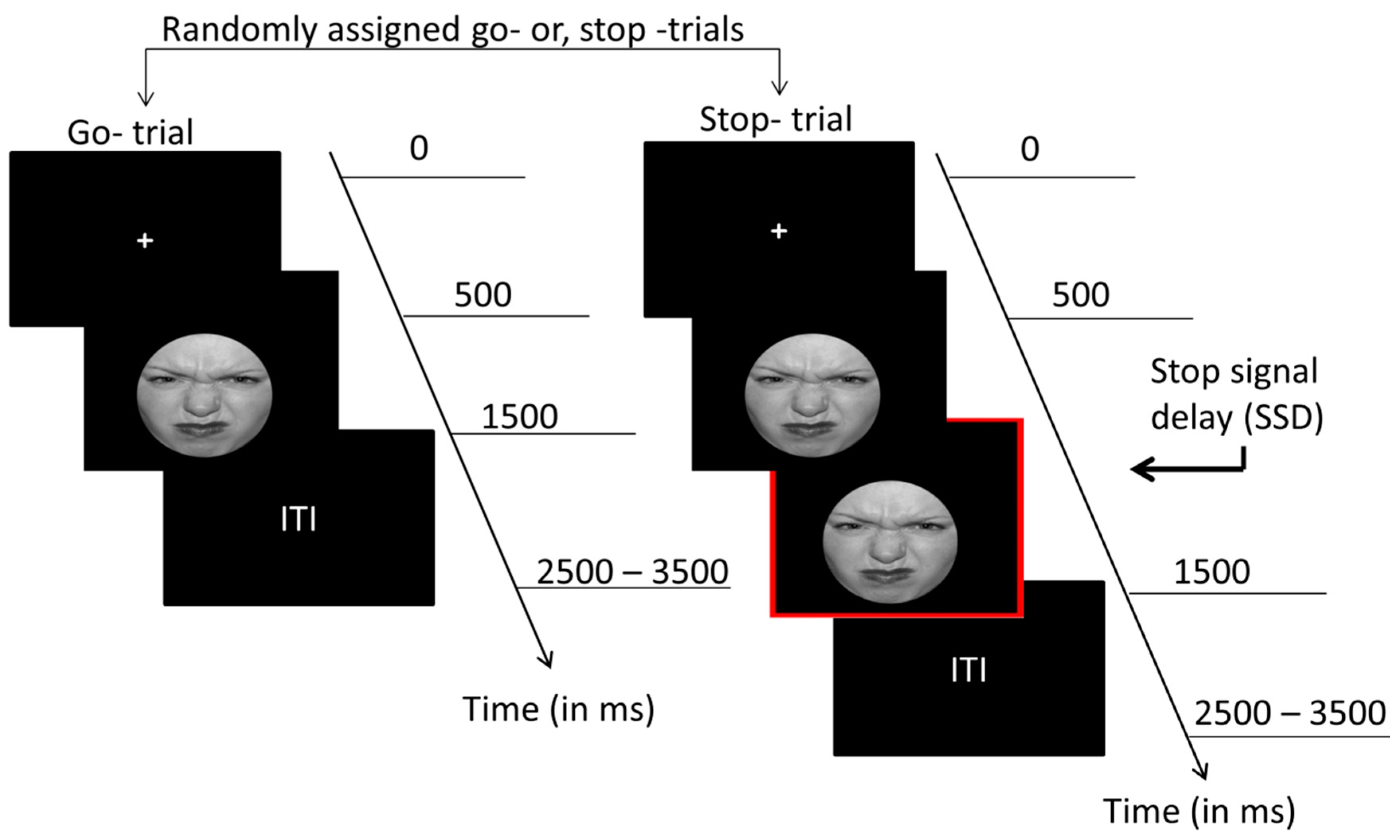
2.2. Experimental Design
2.3. Recording and Analysis
2.3.1. EEG Recording
2.3.2. EEG Analysis
2.3.3. Group-Level Event-Related Spectral Perturbation (ERSP)
2.3.4. Single-Trial ERSP
2.3.5. Hierarchical Drift Diffusion Model (HDDM) Analysis
3. Results
3.1. Behavioral Analysis
3.2. Group Level ERSP Results across Conditions
3.2.1. Stimulus-Locked ERSPs
3.2.2. Response Locked ERSPs
3.3. Exploring Trial-by-Trial Analysis of Correct Go Trials for Happy, Disgust and Neutral Conditions
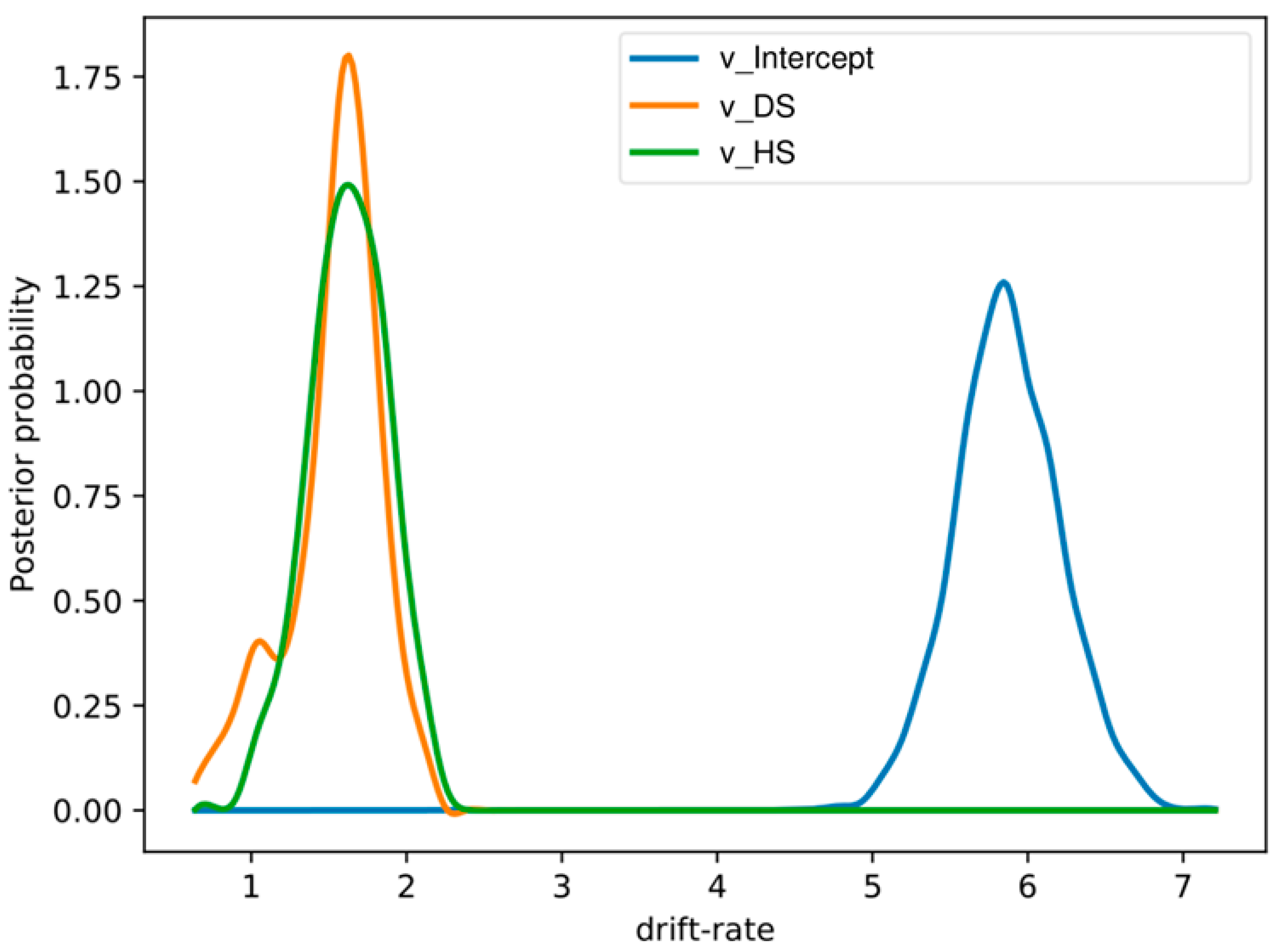
3.4. Drift Diffusion Modeling with Behavioral Data
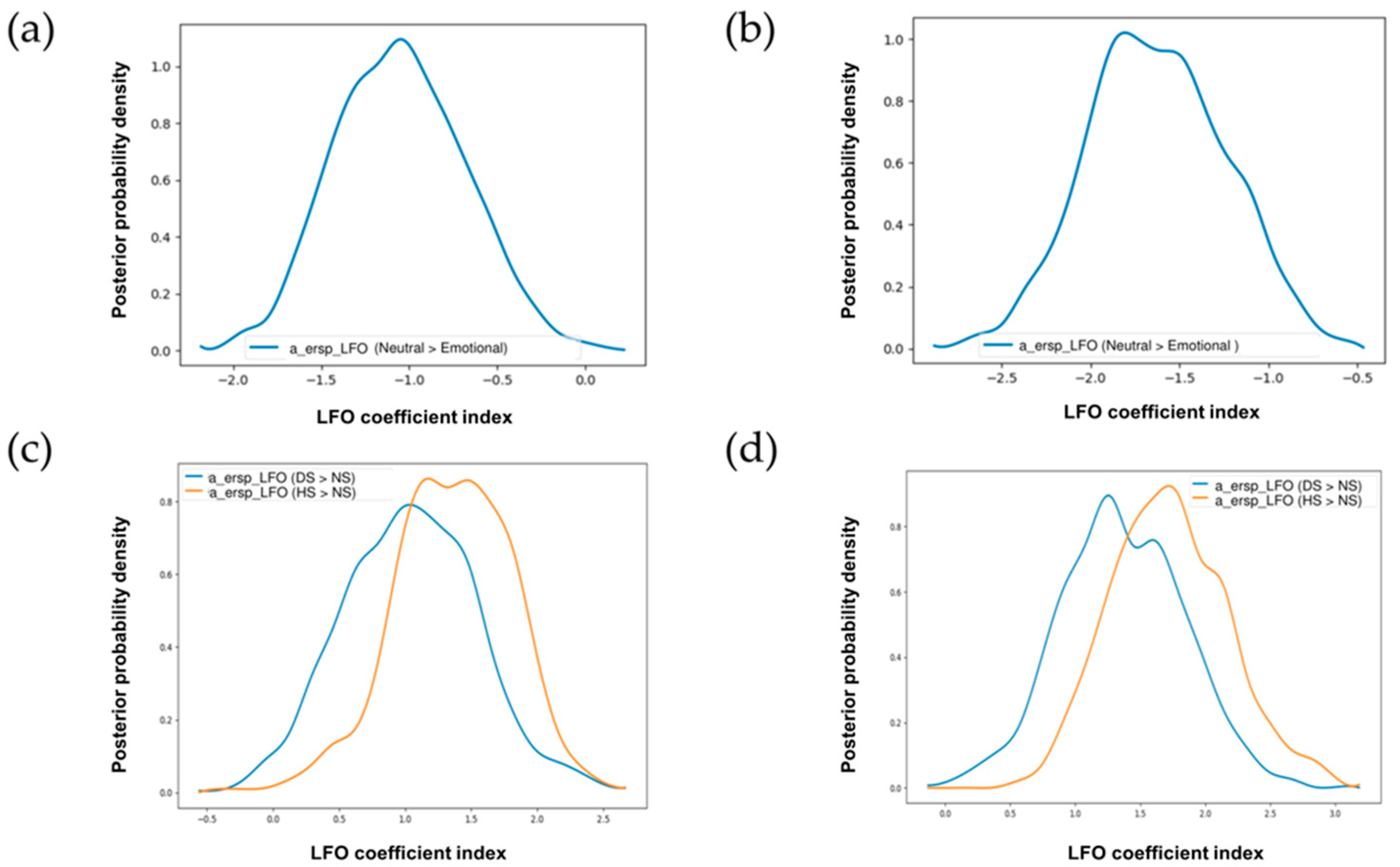
3.5. Exploring Trial-by-Trial Regression Analysis of ERSP Data with HDDM Parameters
3.5.1. Stimulus-Locked Trials
3.5.2. Response-Locked Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Kastner, S.; Ungerleider, L.G. Attentional control of the processing of neutral and emotional stimuli. Cogn. Brain Res. 2002, 15, 31–45. [Google Scholar] [CrossRef]
- Chambers, C.D.; Garavan, H.; Bellgrove, M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 631–646. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.-I.; Miyashita, Y.; Konishi, S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009, 33, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G.D. Proactive adjustments of response strategies in the stop-signal paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 835. [Google Scholar] [CrossRef]
- Ide, J.S.; Shenoy, P.; Angela, J.Y.; Chiang-Shan, R.L. Bayesian prediction and evaluation in the anterior cingulate cortex. J. Neurosci. 2013, 33, 2039–2047. [Google Scholar] [CrossRef]
- Wessel, J.R.; Aron, A.R. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J. Neurosci. 2013, 33, 18481–18491. [Google Scholar] [CrossRef]
- Jahfari, S.; Waldorp, L.; Ridderinkhof, K.R.; Scholte, H.S. Visual information shapes the dynamics of corticobasal ganglia pathways during response selection and inhibition. J. Cogn. Neurosci. 2015, 27, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Stuphorn, V. Neural mechanisms of response inhibition. Curr. Opin. Behav. Sci. 2015, 1, 64–71. [Google Scholar] [CrossRef]
- Boucher, L.; Palmeri, T.J.; Logan, G.D.; Schall, J.D. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol. Rev. 2007, 114, 376. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Stanford, T.R. The countermanding task revisited: Fast stimulus detection is a key determinant of psychophysical performance. J. Neurosci. 2013, 33, 5668–5685. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Stevens, T.; Chambers, C.D. Proactive and reactive stopping when distracted: An attentional account. J. Exp. Psychol. Hum. Percept. Perform. 2014, 40, 1295. [Google Scholar] [CrossRef] [PubMed]
- Erika-Florence, M.; Leech, R.; Hampshire, A. A functional network perspective on response inhibition and attentional control. Nat. Commun. 2014, 5, 4073. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A. Putting the brakes on inhibitory models of frontal lobe function. NeuroImage 2015, 113, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, R.; Brown, E.; Moehlis, J.; Holmes, P.; Cohen, J.D. The physics of optimal decision making: A formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006, 113, 700. [Google Scholar] [CrossRef]
- Ratcliff, R.; McKoon, G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Comput. 2008, 20, 873–922. [Google Scholar] [CrossRef]
- Lambert, C.; Zrinzo, L.; Nagy, Z.; Lutti, A.; Hariz, M.; Foltynie, T.; Draganski, B.; Ashburner, J.; Frackowiak, R. Confirmation of functional zones within the human subthalamic nucleus: Patterns of connectivity and sub-parcellation using diffusion weighted imaging. NeuroImage 2012, 60, 83–94. [Google Scholar] [CrossRef]
- Bogacz, R.; Wagenmakers, E.-J.; Forstmann, B.U.; Nieuwenhuis, S. The neural basis of the speed–accuracy tradeoff. Trends Neurosci. 2010, 33, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.R.; Burman, D.D.; Meyer, J.R.; Lei, Z.; Trommer, B.L.; Davenport, N.D.; Li, W.; Parrish, T.B.; Gitelman, D.R.; Mesulam, M.M. Neural development of selective attention and response inhibition. NeuroImage 2003, 20, 737–751. [Google Scholar] [CrossRef]
- Pessoa, L. To what extent are emotional visual stimuli processed without attention and awareness? Curr. Opin. Neurobiol. 2005, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Onoda, K. Interaction between emotion and attention systems. Front. Neurosci. 2012, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Pourtois, G.; Schettino, A.; Vuilleumier, P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol. Psychol. 2013, 92, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.H.; Holmes, A.J.; Pizzagalli, D.A. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. NeuroImage 2008, 42, 988–997. [Google Scholar] [CrossRef]
- Pawliczek, C.M.; Derntl, B.; Kellermann, T.; Kohn, N.; Gur, R.C.; Habel, U. Inhibitory control and trait aggression: Neural and behavioral insights using the emotional stop signal task. NeuroImage 2013, 79, 264–274. [Google Scholar] [CrossRef]
- Pessoa, L.; Padmala, S.; Kenzer, A.; Bauer, A. Interactions between cognition and emotion during response inhibition. Emotion 2012, 12, 192. [Google Scholar] [CrossRef]
- Yang, S.; Luo, W.; Zhu, X.; Broster, L.S.; Chen, T.; Li, J.; Luo, Y. Emotional content modulates response inhibition and perceptual processing. Psychophysiology 2014, 51, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; De Houwer, J. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn. Emot. 2007, 21, 391–403. [Google Scholar] [CrossRef]
- Yamanaka, K.; Yamamoto, Y. Single-trial EEG power and phase dynamics associated with voluntary response inhibition. J. Cogn. Neurosci. 2010, 22, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Schmiedt-Fehr, C.; Basar-Eroglu, C. Event-related delta and theta brain oscillations reflect age-related changes in both a general and a specific neuronal inhibitory mechanism. Clin. Neurophysiol. 2011, 122, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Nigbur, R.; Ivanova, G.; Stürmer, B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011, 122, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, C.F.; Herrmann, C.S.; Weerda, R.; Huster, R.J. Stimulus-response mappings shape inhibition processes: A combined EEG-fMRI study of contextual stopping. PLoS ONE 2014, 9, e96159. [Google Scholar] [CrossRef] [PubMed]
- Savostyanov, A.N.; Tsai, A.C.; Liou, M.; Levin, E.A.; Lee, J.-D.; Yurganov, A.V.; Knyazev, G.G. EEG-correlates of trait anxiety in the stop-signal paradigm. Neurosci. Lett. 2009, 449, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Plis, S.M.; Lavallee, C.F.; Calhoun, V.D.; Herrmann, C.S. Functional and effective connectivity of stopping. NeuroImage 2014, 94, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.; Tandon, N.; Canolty, R.; Ellmore, T.M.; McEvoy, L.K.; Dreyer, S.; DiSano, M.; Aron, A.R. Intracranial EEG reveals a time-and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 2009, 29, 12675–12685. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.; Poizner, H.; Houser, M.; Gould, S.; Greenhouse, I.; Cai, W.; Strunk, J.; George, J.; Aron, A.R. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: A scalp EEG study in Parkinson’s disease. J. Neurosci. 2011, 31, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Zambrano-Vazquez, L.; Allen, J.J. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology 2012, 49, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Wiecki, T.V.; Cohen, M.X.; Figueroa, C.M.; Samanta, J.; Sherman, S.J.; Frank, M.J. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 2011, 14, 1462. [Google Scholar] [CrossRef] [PubMed]
- Herz, D.M.; Zavala, B.A.; Bogacz, R.; Brown, P. Neural correlates of decision thresholds in the human subthalamic nucleus. Curr. Biol. 2016, 26, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Herz, D.M.; Tan, H.; Brittain, J.-S.; Fischer, P.; Cheeran, B.; Green, A.L.; FitzGerald, J.; Aziz, T.Z.; Ashkan, K.; Little, S. Distinct mechanisms mediate speed-accuracy adjustments in cortico-subthalamic networks. eLife 2017, 6, e21481. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Gagne, C.; Nyhus, E.; Masters, S.; Wiecki, T.V.; Cavanagh, J.F.; Badre, D. fMRI and EEG predictors of dynamic decision parameters during human reinforcement learning. J. Neurosci. 2015, 35, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Herz, D.M.; Brown, P.; Forstmann, B.U.; Zaghloul, K. Frontosubthalamic circuits for control of action and cognition. J. Neurosci. 2016, 36, 11489–11495. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Behrens, T.E.; Smith, S.; Frank, M.J.; Poldrack, R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007, 27, 3743–3752. [Google Scholar] [CrossRef]
- Forstmann, B.U.; Anwander, A.; Schäfer, A.; Neumann, J.; Brown, S.; Wagenmakers, E.-J.; Bogacz, R.; Turner, R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc. Natl. Acad. Sci. USA 2010, 107, 15916–15920. [Google Scholar] [CrossRef]
- Richard Ridderinkhof, K.; Forstmann, B.U.; Wylie, S.A.; Burle, B.; van den Wildenberg, W.P. Neurocognitive mechanisms of action control: Resisting the call of the Sirens. Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 174–192. [Google Scholar] [CrossRef]
- Zavala, B.; Brittain, J.-S.; Jenkinson, N.; Ashkan, K.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Green, A.L.; Aziz, T.; Zaghloul, K. Subthalamic nucleus local field potential activity during the Eriksen flanker task reveals a novel role for theta phase during conflict monitoring. J. Neurosci. 2013, 33, 14758–14766. [Google Scholar] [CrossRef] [PubMed]
- Zavala, B.; Damera, S.; Dong, J.W.; Lungu, C.; Brown, P.; Zaghloul, K.A. Human subthalamic nucleus theta and beta oscillations entrain neuronal firing during sensorimotor conflict. Cereb. Cortex 2015, 27, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Zavala, B.; Tan, H.; Ashkan, K.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Zaghloul, K.; Brown, P. Human subthalamic nucleus–medial frontal cortex theta phase coherence is involved in conflict and error related cortical monitoring. NeuroImage 2016, 137, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R.; Frank, M.J. Reinforcement-based decision making in corticostriatal circuits: Mutual constraints by neurocomputational and diffusion models. Neural Comput. 2012, 24, 1186–1229. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci. 2014, 37, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.N.; Münte, T.F.; Krebs, R.M.; Heinze, H.-J.; Schoenfeld, M.A.; Hopf, J.-M. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cereb. Cortex 2008, 19, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Langford, Z.D.; Krebs, R.M.; Talsma, D.; Woldorff, M.G.; Boehler, C.N. Strategic down-regulation of attentional resources as a mechanism of proactive response inhibition. Eur. J. Neurosci. 2016, 44, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Langford, Z.D.; Schevernels, H.; Boehler, C.N. Motivational context for response inhibition influences proactive involvement of attention. Sci. Rep. 2016, 6, 35122. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Ridderinkhof, K.R.; Scholte, H.S. Spatial frequency information modulates response inhibition and decision-making processes. PLoS ONE 2013, 8, e76467. [Google Scholar] [CrossRef] [PubMed]
- White, C.N.; Congdon, E.; Mumford, J.A.; Karlsgodt, K.H.; Sabb, F.W.; Freimer, N.B.; London, E.D.; Cannon, T.D.; Bilder, R.M.; Poldrack, R.A. Decomposing decision components in the stop-signal task: A model-based approach to individual differences in inhibitory control. J. Cogn. Neurosci. 2014, 26, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Junghöfer, M.; Weike, A.I.; Hamm, A.O. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. NeuroReport 2003, 14, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Senderecka, M. Threatening visual stimuli influence response inhibition and error monitoring: An event-related potential study. Biol. Psychol. 2016, 113, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Senderecka, M. Emotional enhancement of error detection—The role of perceptual processing and inhibition monitoring in failed auditory stop trials. Cogn. Affect. Behav. Neurosci. 2018, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Marcus, D.J.; Westerlund, A.; Casey, B.; Nelson, C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zavala, B.; Tan, H.; Little, S.; Ashkan, K.; Green, A.L.; Aziz, T.; Foltynie, T.; Zrinzo, L.; Zaghloul, K.; Brown, P. Decisions made with less evidence involve higher levels of corticosubthalamic nucleus theta band synchrony. J. Cogn. Neurosci. 2016, 28, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Zavala, B.A.; Tan, H.; Little, S.; Ashkan, K.; Hariz, M.; Foltynie, T.; Zrinzo, L.; Zaghloul, K.A.; Brown, P. Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. J. Neurosci. 2014, 34, 7322–7333. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Woodman, G.F.; Vogel, E.K. Event-related potential studies of attention. Trends Cogn. Sci. 2000, 4, 432–440. [Google Scholar] [CrossRef]
- Wiecki, T.V.; Sofer, I.; Frank, M.J. HDDM: Hierarchical bayesian estimation of the drift-diffusion model in python. Front. Neuroinform. 2013, 7, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Rowe, J.B. Dissociable mechanisms of speed-accuracy tradeoff during visual perceptual learning are revealed by a hierarchical drift-diffusion model. Front. Neurosci. 2014, 8, 69. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Pruessner, J.; Sousa, N.; Almeida, O.F.; Van Dam, A.M.; Rajkowska, G.; Swaab, D.F.; Czéh, B. Neuropathology of stress. Acta Neuropathol. 2014, 127, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, Y.; Ge, Y. Frontal EEG asymmetry and middle line power difference in discrete emotions. Front. Behav. Neurosci. 2018, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Angela, J.Y.; Rao, R.P. A rational decision making framework for inhibitory control. Adv. Neural Inf. Process. Syst. 2010, 23, 2146–2154. [Google Scholar]
- Knyazev, G.; Slobodskoj-Plusnin, J.Y.; Bocharov, A. Event-related delta and theta synchronization during explicit and implicit emotion processing. Neuroscience 2009, 164, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia 2014, 58, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Z.; Ding, C.; Zhang, J.; Fan, L.; Diao, L.; Yang, D. The divergent effects of fear and disgust on inhibitory control: An ERP study. PLoS ONE 2015, 10, e0128932. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.J.; White, C.N.; Kuchinke, L. Individual differences in emotion processing: How similar are diffusion model parameters across tasks? Psychol. Res. 2017, 83, 1172–1183. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Heathcote, A.; Lin, Y.-S.; Reynolds, A.; Strickland, L.; Gretton, M.; Matzke, D. Dynamic models of choice. Behav. Res. Methods 2019, 51, 961–985. [Google Scholar] [CrossRef] [PubMed]


| disgust_go | happy_go | neutral_go | disgust_stop_SSRT | happy_stop_SSRT | neutral_stop_SSRT | v_subj_DS | v_subj_HS | v_subj_NS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| disgust_go | Pearson Correlation | 1 | 0.980 ** | 0.897 ** | 0.604 * | 0.545 * | 0.361 | −0.872 ** | −0.924 ** | −0.690 ** |
| Significance | 0.000 | 0.000 | 0.017 | 0.036 | 0.186 | 0.000 | 0.000 | 0.004 | ||
| happy_go | Pearson Correlation | 0.980 ** | 1 | 0.870 ** | 0.600 * | 0.587 * | 0.336 | −0.902 ** | −0.960 ** | −0.670 ** |
| Significance. (2-tailed) | 0.000 | 0.000 | 0.018 | 0.021 | 0.221 | 0.000 | 0.000 | 0.006 | ||
| neutral_go | Pearson Correlation | 0.897 ** | 0.870 ** | 1 | 0.499 | 0.324 | 0.623 * | −0.793 ** | −0.774 ** | −0.730 ** |
| Significance. (2-tailed) | 0.000 | 0.000 | 0.058 | 0.239 | 0.013 | 0.000 | 0.001 | 0.002 | ||
| disgust_stop_SSRT | Pearson Correlation | 0.604 * | 0.600 * | 0.499 | 1 | 0.637 * | 0.237 | −0.555 * | −0.576 * | −0.435 |
| Significance. (2-tailed) | 0.017 | 0.018 | 0.058 | 0.011 | 0.395 | 0.032 | 0.025 | 0.105 | ||
| happy_stop_SSRT | Pearson Correlation | 0.545 * | 0.587 * | 0.324 | 0.637 * | 1 | 0.203 | −0.583 * | −0.625 * | −0.306 |
| Significance. (2-tailed) | 0.036 | 0.021 | 0.239 | 0.011 | 0.469 | 0.023 | 0.013 | 0.268 | ||
| neutral_stop_SSRT | Pearson Correlation | 0.361 | 0.336 | 0.623 * | 0.237 | 0.203 | 1 | −0.346 | −0.213 | −0.585 * |
| Significance. (2-tailed) | 0.186 | 0.221 | 0.013 | 0.395 | 0.469 | 0.207 | 0.447 | 0.022 | ||
| v_subj_DS | Pearson Correlation | −0.872 ** | −0.902 ** | −0.793 ** | −0.555 * | −0.583 * | −0.346 | 1 | 0.937 ** | 0.764 ** |
| Significance. (2-0tailed) | 0.000 | 0.000 | 0.000 | 0.032 | 0.023 | 0.207 | 0.000 | 0.001 | ||
| v_subj_HS | Pearson Correlation | −0.924 ** | −0.960 ** | −0.774 ** | −0.576 * | −0.625 * | −0.213 | 0.937 ** | 1 | 0.667 ** |
| Significance. (2-0tailed) | 0.000 | 0.000 | 0.001 | 0.025 | 0.013 | 0.447 | 0.000 | 0.007 | ||
| v_subj_NS | Pearson Correlation | −0.690 ** | −0.670 ** | −0.730 ** | −0.435 | −0.306 | −0.585 * | 0.764 ** | 0.667 ** | 1 |
| Significance. (2-tailed) | 0.004 | 0.006 | 0.002 | 0.105 | 0.268 | 0.022 | 0.001 | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, S.; Kuo, C.; Tsai, A.C.-H. Mid-Frontal Theta Modulates Response Inhibition and Decision Making Processes in Emotional Contexts. Brain Sci. 2019, 9, 271. https://doi.org/10.3390/brainsci9100271
Nayak S, Kuo C, Tsai AC-H. Mid-Frontal Theta Modulates Response Inhibition and Decision Making Processes in Emotional Contexts. Brain Sciences. 2019; 9(10):271. https://doi.org/10.3390/brainsci9100271
Chicago/Turabian StyleNayak, Siddharth, ChiiShyang Kuo, and Arthur Chih-Hsin Tsai. 2019. "Mid-Frontal Theta Modulates Response Inhibition and Decision Making Processes in Emotional Contexts" Brain Sciences 9, no. 10: 271. https://doi.org/10.3390/brainsci9100271
APA StyleNayak, S., Kuo, C., & Tsai, A. C.-H. (2019). Mid-Frontal Theta Modulates Response Inhibition and Decision Making Processes in Emotional Contexts. Brain Sciences, 9(10), 271. https://doi.org/10.3390/brainsci9100271




