Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Hippocampal Cell Culture
2.2. In Vitro Trauma
2.3. Immunohistochemistry
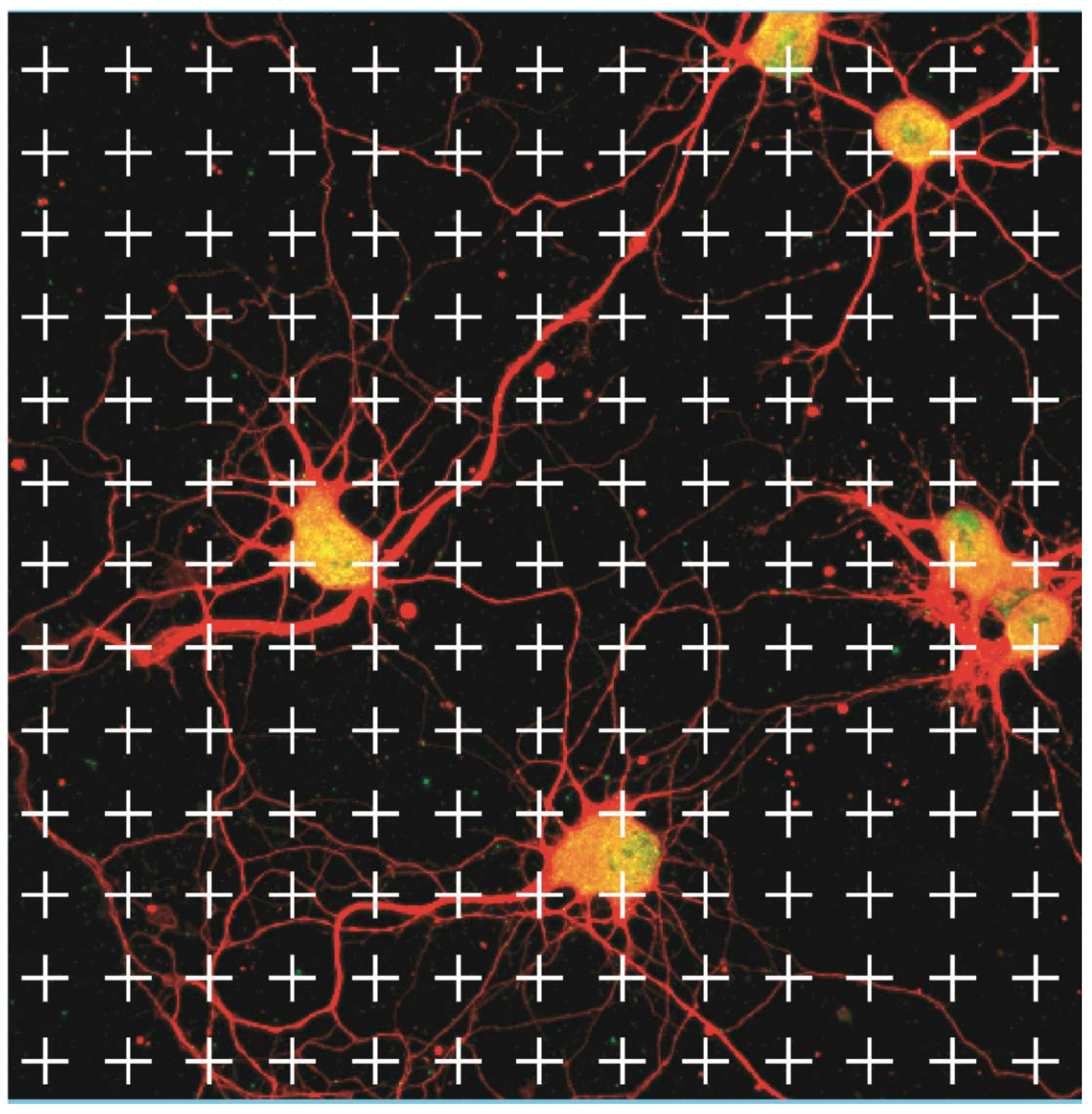
2.4. Microscopy Analysis
2.5. Statistical Analysis
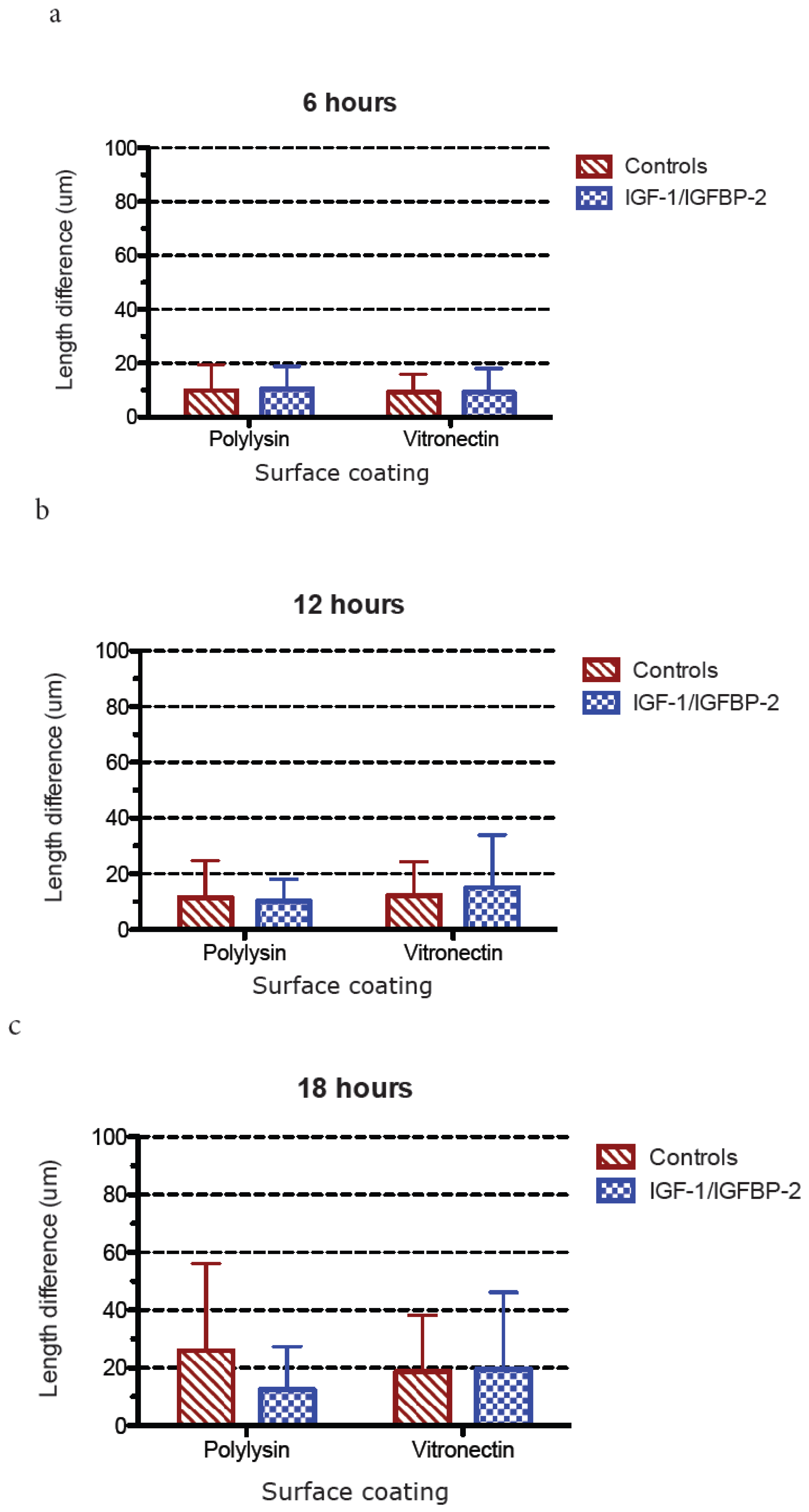
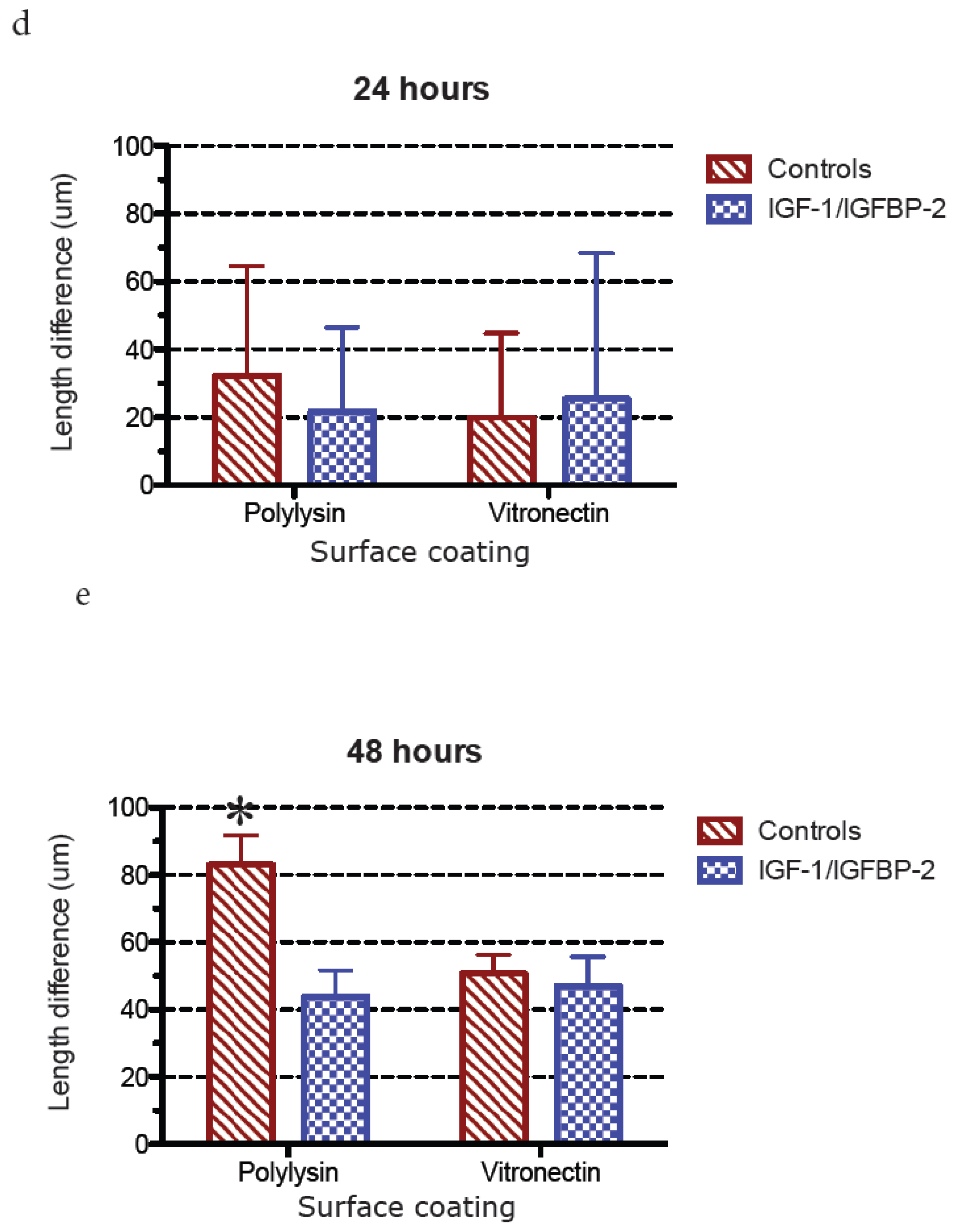
3. Results
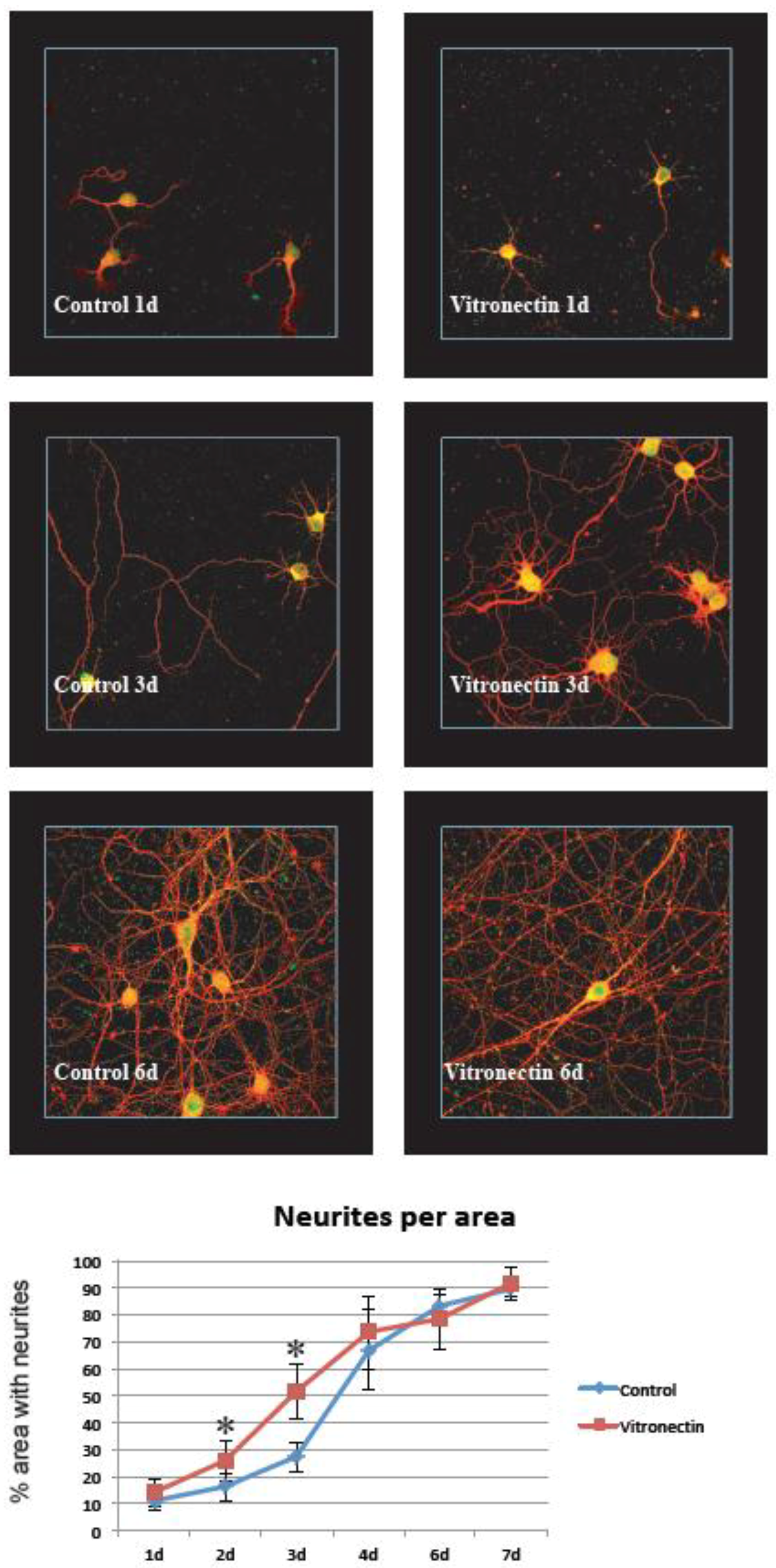
3.1. Neurite Growth on Poly-L-lysin Compared to Vitronectin
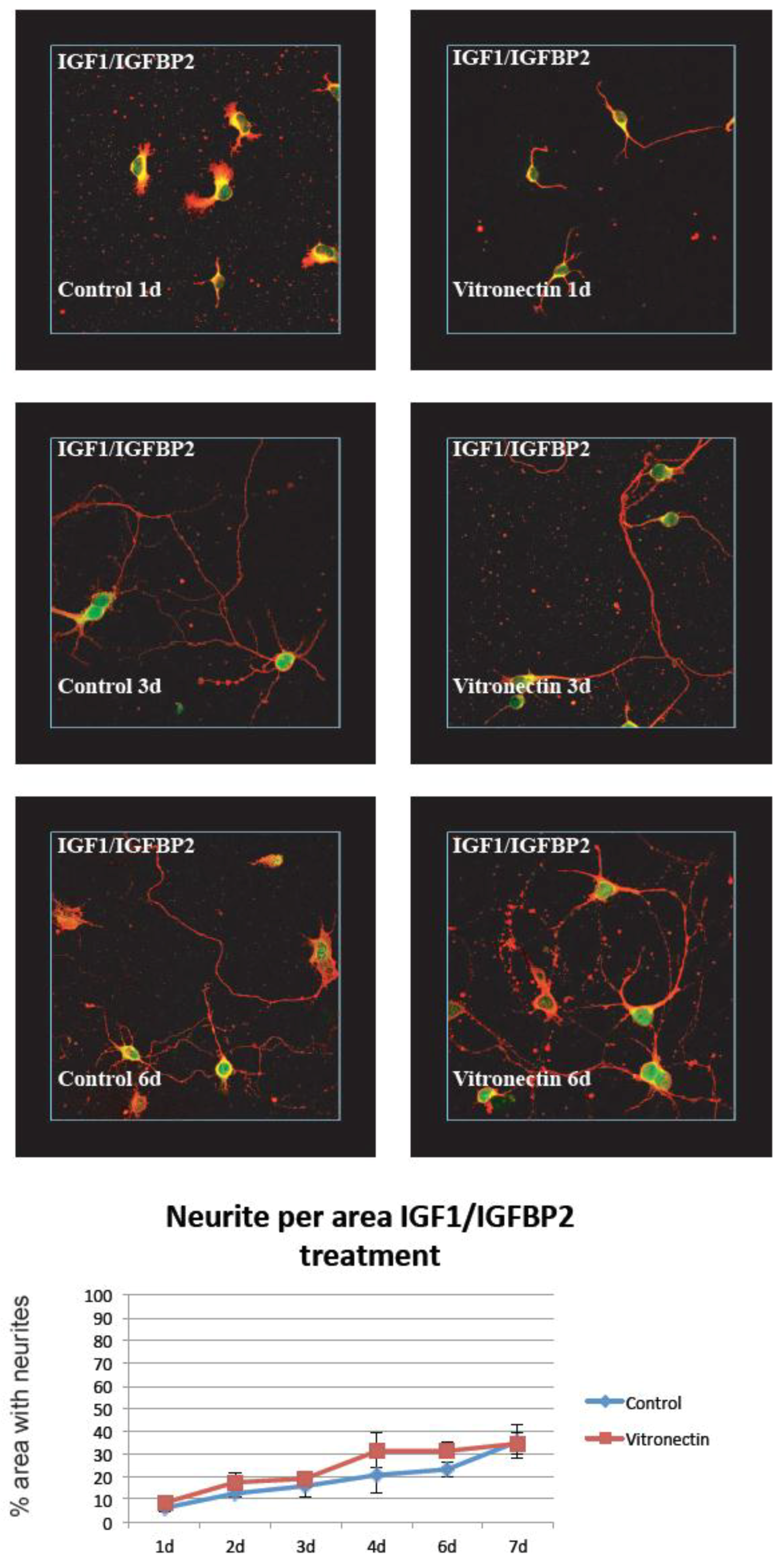
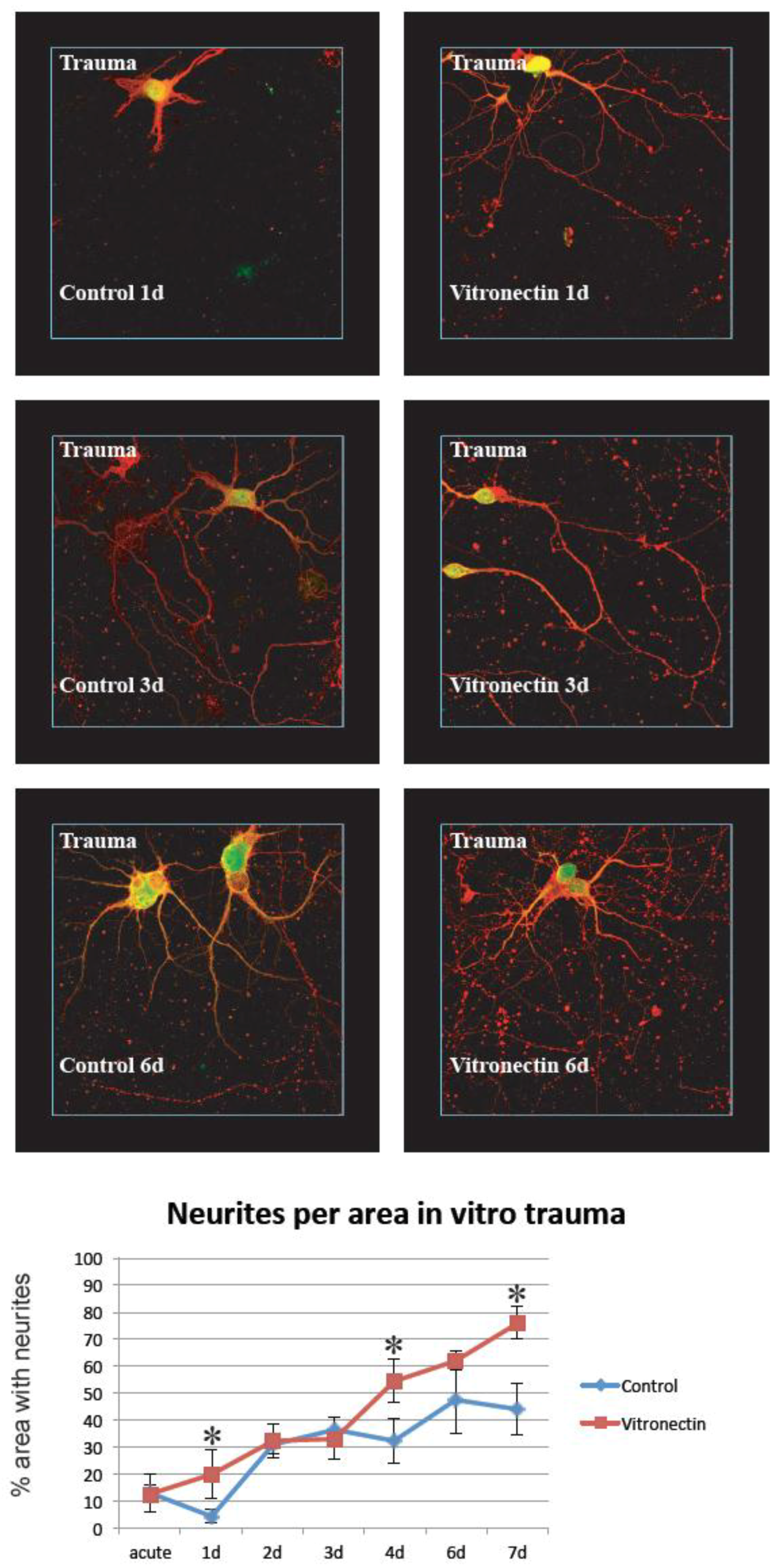
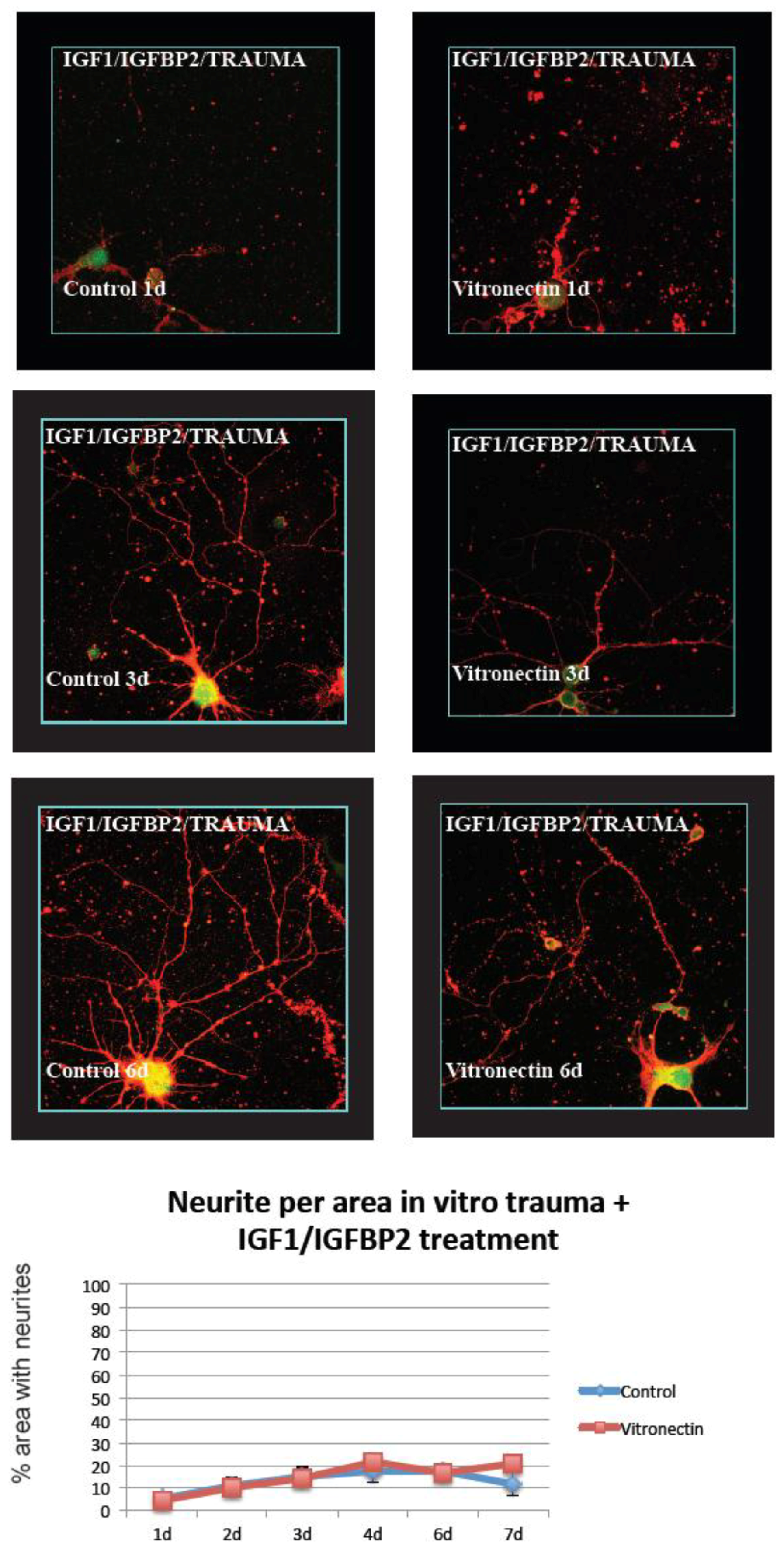
3.2. Neurite Growth after In Vitro Trauma
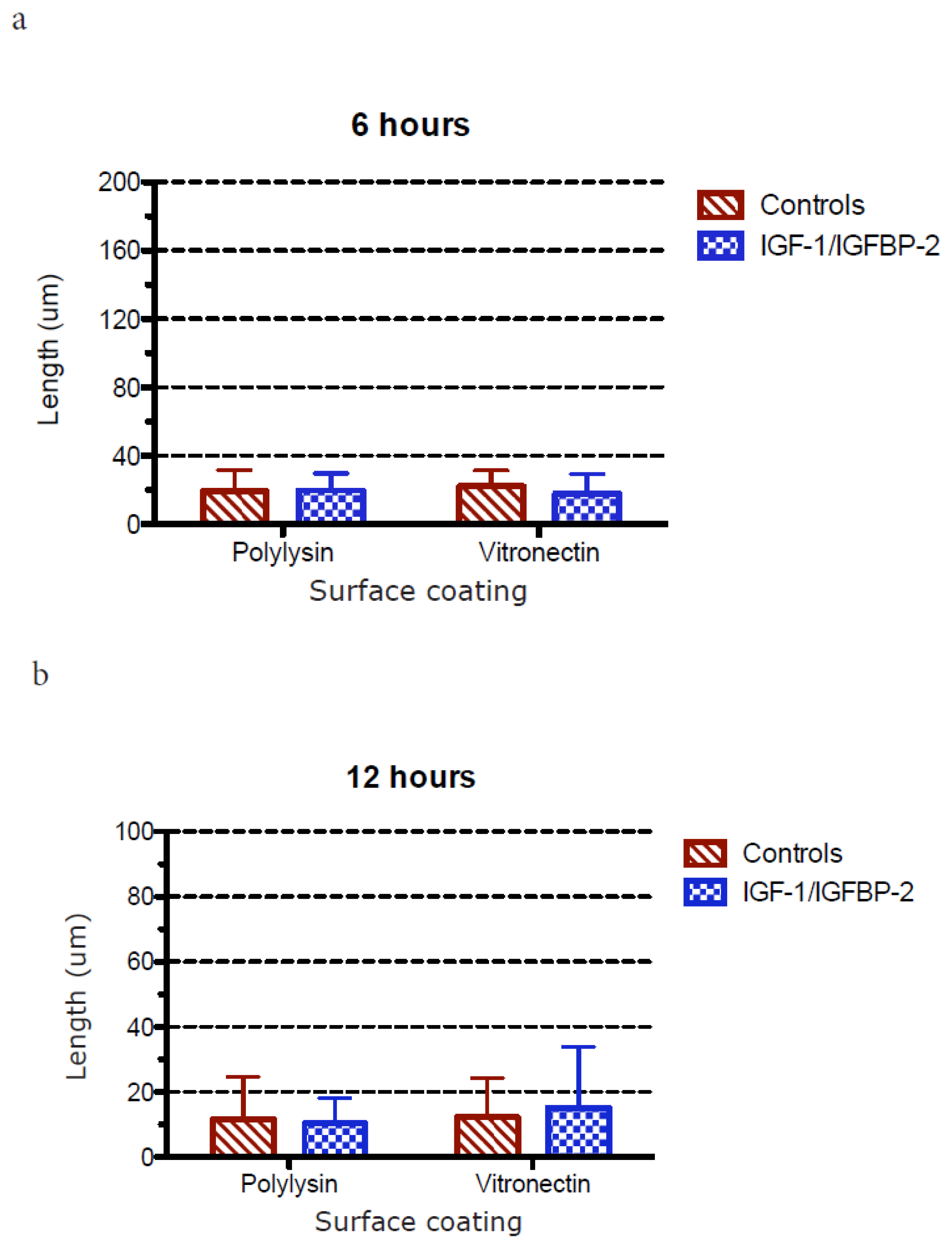
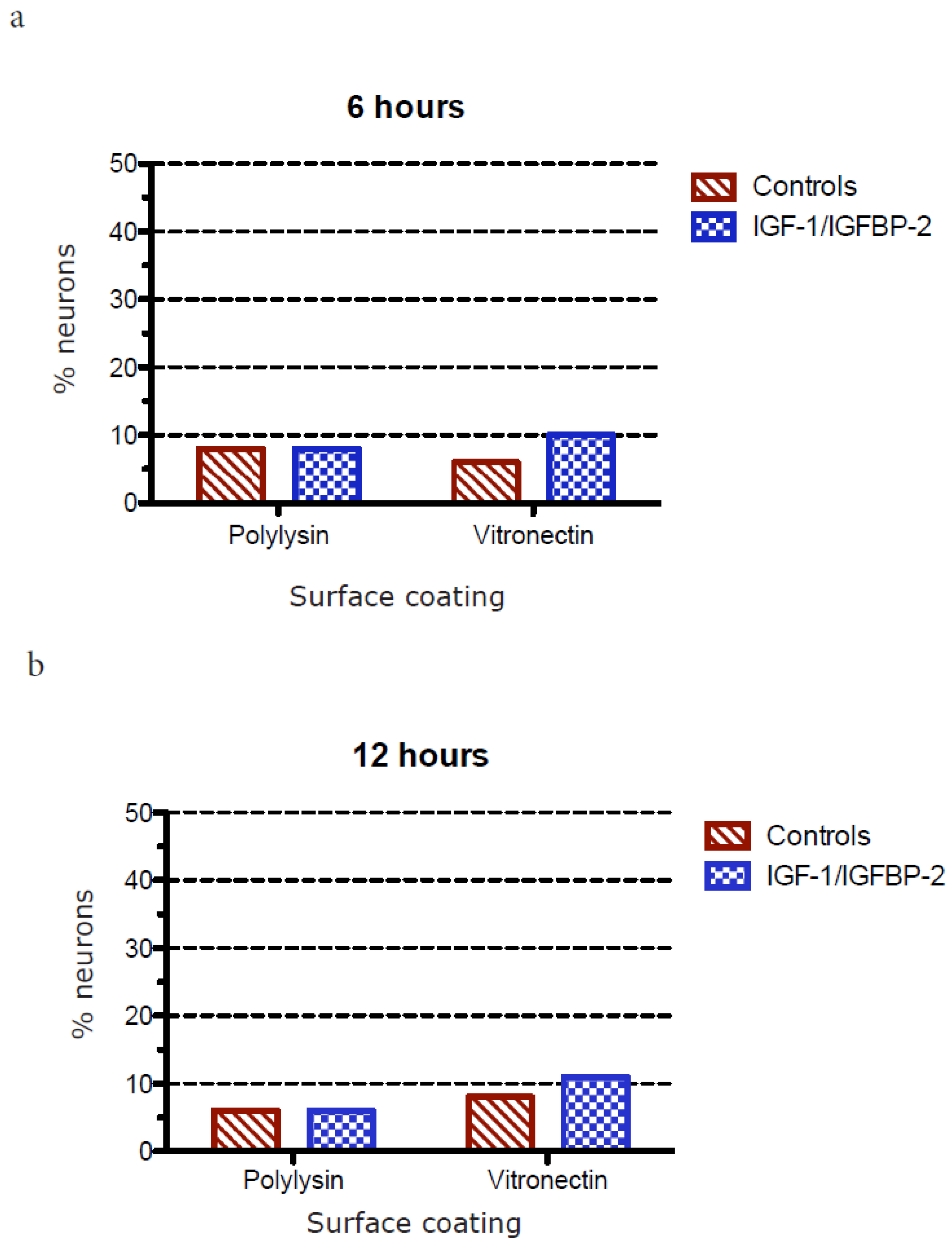
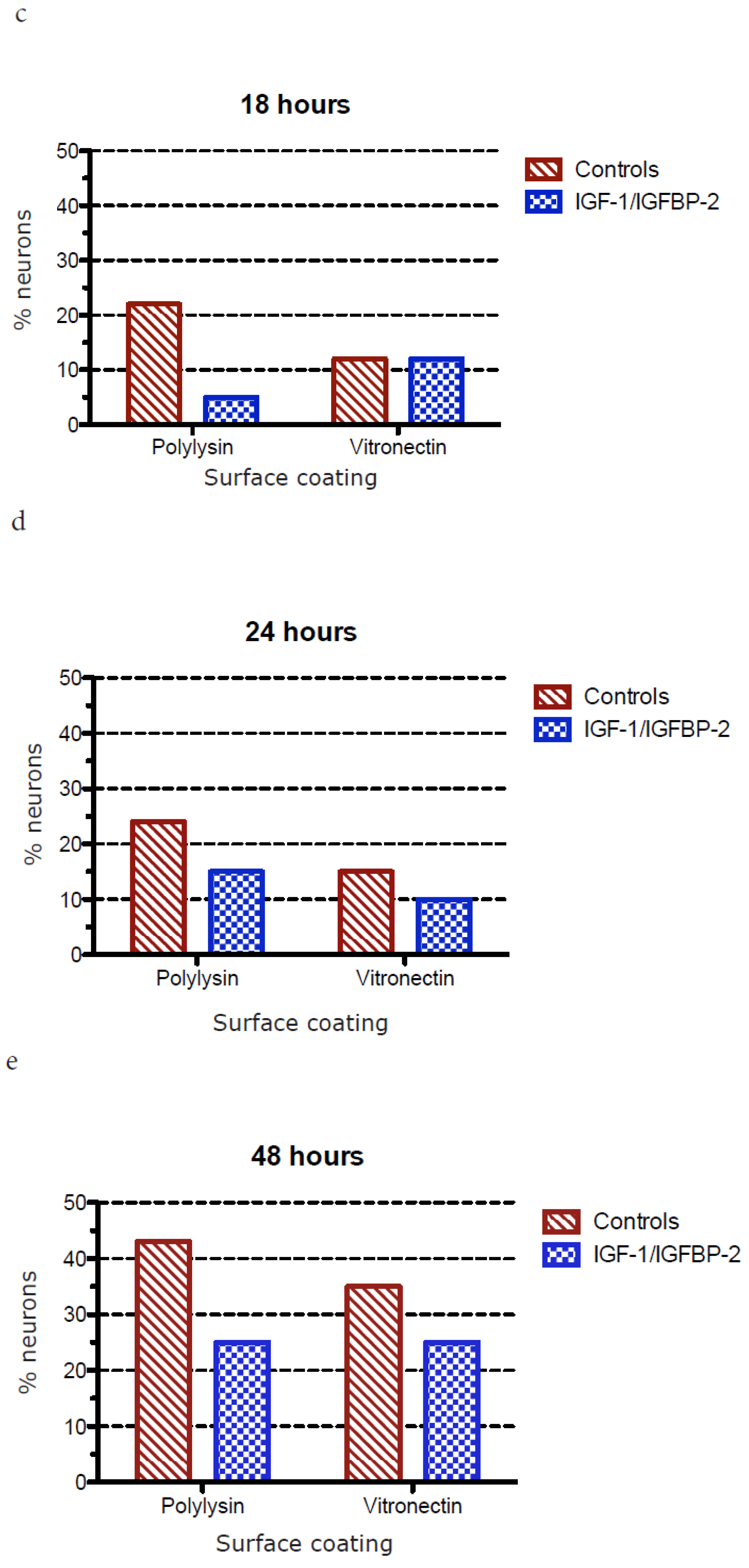
3.3. Measurements of Neurite Polarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandberg Nordqvist, A.C.; von Holst, H.; Holmin, S.; Sara, V.R.; Bellander, B.M.; Schalling, M. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and -4 mRNAs following cerebral contusion. Brain Res. Mol. Brain Res. 1996, 38, 285–293. [Google Scholar] [CrossRef]
- Schober, M.E.; Ke, X.; Block, B.P.; Requena, D.F.; McKnight, R.; Lane, R.H. Traumatic brain injury increased IGF-1B mRNA and altered IGF-1 exon 5 and promoter region epigenetic characteristics in the rat pup hippocampus. J. Neurotrauma 2012, 29, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.A.; Shen-Orr, Z.; Lowe, W.L., Jr.; Roberts, C.T., Jr.; LeRoith, D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res. Mol. Brain Res. 1991, 10, 43–48. [Google Scholar] [CrossRef]
- Madathil, S.K.; Evans, H.N.; Saatman, K.E. Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 2010, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/cns.12866. [CrossRef] [PubMed]
- Pons, S.; Torres-Aleman, I. Insulin-like growth factor-I stimulates dephosphorylation of ikappa B through the serine phosphatase calcineurin (protein phosphatase 2B). J. Biol. Chem. 2000, 275, 38620–38625. [Google Scholar] [CrossRef] [PubMed]
- Clawson, T.F.; Vannucci, S.J.; Wang, G.M.; Seaman, L.B.; Yang, X.L.; Lee, W.H. Hypoxia-ischemia-induced apoptotic cell death correlates with IGF-1 mRNA decrease in neonatal rat brain. Biol. Signals Recept. 1999, 8, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Wang, G.M.; Yang, X.L.; Seaman, L.B.; Vannucci, S.I. Perinatal hypoxia-ischemia decreased neuronal but increased cerebral vascular endothelial IGFBP3 expression. Endocrine 1999, 11, 181–188. [Google Scholar] [CrossRef]
- Breese, C.R.; D’Costa, A.; Rollins, Y.D.; Adams, C.; Booze, R.M.; Sonntag, W.E.; Leonard, S. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J. Comp. Neurol. 1996, 369, 388–404. [Google Scholar] [CrossRef]
- Morel, G.R.; Leon, M.L.; Uriarte, M.; Reggiani, P.C.; Goya, R.G. Therapeutic potential of IGF-1 on hippocampal neurogenesis and function during aging. Neurogenesis (Austin) 2017, 4, e1259709. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.W.; Saatman, K.E. Central Infusion of Insulin-Like Growth Factor-1 Increases Hippocampal Neurogenesis and Improves Neurobehavioral Function after Traumatic Brain Injury. J. Neurotrauma. 2018, 35, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Contreras, P.C.; Smith, D.H.; Raghupathi, R.; McDermott, K.L.; Fernandez, S.C.; Sanderson, K.L.; Voddi, M.; McIntosh, T.K. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 1997, 147, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Loddick, S.A.; Liu, X.J.; Lu, Z.X.; Liu, C.; Behan, D.P.; Chalmers, D.C.; Foster, A.C.; Vale, W.W.; Ling, N.; De Souza, E.B. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc. Natl. Acad. Sci. USA 1998, 95, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Hatton, J.; Rapp, R.P.; Kudsk, K.A.; Brown, R.O.; Luer, M.S.; Bukar, J.G.; Chen, S.A.; McClain, C.J.; Gesundheit, N.; Dempsey, R.J.; et al. Intravenous insulin-like growth factor-I (IGF-1) in moderate-to-severe head injury: a phase II safety and efficacy trial. J. Neurosurg. 1997, 86, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.A.; Aberg, N.D.; Hedbacker, H.; Oscarsson, J.; Eriksson, P.S. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000, 20, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Borasio, G.D.; Robberecht, W.; Leigh, P.N.; Emile, J.; Guiloff, R.J.; Jerusalem, F.; Silani, V.; Vos, P.E.; Wokke, J.H.; Dobbins, T. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-1 Study Group. Neurology 1998, 51, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, N.; de Vos, R.A.; De Keyser, J. Free insulin-like growth factor (IGF)-I and IGF binding proteins 2, 5, and 6 in spinal motor neurons in amyotrophic lateral sclerosis. Lancet 2003, 361, 1007–1011. [Google Scholar] [CrossRef]
- Yoshimura, T.; Arimura, N.; Kaibuchi, K. Signaling networks in neuronal polarization. J. Neurosci. 2006, 26, 10626–10630. [Google Scholar] [CrossRef] [PubMed]
- Ozdinler, P.H.; Macklis, J.D. IGF-1 specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 2006, 9, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.; Dupraz, S.; Laurino, L.; Bollati, F.; Bisbal, M.; Cáceres, A.; Pfenninger, K.H.; Quiroga, S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat. Neurosci. 2006, 9, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, S.E.; Doe, C.Q. Microtubule-induced cortical cell polarity. Genes Dev. 2007, 21, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, S.L.; Barnard, Z.; Upton, Z.; Harkin, D.G. Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF-1 and HGF, and facilitates serum-free cultivation. Exp. Eye Res. 2006, 83, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Van Lonkhuyzen, D.R.; Hollier, B.G.; Shooter, G.K.; Leavesley, D.I.; Upton, Z. Chimeric vitronectin: insulin-like growth factor proteins enhance cell growth and migration through co-activation of receptors. Growth Factors 2007, 25, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Marti, E. Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation. Development 2000, 127, 333–342. [Google Scholar] [PubMed]
- Hashimoto, K.; Sakane, F.; Ikeda, N.; Akiyama, A.; Sugahara, M.; Miyamoto, Y. Vitronectin promotes the progress of the initial differentiation stage in cerebellar granule cells. Mol. Cell Neurosci. 2016, 70, 76–85. [Google Scholar] [CrossRef] [PubMed]
- von Gertten, C.; Flores Morales, A.; Holmin, S.; Mathiesen, T.; Nordqvist, A.C. Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci. 2005, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Risling, M.; Malm, E.; Sondén, A.; Bolling, M.F.; Sköld, M.K. Cellular High-Energy Cavitation Trauma - Description of a Novel In Vitro Trauma Model in Three Different Cell Types. Front Neurol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Sonden, A.; Svensson, B.; Roman, N.; Ostmark, H.; Brismar, B.; Palmblad, J.; Kjellström, B.T. Laser-induced shock wave endothelial cell injury. Lasers Surg. Med. 2000, 26, 364–375. [Google Scholar] [CrossRef]
- Sonden, A.; Svensson, B.; Roman, N.; Brismar, B.; Palmblad, J.; Kjellström, B.T. Mechanisms of shock wave induced endothelial cell injury. Lasers Surg. Med. 2002, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kricker, J.A.; Towne, C.L.; Firth, S.M.; Herington, A.C.; Upton, Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology 2003, 144, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Kricker, J.A.; Hyde, C.E.; Van Lonkhuyzen, D.R.; Hollier, B.G.; Shooter, G.K.; Leavesley, D.I.; Herington, A.C.; Upton, Z. Mechanistic investigations into interactions between IGF-1 and IGFBPs and their impact on facilitating cell migration on vitronectin. Growth Factors 2010, 28, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Hollier, B.G.; Manton, K.J.; Satyamoorthy, K.; Leavesley, D.I.; Upton, Z. Insulin-like growth factor-I: vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology 2011, 152, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Schütt, B.S.; Andaloro, E.; Ymer, SI.; Hoeflich, A.; Ranke, M.B.; Bach, L.A.; Werther, G.A. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology 2005, 146, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh Tafreshi, A.; Talebi, F.; Ghorbani, S.; Bernard, C.; Noorbakhsh, F. Altered expression of IGF-1 system in neurons of the inflamed spinal cord during acute experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2017, 525, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997, 8, 45–62. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Gockerman, A.; Busby, W.H., Jr.; Camacho-Hubner, C.; Clemmons, D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-1. J. Cell Biol. 1993, 121, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front Endocrinol (Lausanne) 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergen, K.; Frödin, M.; Von Gertten, C.; Sandberg-Nordqvist, A.-C.; Sköld, M.K. Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment. Brain Sci. 2018, 8, 151. https://doi.org/10.3390/brainsci8080151
Bergen K, Frödin M, Von Gertten C, Sandberg-Nordqvist A-C, Sköld MK. Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment. Brain Sciences. 2018; 8(8):151. https://doi.org/10.3390/brainsci8080151
Chicago/Turabian StyleBergen, K., M. Frödin, C. Von Gertten, A. -C. Sandberg-Nordqvist, and M. K. Sköld. 2018. "Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment" Brain Sciences 8, no. 8: 151. https://doi.org/10.3390/brainsci8080151
APA StyleBergen, K., Frödin, M., Von Gertten, C., Sandberg-Nordqvist, A.-C., & Sköld, M. K. (2018). Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment. Brain Sciences, 8(8), 151. https://doi.org/10.3390/brainsci8080151



