The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies
2.3. Cells, Plasmids, Transfection, and Reagents
2.4. Immunofluorescence
2.5. Immunoblotting
2.6. Co-Immunoprecipitation Assay
2.7. Small Interfering RNAs
2.8. Herpes Simplex Virus Type 1 (HSV-1)-Based Amplicon Vectors
2.9. Stereotaxic Procedures
2.10. Binge Drinking Paradigm
2.11. Kelley Nicotine Sensitization Paradigm
2.12. Statistics
3. Results
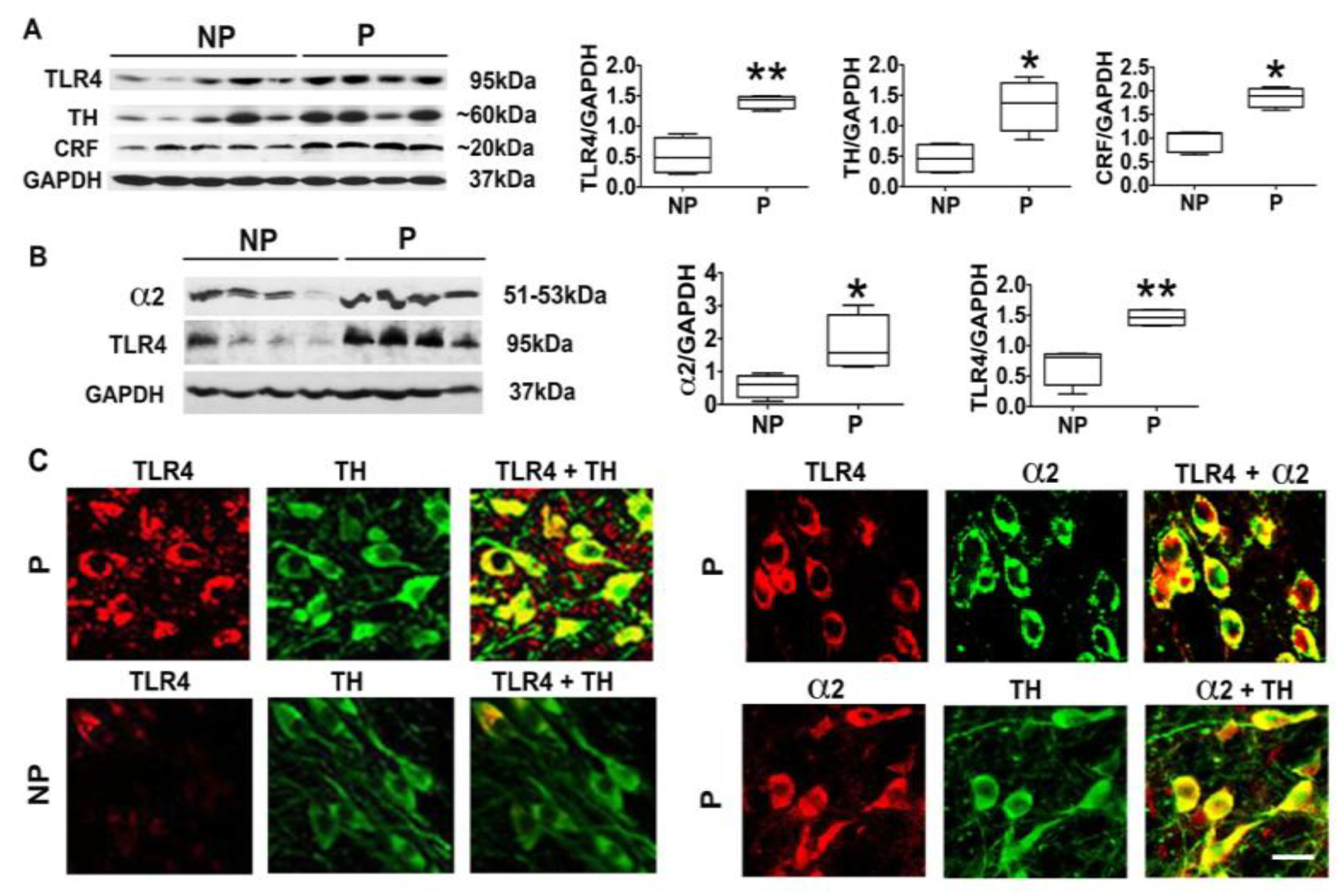
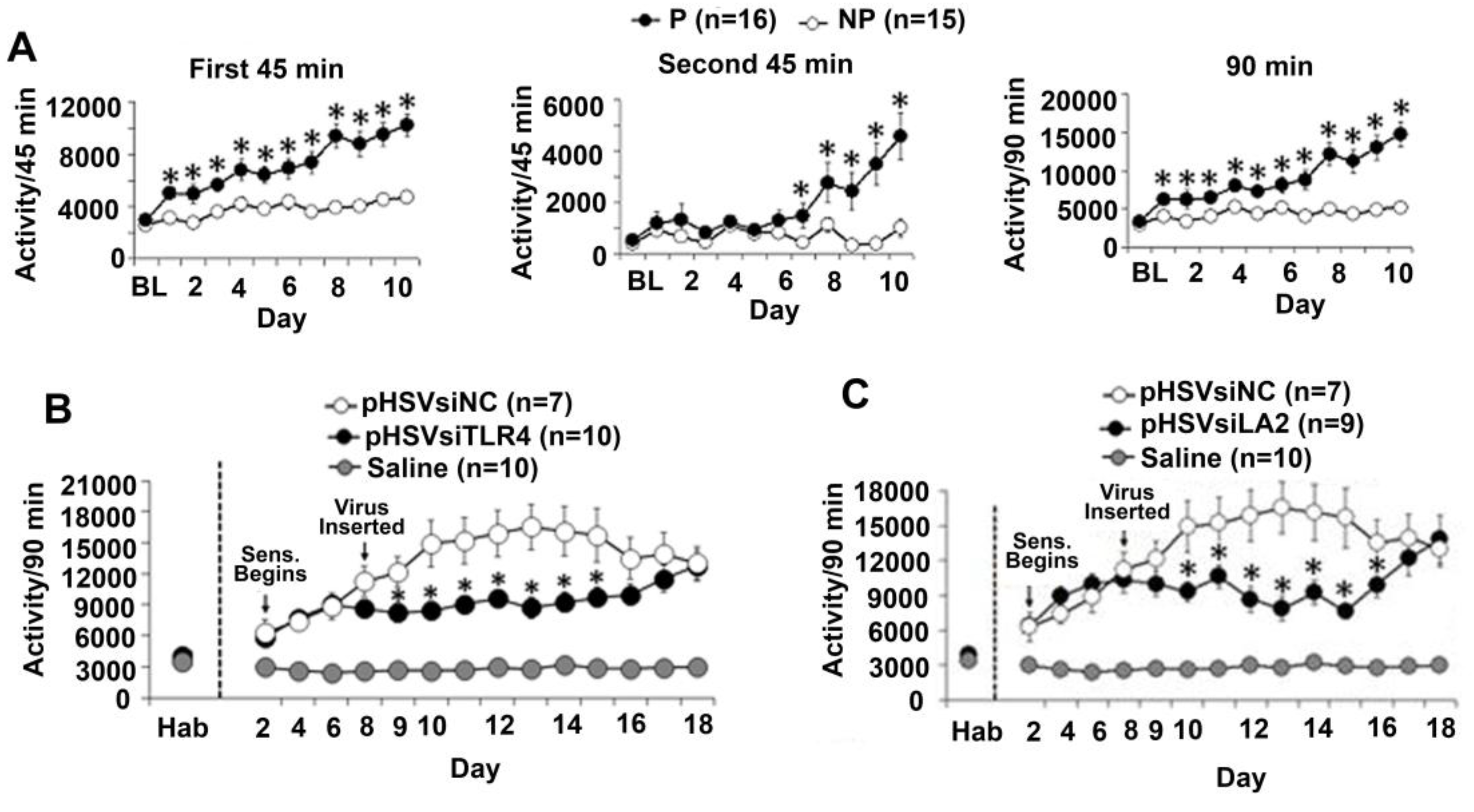
3.1. The P Rats Have Larger Numbers of VTA-Located TH+ Neurons that Co-Express TLR4 and α2 Than the NP Rats
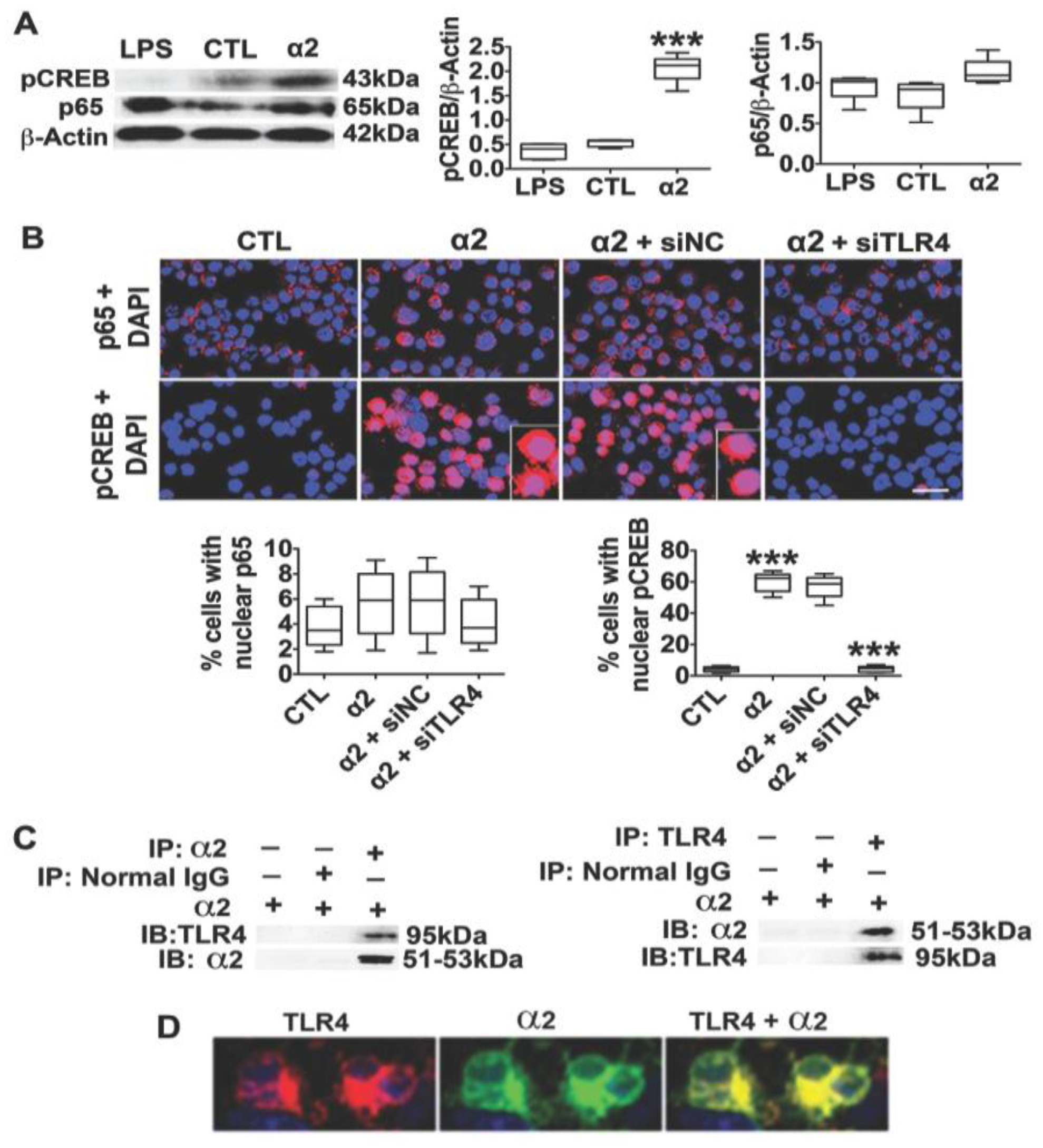
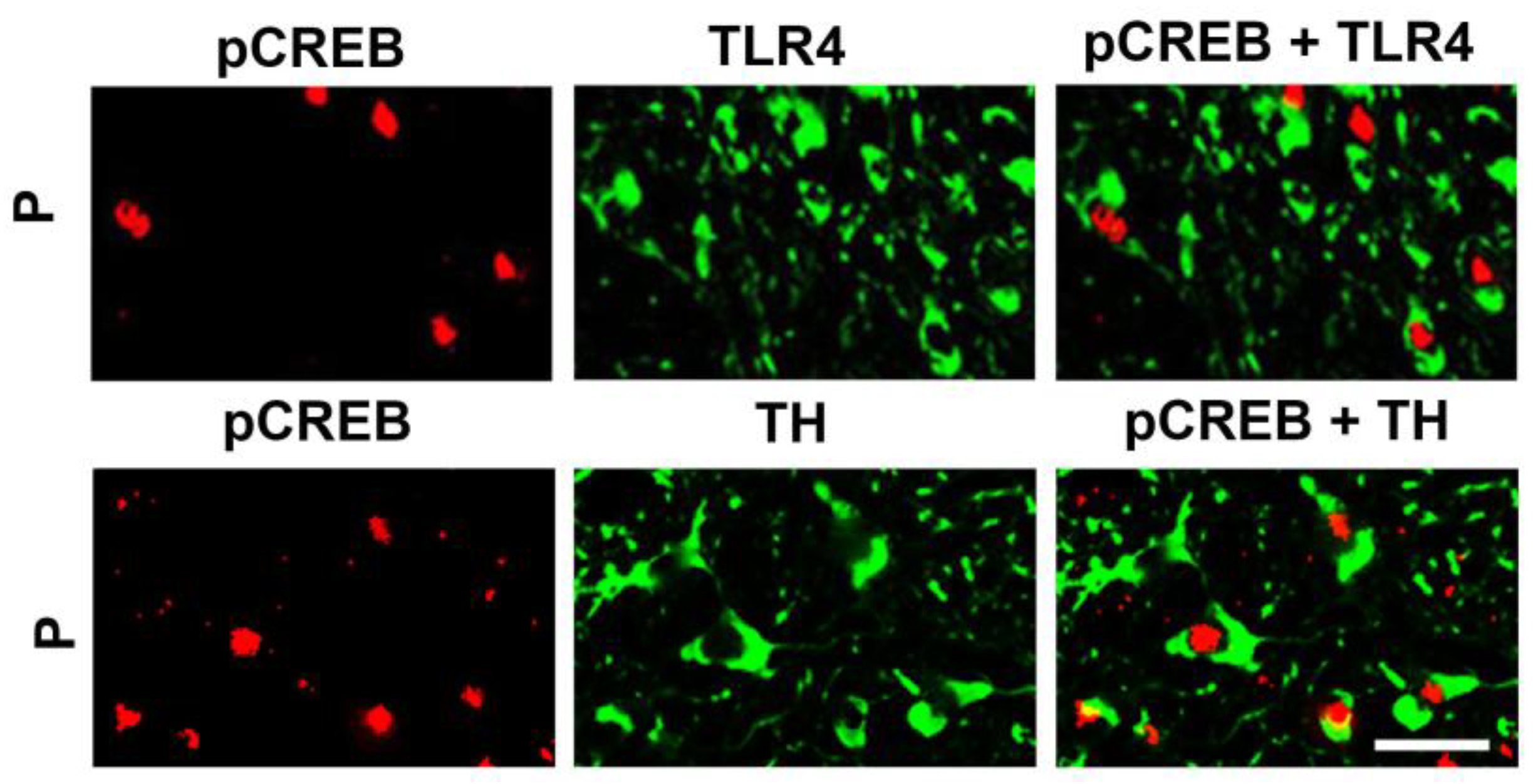
3.2. The Neuronal TLR4 Signal is Activated by α2 and it Culminates in Activated CREB, but not NF-kB
3.3. α2+ Binds TLR4, Likely at the Cell Surface
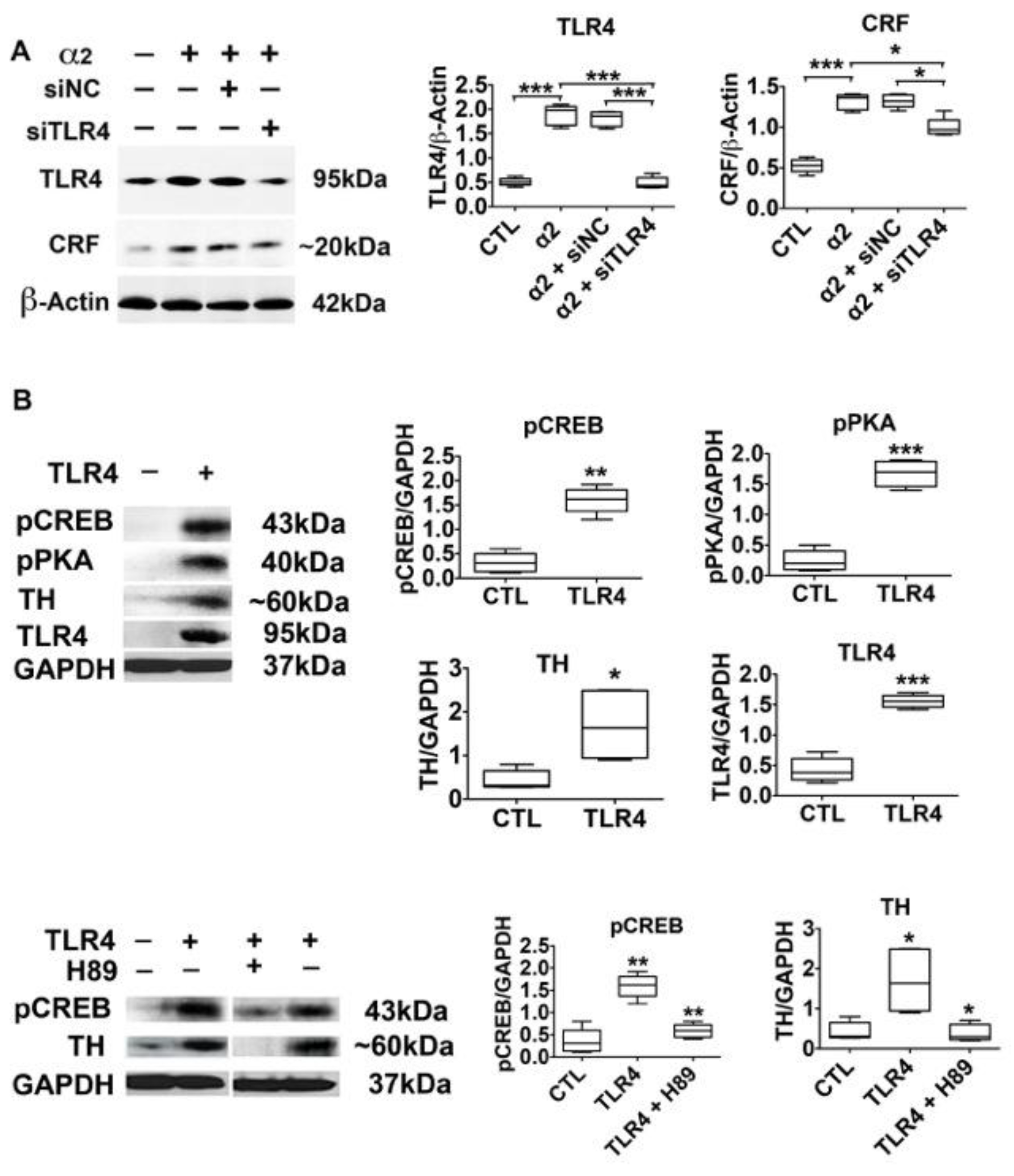
3.4. The α2-Activated TLR4 Signal Contributes to CRF and TH Expression
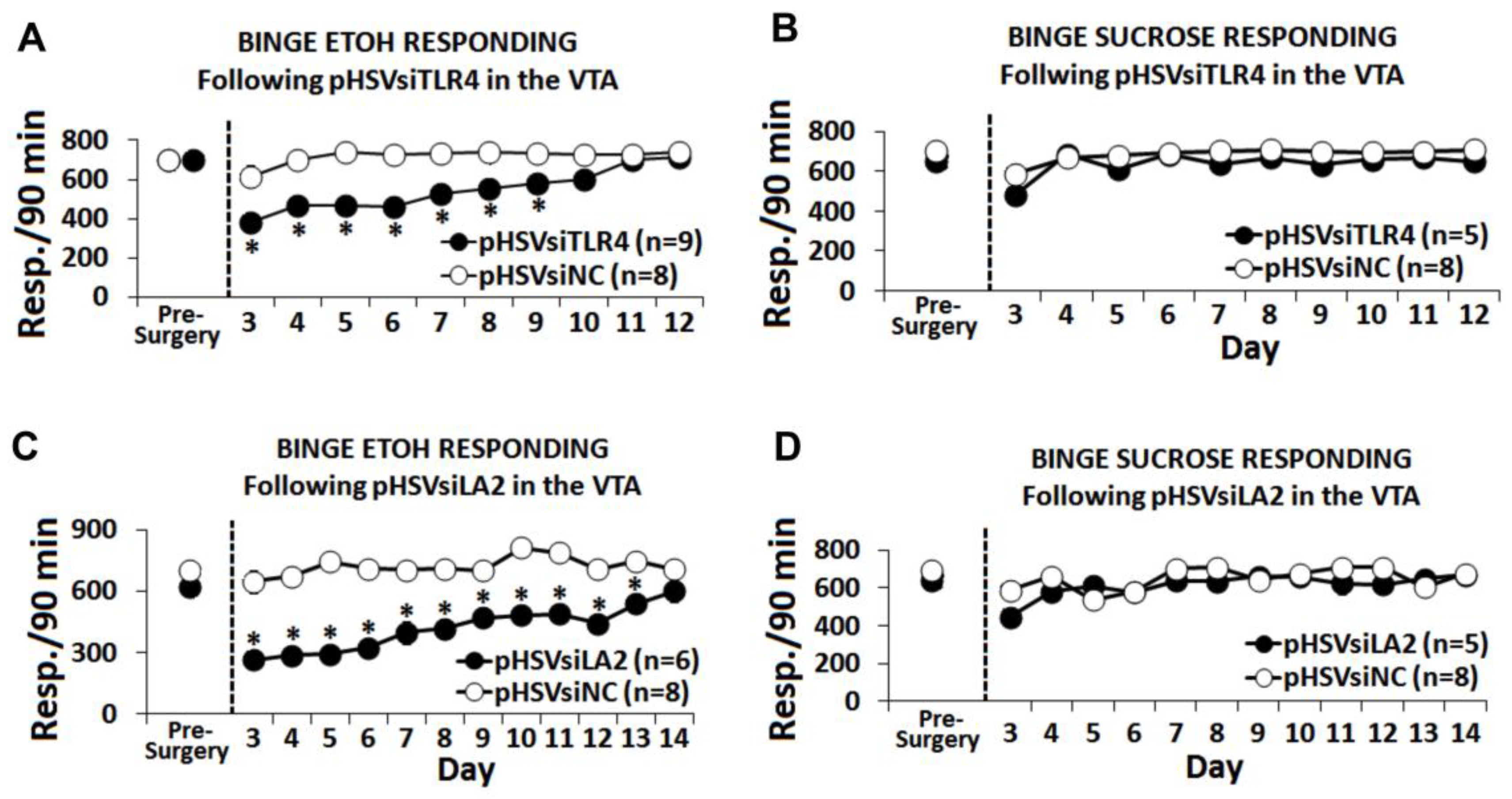
3.5. The α2/TLR4 Signal Controls Binge Drinking
3.6. The α2/TLR4 Signal in the VTA Controls Nicotine Sensitization
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Town, M.; Naimi, T.S.; Mokdad, A.H.; Brewer, R.D. Health care access among U.S. adults who drink alcohol excessively: Missed opportunities for prevention. Prev. Chronic Dis. 2006, 3, A53. [Google Scholar] [PubMed]
- US Department of Health and Human Services. National Institute on Alcohol Abuse and Alcoholism NIAAA Council approves definition of binge drinking. In NIAAA Newsletter; US Department of Health and Human Services: Washington, DC, USA, 2004. [Google Scholar]
- Ducci, F.; Enoch, M.A.; Funt, S.; Virkkunen, M.; Albaugh, B.; Goldman, D. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol 2007, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; Dick, D.M.; Xuei, X.; Tian, H.; Almasy, L.; Bauer, L.O.; Crowe, R.R.; Goate, A.; Hesselbrock, V.; Jones, K.; et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 2004, 74, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Chikritzhs, T.N.; Jonas, H.A.; Stockwell, T.R.; Heale, P.F.; Dietze, P.M. Mortality and life-years lost due to alcohol: A comparison of acute and chronic causes. Med. J. Aust. 2001, 174, 281–284. [Google Scholar] [PubMed]
- Naimi, T.S.; Brewer, R.D.; Mokdad, A.; Denny, C.; Serdula, M.K.; Marks, J.S. Binge drinking among US adults. JAMA 2003, 289, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ling, P.M. Impact of alcohol use and bar attendance on smoking and quit attempts among young adult bar patrons. Am. J. Public Health 2013, 103, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Taylor, R.E.; Tizabi, Y. Positive and negative effects of alcohol and nicotine and their interactions: A mechanistic review. Neurotox. Res. 2012, 21, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Nurnberger, J.I., Jr.; Wiegand, R.; Bucholz, K.; O’Connor, S.; Meyer, E.T.; Reich, T.; Rice, J.; Schuckit, M.; King, L.; Petti, T.; et al. A family study of alcohol dependence: Coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch. Gen. Psychiatry 2004, 61, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.E.; Scherrer, J.F.; Bucholz, K.K.; Todorov, A.; Heath, A.C.; Jacob, T.; True, W.R. Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug Alcohol Depend. 2007, 87, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.R.; Getachew, B.; Oster, S.M.; Dhaher, R.; Ding, Z.M.; Bell, R.L.; McBride, W.J.; Rodd, Z.A. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol. Clin. Exp. Res. 2012, 36, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Buck, C.L.; Cohen, A.; Edwards, S.; Park, P.E.; Schlosburg, J.E.; Schmeichel, B.; Vendruscolo, L.F.; Wade, C.L.; Whitfield, T.W., Jr.; et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014, 76 Pt B, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Walter, T.J.; Coleman, L.G., Jr.; Vetreno, R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology 2017, 234, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.M.; Smith, G.; Olausson, P.; Mitchell, S.H.; Leeman, R.F.; O’Malley, S.S.; Sher, K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict. Biol. 2010, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Caswell, A.J.; Celio, M.A.; Morgan, M.J.; Duka, T. Impulsivity as a Multifaceted Construct Related to Excessive Drinking Among UK Students. Alcohol Alcohol. 2016, 51, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Rodd, Z.A.; Lumeng, L.; Murphy, J.M.; McBride, W.J. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict. Biol. 2006, 11, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, B.G.; Grahame, N.J. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol. Clin. Exp. Res. 2009, 33, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, S.W.; Czachowski, C.L. Increased delay discounting tracks with a high ethanol-seeking phenotype and subsequent ethanol seeking but not consumption. Alcohol. Clin. Exp. Res. 2014, 38, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, A.R.; Kelly, T.; Puche, A.; Esoga, C.; June, H.L., Jr.; Elnabawi, A.; Merchenthaler, I.; Sieghart, W.; June, H.L., Sr.; et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc. Natl. Acad. Sci. USA 2011, 108, 4465–4470. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Liu, J.; Warnock, K.T.; Bell, K.A.; Balan, I.; Bollino, D.; Puche, A.; Aurelian, L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 2015, 40, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Aurelian, L.; Warnock, K.T.; Balan, I.; Puche, A.; June, H. TLR4 signaling in VTA dopaminergic neurons regulates impulsivity through tyrosine hydroxylase modulation. Transl. Psychiatry 2016, 6, e815. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, P.M.; Sterling, C.R.; Tank, A.W. Induction of tyrosine hydroxylase mRNA by nicotine in rat midbrain is inhibited by mifepristone. J. Neurochem. 2009, 109, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Nisell, M.; Nomikos, G.G.; Svensson, T.H. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Pharmacol. Toxicol. 1995, 76, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Zhang, J.; Corrigall, W.A. Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: In vivo microdialysis study. Neurosci. Lett. 2003, 348, 61–64. [Google Scholar] [CrossRef]
- Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; Aurelian, L. Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity. Brain. Behav. Immun. 2018, 69, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF family of neuropeptides and their receptors—Mediators of the central stress response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.J.; Reed, C.; Pastor, R. Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation. Genes Brain Behav. 2015, 14, 98–135. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.; Wang, A.; Harding, S.; Juzytsch, W.; Shaham, Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology 2003, 168, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.; Li, Z.; Funk, D.; Shram, M.; Li, T.K.; Shaham, Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J. Neurosci. 2006, 26, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, M.; Dani, J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011, 34, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000, 393, 295–314. [Google Scholar] [CrossRef]
- Gonzales, R.A.; Job, M.O.; Doyon, W.M. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol. Ther. 2004, 103, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Okun, E.; Griffioen, K.J.; Mattson, M.P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011, 34, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Doyon, W.M.; Dong, Y.; Ostroumov, A.; Thomas, A.M.; Zhang, T.A.; Dani, J.A. Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 2013, 79, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Matsushita, N.; Kobayashi, K.; Kobayashi, K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J. Neurochem. 2004, 89, 7–14. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Li, T.K. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit. Rev. Neurobiol. 1998, 12, 339–369. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Nelson, J.W.; Zhu, J.; Smith, C.C.; Kulka, M.; Aurelian, L. ATP and SH3 binding sites in the protein kinase of the large subunit of herpes simplex virus type 2 of ribonucleotide reductase (ICP10). J. Biol. Chem. 1996, 271, 17021–17027. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Yu, Y.X.; Kulka, M.; Aurelian, L. A novel human gene similar to the protein kinase (PK) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) codes for a serine-threonine PK and is expressed in melanoma cells. J. Biol. Chem. 2000, 275, 25690–25699. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, A.; Aramuni, G.; Meyer, G.; Soykan, T.; Hoon, M.; Papadopoulos, T.; Zhang, M.; Paarmann, I.; Fuchs, C.; Harvey, K.; et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 2009, 63, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Glauser, D.L.; Heid, I.; Turkeri, G.; Hilbe, M.; Jacobs, A.H.; Ackermann, M.; Fraefel, C. Herpes simplex virus 1 amplicon vector-mediated siRNA targeting epidermal growth factor receptor inhibits growth of human glioma cells in vivo. Mol. Ther. 2005, 12, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.C.; Foster, K.L.; McKay, P.F.; Carroll, M.R.; Seyoum, R.; Woods, J.E., II; Grey, C.; Jones, C.M.; McCane, S.; Cummings, R.; et al. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J. Neurosci. 2002, 22, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Rodd, Z.A.; Engleman, E.A.; Toalston, J.E.; McBride, W.J. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: Modeling adolescent and adult binge-like drinking. Alcohol 2014, 48, 225–234. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Eiler, W.J.A. Dopaminergic and GABAergic regulation of alcohol-motivated behaviors: novel neuroanatomical substrates. In Handbook of Contemporary Neuropharmacology; John Wiley & Sons, Inc.: Francisco, CA, USA, 2007. [Google Scholar]
- Stephens, D.N.; Duka, T. Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.E.; Holahan, M.R.; Landry, C.F.; Kelley, A.E. Morphine-associated environmental cues elicit conditioned gene expression. Synapse 2000, 37, 146–158. [Google Scholar] [CrossRef]
- DiFranza, J.R.; Wellman, R.J. Sensitization to nicotine: How the animal literature might inform future human research. Nicot. Tob. Res. 2007, 9, 9–20. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Kimpel, M.W.; McClintick, J.N.; Ding, Z.M.; Hauser, S.R.; Edenberg, H.J.; Bell, R.L.; Rodd, Z.A. Changes in gene expression within the ventral tegmental area following repeated excessive binge-like alcohol drinking by alcohol-preferring (P) rats. Alcohol 2013, 47, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Gondre-Lewis, M.C.; Warnock, K.T.; Wang, H.; June, H.L., Jr.; Bell, K.A.; Rabe, H.; Tiruveedhula, V.V.; Cook, J.; Luddens, H.; Aurelian, L.; et al. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress 2016, 19, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Theberge, F.R.; Li, X.; Kambhampati, S.; Pickens, C.L.; St Laurent, R.; Bossert, J.M.; Baumann, M.H.; Hutchinson, M.R.; Rice, K.C.; Watkins, L.R.; et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry 2013, 73, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Bonci, A.; Bernardi, G.; Grillner, P.; Mercuri, N.B. The dopamine-containing neuron: Maestro or simple musician in the orchestra of addiction? Trends Pharmacol. Sci. 2003, 24, 172–177. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Piras, V.; Choi, S.; Akira, S.; Tomita, M.; Giuliani, A.; Selvarajoo, K. Emergent genome-wide control in wildtype and genetically mutated lipopolysaccarides-stimulated macrophages. PLoS ONE 2009, 4, e4905. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Yamazaki, T.; Murakami, Y.; Fukui, R.; Yamai, I.; Ichimonji, I.; Nakashima, A.; Nagaoka, F.; Takagi, H.; Miyake, K.; et al. C4b-binding protein negatively regulates TLR4/MD-2 response but not TLR3 response. FEBS Lett. 2017, 591, 1732–1741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandrashekaran, I.R.; Norton, R.S.; Schmitz-Peiffer, C. Characterisation of peptide interactions that regulate PKCepsilon activation. FEBS Lett. 2018, 592, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Faraz, M.; Herdenberg, C.; Holmlund, C.; Henriksson, R.; Hedman, H. A protein interaction network centered on leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) regulates growth factor receptors. J. Biol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Suda, T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr. J. 2009, 56, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.Y.; Husnain, O.; Stafford, R.; Howard, M.; Gujar, A.S.; Moradiya, V.; Patel, K.K.; Sihotra, S. Hyperphosphorylation of CREB in human dopaminergic neurons: A kinetic study of cellular distribution of total CREB and phospho-CREB following oxidative stress. Neuroreport 2013, 24, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Banerjee, K.; Baker, H.; Cave, J.W. Nucleotide sequence conservation of novel and established cis-regulatory sites within the tyrosine hydroxylase gene promoter. Front. Biol. (Beijing) 2015, 10, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.J.; Crews, L.; Roffler-Tarlov, S.; Chikaraishi, D.M. 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Mol. Brain Res. 1999, 74, 1–14. [Google Scholar] [CrossRef]
- Gueorguiev, V.D.; Cheng, S.Y.; Sabban, E.L. Prolonged activation of cAMP-response element-binding protein and ATF-2 needed for nicotine-triggered elevation of tyrosine hydroxylase gene transcription in PC12 cells. J. Biol. Chem. 2006, 281, 10188–10195. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; Palmer, T.M. Regulating gene transcription in response to cyclic AMP elevation. Cell Sign. 2008, 20, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Kanter, J.R.; Jones, K.C.; Taylor, S.S. Phosphorylation of the catalytic subunit of protein kinase A. Autophosphorylation versus phosphorylation by phosphoinositide-dependent kinase-1. J. Biol. Chem. 2002, 277, 47878–47884. [Google Scholar] [CrossRef] [PubMed]
- Corrigall, W.A. Nicotine self-administration in animals as a dependence model. Nicot. Tob. Res. 1999, 1, 11–20. [Google Scholar] [CrossRef]
- Uhl, G.R.; Drgon, T.; Johnson, C.; Fatusin, O.O.; Liu, Q.R.; Contoreggi, C.; Li, C.Y.; Buck, K.; Crabbe, J. “Higher order” addiction molecular genetics: Convergent data from genome-wide association in humans and mice. Biochem. Pharmacol. 2008, 75, 98–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ducci, F.; Goldman, D. Genetic approaches to addiction: Genes and alcohol. Addiction 2008, 103, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Benavidez, J.M.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R.A. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25 (Suppl. 1), S92–S105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lousberg, E.L.; Moldenhauer, L.M.; Hayball, J.D.; Coller, J.K.; Rice, K.C.; Watkins, L.R.; Somogyi, A.A.; Hutchinson, M.R. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br. J. Pharmacol. 2012, 165, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Bajo, M.; Montgomery, S.E.; Cates, L.N.; Nadav, T.; Delucchi, A.M.; Cheng, K.; Yin, H.; Crawford, E.F.; Roberts, A.J.; Roberto, M. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol Alcohol. 2016, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Alfonso-Loeches, S.; Guerri, C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol. Clin. Exp. Res. 2016, 40, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Black, M.; Chernis, J.; Da Costa, A.; Mayfield, J.; Harris, R.A. Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol. Clin. Exp. Res. 2017, 41, 516–530. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Dayhoff-Brannigan, M.; Cheng, W.C.; Gilbert, C.E.; Sing, C.N.; Diny, N.L.; Wheelan, S.J.; Dunham, M.J.; Boeke, J.D.; Pineda, F.J.; et al. Genome-wide consequences of deleting any single gene. Mol. Cell 2013, 52, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 1–10. [Google Scholar]
- Harris, R.A.; Bajo, M.; Bell, R.L.; Blednov, Y.A.; Varodayan, F.P.; Truitt, J.M.; de Guglielmo, G.; Lasek, A.W.; Logrip, M.L.; Vendruscolo, L.F.; et al. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J. Neurosci. 2017, 37, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, J.; McNally, A.; Guo, R.; Vandivier, R.W.; Simonian, P.L.; Burnham, E.L. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L507–L518. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Cardell, L.O. Nicotine exaggerates LPS-induced airway hyperreactivity via JNK-mediated up-regulation of Toll-like receptor 4. Am. J. Respir. Cell Mol. Biol. 2014, 51, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lacey, M.G. Neurotransmitter receptors and ionic conductances regulating the activity of neurones in substantia nigra pars compacta and ventral tegmental area. Prog. Brain Res. 1993, 99, 251–276. [Google Scholar] [PubMed]
- Aurelian, L. Herpes simplex viruses: General features. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H., Eds.; Elsevier: New York, NY, USA, 2014; pp. 383–397. [Google Scholar]
- Berges, B.K.; Wolfe, J.H.; Fraser, N.W. Transduction of brain by herpes simplex virus vectors. Mol. Ther. 2007, 15, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Braun, E.; Tsalenchuck, Y.; Panet, A.; Steiner, I. Restrictions that control herpes simplex virus type 1 infection in mouse brain ex vivo. J. Gen. Virol. 2011, 92 Pt 10, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.; Bowers, W.J. Targeting the central nervous system with herpes simplex virus/Sleeping Beauty hybrid amplicon vectors. Curr. Gene Ther. 2011, 11, 332–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiandaca, M.S.; Bankiewicz, K.S.; Federoff, H.J. Gene therapy for the treatment of Parkinson’s disease: The nature of the biologics expands the future indications. Pharmaceuticals (Basel) 2012, 5, 553–590. [Google Scholar] [CrossRef] [PubMed]
- Manservigi, R.; Argnani, R.; Marconi, P. HSV Recombinant Vectors for Gene Therapy. Open Virol. J. 2010, 4, 123–156. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.; Gyure, K.A.; Pereira, E.F.; Aurelian, L. Herpes simplex virus type 1-induced encephalitis has an apoptotic component associated with activation of c-Jun N-terminal kinase. J. Neurovirol. 2003, 9, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y. Stable CNS gene delivery with Sleeping Beauty armed with a high-capacity HSV virion. Mol. Ther. 2006, 13, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Chiocca, E.A.; Saeki, Y. Stable transgene expression from HSV amplicon vectors in the brain: Potential involvement of immunoregulatory signals. Mol. Ther. 2008, 16, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.W.; Smith, R.M.; Pari, G.; Wobeser, W.; Rossiter, J.P.; Jackson, A.C. Herpes simplex encephalitis. Can. J. Neurol. Sci. 2005, 32, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cighetti, R.; Peri, F. Molecular simplification of lipid a structure: TLR4-modulating cationic and anionic amphiphiles. Mol. Immunol. 2015, 63, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.J.; Walters, C.L.; Damaj, M.I. Beta 2 subunit-containing nicotinic receptors mediate acute nicotine-induced activation of calcium/calmodulin-dependent protein kinase II-dependent pathways in vivo. J. Pharmacol. Exp. Ther. 2009, 330, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Lowery-Gionta, E.G.; Navarro, M.; Li, C.; Pleil, K.E.; Rinker, J.A.; Cox, B.R.; Sprow, G.M.; Kash, T.L.; Thiele, T.E. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J. Neurosci. 2012, 32, 3405–3413. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.R.; Miczek, K.A. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology 2014, 231, 1557–1580. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Beattie, M.; O’Buckley, T.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Signaling in Immune Cells and Rat Brain. Neuropsychopharmacology. Submitted.
- Middleton, L.S.; Apparsundaram, S.; King-Pospisil, K.A.; Dwoskin, L.P. Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur. J. Pharmacol. 2007, 554, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kirch, D.G.; Alho, A.M.; Wyatt, R.J. Hypothesis: A nicotine-dopamine interaction linking smoking with Parkinson’s disease and tardive dyskinesia. Cell Mol. Neurobiol. 1988, 8, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, J.; Galaz, P.; Araya, K.A.; Slater, P.G.; Blanco, E.H.; Campusano, J.M.; Ciruela, F.; Gysling, K. Dopamine D1 and corticotrophin-releasing hormone type-2alpha receptors assemble into functionally interacting complexes in living cells. Br. J. Pharmacol. 2014, 171, 5650–5664. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Tarakanov, A.; Romero Fernandez, W.; Ferraro, L.; Tanganelli, S.; Filip, M.; Agnati, L.F.; Garriga, P.; Diaz-Cabiale, Z.; Borroto-Escuela, D.O. Diversity and Bias through Receptor-Receptor Interactions in GPCR Heteroreceptor Complexes. Focus on Examples from Dopamine D2 Receptor Heteromerization. Front. Endocrinol. (Lausanne) 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Banus, S.; Reijmerink, N.; Reimerink, J.; Stelma, F.F.; Koppelman, G.H.; Thijs, C.; Postma, D.S.; Kerkhof, M. Association of interacting genes in the toll-like receptor signaling pathway and the antibody response to pertussis vaccination. PLoS ONE 2008, 3, e3665. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Whitby, L.R.; Surakattula, M.M.; Huang, H.; Shi, H.; Choi, J.H.; Wang, K.W.; et al. TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc. Natl. Acad. Sci. USA 2016, 113, E884–E893. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Crews, F.T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE 2014, 9, e87915. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, M.F.; Boelens, W.C.; Joosten, L.A.; Abdollahi-Roodsaz, S.; Geurts, J.; Wunderink, L.U.; Schreurs, B.W.; van den Berg, W.B.; Radstake, T.R. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J. Immunol. 2006, 176, 7021–7027. [Google Scholar] [CrossRef] [PubMed]
- Klasener, K.; Yang, J.; Reth, M. Study B Cell Antigen Receptor Nano-Scale Organization by In Situ Fab Proximity Ligation Assay. Methods Mol. Biol. 2018, 1707, 171–181. [Google Scholar] [PubMed]
- Morud, J.; Adermark, L.; Perez-Alcazar, M.; Ericson, M.; Soderpalm, B. Nicotine produces chronic behavioral sensitization with changes in accumbal neurotransmission and increased sensitivity to re-exposure. Addict. Biol. 2016, 21, 397–406. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; June, H.; Aurelian, L. The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sci. 2018, 8, 72. https://doi.org/10.3390/brainsci8040072
Balan I, Warnock KT, Puche A, Gondre-Lewis MC, June H, Aurelian L. The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sciences. 2018; 8(4):72. https://doi.org/10.3390/brainsci8040072
Chicago/Turabian StyleBalan, Irina, Kaitlin T. Warnock, Adam Puche, Marjorie C. Gondre-Lewis, Harry June, and Laure Aurelian. 2018. "The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse" Brain Sciences 8, no. 4: 72. https://doi.org/10.3390/brainsci8040072
APA StyleBalan, I., Warnock, K. T., Puche, A., Gondre-Lewis, M. C., June, H., & Aurelian, L. (2018). The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sciences, 8(4), 72. https://doi.org/10.3390/brainsci8040072



