Sex Differences in Behavioral Outcomes Following Temperature Modulation During Induced Neonatal Hypoxic Ischemic Injury in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
Induction of Hypoxia Ischemia
| Treatment Groups | Temperature Before Hypoxia (°C; mean ± SE) | Temperature After Hypoxia (°C; mean ± SE) |
|---|---|---|
| Male HI normothermic (n = 9) | 36.61 ± 0.28 | 37.04°C ± 0.49 |
| Male HI hypothermic (n = 10) | 36.69 ± 0.26 | 35.76°C ± 0.47 |
| Male Sham (pooled; n = 12) | 36.79 ± 0.24 | 37.55°C ± 0.43 |
| Female HI normothermic (n = 10) | 36.42 ± 0.26 | 37.29°C± 0.47 |
| Female HI hypothermic (n = 10) | 36.53 ± 0.26 | 35.98°C ± 0.47 |
| Female Sham (pooled; n = 12) | 36.76 ± 0.24 | 37.38°C ± 0.43 |
2.2. Behavioral Testing
2.2.1. Sensorimotor Task: Rota-Rod (P30–P32)
2.2.2. Auditory Discrimination: Startle Reduction Paradigm
2.2.3. Auditory Discrimination: Behavioral Testing Apparatus
2.2.4. Auditory Discrimination: Normal Single Tone (NST; P32)
2.2.5. Auditory Discrimination: Silent Gap (SG) Procedure (0–100; P33–P37)
2.2.6. Learning/Memory Assessments: Water Maze and Water Escape
2.2.7. Learning/Memory Assessments: Morris Water Maze (MWM; P73–P76)
2.2.8. Learning/Memory Assessments: Non-Spatial Water Maze (P80–P83)
2.3. Statistical Analyses
3. Results
3.1. Temperature Analysis
3.2. Rota-Rod
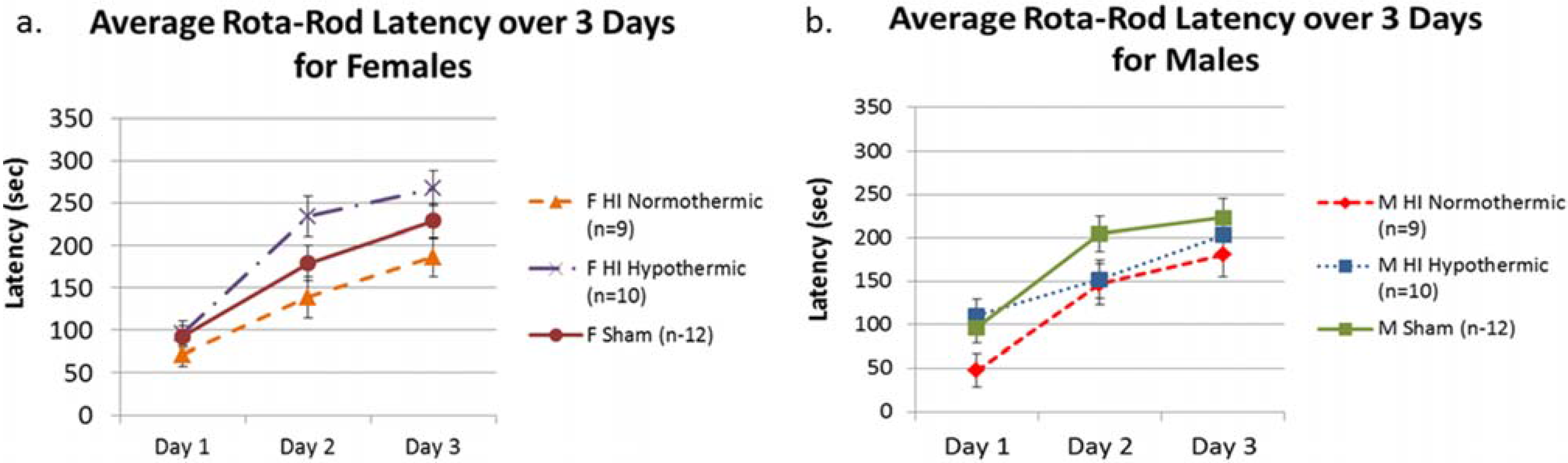
3.3. NST
3.4. SG 0–100
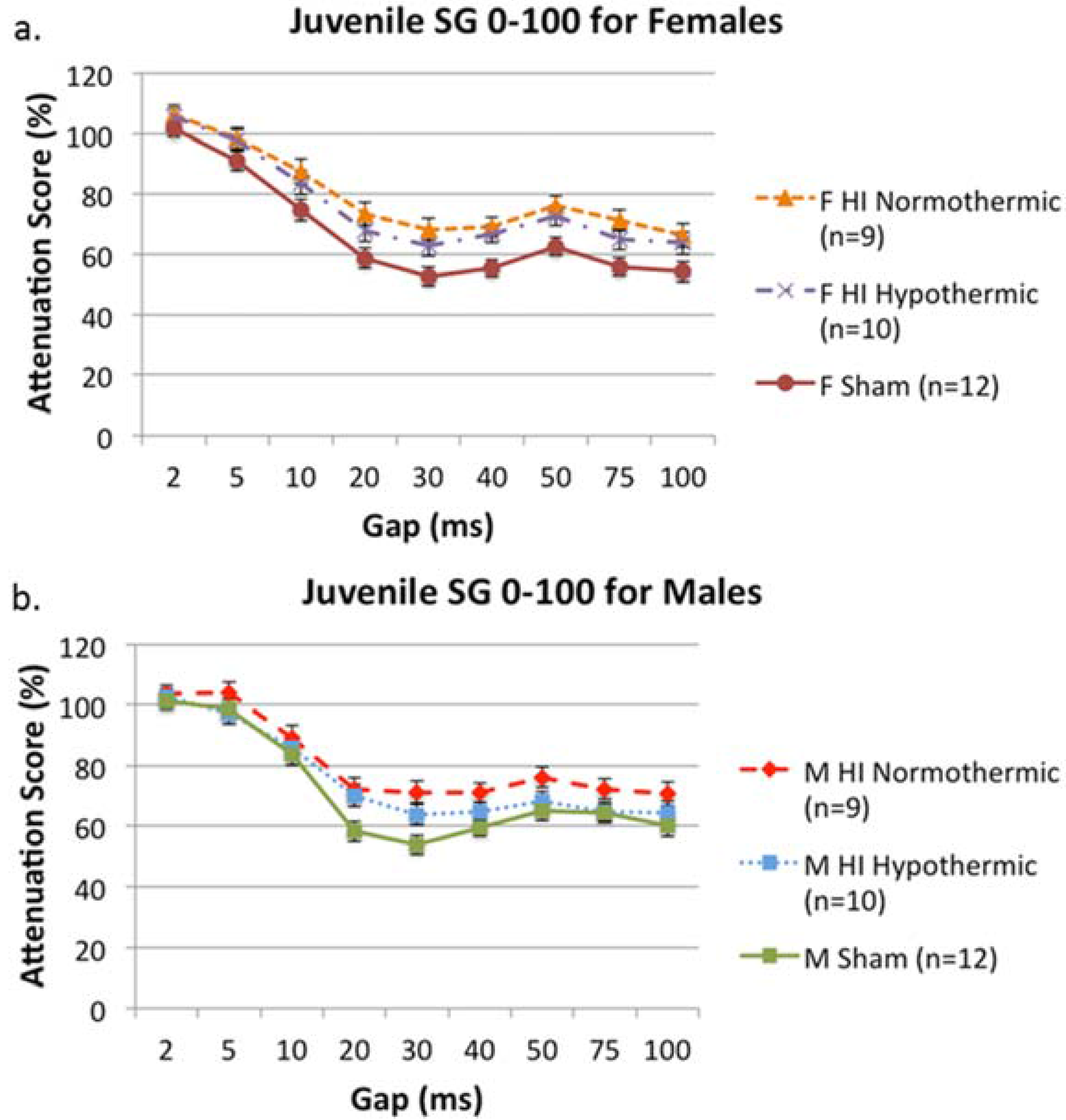
3.5. MWM

3.6. Non-Spatial Maze
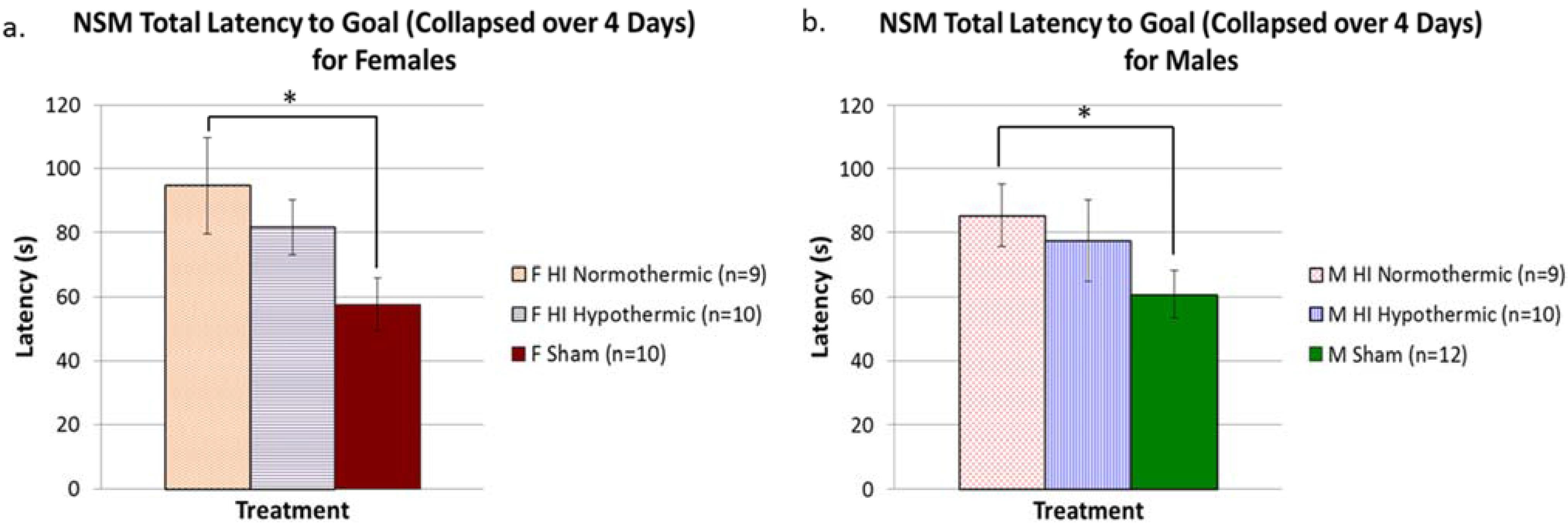
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vannucci, S.J.; Hagberg, H. Hypoxia-ischemia in the immature brain. J. Exp. Biol. 2004, 207, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Y.; Castillo, M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics: Rev. Publ. Radiol. Soc. N. Am. 2008, 28, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.D.; Bennet, L.; Davidson, J.; Dean, J.M.; George, S.; Emerald, B.S.; Gunn, A.J. Destruction and reconstruction: Hypoxia and the developing brain. Birth Defects Res. Part C Embryo Today: Rev. 2007, 81, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; Huppi, P.S.; Warfield, S.; Kikinis, R.; Zientara, G.P.; Barnes, P.D.; Jolesz, F.; Volpe, J.J. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann. Neurol. 1999, 46, 755–760. [Google Scholar] [CrossRef]
- Billiards, S.S.; Pierson, C.R.; Haynes, R.L.; Folkerth, R.D.; Kinney, H.C. Is the late preterm infant more vulnerable to gray matter injury than the term infant? Clin. Perinatol. 2006, 33, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, A.; Wilson, M.A.; Johnston, M. VHypoxic-ischemic encephalopathy in the term infant. Clin. Perinatol. 2009, 36, 835–858. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.S.; Cowan, F.M. Evolving understanding of hypoxic-ischemic encephalopathy in the term infant. Semin. Pediatr. Neurol. 2009, 16, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.S.; Magalhaes, L.C.; Alves, C.R. Effect of preterm birth on motor development, behavior, and school performance of school-age children: A systematic review. J. Pediatr. 2014, 90, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Mishina, J.; Yonemoto, N.; Kusuda, S.; Fujimura, M.; NICU Network, J. Neonatal correlates of adverse outcomes in very low-birthweight infants in the NICU network. Pediatr. Int.: Off. J. Jpn. Pediatr. Soc. 2011, 53, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Orton, J. Cerebral palsy and developmental coordination disorder in children born preterm. Semin. Fetal Neonatal Med. 2014, 19, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Costello, D.W. Trends in the rates of cerebral palsy associated with neonatal intensive care of preterm children. Clin. Obstet. Gynecol. 2008, 51, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Potharst, E.S.; van Wassenaer-Leemhuis, A.G.; Houtzager, B.A.; Livesey, D.; Kok, J.H.; Last, B.F.; Oosterlaan, J. Perinatal risk factors for neurocognitive impairments in preschool children born very preterm. Dev. Med. Child Neurol. 2013, 55, 178–184. [Google Scholar] [CrossRef] [PubMed]
- De Kleine, M.J.; den Ouden, A.L.; Kollee, L.A.; Nihuis-van der Sanden, M.W.; Sondaar, M.; van Kessel-Feddema, B.J.; Knuijt, S.; van Baar, A.L.; Ilsen, A.; Breaur-Pieterse, R.; et al. Development and evaluation of a follow up assessment of preterm infants at 5 years of age. Arch. Dis. Child 2003, 88, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Howe, T.H.; Sheu, C.F.; Wang, T.N.; Hsu, Y.W.; Wang, L.W. Neuromotor outcomes in children with very low birth weight at 5 yrs of age. Am. J. Phys. Med. Rehabil. 2011, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Yoon, S.W.; Mackay, M.; Petri-Thoma, J.; Rogers, M.; Synnes, A.R. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch. Dis. Child. 2013, 98, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Van Hus, J.W.; Potharst, E.S.; Jeukens-Visser, M.; Kok, J.H.; van Wassenaer-Leemhuis, A.G. Mmotor impairment in very preterm-born children: Links with other developmental deficits at 5 years of age. Dev. Med. Child. Neurol. 2014, 56, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.P.; Dawson, L.; Smith, L.M.; Gasson, N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008, 27, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Fitch, R.H.; Alexander, M.L.; Threlkeld, S.W. Early neural disruption and auditory processing outcomes in rodent models: Implications for developmental language disability. Front. Syst. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Benasich, A.A.; Choudhury, N.; Friedman, J.T.; Realpe-Bonilla, T.; Chojnowska, C.; Gou, Z. The infant as a prelinguistic model for language learning impairments: Predicting from event-related potentials to behavior. Neuropsychologia 2006, 44, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Leppanen, P.H.; Leevers, H.J.; Benasich, A.A. Infant information processing and family history of specific language impairment: Converging evidence for RAP deficits from two paradigms. Dev. Sci. 2007, 10, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.L.S.; Jakobson, L.S.; Frisk, V.; Ushycky, I. Auditory temporal processing deficits in children with periventricular brain injury. Brain Lang. 2002, 80, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Ment, L.R.; Schneider, K.C.; Katz, K.H.; Allan, W.C.; Vohr, B.R. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics 2009, 123, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Mantilla, S.; Choudhury, N.; Leevers, H.; Benasich, A.A. Understanding language and cognitive deficits in very low birth weight children. Dev. Psychobiol. 2008, 50, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Van Lierde, K.M.; Roeyers, H.; Boerjan, S.; de Groote, I. Expressive and receptive language characteristics in three-year-old preterm children with extremely low birth weight. Folia Phoniatr. Logop. 2009, 61, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Alexander, M.; Rosenkrantz, T.S.; Sadek, M.L.; Fitch, R.H. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp. Neurol. 2014, 254, 54–67. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, C.; Warren, J.E.; Eisner, F.; Marshall, C.R.; Shanmugalingam, P.; Scott, S.K. Neural correlates of sublexical processing in phonological working memory. J. Cogn. Neurosci. 2011, 23, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Gadian, D.G.; Aicardi, J.; Watkins, K.E.; Porter, D.A.; Mishkin, M.; Vargha-Khadem, F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain: J. Neurol. 2000, 123, 499–507. [Google Scholar] [CrossRef]
- Curtis, W.J.; Zhuang, J.; Townsend, E.L.; Hu, X.; Nelson, C.A. Memory in early adolescents born prematurely: A functional magnetic resonance imaging investigation. Dev. Neuropsychol. 2006, 29, 341–377. [Google Scholar] [CrossRef] [PubMed]
- Donders, J.; Hoffman, N.M. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology 2002, 16, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, M.D.; Raz, S.; Sander, C.J. Neonatal hypoxic risk in preterm birth infants: The influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology 2011, 15, 411–420. [Google Scholar] [CrossRef]
- Rutter, M.; Caspi, A.; Moffitt, T.E. Using sex differences in psychopathology to study causal mechanisms: Unifying issues and research strategies. J. Child Psychol. Psychiatr. Allied Discip. 2003, 44, 1092–1115. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef]
- Raz, S.; Debastos, A.K.; Newman, J.B.; Batton, D. Extreme prematurity and neuropsychological outcome in the preschool years. J. Int. Neuropsychol. Soc. JINS 2010, 16, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, G.J.; O’Callaghan, M.J.; Mohay, H.A.; Rogers, Y.M. Gender differences in cognitive abilities at 2 years in ELBW infants Extremely low birth weight. Early Hum. Dev. 2000, 60, 115–122. [Google Scholar] [CrossRef]
- Kent, A.L.; Wright, I.M.; Abdel-Latif, M.E.; New South Wales and Australian Capital Territory Neonatal Intensive Care Units Audit Group. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics 2012, 129, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Reiss, A.L.; Vohr, B.; Watson, C.; Schneider, K.C.; Katz, K.H.; Ment, L.R. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J. Pediatr. 2008, 152, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Mansson, J.; Fellman, V.; Stjernqvist, K.; The EXPRESS Study Group. Extremely preterm birth affects boys more and socio-economic and neonatal variables pose sex-specific risks. Acta Paediatr. 2015, 104, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Elsmen, E.; Hansen Pupp, I.; Hellstrom-Westas, L. Preterm male infants need more initial respiratory and circulatory support than female infants. Acta Paediatr. 2004, 93, 529–533. [Google Scholar] [PubMed]
- McCarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.T.; McCullough, L.D. Pathways to ischemic neuronal cell death: Are sex differences relevant? J. Transl. Med. 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; Li, J.; Siegel, C.; Yuan, R.; McCullough, L.D. Sex differences in caspase activation after stroke. Stroke 2009, 40, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Manwani, B.; McCullough, L.D. Sexual dimorphism in ischemic stroke: Lessons from the laboratory. Women’s Health 2011, 7, 319–339. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.D.; Zeng, Z.; Blizzard, K.K.; Debchoudhury, I.; Hurn, P.D. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005, 25, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Bayir, H.; Lai, Y.; Zhang, X.; Kochanek, P.M.; Watkins, S.C.; Graham, S.H.; Clark, R.S. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. Sep. 2004, 279, 38563–38570. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal encephalopathy: Pre-clinical studies in neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E., III; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Vannucci, S.J. A model of perinatal hypoxic-ischemic brain damage. Ann. N.Y. Acad. Sci. 1997, 835, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Connor, J.R.; Mauger, D.T.; Palmer, C.; Smith, M.B.; Towfighi, J.; Vannucci, S.J. Rat model of perinatal hypoxic-ischemic brain damage. J. Neurosci. Res. 1999, 55, 158–163. [Google Scholar] [CrossRef]
- Vannucci, R.C. Hypoxic-ischemic encephalopathy. Am. J. Perinatol. 2000, 17, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Hill, C.A.; Alexander, M.; Szalkowski, C.E.; Chrobak, J.J.; Rosenkrantz, T.S.; Fitch, R.H. Spatial working memory deficits in male rats following neonatal hypoxic ischemic brain injury can be attenuated by task modifications. Brain Sci. 2014, 4, 240–272. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Garbus, H.; Rosenkrantz, T.S.; Fitch, R.H. Dissociation in the effects of induced neonatal hypoxia ischemia on rapid auditory processing and spatial working memory in male rats. Dev. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.M.; Peiffer, A.M.; Rosen, G.D.; Fitch, R.H. Auditory processing deficits in rats with neonatal hypoxic-ischemic injury. Int. J. Dev. Neurosci. 2005, 4, 351–362. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.M.; Threlkeld, S.W.; Fitch, R.H. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Res. 2006, 1087, 190–195. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.M.; Threlkeld, S.W.; Fitch, R.H. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007, 1132, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Threlkeld, S.W.; Fitch, R.H. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int. J. Dev. Neurosci. 2011, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Alexander, M.L.; McCullough, L.D.; Fitch, R.H. Inhibition of X-linked inhibitor of apoptosis with embelin differentially affects male versus female behavioral outcome following neonatal hypoxia-ischemia in rats. Dev. Neurosci. 2011, 33, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.L.; Hill, C.A.; Rosenkrantz, T.S.; Fitch, R.H. Evaluation of the therapeutic benefit of delayed administration of erythropoietin following early hypoxic-ischemic injury in rodents. Dev. Neurosci. 2012, 34, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.L.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013, 3, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Garbus, H.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Behavioral and histological outcomes following neonatal HI injury in a preterm (P3) and term (P7) rodent model. Behav. Brain Res. 2013, 259, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Rosen, G.D.; Fitch, R.H. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport 2002, 13, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Rosen, G.D.; Fitch, R.H. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res. Dev. Brain Res. 2004, 148, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Drury, P.P.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Semin. Fetal Neonatal Med. 2010, 15, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Shalak, L.; Corbett, R.J.T. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics 2001, 108, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Corbett, R.J.; Burns, D.; Sterett, R. Neonatal ischemic neuroprotection by modest hypothermia is associated with attenuated brain acidosis. Stroke 1995, 26, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; McDonald, S.A.; Shankaran, S.; Stephens, B.E.; Vohr, B.R.; Guillet, R.; Higgins, R.D.; Das, A.; Extended Hypothermia Follow-Up Subcommittee of the National Institute of Child Health and Human Development Neonatal Research Network. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann. Neurol. 2013, 73, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sinha, B.; Pandya, R.S.; Lin, N.; Popp, A.J.; Li, J.; Yao, J.; Wang, X. Therapeutic hypothermia as a neuroprotective strategy in neonatal hypoxic-ischemic brain injury and traumatic brain injury. Curr. Mol. Med. 2012, 12, 1282–1296. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 monthes of age after moderate hypothermia for perinatal hypoxic ischemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010, 340. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Karibe, H.; Chen, J.; Zarow, G.J.; Graham, S.H.; Weinstein, P.R. Delayed induction of mild hypothermia to reduce infarct volume after temporary middle cerebral artery occlusion in rats. J. Neurosurg. 1994, 80, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Meden, P.; Overgaard, K.; Pederson, H.; Boysen, G. The influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res. 1994, 647, 131–138. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Xu, F.; Qiu, L.; Cheng, X.; Simbruner, G.; Blomgren, K. Intraischemic mild hypothermia prevents neuronal cell death and tissue loss after neonatal cerebral hypoxia-ischemia. Eur. J. Neurosci. 2006, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kerenyi, A.; Kelen, D.; Faulkner, S.D.; Bainbridge, A.; Chandrasekaran, M.; Cady, E.B.; Golay, X.; Robertson, N.J. Systemic effects of whole-body cooling to 35 °C, 33.5 °C, and 30 °C in a piglet model of perinatal asphyxia: Implications for therapeutic hypothermia. Pediatr. Res. 2012, 71, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; van Bel, F.; van der Kooij, M.A.; Heijnen, C.J.; Groenendaal, F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr. Res. 2013, 73, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Hagberg, H.; Loberg, E.M.; Bagenholm, R.; Thoresen, M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: Short- and long-term outcome. Pediatr. Res. 1998, 43, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, A.; Nakajima, W.; Ishida, A.; Yasuoka, N.; Kawamura, M.; Miura, S.; Takada, G. Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain Dev. 2005, 27, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Fitch, R.H.; Threlkeld, S.W.; McClure, M.M.; Peiffer, A.M. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res. Bull. 2008, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Renolleau, S.; Fau, S.; Goyenvalle, C.; Joly, L.M.; Chauvier, D.; Jacotot, E.; Charriaut-Marlangue, C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: A role for gender. J. Neurochem. 2007, 100, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Corbett, R.J.; Sterett, R.; Burns, D.K.; Tollefsbol, G.; Garcia, D. Modest hypothermia provides partial neuroprotection for ischemic neonatal brain. Pediatr Res. 1994, 35, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.Y.; Gonzalez, F.F.; Sheldon, R.A.; Ferriero, D.M. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr. Res. 2013, 73, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Woo, C.W.; Kim, S.T.; Kim, K.S. Long-term neuroprotective effect of postischemic hypothermia in a neonatal rat model of severe hypoxic ischemic encephalopathy: A comparative study on the duration and depth of hypothermia. Pediatr. Res. 2010, 68, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lubics, A.; Reglodi, D.; Tamas, A.; Kiss, P.; Szalai, M.; Szalontay, L.; Lengvari, I. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav. Brain Res. 2005, 157, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Aoo, N.; Harada, K.; Sakamoto, Y.; Akitake, Y.; Irie, K.; Mishima, K.; Ikeda, T.; Fujiwara, M. Sex differences in the benefits of rehabilitative training during adolescence following neonatal hipoxia-ischemia in rats. Exp. Neurol. 2010, 226, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, J.; Cheung, P.Y.; Chen, C. Long-term cognitive impairment and myelination in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009, 8, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Almli, C.R. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp. Neurol. 2000, 166, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Arteni, N.S.; Salgueiro, J.; Torres, I.; Achaval, M.; Netto, C.A. Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain Res. 2003, 973, 171–178. [Google Scholar] [CrossRef]
- Kumral, A.; Uysal, N.; Tugyan, K.; Sonmez, A.; Yilmaz, O.; Gokmen, N.; Genc, K. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav. Brain Res. 2004, 153, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.P.; Nedelcu, J.; Martin, E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr. Res. 2002, 51, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Corbett, D.; Thornhill, J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol. 2000, 10, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Green, E.J.; Dietrich, W.D.; van Dijk, R.; Busto, R.; Markgraf, C.G.; McCabe, P.M.; Ginsberg, M.D.; Schneiderman, N. Protective effects of brain hypothermia on behavaior and histopathology following global cerebral ischemia in rats. Brain Res. 1992, 580, 197–204. [Google Scholar] [CrossRef]
- Barone, F.C.; Feuerstein, G.Z.; White, R.F. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci. Biobehav. 1997, 21, 31–44. [Google Scholar] [CrossRef]
- Wang, R.H.; Schorer-Apelbaum, D.; Weinstock, M. Testosterone mediates sex difference in hypothermia and cholinesterase inhibition by rivastigmine. Eur. J. Pharmacol. 2001, 433, 73–79. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Baron-Cohen, S. Fetal testosterone and sex differences in typical social development and in autism. J. Child Neurol. 2006, 21, 825–845. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.N.; Evangelou, I.; Fatemi, A.; Vezina, G.; Mccarter, R.; Glass, P.; Limperpoulos, C. White matter tract integrity and developmental outcomes in newborn infants with hypoxic-ischemic encephalopathy treated with hypothermia. Dev. Med. Child Neurol. 2015, 57, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Li, J.; Ma, S.M.; Yang, Y.; Zhou, W.H. Effects of hypothermia on oligodendrocyte precursor cell proliferation, differentiation, and maturation following hypoxia ischemia in vivo and in vitro. Exp. Neurol. 2013, 247, 720–729. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.L.; Garbus, H.; Rosenkrantz, T.S.; Fitch, R.H. Sex Differences in Behavioral Outcomes Following Temperature Modulation During Induced Neonatal Hypoxic Ischemic Injury in Rats. Brain Sci. 2015, 5, 220-240. https://doi.org/10.3390/brainsci5020220
Smith AL, Garbus H, Rosenkrantz TS, Fitch RH. Sex Differences in Behavioral Outcomes Following Temperature Modulation During Induced Neonatal Hypoxic Ischemic Injury in Rats. Brain Sciences. 2015; 5(2):220-240. https://doi.org/10.3390/brainsci5020220
Chicago/Turabian StyleSmith, Amanda L., Haley Garbus, Ted S. Rosenkrantz, and Roslyn Holly Fitch. 2015. "Sex Differences in Behavioral Outcomes Following Temperature Modulation During Induced Neonatal Hypoxic Ischemic Injury in Rats" Brain Sciences 5, no. 2: 220-240. https://doi.org/10.3390/brainsci5020220
APA StyleSmith, A. L., Garbus, H., Rosenkrantz, T. S., & Fitch, R. H. (2015). Sex Differences in Behavioral Outcomes Following Temperature Modulation During Induced Neonatal Hypoxic Ischemic Injury in Rats. Brain Sciences, 5(2), 220-240. https://doi.org/10.3390/brainsci5020220





