1. Introduction
The idea that cognition and perception are anticipatory in nature has recently regained scholarly attention [
1,
2]. Humans continuously make predictions concerning upcoming events or stimuli and match these predictions to the actual input. When expectations are not met, the resulting error signal is used to extract important information and adjust expectations. The ability to predict therefore underlies the ability to learn and adapt. Predictive processing is an essential part of perception, action and cognition [
1,
2], including language processing [
3,
4,
5,
6,
7,
8,
9,
10]. Therefore, a growing need exists for experimental techniques and designs that can tap into predictive processing. Event-related-potentials (ERPs) are an appropriate method, since this technique enables the continuous recording of brain activation during cognitive processing.
One class of ERP components associated with anticipatory processing are stimulus preceding negativities (SPNs) [
11]. SPNs are a collection of negativities whose scalp distribution varies depending on the task and type of information that is expected [
12,
13]. SPNs are typically elicited in delay paradigms. In these paradigms, a particular stimulus is temporally separated from a subsequent stimulus, which can occur with a varying degree of predictability. SPNs have typically been observed in the period: before a stimulus that conveys feedback on the performance of the preceding stimulus; before a stimulus that conveys instructions; before a probe to which previous stimuli or task outcomes need to be compared; or before a stimulus that is expected to be associated with a strong emotion [
13]. The SPN is not an index of expectancy of stimuli in general, however. First, not all stimuli that are highly predictable give rise to an SPN. For instance, the expected occurrence of an instruction stimulus at a particular point in a trial does not [
14]. Second, the SPN can be larger when the nature of the following stimulus is less, rather than more, predictable [
15,
16,
17]. One interpretation is that the SPN is larger when the participant expects the upcoming stimulus to be more emotionally informative [
16,
18].
Another ERP response associated with anticipation is an omission-related negativity, also regarded as a mismatch negativity (MMN) and/or an N2 component [
19,
20,
21]. This component has been observed in auditory paradigms in which an expected stimulus is occasionally either briefly delayed or omitted and replaced with a brief period of silence. The omission-related negativity can be modulated by the degree of expectation: Its amplitude is larger when the omitted stimulus is more strongly expected [
22,
23]. Omission-related effects have also been reported in studies using visual presentation in which the temporal regularity of stimulus presentation is occasionally disrupted [
24,
25]; to our knowledge, however, no omission studies have manipulated the predictability of the upcoming stimulus in a visual paradigm.
The present study aimed to test whether anticipation-related effects could be elicited in a delay paradigm in which pre-learned visual sequences were used and no explicit feedback was provided. Participants were presented with letter sequences, which could be either in alphabetical order, in which the next letter was 100% predictable, or in a random order, in which the next letter was about 5% predictable. In both types of sequence, half of the trials had a lengthened inter stimulus interval (ISI) between two of the letters, creating a delay. The task was an end-of-trial letter recognition task, which was unrelated to the predictability of the sequence or the presence of a delay. If participants form predictions of the next letter on the basis of the preceding letters, the delay would either strengthen the expectation of the next letter or, alternatively, violate the expectation of the presentation of a particular letter at a certain point in time. In either case, we expected differences in amplitude between the predictable and random sequences.
In addition, we were interested in the effect of predictability and delay on the degree to which the next letter was pre-activated. One interpretation of anticipatory processing is that the perception of upcoming stimuli is facilitated by the pre-activation of the relevant sensory and cognitive systems. This facilitation may be reflected in the modulation of early sensory ERP components when the expected stimulus occurs. The P2 is a component typically occurring between 100 and 300 ms. The P2 has been associated with early perceptual processes, which can be modulated by top-down information. In the visual domain, a larger P2 amplitude has been associated with the ease in which relevant (pre-specified, target-related) visual features, such as a particular color or size, can be detected [
26]. In the language domain, the P2 is larger for repeated words [
27,
28] and larger for plausible words in a highly-constraining
versus a weakly-constraining semantic context [
29,
30]. Evans and Federmeier [
27] (p. 305) suggest that, since the P2 is sensitive to word repetition, “it may reflect the process of comparing visual input with either stored knowledge or generated expectations”. In addition, the P300, and in particular the P3b, has been found to be larger for stimuli that are highly expected in a context and has been interpreted as indexing the matching of expectation, that is, the match of the actual stimulus with a pre-activated item [
31,
32,
33].
We therefore expected a larger P2 and/or P3b for elements in predictive versus non-predictive sequences. If a lengthened ISI makes the prediction stronger and leads to more pre-activation of the anticipated element, we expected a larger P2/P3b after a delay than without a delay, especially for predictive sequences.
2. Methods
2.1. Participants
Nineteen native speakers of American English were recruited from the University of Florida community. Data from one participant were excluded because of chance performance on the end-of-trial probe verification task, leaving a total of 18 participants (age 18–30, mean 20.9; 4 men). All, except for one, participants were right-handed. All had normal or corrected-to-normal vision, had no reading problems and were neurologically healthy according to a self-report. All participants were able to generate the letters of the Roman alphabet in the correct order when asked before the study. Participants were either paid $10.00 per hour or received course credit for their participation. All participants gave written informed consent as per the University of Florida Institutional Review Board procedures (Protocol #2013-U-0768, approved 6/19/2013).
2.2. Materials
Stimuli consisted of 180 sequences of upper-case letters of the Roman alphabet. First, 45 predictive, alphabetical sequences (e.g., “E”, “F”, “G”, “H”, “I”, “J”, “K”) were formed. Sequences were between 7 and 10 letters long. Random sequences were derived from the predictive sequences, such that the random and predictive sequences did not differ in the number and distribution of particular letters over the positions in the sequence. When creating random sequences, strings of letters were avoided that may form known acronyms or other recognizable patterns (e.g., “OMG”, “LOL”, etc.). From these 90 no-delay sequences, 90 delay trials were created by adding a delay (that is, a lengthened ISI) after the fourth to eighth letter (nine instances for each of these five positions for the predictive and random conditions). Care was taken that the position of the delay in the predictive sequences did not occur in a place where a pause may feel natural due to the pauses in the “ABC Song” (after “G”, “P”, “S”, “V”, and “X”) and that the delay was never in a pre-final position. This was to prevent participants from expecting a delay in a certain position, and to encourage them to keep processing the stimuli even after the delay. Each participant saw all 180 trials (45 for each of the 4 conditions: predictive delay; random delay; predictive no delay; random no delay). Each of the trials was followed by a letter prompt. In half of the trials, equally distributed across conditions, the prompt letter was a member of the sequence (“yes” trials); in the other half, it was not (“no” trials). Prompt letters in the “yes” trials were equally drawn from the various letter positions, with the position balanced equally over the random and predictive sequences.
2.3. Procedure
Participants were seated comfortably in a sound-attenuating room, 1 m away from an SVGA monitor. Letters were presented in Courier New 24 point font, white letters on a black background. Each sequence began with a fixation presented in the center of the screen for 1000 ms, followed by a blank screen of 200 ms. Letters were presented individually for 300 ms followed by a blank screen (ISI) of 200 ms. In the conditions containing a delay, one of the letters in Positions 5 through 9 was followed by an ISI of 500 ms rather than 200 ms, thus creating a delay of 300 ms relative to the expected onset time. The final letter of each sequence was presented with a period. After a blank screen of 1000 ms, a prompt letter was presented. At this point, participants indicated whether or not that letter had occurred in the preceding sequence by pressing the left (“yes”) or right (“no”) trigger button on a game pad. After responding to the prompt, the message “Press to continue” remained on the screen until the participant pressed a button on the gamepad to proceed to the next trial.
Participants were instructed to limit movements and blinking to the pauses at the end of each sequence (during the prompt or after). Upon the completion of a practice block, which consisted of six sequences (three alphabetic, three random, none containing a disruption), participants completed three blocks, each consisting of 60 sequences. Each block contained 15–16 trials from each of the four conditions, in a randomized order. The order of the three blocks was varied such that no more than two participants saw the blocks in a given order.
2.4. EEG Recording
EEG was recorded with 64 Ag/AgCl electrodes mounted in an elastic cap (Waveguard 64). The following electrode positions were used: on the midline (FPz, Fz, FCz, Cz, CPz, Pz, POz, Pz) and lateral sites (FP1/2, AF3/4/7/8, F1-8, FC1-6, FT7/8, C1-6, T7/8, CP1-6, TP7/8, P1-8, PO3-8, O1/2). Horizontal EOG was recorded with two Ag/AgCl electrodes attached to the left and right outer canthi of both eyes, and vertical EOG was recorded with Ag/AgCl electrodes above and below the right eye. Additional electrodes were placed on the left and right mastoids. Impedances never exceeded 5 kΩ at all times for all electrodes. EEG was acquired using an ANT REFA 8-72 amplifier, at a sampling rate of 512 Hz, referenced to the left mastoid.
2.5. Analysis
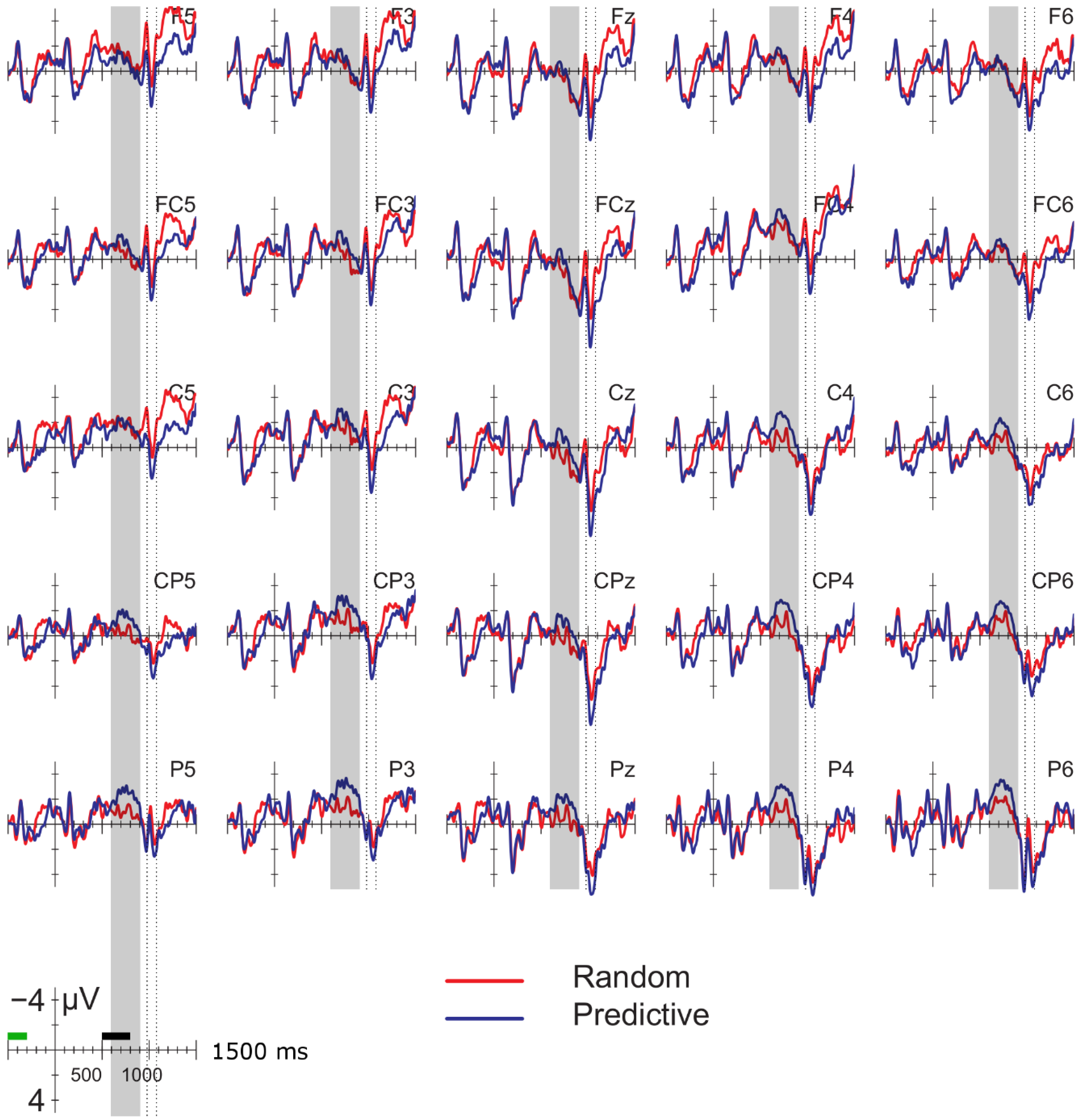
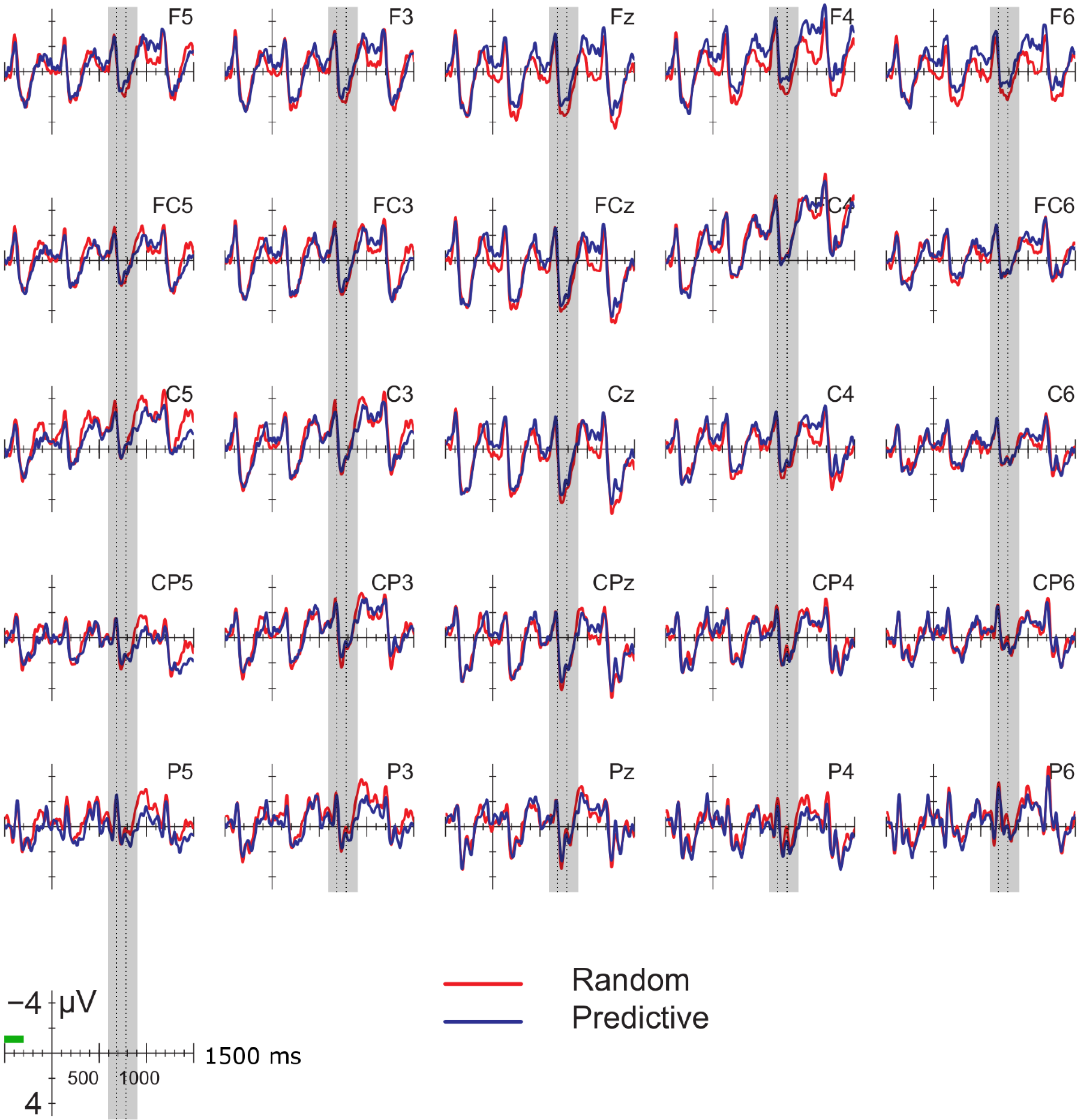
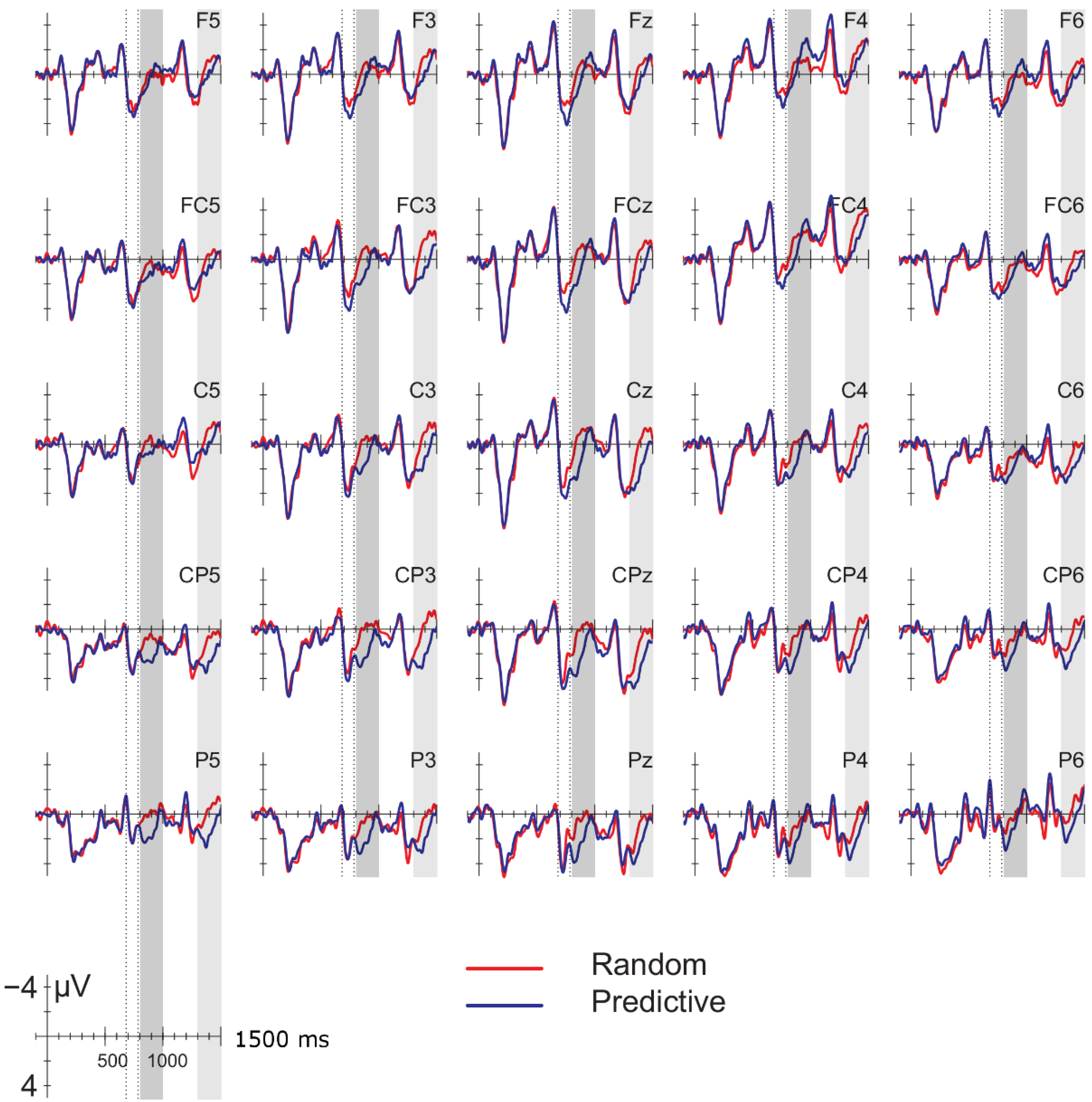
Data were filtered offline using a 0.01–30 Hz band pass filter and arithmetically re-referenced to the mean of both mastoids. Trials with excessive eye movements (standard deviation exceeding 20 µV for horizontal EOG; greater than 30 for vertical EOG in a 200-ms window) and trials in which one of the channels was malfunctioning as determined by visual inspection were rejected from analysis. The rejection rate was equally distributed among the four conditions (predictive, delay 16.5%; predictive, no delay, 16.5%; random, delay 16.4%; random, no delay 16.2%). ERPs were time-locked to the onset of the presentation of the letter preceding the potential delay. Epochs spanned 2000 ms, from −500 to 1500 ms, relative to stimulus onset. As will be explained below, the 200-ms time window immediately preceding the pre-delay letter may have been confounded by pre-existing differences between the conditions. For this reason, we report an analysis using the −500 to −300-ms interval preceding the onset of the pre-delay letter as the baseline. This window coincided with the first 200 ms after onset of the second letter preceding the potential delay. To investigate the effects of random versus predictive sequences, without contamination of the nature or the preceding stimuli or presence of a delay, we also investigated the ERP for the first three letters in the trial, using the 100-ms interval preceding the onset as a baseline (a longer baseline was too contaminated with eye movements spilling over from the preceding fixation). Epochs were 1600 ms in length, from −100 to 1500 ms from the onset of the first letter.
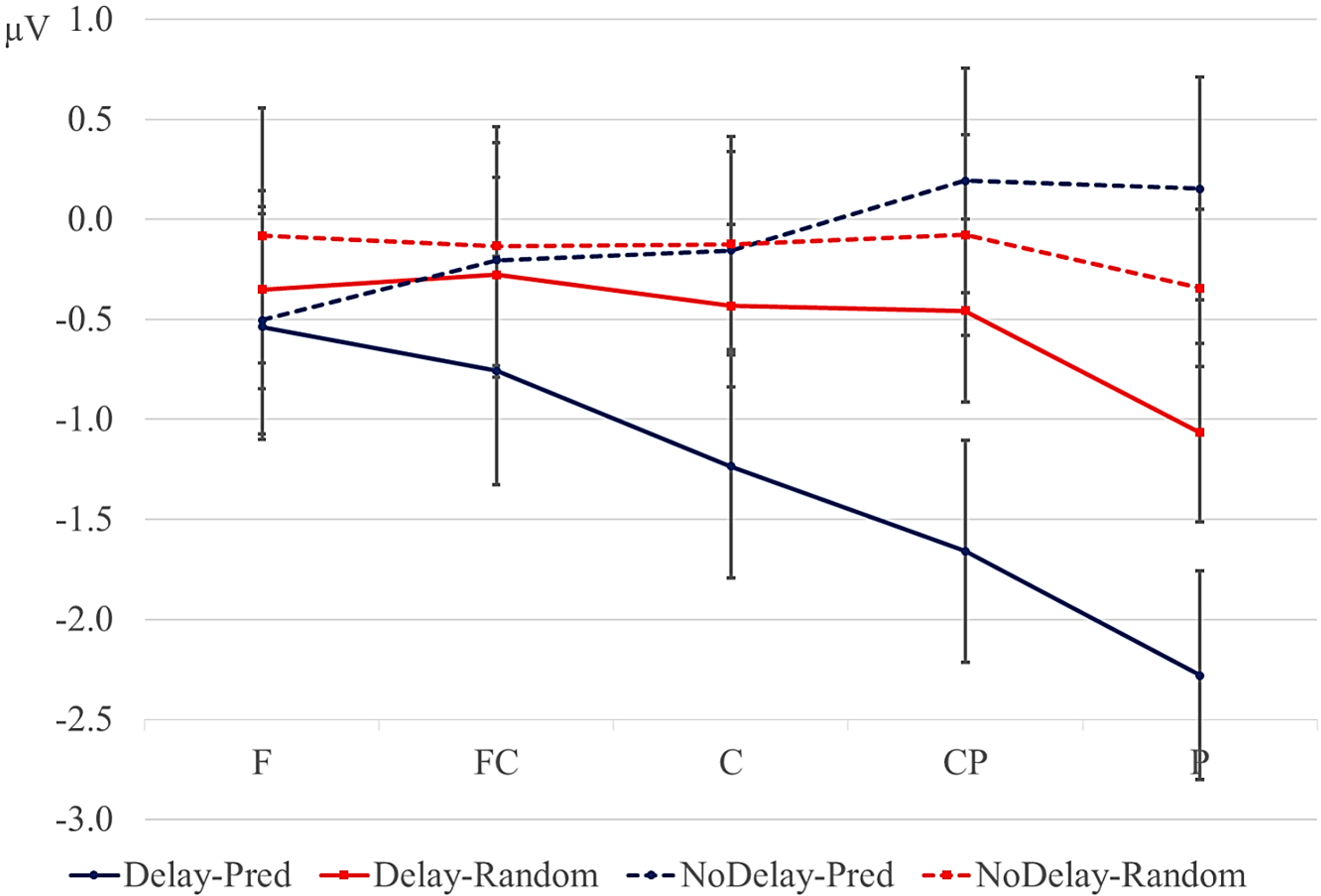
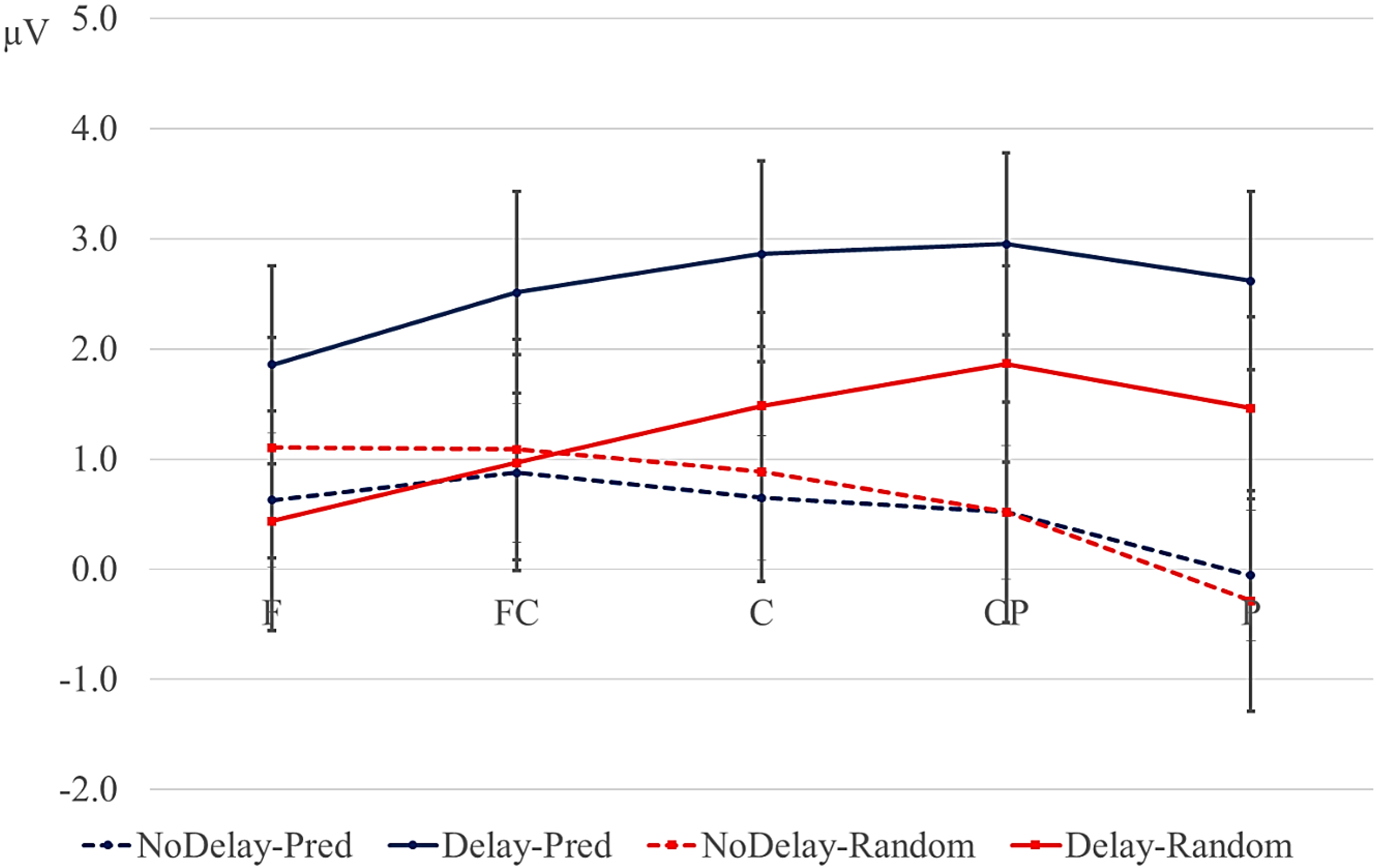
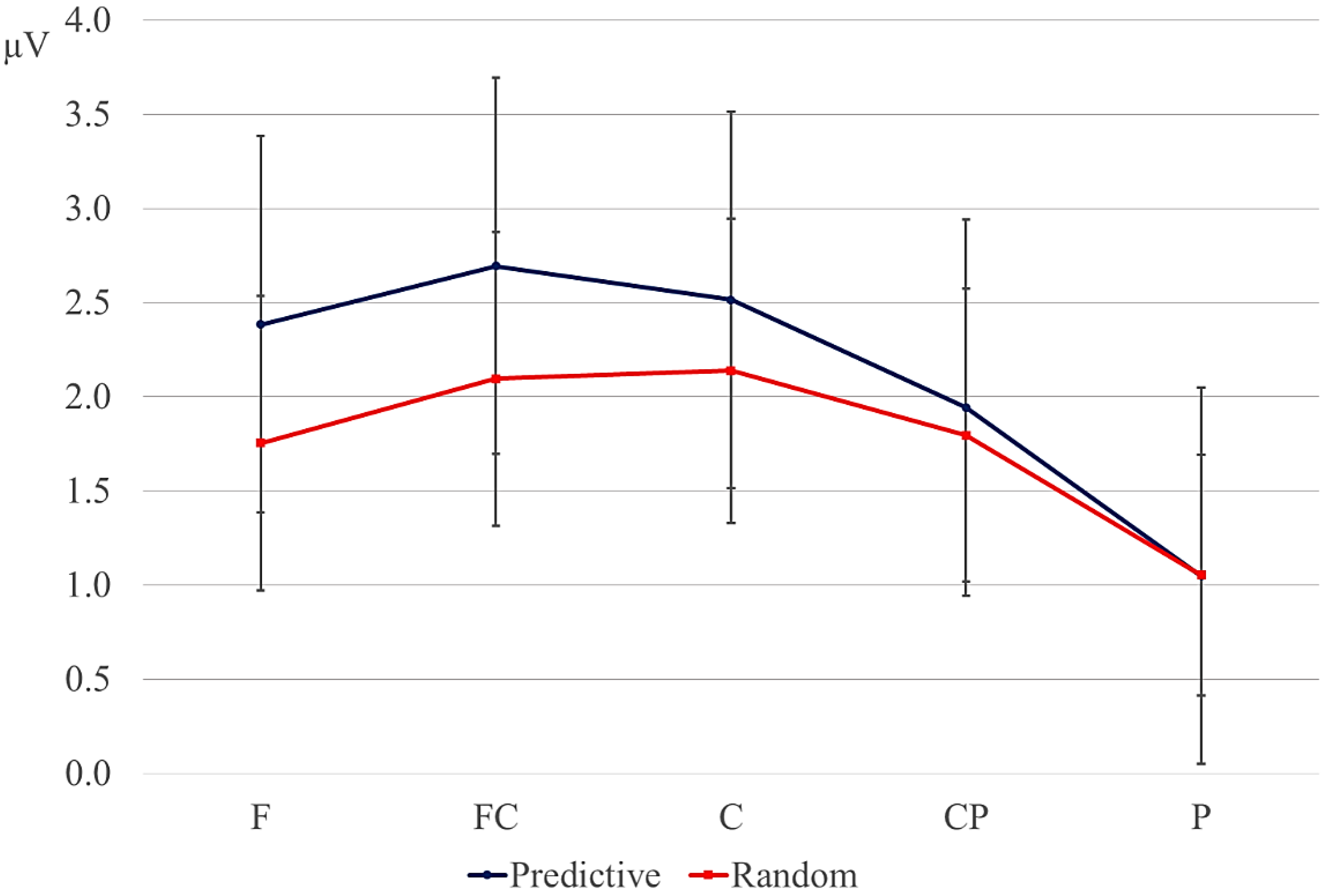
Statistical analyses were performed on the mean of five of the midline electrodes (Fz, FCz, Cz, CPz, Pz) and 30 lateral electrodes grouped into 10 regions (left frontal: F1/3/5; right frontal: F2/4/6; left fronto-central: FC1/3/5; right fronto-central: FC2/4/6; left central: C2/4/6; right central: C1/3/5; left centro-parietal: CP2/4/6; right centro-parietal: CP1/3/5; left parietal: P2/4/6; and right parietal: P1/3/5). Critical time windows were: (1) −300 to 0 ms, 0–300 ms and 300–500 ms relative to the onset of the pre-delay letter; this was to examine potential differences between the conditions before the onset of the delay; (2) 600 to 900 ms after onset of the pre-delay letter and corresponding letter in the no-delay conditions; this interval captured the delay-related ERPs; (3) the P2/P3b, which was defined as the 100-ms window surrounding the peak latency averaged over midline sites. This corresponded to the 680–780-ms window from the start of the pre-delay letter for the no-delay conditions and the 980–1080-ms window for the delay conditions. The critical time windows for the analysis of the first three letters of each trial were: (1) 690–780-ms window from the onset of the first letter, capturing the P2/P3 to the second letter; (2) the mean amplitude between 800 and 1000 ms; and (3) 1300–1500 ms after the onset of the first letter, based on visual inspection. SPSS GLM repeated measures analyses were performed on the mean amplitude in the time windows defined, separately for midline and lateral sites. Within-subject factors were order (2 levels: predictive/random), delay (2 levels: delay/no delay), when applicable, and anteriority (5 levels: frontal, fronto-central, central, centro-parietal, parietal). Lateral analyses were performed with the addition of hemisphere (2 levels: left/right) as a factor. Significant interactions involving the factors delay or order were followed up with ANOVAs on the relevant subset of the data. For effects involving more than one degree of freedom, the Greenhouse–Geisser correction was applied to protect against Type I error due to a violation of sphericity [
34].
Accuracy for the end-of-trial probe verification task and response times to accurate responses were analyzed with an SPSS GLM repeated measures with order (2) and delay (2) as within-subjects factors. Outliers in reaction times were omitted when they exceeded the median plus 3-times the median absolute deviation [
35]. This affected 8.3% of all data points.
5. Conclusions
Most previous studies on anticipation in sequential processing have used paradigms in which the participant learns to associate a particular stimulus with a particular outcome and in which explicit feedback is provided. We have shown that an anticipation-related negativity can be elicited by stimuli in sequences that are based on long-term associations and in which no feedback is provided. In addition, our results suggests that some features of the anticipated stimulus are pre-activated during the delay period.
The present paradigm can be applied to the study of predictive processing during sentence comprehension. In contrast to the delay paradigms used in non-language studies, most studies on anticipation in language processing have focused on ERPs elicited by words that violate or meet context-based predictions [
47,
48,
49,
50,
51,
52]. ERPs at these word positions also reflect integration and revision processes, which makes it hard to distinguish predictive processing from other processes. The delay paradigm can be easily applied to sentence processing [
17]: the next word in a sentence can be occasionally delayed, enabling the tracking of anticipatory processes separately from integration, while still encouraging readers to process the materials following the delay, while avoiding anomalies inherent to omission [
22] and violation paradigms [
47,
48,
49,
50,
51,
52]. We are currently applying the delay paradigm to sentence processing in which the cloze probability of the post-delay word is manipulated [
53]. In addition, by manipulating the length of the delay, one can yield a better view of the time needed for certain predictions to be generated and whether there are differences in populations in this respect, such as between second-language learners with different levels of proficiency. In sum, the delay paradigm may be a fruitful method to determine the nature and time course of anticipations during the processing of sequential stimuli, be it linguistic or otherwise.