The Loss of α- and β-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Brain Samples and Ethics Statement
2.2. Rats
2.3. Brain Tissue Homogenization
2.4. One-Dimensional Polyacrylamide Gel Electrophoresis (1-D PAGE)
2.5. Western (Immuno) Blotting
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistics
3. Results
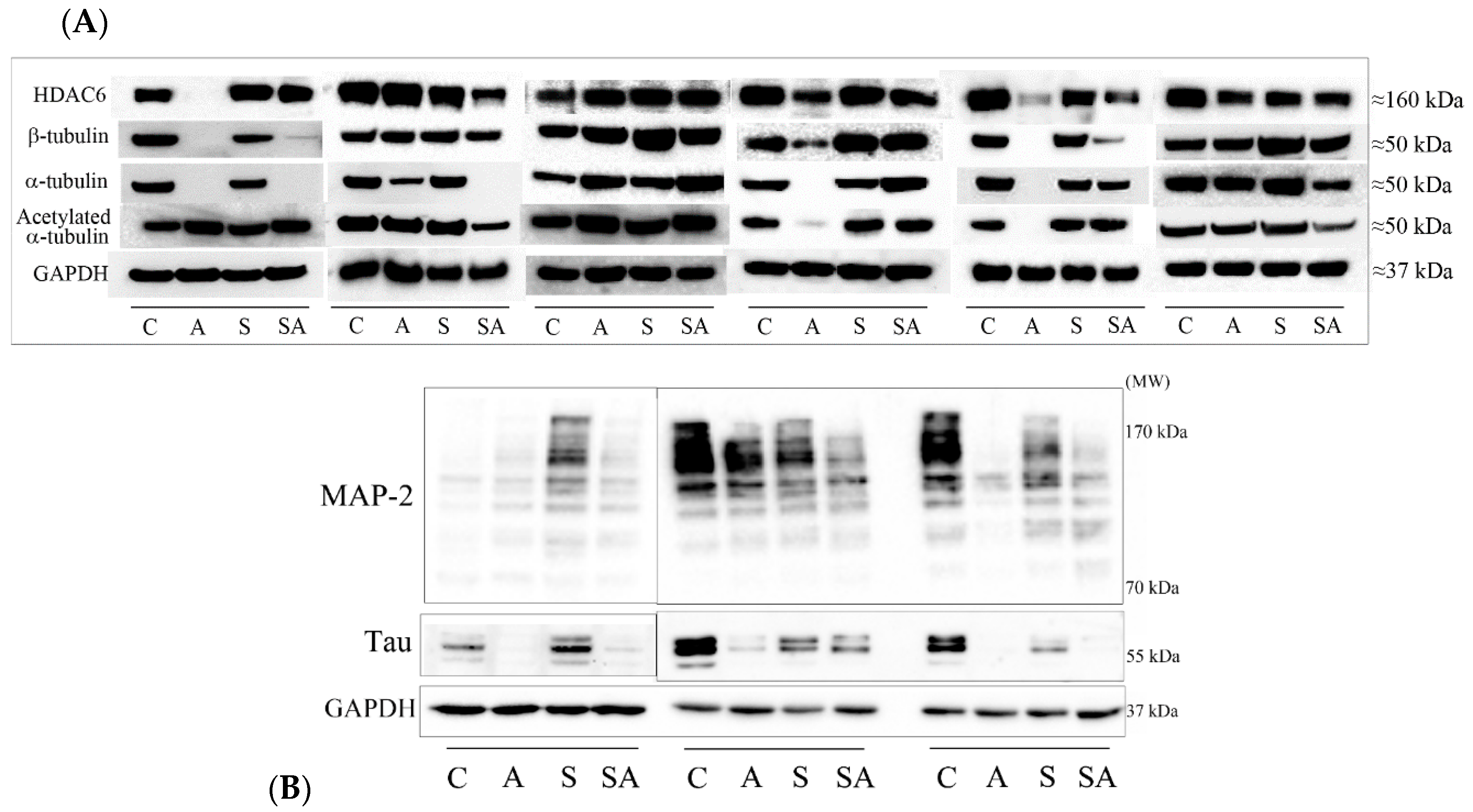
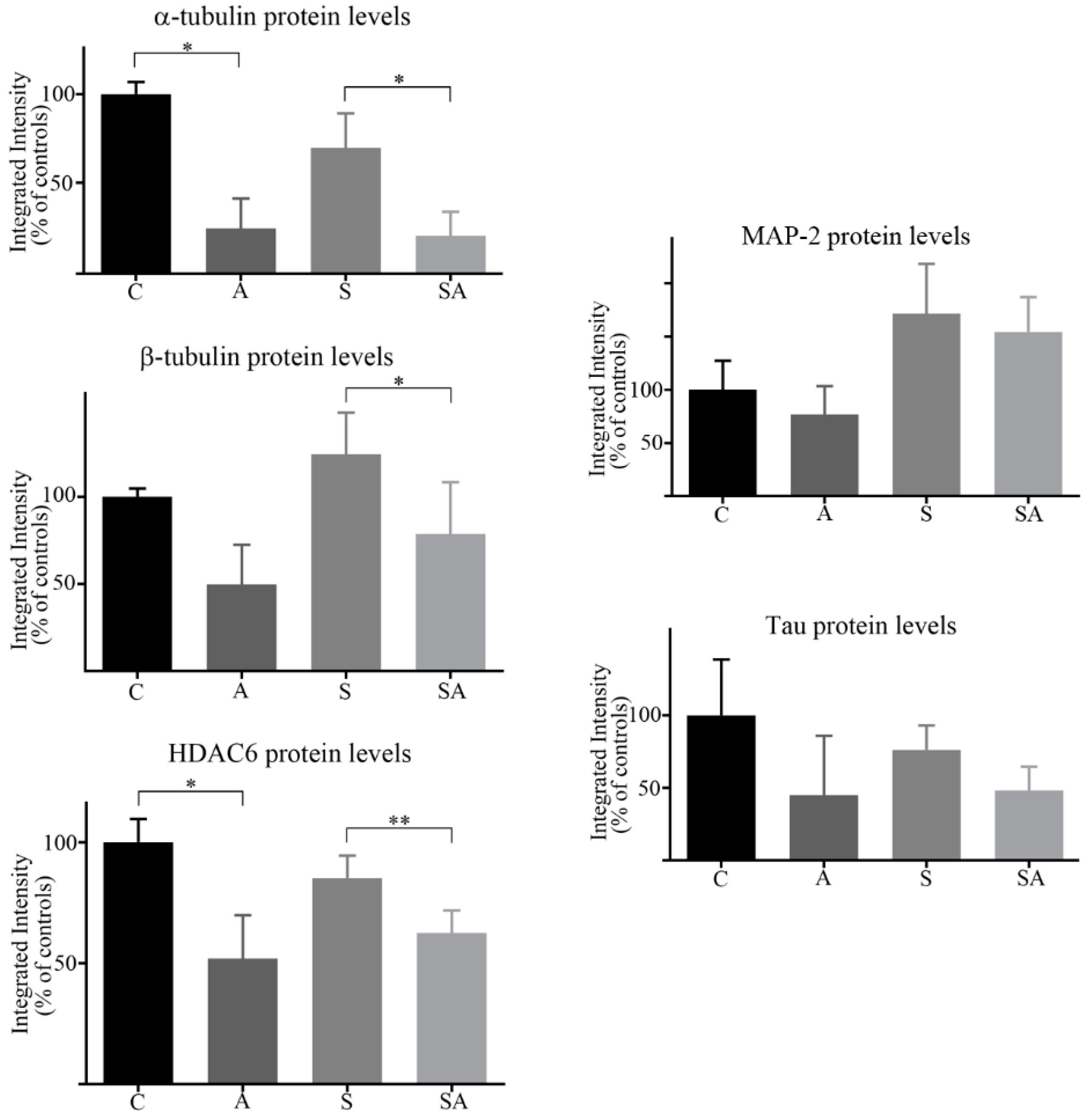
3.1. Reduced α- and β-Tubulins and Microtubule-Associated Proteins in the PFC of Alcoholic Subjects
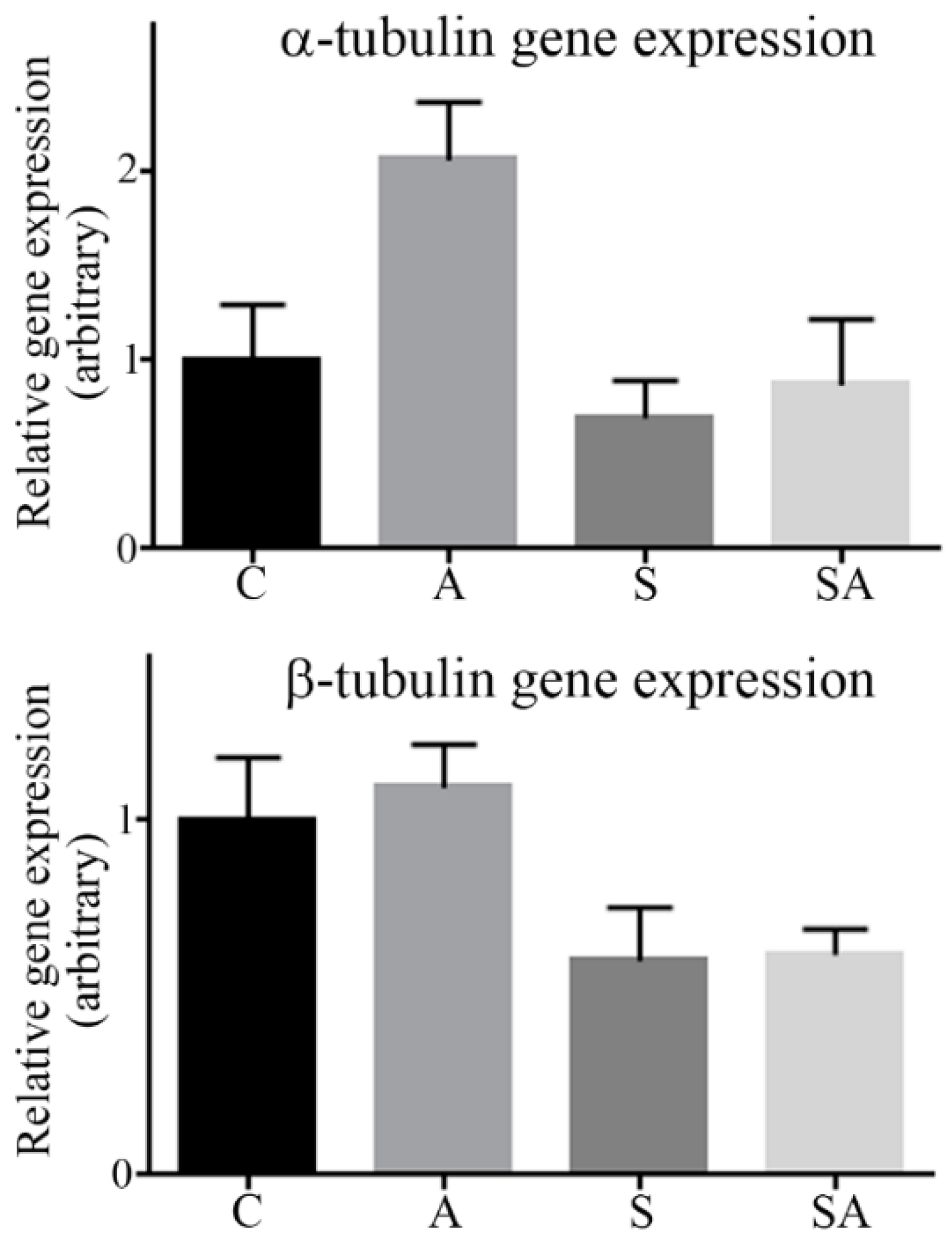
3.2. α- and β-Tubulin Gene Expression Were Moderately Increased in Alcoholic Cohorts
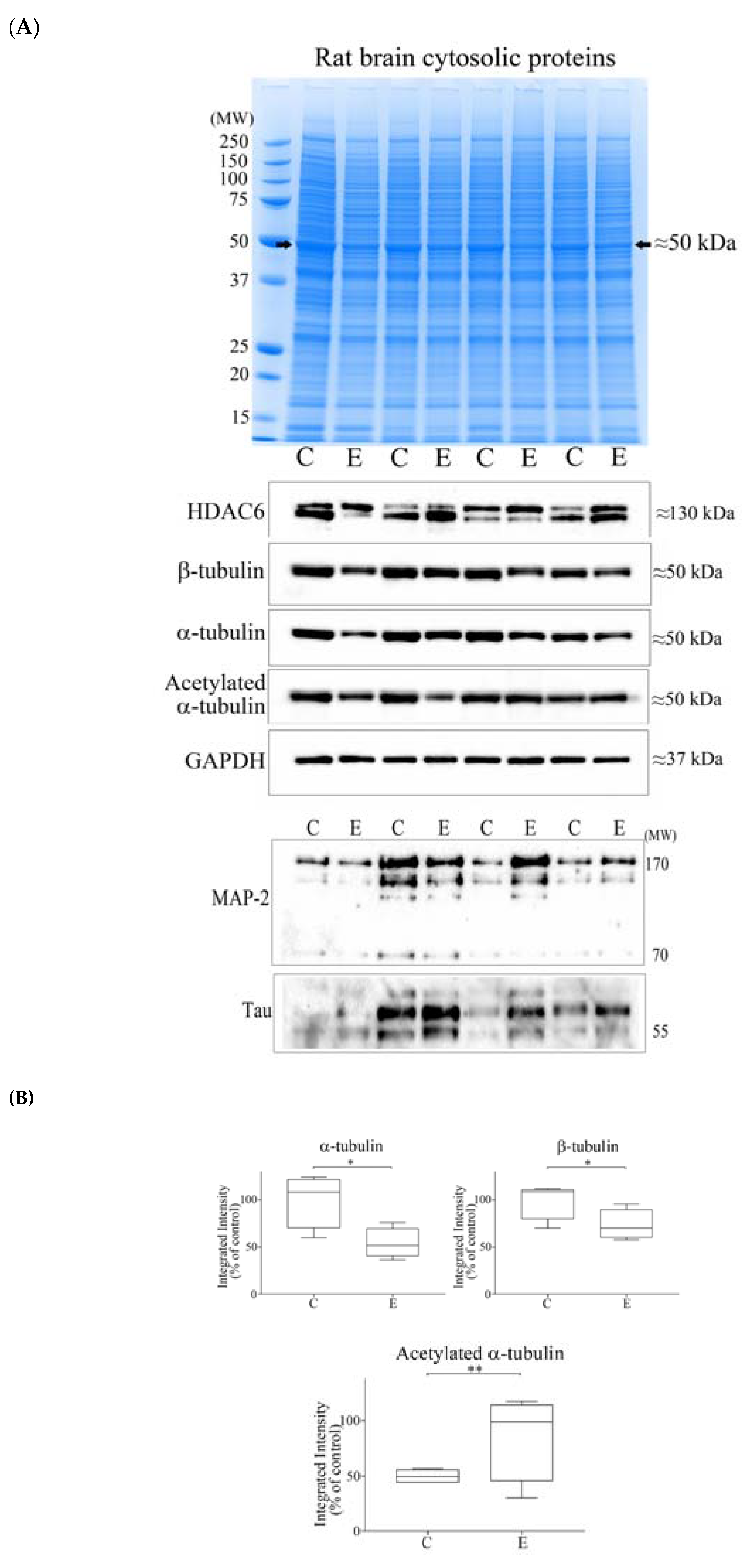
3.3. Alcohol Consumption Modelled In Vivo
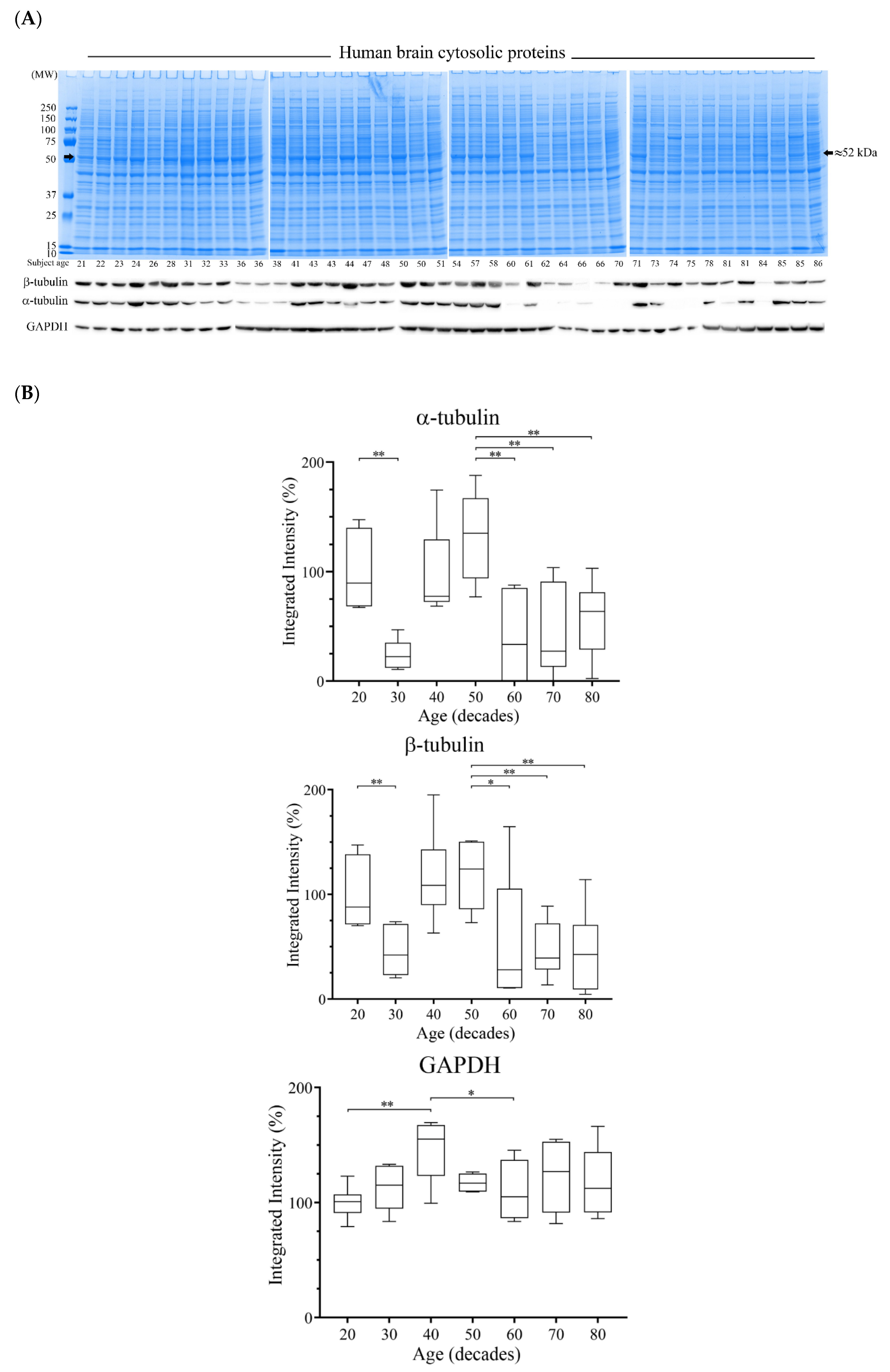
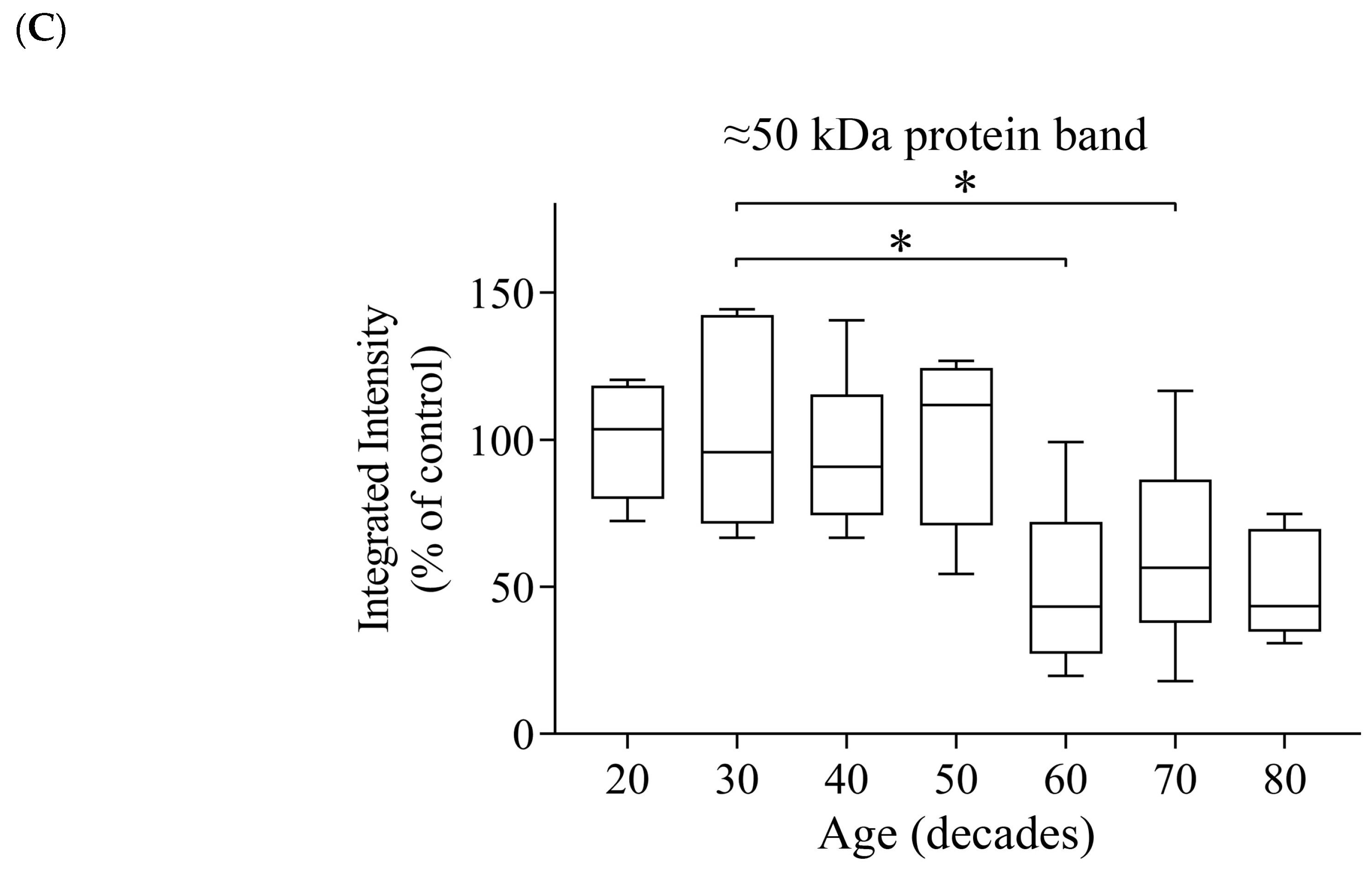
3.4. Reductions of α- and β-Tubulin Protein Levels during Ageing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connor, J.P.; Haber, P.S.; Hall, W.D. Alcohol use disorders. Lancet 2016, 387, 988–998. [Google Scholar] [CrossRef]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Global Status Report on Alcohol and Health 2014. World Health Organization: Geneva, Switzerland, 2014. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 8 May 2014).
- Harper, C. The neurotoxicity of alcohol. Hum. Exp. Toxicol. 2007, 26, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Brust, J.C. Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: A review. Int. J. Environ. Res. Public Health 2010, 7, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- Kandel, D.B.; Huang, F.Y.; Davies, M. Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend. 2001, 64, 233–241. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Koob, G.F. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res. Health 2008, 31, 185–195. [Google Scholar] [PubMed]
- Erdozain, A.M.; Callado, L.F. Neurobiological alterations in alcohol addiction: A review. Adicciones 2014, 26, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009, 44, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Erdozain, A.M.; Morentin, B.; Bedford, L.; King, E.; Tooth, D.; Brewer, C.; Wayne, D.; Johnson, L.; Gerdes, H.K.; Wigmore, P.; et al. Alcohol-related brain damage in humans. PLoS ONE 2014, 9, e93586. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Pakkenberg, B. Do alcoholics drink their neurons away? Lancet 1993, 342, 1201–1204. [Google Scholar] [PubMed]
- Kril, J.J.; Halliday, G.M.; Svoboda, M.D.; Cartwright, H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience 1997, 79, 983–998. [Google Scholar] [CrossRef]
- Harper, C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J. Neuropathol. Exp. Neurol. 1998, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kril, J.J.; Halliday, G.M. Brain shrinkage in alcoholics: A decade on and what have we learned? Prog. Neurobiol. 1999, 58, 381–387. [Google Scholar] [CrossRef]
- Skuja, S.; Groma, V.; Smane, L. Alcoholism and cellular vulnerability in different brain regions. Ultrastruct. Pathol. 2012, 36, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Whittom, A.; Villarreal, A.; Soni, M.; Owusu-Duku, B.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A.; Miguel-Hidalgo, J.J. Markers of apoptosis induction and proliferation in the orbitofrontal cortex in alcohol dependence. Alcohol. Clin. Exp. Res. 2014, 38, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.G.; Kril, J.J. Neuropathology of alcoholism. Alcohol Alcohol. 1990, 25, 207–216. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Sullivan, E.V.; Mathalon, D.H.; Lim, K.O. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol. Clin. Exp. Res. 1997, 21, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Nakazaki, S.; Hirai, S.; Saeki, N.; Yamaura, A.; Kusaka, T. Alcohol consumption and frontal lobe shrinkage: Study of 1432 non-alcoholic subjects. J. Neurol. Neurosurg. Psychiatry 2001, 71, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J.; Rajkowska, G. Comparison of prefrontal cell pathology between depression alcohol dependence. J. Psychiatr. Res. 2003, 37, 411–420. [Google Scholar] [CrossRef]
- Makris, N.; Oscar-Berman, M.; Jaffin, S.K.; Hodge, S.M.; Kennedy, D.N.; Caviness, V.S.; Marinkovic, K.; Breiter, H.C.; Gasic, G.P.; Harris, G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry 2008, 64, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Momenan, R.; Steckler, L.E.; Saad, Z.S.; van Rafelghem, S.; Kerich, M.J.; Hommer, D.W. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012, 204, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Monnig, M.A.; Tonigan, J.S.; Yeo, R.A.; Thoma, R.J.; McCrady, B.S. White matter volume in alcohol use disorders: A meta-analysis. Addict. Biol. 2013, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dai, Z.; Zhong, J.; Zhu, Y.; Shi, H.; Pan, P. Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015, 153, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, F.; Zhang, H.; Zeng, J.; Chen, T.; Wang, S.; Jia, Z.; Gong, Q. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: A voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2016, 66, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Martindale, S.L.; Hurley, R.A.; Taber, K.H. Chronic alcohol use and sleep homeostasis: Risk factors and neuroimaging of recovery. J. Neuropsychiatry Clin. Neurosci. 2017, 29, A6-5. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Gazdzinski, S.; Meyerhoff, D.J. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol. 2007, 42, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.K.; Thompson, J.; Chen, L.; Dagda, M.; Dertien, J.; Dossou, K.S.; Moaddel, R.; Bergeson, S.E.; Kruman, I.I. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity. PLoS ONE 2014, 9, e106945. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Nixon, K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009, 44, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Rosenbloom, M.J.; Chu, W.; Sassoon, S.A.; Rohlfing, T.; Pohl, K.M.; Zahr, N.M.; Sullivan, E.V. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: A controlled longitudinal DTI study. Lancet Psychiatry 2014, 1, 202–212. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Mon, A.; Gazdzinski, S.; Yeh, P.H.; Meyerhoff, D.J. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict. Biol. 2015, 20, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Putzke, J.; De Beun, R.; Schreiber, R.; De Vry, J.; Tölle, T.R.; Zieglgänsberger, W.; Spanagel, R. Long-term alcohol self-administration and alcohol withdrawal differentially modulate microtubule-associated protein 2 (MAP2) gene expression in the rat brain. Brain Res. Mol. Brain Res. 1998, 62, 196–205. [Google Scholar] [CrossRef]
- Evrard, S.G.; Brusco, A. Ethanol effects on the cytoskeleton of nerve tissue cells. In Cytoskeleton of the Nervous System; Springer Science Press: Berlin/Heidelberg, Germany, 2011; Chapter 29; pp. 697–758. [Google Scholar]
- Erdozain, A.M.; Rubio, M.; Valdizan, E.M.; Pazos, A.; Meana, J.J.; Fernández-Ruiz, J.; Alexander, S.P.; Callado, L.F. The endocannabinoid system is altered in the post-mortem prefrontal cortex of alcoholic subjects. Addict. Biol. 2015, 20, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Rogers, D.D., II; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine. Biochem. Pharmacol. 2005, 70, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Leggate, M.; Carter, W.G.; Evans, M.J.; Vennard, R.A.; Sribala-Sundaram, S.; Nimmo, M.A. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J. Appl. Physiol. 2012, 112, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.G.; Vigneswara, V.; Newlaczyl, A.; Wayne, D.; Ahmed, B.; Saddington, S.; Brewer, C.; Raut, N.; Gerdes, H.K.; Erdozain, A.M.; et al. Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury. Biochem. Biophys. Res. Commun. 2015, 458, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, R.F. A hypothesis on the origin and evolution of tubulin. Int. Rev. Cell Mol. Biol. 2013, 302, 41–185. [Google Scholar] [PubMed]
- Conde, C.; Caceres, A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009, 10, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, R.F. Multiple forms of tubulin: Different gene products and covalent modifications. Int. Rev. Cytol. 1998, 178, 207–275. [Google Scholar] [PubMed]
- Leandro-García, L.J.; Leskelä, S.; Landa, I.; Montero-Conde, C.; López-Jiménez, E.; Letón, R.; Cascón, A.; Robledo, M.; Rodríguez-Antona, C. Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton 2010, 67, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Verhey, K.J.; Gaertig, J. The tubulin code. Cell Cycle 2007, 6, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Brady, S.T. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trend Cell Biol. 2015, 25, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Díaz-Nido, J.; Avila, J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 2000, 61, 133–168. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef] [PubMed]
- Noraberg, J.; Zimmer, J. Ethanol induces MAP2 changes in organotypic hippocampal slice cultures. Neuroreport 1998, 9, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, P.; Vega, M.D.; Evrard, S.G.; Ramos, A.J.; Brusco, A. Alcohol exposure during adulthood induces neuronal and astroglial alterations in the hippocampal CA-1 area. Ann. N. Y. Acad. Sci. 2002, 965, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Esteban-Pretel, G.; Marín, M.P.; Ponsoda, X.; Ballestín, R.; Canales, J.J.; Renau-Piqueras, J. Chronic ethanol exposure alters the levels, assembly, and cellular organization of the actin cytoskeleton and microtubules in hippocampal neurons in primary culture. Toxicol. Sci. 2010, 118, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Oguchi, K.; Okabe, S.; Kuno, J.; Terada, S.; Ohshima, T.; Sato-Yoshitake, R.; Takei, Y.; Noda, T.; Hirokawa, N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 1994, 369, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Teng, J.; Takei, Y.; Oguchi, K.; Hirokawa, N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell. Biol. 2002, 158, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.F.; Yao, T.P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Shan, B. Analysis of expression and functions of histone deacetylase 6 (HDAC6). Methods Mol. Biol. 2016, 1436, 85–94. [Google Scholar] [PubMed]
- Skultetyova, L.; Ustinova, K.; Kutil, Z.; Novakova, Z.; Pavlicek, J.; Mikesova, J.; Trapl, D.; Baranova, P.; Havlinova, B.; Hubalek, M.; et al. Human histone deacetylase 6 shows strong preference for tubulin dimers over assembled microtubules. Sci. Rep. 2017, 7, 11547. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Jennett, R.B.; Sorrell, M.F.; Tuma, D.J. Acetaldehyde substoichiometrically inhibits bovine neurotubulin polymerization. J. Clin. Investig. 1989, 84, 337–341. [Google Scholar] [CrossRef]
- Lee, J.Y.; Koga, H.; Kawaguchi, Y.; Tang, W.; Wong, E.; Gao, Y.S.; Pandey, U.B.; Kaushik, S.; Tresse, E.; Lu, J.; et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010, 29, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pires, C.; Zwick, V.; Nurisso, A.; Schenker, E.; Carrupt, P.; Cuendet, M. HDAC6 as a target for neurodegenerative diseases: What makes it different from the other HDACs? Mol. Neurodegener. 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Lewohl, J.M.; Van Dyk, D.D.; Craft, G.E.; Innes, D.J.; Mayfield, R.D.; Cobon, G.; Harris, R.A.; Dodd, P.R. The application of proteomics to the human alcoholic brain. Ann. N. Y. Acad. Sci. 2004, 1025, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Butler, T.R.; Prendergast, M.A. Ethanol impairs microtubule formation via interactions at a microtubule associated protein-sensitive site. Alcohol 2013, 47, 539–543. [Google Scholar] [CrossRef]
- Najbauer, J.; Orpiszewski, J.; Aswad, D.W. Molecular aging of tubulin: Accumulation of isoaspartyl sites in vitro and in vivo. Biochemistry 1996, 35, 5183–5190. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Lowenson, J.D.; Powell, C.D.; Thakur, M.; Bailey, K.; Clarke, S.; Ray, D.E.; Carter, W.G. Proteomic identification of novel substrates of a protein isoaspartyl methyltransferase repair enzyme. J. Biol. Chem. 2006, 281, 32619–32629. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.M.; Gilman, J.M. The effects of acute alcohol administration on the human brain: Insights from neuroimaging. Neuropharmacology 2014, 84, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Walhovd, K.B.; Fjell, A.M.; Reinvang, I.; Lundervold, A.; Dale, A.M.; Eilertsen, D.E.; Quinn, B.T.; Salat, D.; Makris, N.; Fischl, B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 2005, 26, 1261–1270, discussion 1275–1268. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Gunning, F.M.; Head, D.; Dupuis, J.H.; McQuain, J.; Briggs, S.D.; Loken, W.J.; Thornton, A.E.; Acker, J.D. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb. Cortex 1997, 7, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, D.J.; Van Boxtel, M.P.; Pruessner, J.C.; Hofman, P.; Evans, A.C.; Jolles, J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb. Cortex 2004, 14, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Cowell, P.E.; Sluming, V.A.; Wilkinson, I.D.; Cezayirli, E.; Romanowski, C.A.; Webb, J.A.; Keller, S.S.; Mayes, A.; Roberts, N. Effects of sex and age on regional prefrontal brain volume in two human cohorts. European. J. Neurosci. 2007, 25, 307–318. [Google Scholar]
- Farokhian, F.; Yang, C.; Beheshti, I.; Matsuda, H.; Wu, S. Age-related gray and white matter changes in normal adult brains. Aging Dis. 2017, 8, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Guggenmos, M.; Schmack, K.; Sekutowicz, M.; Garbusow, M.; Sebold, M.; Sommer, C.; Smolka, M.N.; Wittchen, H.U.; Zimmermann, U.S.; Heinz, A.; et al. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Trans. Psychiatry 2017, 7, 1279. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Santelli, L.; Tomassini, V.; Bosnell, R.; Smith, S.; De Stefano, N.; Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 2010, 51, 943–951. [Google Scholar] [CrossRef] [PubMed]
| Case | Gender (F/M) | Age (Years) | PMD (h) | Psychiatric Diagnose | Etiology of Death | Cause of Death | Ethanol in Blood (mg/mL) | Other Drugs in Blood |
|---|---|---|---|---|---|---|---|---|
| C1 | F | 66 | 15 | Control | Accidental | Run over | 0 | (−) |
| A1 | F | 51 | 8 | Alcoholism | Natural | CRF | 2.98 | Nordiazepam |
| S1 | F | 58 | 48 | Major Depression | Suicidal | Drowning | 0 | (−) |
| SA1 | F | 57 | 24 | Alcoholism | Suicidal | Drug intoxication | 0.23 | Methanol |
| C2 | M | 71 | 19 | Control | Natural | CRF | 0 | Nordiazepam |
| A2 | M | 68 | 15 | Alcoholism | Natural | CRF | 0 | (−) |
| S2 | M | 65 | 19 | Obsessive Disorder | Suicidal | Jumping | 0 | Nordiazepam |
| SA2 | M | 71 | 19 | Alcoholism | Suicidal | Hanging | 0.55 | (−) |
| C3 | M | 48 | 7 | Control | Accidental | Traffic | 0 | (−) |
| A3 | M | 50 | 24 | Alcoholism | Natural | CRF | 0 | (−) |
| S3 | M | 50 | 6 | Bipolar Disorder | Suicidal | Jumping | 0 | Diazepam |
| SA3 | M | 50 | 3 | Alcoholism | Suicidal | Hanging | 0 | (−) |
| C4 | M | 52 | 18 | Control | Accidental | Crushed | 0 | (−) |
| A4 | M | 57 | 12 | Alcoholism | Natural | CRF | 3.37 | (−) |
| S4 | M | 59 | 29 | Major Depression | Suicidal | Hanging | 0 | Citalopram, Nordiazepam |
| SA4 | M | 55 | 24 | Alcoholism | Suicidal | Drug intoxication | 2.4 | (−) |
| C5 | M | 40 | 18 | Control | Accidental | Jumping | 0.56 | (−) |
| A5 | M | 43 | 4 | Alcoholism | Natural | CRF | 1.64 | Chlormethiazole, Metamizole |
| S5 | M | 44 | 15 | Anxiety Disorder | Suicidal | Hanging | 0 | (−) |
| SA5 | M | 40 | 21 | Alcoholism | Suicidal | Run over | 0 | (−) |
| C6 | F | 36 | 9 | Control | Natural | Heart failure | 0 | (−) |
| A6 | F | 43 | 35 | Alcoholism | Natural | Hemorrhage | 0 | Metamizole, Fluoxetine |
| S6 | F | 48 | 9 | Anxiety Disorder | Suicidal | Jumping | 0 | (−) |
| SA6 | F | 50 | 6 | Alcoholism | Suicidal | Jumping | 0 | Hydrochlorothiazide |
| C7 | M | 66 | 50 | Control | Accidental | Traffic | 0 | (−) |
| A7 | M | 71 | 17 | Alcoholism | Accidental | Traffic | 0.35 | (−) |
| S7 | M | 67 | 8 | Major Depression | Suicidal | Sharp weapon | 0 | (−) |
| SA7 | M | 69 | 24 | Alcoholism | Suicidal | Jumping | 0.38 | (−) |
| C8 | M | 37 | 21 | Control | Accidental | Cranoencephalic trauma | 0 | (−) |
| A8 | M | 39 | 19 | Alcoholism | Natural | Hemorrhage | 0.44 | (−) |
| S8 | M | 40 | 15 | Personality Disorder | Suicidal | Jumping | 0 | Diazepam |
| SA8 | M | 37 | 68 | Alcoholism | Suicidal | Hanging | 0.9 | Diazepam |
| C9 | M | 54 | 23 | Control | Accidental | Jumping | 0 | (−) |
| A9 | M | 53 | 12 | Alcoholism | Natural | Suffocation | 0 | (−) |
| S9 | M | 50 | 29 | Major Depression | Suicidal | Hanging | 0 | Diazepam |
| SA9 | M | 50 | 70 | Alcoholism | Suicidal | Hanging | 2.93 | (−) |
| C10 | M | 42 | 27 | Control | Accidental | Traffic | 0 | (−) |
| A10 | M | 42 | 20 | Alcoholism | Natural | Hemorrhage | 0 | (−) |
| S10 | M | 41 | 50 | Major Depression | Suicidal | Hanging | 0 | (−) |
| SA10 | M | 41 | 78 | Alcoholism | Suicidal | Hanging | 3.4 | (−) |
| C11 | M | 47 | 26 | Control | Accidental | Work accident | 0 | (−) |
| A11 | M | 46 | 16 | Alcoholism | Accidental | Suffocation | 0.97 | (−) |
| S11 | M | 46 | 47 | Anxiety Disorder | Suicidal | Hanging | 0 | (−) |
| SA11 | M | 44 | 5 | Alcoholism | Suicidal | Jumping | 2.47 | Diazepam |
| Controls | Alcoholic Subjects | Suicide Subjects | Suicide Alcoholic Subjects | |
|---|---|---|---|---|
| Age (years) | 50 ± 4 | 51 ± 3 | 52 ± 3 | 51 ± 3 |
| PMD (h) | 21 ± 3 | 17 ± 3 | 25 ± 5 | 31 ± 8 |
| Gender (F/M) | 2F/9M | 2F/9M | 2F/9M | 2F/9M |
| Case | Gender (F/M) | Age (Years) | PMD (h) | Cause of Death |
|---|---|---|---|---|
| 1 | M | 21 | 15 | Accident/Traffic |
| 2 | F | 22 | 24 | Accident/Fall from height |
| 3 | M | 23 | 17 | Accident/Work |
| 4 | M | 24 | 20 | Accident/Traffic |
| 5 | F | 26 | 5 | Accident/Traffic |
| 6 | M | 28 | 5 | Accident/Fall from height |
| 7 | M | 31 | 13 | Accident/Traffic |
| 8 | M | 32 | 27 | Accident/Traffic |
| 9 | M | 33 | 13 | Accident/Traffic |
| 10 | M | 36 | 18 | Accident/Work |
| 11 | M | 36 | 23 | Accident/Work |
| 12 | F | 38 | 22 | Accident/Traffic |
| 13 | M | 41 | 14 | Natural/Heart Attack |
| 14 | M | 43 | 10 | Accident/Traffic |
| 15 | F | 43 | 28 | Accident/Train |
| 16 | F | 44 | 9 | Natural/CRF |
| 17 | M | 47 | 15 | Accident/Traffic |
| 18 | F | 48 | 9 | Accident/Fall from height |
| 19 | F | 50 | 11 | Natural/CRF |
| 20 | F | 50 | 11 | Natural/CRF |
| 21 | M | 51 | 18 | Accident/Traffic |
| 22 | M | 54 | 24 | Accident/Traffic |
| 23 | F | 57 | 14 | Natural/CRF |
| 24 | M | 58 | 16 | Accident/Traffic |
| 25 | F | 60 | 27 | Accident/Traffic |
| 26 | M | 61 | 23 | Accident/Traffic |
| 27 | M | 62 | 19 | Accident/Work |
| 28 | F | 64 | 19 | Natural/CRF |
| 29 | F | 66 | 20 | Natural/CRF |
| 30 | M | 66 | 18 | Natural/Tumor |
| 31 | F | 70 | 7 | Accident/Traffic |
| 32 | M | 71 | 21 | Natural/CRF |
| 33 | M | 73 | 19 | Accident/Traffic |
| 34 | F | 74 | 21 | Accident/Traffic |
| 35 | F | 75 | 18 | Accident/Traffic |
| 36 | M | 78 | 29 | Accident/Traffic |
| 37 | F | 81 | 14 | Accident/Traffic |
| 38 | M | 81 | 21 | Accident/Traffic |
| 39 | F | 84 | 18 | Natural/CRF |
| 40 | M | 85 | 19 | Natural/CRF |
| 41 | M | 85 | 10 | Natural/CRF |
| 42 | F | 86 | 18 | Natural/CRF |
| α-Tubulin | β-Tubulin | GAPDH | |
|---|---|---|---|
| All subjects | 42 | 42 | 42 |
| Spearman’s Correlation Coefficient | −0.225 | −0.350 * | 0.068 |
| Significance (two-tailed) | 0.153 | 0.023 | 0.67 |
| Male Subjects | 24 | 24 | 24 |
| Spearman’s Correlation Coefficient | −0.107 | −0.247 | 0.303 |
| Significance (two-tailed) | 0.617 | 0.244 | 0.15 |
| Female Subjects | 18 | 18 | 18 |
| Spearman’s Correlation Coefficient | −0.392 | −0.510 * | −0.274 |
| Significance (two-tailed) | 0.107 | 0.031 | 0.272 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labisso, W.L.; Raulin, A.-C.; Nwidu, L.L.; Kocon, A.; Wayne, D.; Erdozain, A.M.; Morentin, B.; Schwendener, D.; Allen, G.; Enticott, J.; et al. The Loss of α- and β-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing. Brain Sci. 2018, 8, 175. https://doi.org/10.3390/brainsci8090175
Labisso WL, Raulin A-C, Nwidu LL, Kocon A, Wayne D, Erdozain AM, Morentin B, Schwendener D, Allen G, Enticott J, et al. The Loss of α- and β-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing. Brain Sciences. 2018; 8(9):175. https://doi.org/10.3390/brainsci8090175
Chicago/Turabian StyleLabisso, Wajana L., Ana-Caroline Raulin, Lucky L. Nwidu, Artur Kocon, Declan Wayne, Amaia M. Erdozain, Benito Morentin, Daniela Schwendener, George Allen, Jack Enticott, and et al. 2018. "The Loss of α- and β-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing" Brain Sciences 8, no. 9: 175. https://doi.org/10.3390/brainsci8090175
APA StyleLabisso, W. L., Raulin, A.-C., Nwidu, L. L., Kocon, A., Wayne, D., Erdozain, A. M., Morentin, B., Schwendener, D., Allen, G., Enticott, J., Gerdes, H. K., Johnson, L., Grzeskowiak, J., Drizou, F., Tarbox, R., Osna, N. A., Kharbanda, K. K., Callado, L. F., & Carter, W. G. (2018). The Loss of α- and β-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing. Brain Sciences, 8(9), 175. https://doi.org/10.3390/brainsci8090175








