Exercise-Induced Fatigue in One Leg Does Not Impair the Neuromuscular Performance in the Contralateral Leg but Improves the Excitability of the Ipsilateral Corticospinal Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
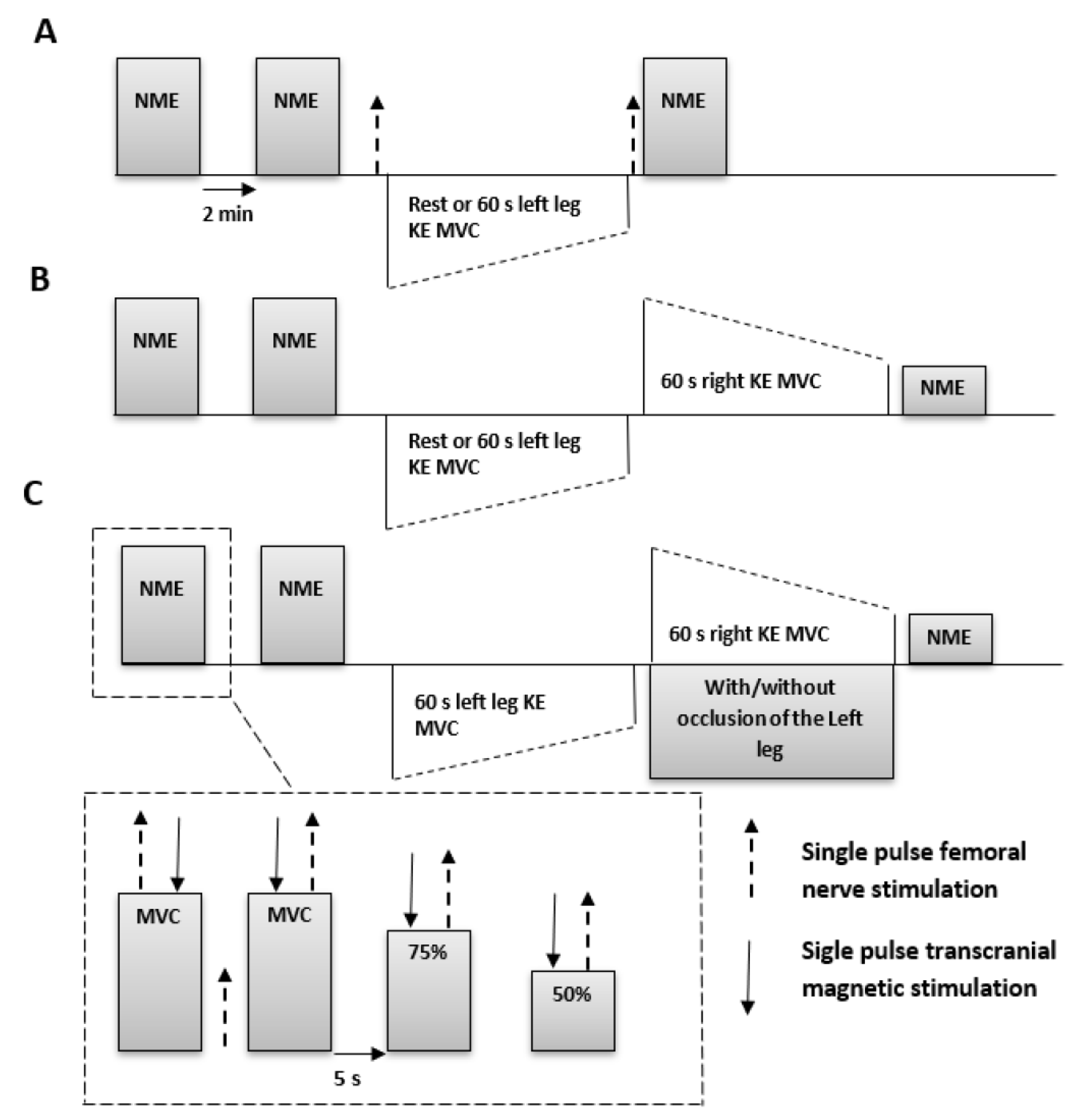
2.2. Experimental Setup and Procedures
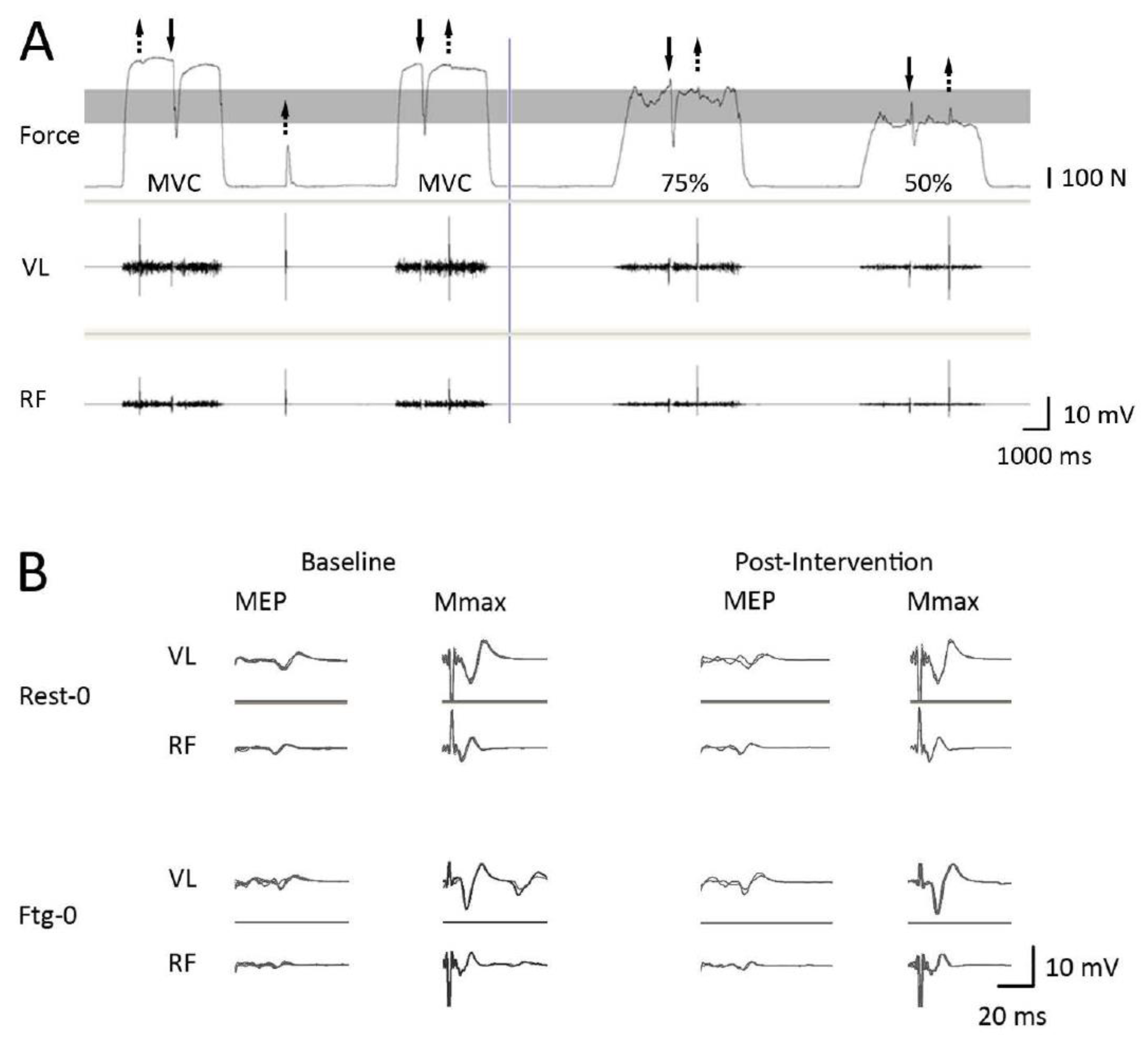
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
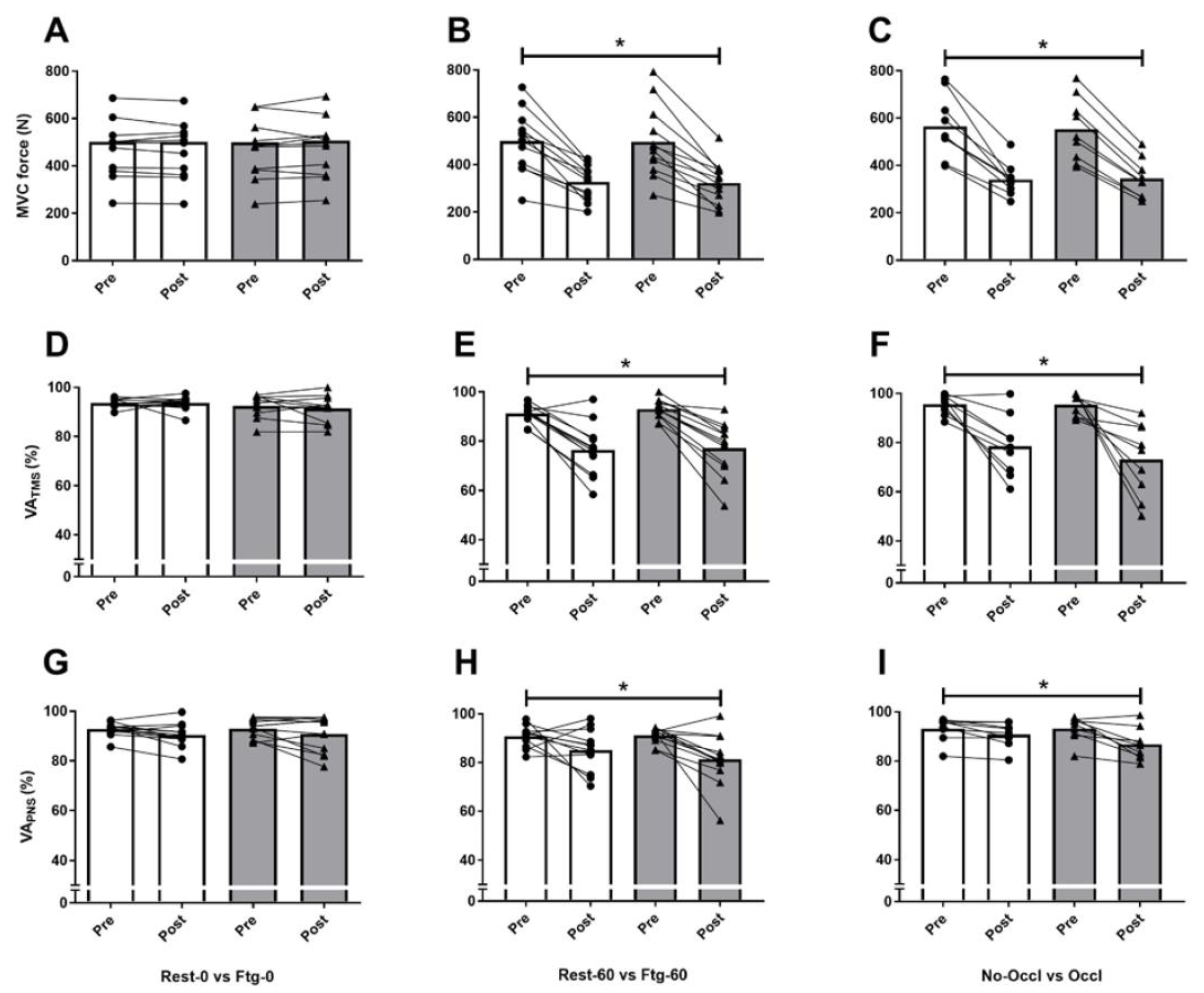
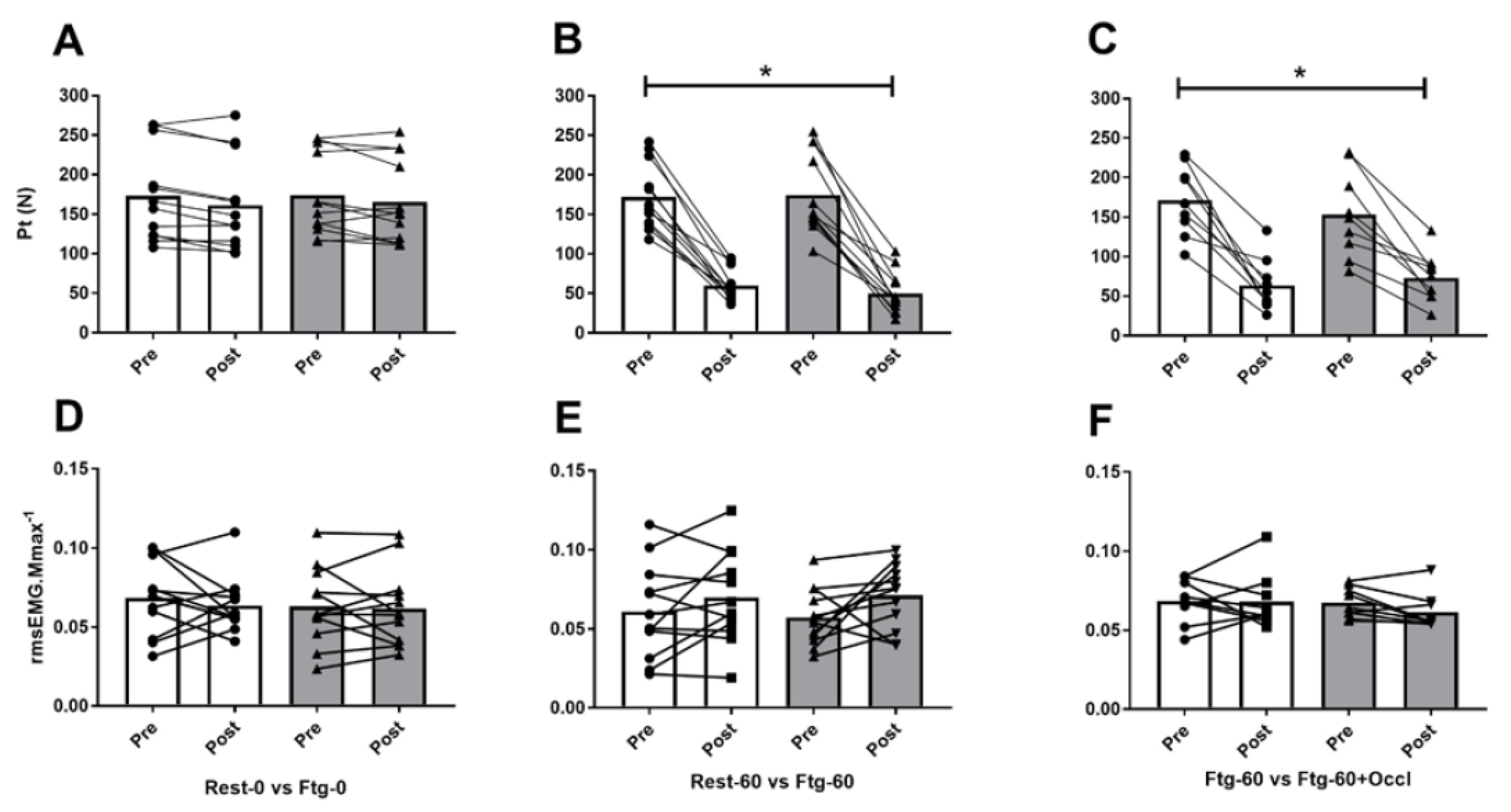
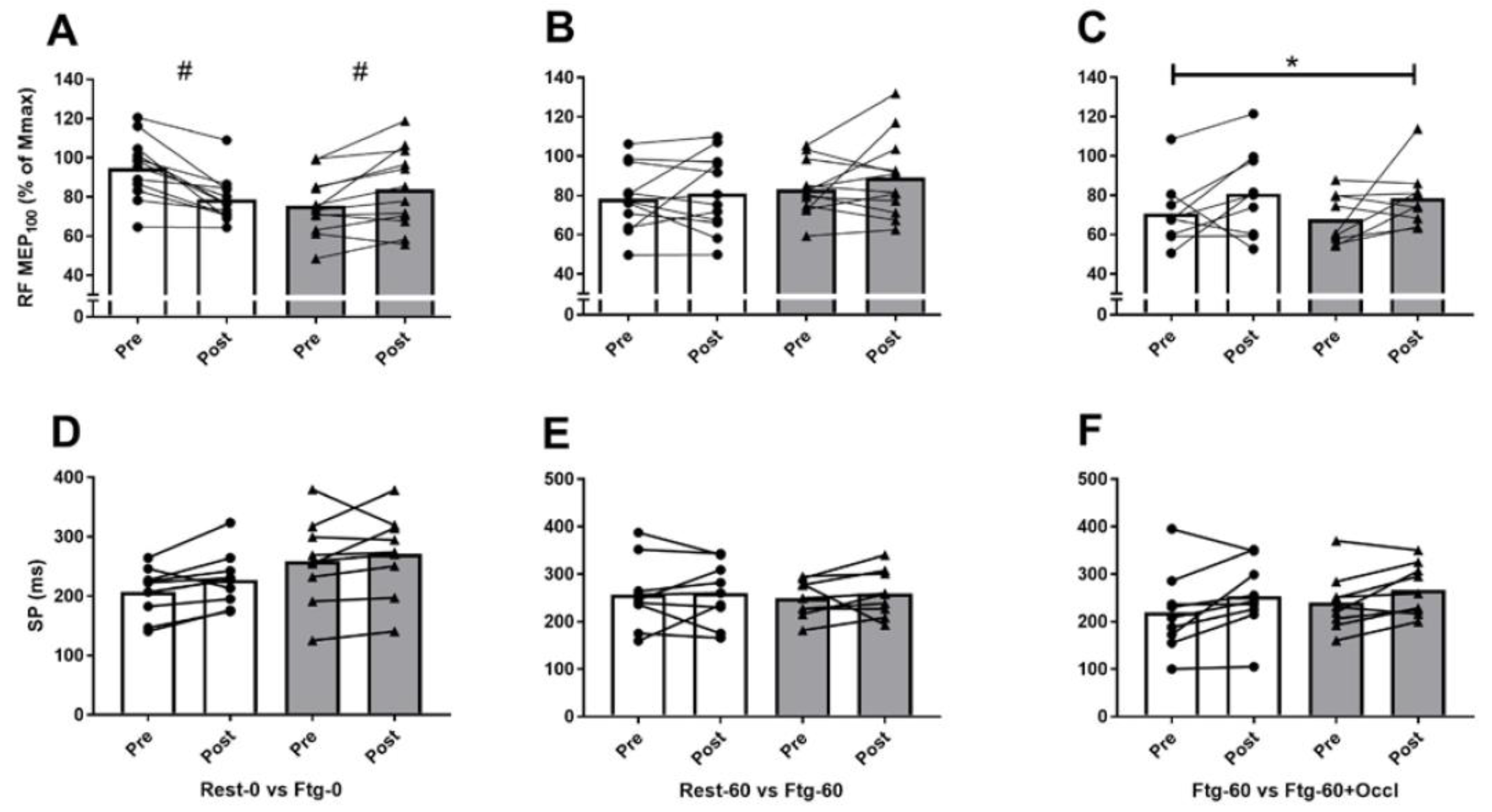
3.1. Experiment A
3.2. Experiment B
3.3. Experiment C
4. Discussion
4.1. MVC Force and Central Motor Drive
4.2. Corticospinal Excitability and Inhibition
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halperin, I.; Chapman, D.W.; Behm, D.G. Non-local muscle fatigue: Effects and possible mechanisms. Eur. J. Appl. Physiol. 2015, 115, 2031–2048. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.G.; Rattey, J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch. 2007, 454, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Šambaher, N.; Aboodarda, S.J.; Behm, D.G. Bilateral Knee Extensor Fatigue Modulates Force and Responsiveness of the Corticospinal Pathway in the Non-fatigued, Dominant Elbow Flexors. Front. Hum. Neurosci. 2016, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Halperin, I.; Aboodarda, S.J.; Behm, D.G. Knee extension fatigue attenuates repeated force production of the elbow flexors. Eur. J. Sport Sci. 2014, 14, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Weavil, J.C.; Venturelli, M.; Garten, R.S.; Rossman, M.J.; Richardson, R.S.; Gmelch, B.S.; Morgan, D.E.; Amann, M. Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J. Physiol. 2014, 592, 5011–5024. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Layec, G.; Rossman, M.J.; Richardson, R.S. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J. Appl. Physiol. (1985) 2013, 115, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Aboodarda, S.J.; Šambaher, N.; Millet, G.Y.; Behm, D.G. Knee extensors neuromuscular fatigue changes the corticospinal pathway excitability in biceps brachii muscle. Neuroscience 2017, 340, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.S.; Fitzpatrick, S.C.; Gandevia, S.C.; Taylor, J.L. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J. Appl. Physiol. (1985) 2015, 118, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Prieske, O.; Aboodarda, S.J.; Benitez Sierra, J.A.; Behm, D.G.; Granacher, U. Slower but not faster unilateral fatiguing knee extensions alter contralateral limb performance without impairment of maximal torque output. Eur. J. Appl. Physiol. 2017, 117, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Chaubet, V.; Maitre, J.; Dumitrescu, M.; Borel, L. Disturbance of contralateral unipedal postural control after stimulated and voluntary contractions of the ipsilateral limb. Neurosci. Res. 2010, 68, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Regueme, S.C.; Barthèlemy, J.; Nicol, C. Exhaustive stretch-shortening cycle exercise: No contralateral effects on muscle activity in maximal motor performances. Scand. J. Med. Sci. Sports 2007, 17, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Aboodarda, S.J.; Copithorne, D.B.; Power, K.E.; Drinkwater, E.; Behm, D.G. Elbow flexor fatigue modulates central excitability of the knee extensors. Appl. Physiol. Nutr. Metab. 2015, 40, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Mills, D.E.; Brown, P.I.; Sharpe, G.R. Prior upper body exercise reduces cycling work capacity but not critical power. Med. Sci. Sports Exerc. 2014, 46, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Halperin, I.; Copithorne, D.; Behm, D.G. Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Appl. Physiol. Nutr. Metab. 2014, 39, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.P.; Rybicki, K.J.; Waldrop, T.G.; Ordway, G.A. Effect of ischemia on responses of group III and IV afferents to contraction. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Hayward, L.; Wesselmann, U.; Rymer, W.Z. Effects of muscle fatigue on mechanically sensitive afferents of slow conduction velocity in the cat triceps surae. J. Neurophysiol. 1991, 65, 360–370. [Google Scholar] [CrossRef] [PubMed]
- van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.G.; van Cingel, R.E.H. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening questionnaire before TMS: An update. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Jamnik, V.; Bredin, S.; Gledhill, N. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+): English North America Version. Health Fit. J. Canada 2011, 4, 18–20. [Google Scholar]
- Vernillo, G.; Temesi, J.; Martin, M.; Millet, G.Y. Mechanisms of Fatigue and Recovery in Upper versus Lower Limbs in Men. Med. Sci. Sports Exerc. 2018, 50, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Behm, D.G.; St-Pierre, D.M.; Perez, D. Muscle inactivation: Assessment of interpolated twitch technique. J. Appl. Physiol. 1996, 81, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, V.; Komi, P.V. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J. Appl. Physiol. 1998, 84, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.; Taylor, J.L.; Gandevia, S.C. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J. Physiol. 2003, 551, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.; Taylor, J.L.; Gandevia, S.C. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J. Appl. Physiol. (1985) 2004, 97, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; pp. xxi, 567. [Google Scholar]
- Arora, S.; Budden, S.; Byrne, J.M.; Behm, D.G. Effect of unilateral knee extensor fatigue on force and balance of the contralateral limb. Eur. J. Appl. Physiol. 2015, 115, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Grabiner, M.D.; Owings, T.M. Effects of eccentrically and concentrically induced unilateral fatigue on the involved and uninvolved limbs. J. Electromyogr. Kinesiol. 1999, 9, 185–189. [Google Scholar] [CrossRef]
- Aboodarda, S.J.; Šambaher, N.; Behm, D.G. Unilateral elbow flexion fatigue modulates corticospinal responsiveness in non-fatigued contralateral biceps brachii. Scand. J. Med. Sci. Sports 2016, 26, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.; Taylor, J.L.; Gandevia, S.C. Measurement of voluntary activation based on transcranial magnetic stimulation over the motor cortex. J. Appl. Physiol. (1985) 2016, 121, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Sadamoto, T.; Bonde-Petersen, F.; Suzuki, Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Taylor, J.L.; Petersen, N.T.; Russell, G.; Gandevia, S.C. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J. Neurophysiol. 2003, 90, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Wassermann, E.M.; Ikoma, K.; Mercuri, B.; Hallett, M. Characterization of postexercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation. Neurology 1996, 46, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Neto, J.P.; Araújo, V.P.; Carneiro, C.R. Postexercise facilitation of motor evoked potentials elicited by ipsilateral voluntary contraction. Muscle Nerve 1999, 22, 1710–1712. [Google Scholar] [CrossRef]
- Heckman, C.J.; Hyngstrom, A.S.; Johnson, M.D. Active properties of motoneurone dendrites: Diffuse descending neuromodulation, focused local inhibition. J. Physiol. 2008, 586, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Watanabe, Y. Supraspinal regulation of physical fatigue. Neurosci. Biobehav. Rev. 2012, 36, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L. Stimulation at the cervicomedullary junction in human subjects. J. Electromyogr. Kinesiol. 2006, 16, 215–223. [Google Scholar] [CrossRef]
| Variables | Experiment A | Experiment B | Experiment C | ||||
|---|---|---|---|---|---|---|---|
| Rest-0 | Ftg-0 | Rest-60 | Ftg-60 | Ftg-60 | Ftg-60-Occl | ||
| MVC force (N) | Baseline | 501.7 ± 159.9 | 499.9 ± 158.7 | 507.1 ± 148.2 | 495.3 ± 150.3 | 555.0 ± 128.8 | 573.9 ± 144.1 |
| Post-test | 501.1 ± 170.7 | 506.3 ± 158.6 | 334.8 ± 88.9 | 322.2 ± 89.9 | 349.9 ± 71.1 | 363.6 ± 96.7 | |
| rmsEMG∙Mmax−1 ratio (rmsEMG100) | Baseline | 0.074 ± 0.031 | 0.070 ± 0.037 | 0.067 ± 0.034 | 0.066 ± 0.026 | 0.063 ± 0.030 | 0.068 ± 0.026 |
| Post-test | 0.069 ± 0.028 | 0.069 ± 0.044 | 0.066 ± 0.026 | 0.078 ± 0.029 | 0.066 ± 0.031 | 0.072 ± 0.038 | |
| PT (N) | Baseline | 173.2 ± 54.4 | 173.7 ± 51.7 | 171.8 ± 41.7 | 174.5 ± 50.4 | 171.5 ± 44.3 | 153.4 ± 54.5 |
| Post-test | 161.4 ± 59.1 | 165.6 ± 52.9 | 59.9 ± 20.2 | 49.8 ± 26.1 | 63.5 ± 33.2 | 72.8 ± 31.1 | |
| VAPNS (%) | Baseline | 92.7 ± 2.9 | 93.1 ± 3.8 | 90.7 ± 4.5 | 91.1 ± 3.2 | 93.1 ± 5.1 | 93.2 ± 5.2 |
| Post-test | 90.6 ± 4.9 | 90.7 ± 7.5 | 85.1 ± 9.1 | 81.2 ± 11.2 | 90.6 ± 5.2 | 86.7 ± 6.7 | |
| VATMS (%) | Baseline | 93.6 ± 1.9 | 92.6 ± 5.2 | 91.1 ± 4.2 | 93.1 ± 4.4 | 94.8 ± 5.5 | 85.4 ± 4.5 |
| Post-test | 93.4 ± 3.1 | 91.2 ± 6.1 | 73.3 ± 18.3 | 76.6 ± 12.6 | 78.4 ± 12.2 | 70.1 ± 17.1 | |
| RF MEP∙Mmax−1 (MEP100) | Baseline | 0.93 ± 0.18 | 0.80 ± 0.21 | 0.77 ± 0.18 | 0.83 ± 0.14 | 0.70 ± 0.16 | 0.67 ± 0.12 |
| Post-test | 0.82 ± 0.18 # | 0.90 ± 0.25 # | 0.79 ± 0.21 | 0.88 ± 0.21 | 0.80 ± 0.22 | 0.78 ± 0.22 | |
| RF MEP∙Mmax−1 (MEP75) | Baseline | 0.94 ± 0.19 | 0.84 ± 0.23 | 0.87 ± 0.20 | 0.92 ± 0.15 | 0.82 ± 0.15 | 0.81 ± 0.15 |
| Post-test | 0.87 ± 0.22 | 0.82 ± 0.20 | 0.83 ± 0.22 | 0.89 ± 0.11 | 0.92 ± 0.24 | 0.84 ± 0.33 | |
| RF MEP∙Mmax−1 (MEP50) | Baseline | 0.96 ± 0.15 | 0.90 ± 0.19 | 0.89 ± 0.18 | 0.97 ± 0.13 | 0.89 ± 0.14 | 0.81 ± 09 |
| Post-test | 0.95 ± 0.16 | 0.88 ± 0.19 | 0.87 ± 0.14 | 0.88 ± 0.22 | 0.97 ± 0.34 | 0.79 ± 0.28 | |
| RF SP100 (ms) | Baseline | 206.7 ± 45.2 | 258.5 ± 78.3 | 257.5 ± 78.6 | 249.3 ± 41.6 | 219.1 ± 84.6 | 240.6 ± 60.5 |
| Post-test | 227.3 ± 50.0 | 270.9 ± 74.7 | 260.1 ± 69.7 | 259.3 ± 52.0 | 253.4 ± 75.3 | 267.4 ± 53.7 | |
| RF SP75 (ms) | Baseline | 211.4 ± 48.3 | 256.8 ± 59.6 | 267.7 ± 64.0 | 252.0 ± 54.3 | 214.1 ± 77.7 | 208.5 ± 96.8 |
| Post-test | 226.1 ± 67.3 | 270.3 ± 77.0 | 254.1 ± 70.3 | 254.9 ± 56.5 | 231.6 ± 67.5 | 250.2 ± 55.9 | |
| RF SP50 (ms) | Baseline | 231.6 ± 40.9 | 276.4 ± 61.4 | 270.0 ± 69.1 | 273.1 ± 45.9 | 207.3 ± 71.8 | 213.1 ± 47.4 |
| Post-test | 248.9 ± 63.2 | 269.8 ± 61.2 | 256.9 ± 61.2 | 261.5 ± 55.6 | 248.5 ± 74.6 | 253.2 ± 77.3 | |
| BF MEP100 (mV.s) | Baseline | 0.009 ± 0.005 | 0.010 ± 0.008 | 0.007 ± 0.004 | 0.009 ± 0.005 | 0.008 ± 0.005 | 0.008 ± 0.004 |
| Post-test | 0.007 ± 0.006 | 0.009 ± 0.006 | 0.007 ± 0.005 | 0.007 ± 0.005 | 0.008 ± 0.005 | 0.006 ± 0.005 | |
| BF MEP75 (mV.s) | Baseline | 0.007 ± 0.005 | 0.009 ± 0.008 | 0.007 ± 0.004 | 0.009 ± 0.006 | 0.007 ± 0.005 | 0.008 ± 0.005 |
| Post-test | 0.007 ± 0.006 | 0.009 ± 0.007 | 0.005 ± 0.005 | 0.007 ± 0.007 | 0.006 ± 0.004 | 0.006 ± 0.005 | |
| BF MEP50 (mV.s) | Baseline | 0.007 ± 0.006 | 0.009 ± 0.008 | 0.007 ± 0.006 | 0.007 ± 0.003 | 0.007 ± 0.006 | 0.006 ± 0.004 |
| Post-test | 0.006 ± 0.006 | 0.008 ± 0.007 | 0.004 ± 0.002 | 0.005 ± 0.003 | 0.005 ± 0.003 | 0.004 ± 0.002 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboodarda, S.J.; Zhang, C.X.Y.; Sharara, R.; Cline, M.; Millet, G.Y. Exercise-Induced Fatigue in One Leg Does Not Impair the Neuromuscular Performance in the Contralateral Leg but Improves the Excitability of the Ipsilateral Corticospinal Pathway. Brain Sci. 2019, 9, 250. https://doi.org/10.3390/brainsci9100250
Aboodarda SJ, Zhang CXY, Sharara R, Cline M, Millet GY. Exercise-Induced Fatigue in One Leg Does Not Impair the Neuromuscular Performance in the Contralateral Leg but Improves the Excitability of the Ipsilateral Corticospinal Pathway. Brain Sciences. 2019; 9(10):250. https://doi.org/10.3390/brainsci9100250
Chicago/Turabian StyleAboodarda, Saied Jalal, Cindy Xin Yu Zhang, Ruva Sharara, Madeleine Cline, and Guillaume Y Millet. 2019. "Exercise-Induced Fatigue in One Leg Does Not Impair the Neuromuscular Performance in the Contralateral Leg but Improves the Excitability of the Ipsilateral Corticospinal Pathway" Brain Sciences 9, no. 10: 250. https://doi.org/10.3390/brainsci9100250
APA StyleAboodarda, S. J., Zhang, C. X. Y., Sharara, R., Cline, M., & Millet, G. Y. (2019). Exercise-Induced Fatigue in One Leg Does Not Impair the Neuromuscular Performance in the Contralateral Leg but Improves the Excitability of the Ipsilateral Corticospinal Pathway. Brain Sciences, 9(10), 250. https://doi.org/10.3390/brainsci9100250





