Potential for Cell-Mediated Immune Responses in Mouse Models of Pelizaeus-Merzbacher Disease
Abstract
:1. Introduction
2. Results and Discussion
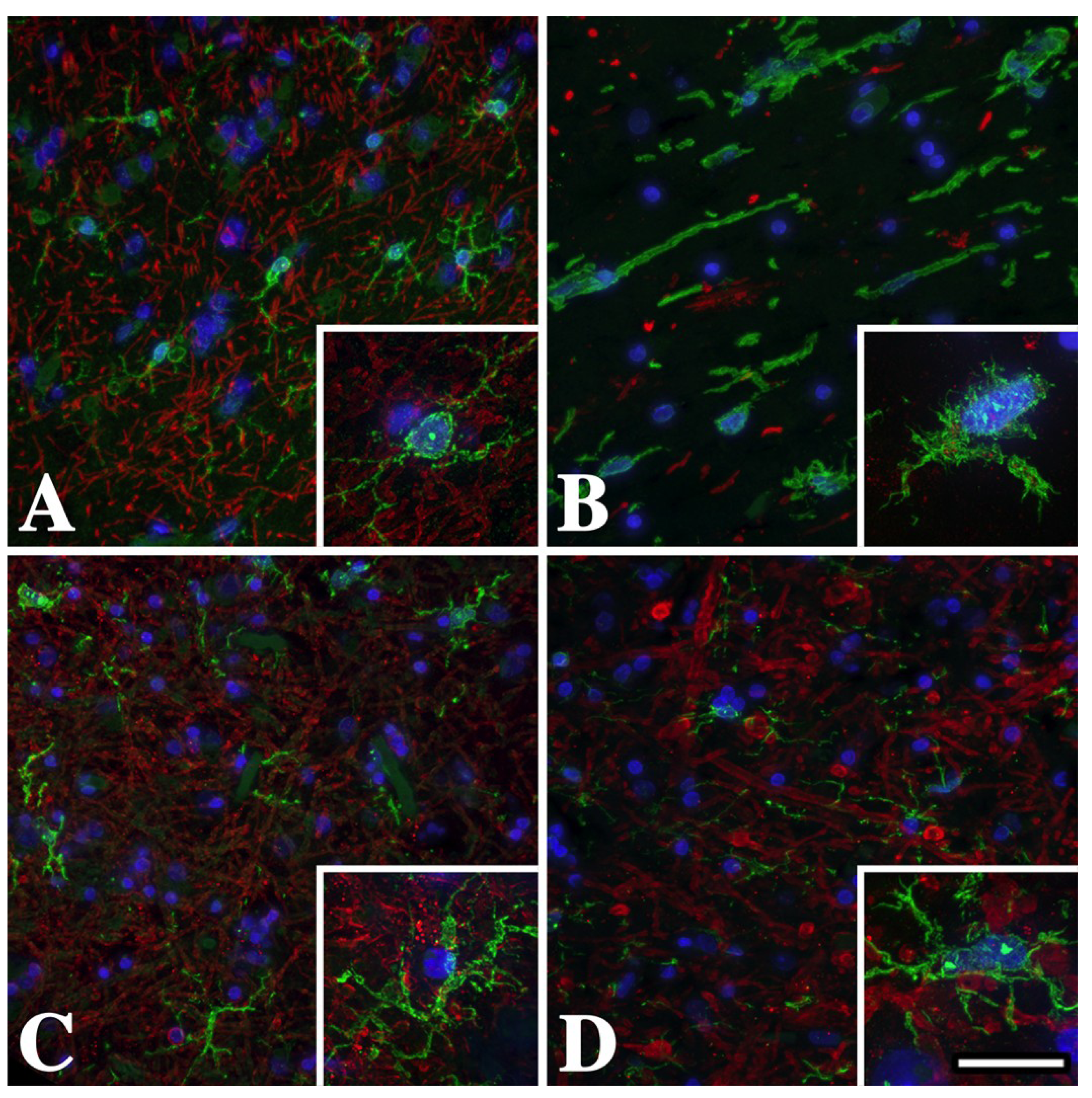
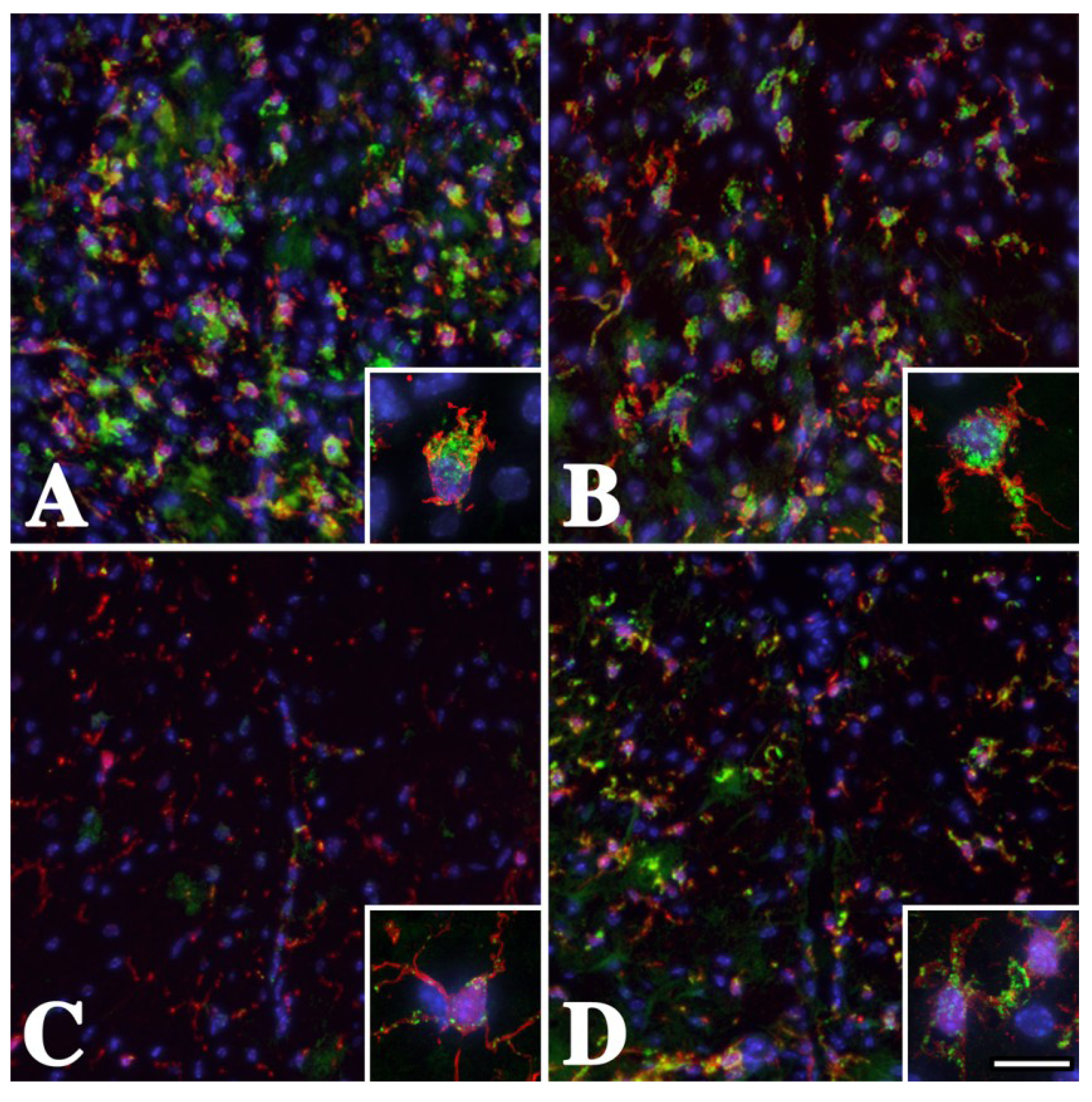
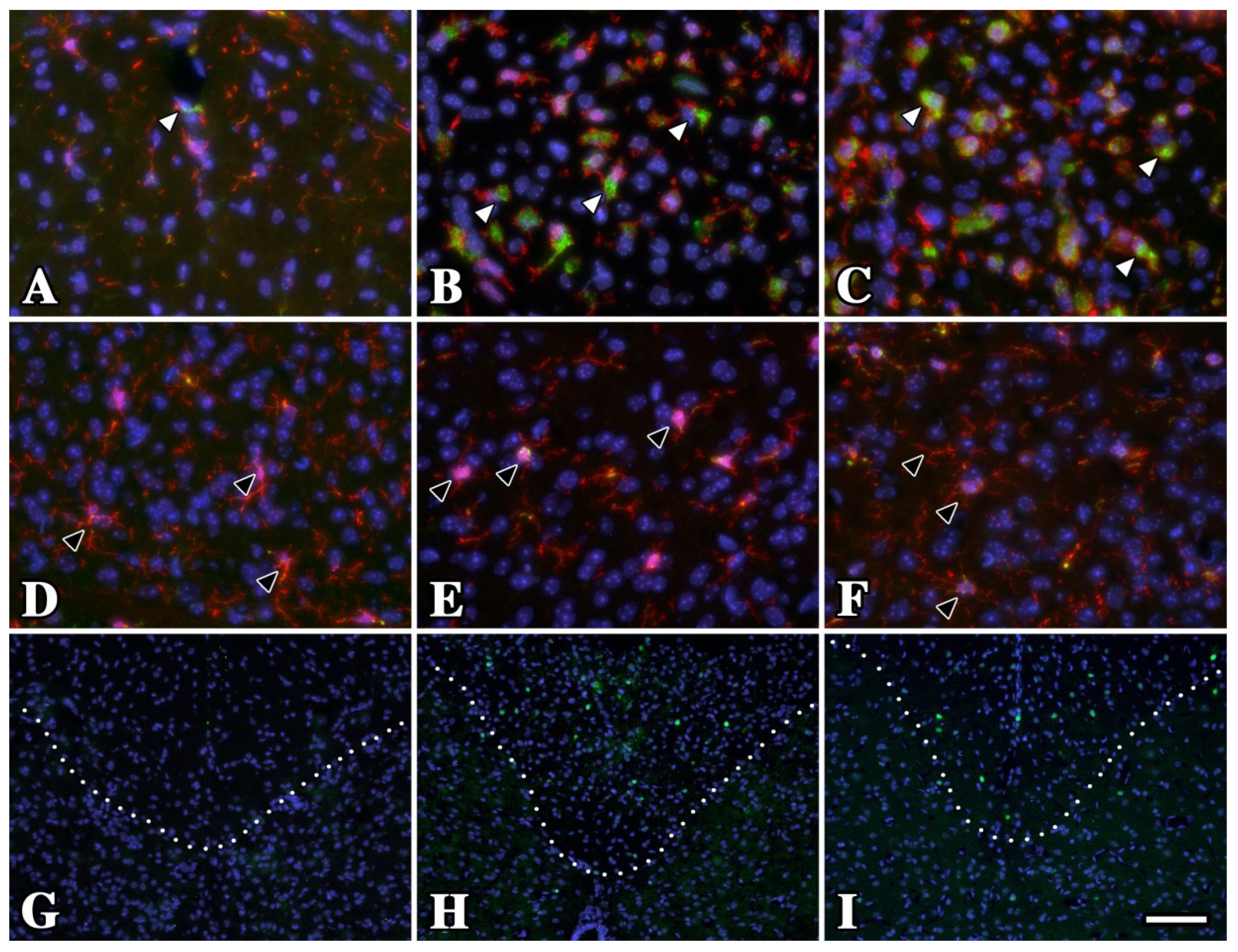
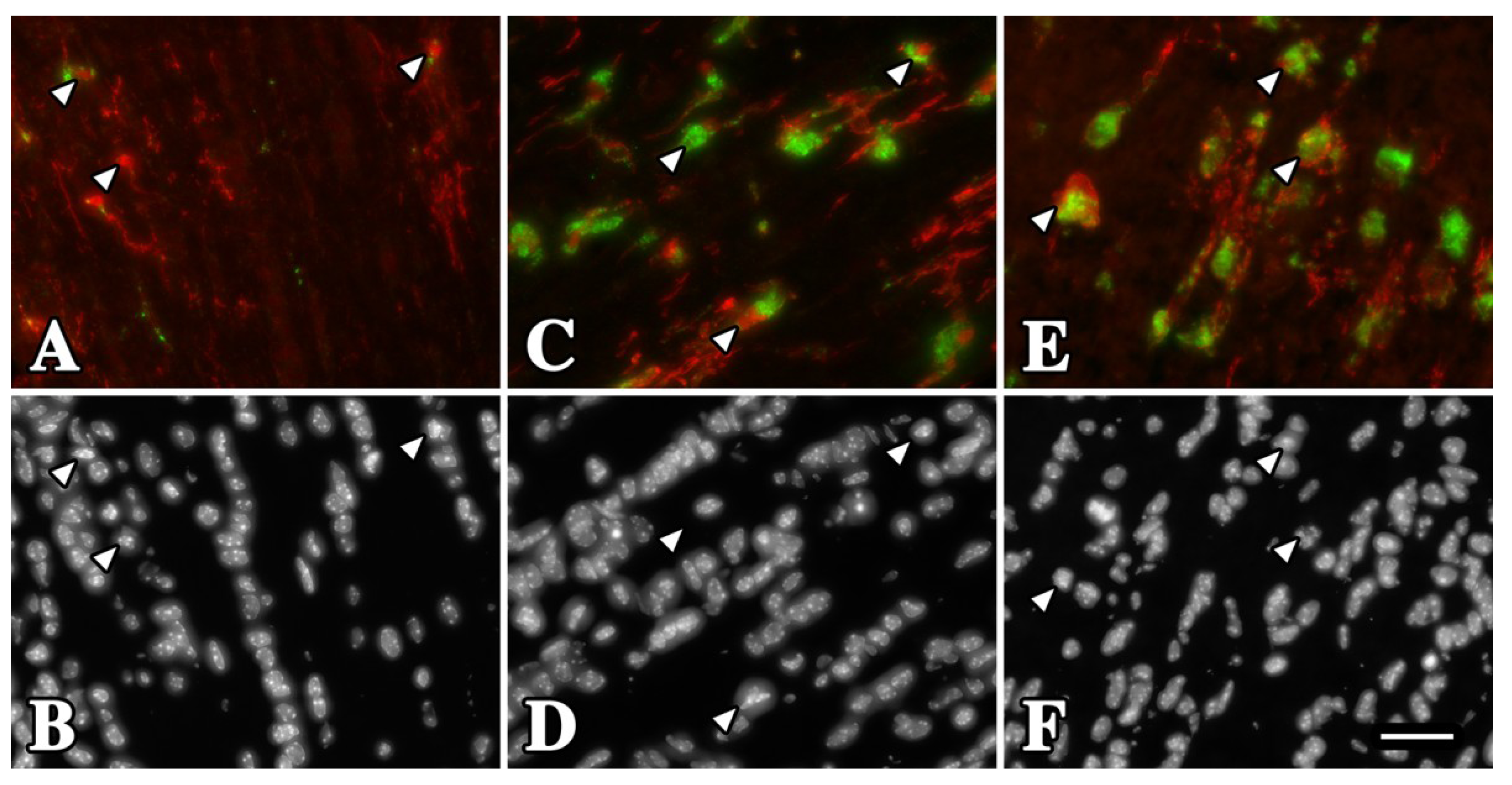
2.1. Microglia/Macrophage Activation Is Variable in Pelizaeus-Merzbacher Disease Patients
2.2. Microglia/Macrophage Activation Varies with Disease Severity in Plp1 Mutant Mice
| Genotype | Proportion of CD68+/Iba-1+ cells (%) a | # Mice |
|---|---|---|
| Wild type | 22 ± 1.9 | 3 |
| rsh | 90 ± 1.0 | 3 |
| msd | 70 ± 4.1 | 3 |
| Plp1#66 b | 82 | 1 |
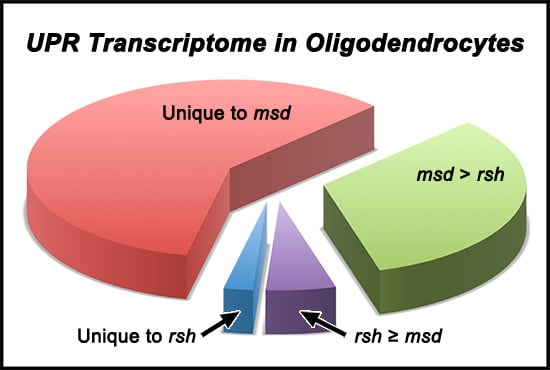
2.3. Optic Nerve Microarray Analysis in rsh and msd Mice Reveal Similarities in Expression Profiles
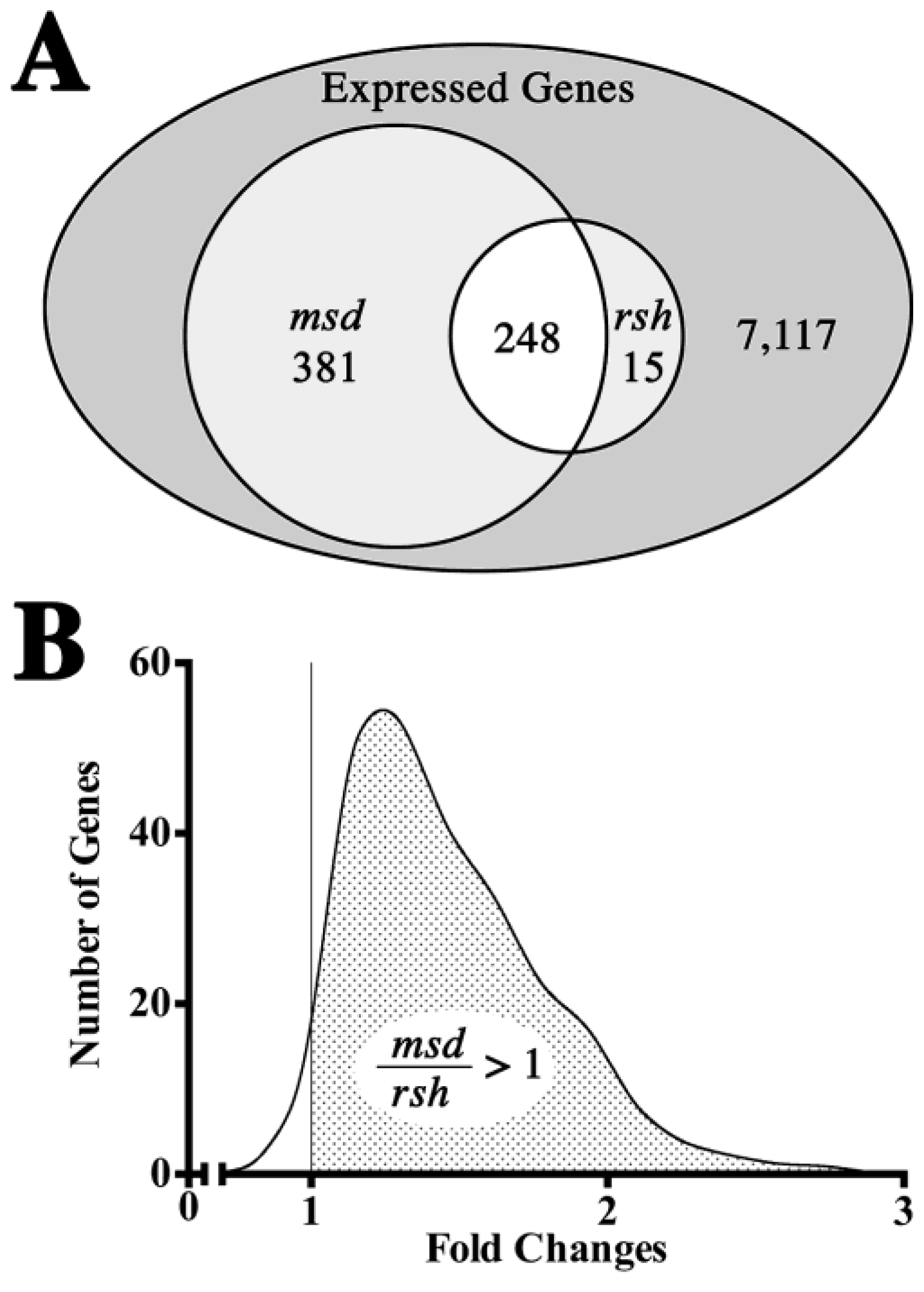
2.4. Validation of Microarray Gene Expression Data for UPR and Myelin Genes
| Function | Gene | Expression Fold-Change Compared to Controls | ||||
|---|---|---|---|---|---|---|
| Microarrays (
FDR < 0.1) P16 Optic Nerve | Taqman PCR/Northern Blot P16 Spinal Cord | |||||
| rsh | msd | rsh | msd | |||
| Myelin | Plp1 | 0.44 ± 0.08 | 0.23 ± 0.05 | 0.31 ± 0.03 a | 0.08 ± 0.01 a | |
| Mbp | 0.56 ± 0.08 | 0.39 ± 0.03 | 0.42 ± 0.05 a | 0.17 ± 0.01 a | ||
| Mog | 0.59 ± 0.07 | 0.37 ± 0.03 | 0.35 ± 0.02 a | 0.08 ± 0.01 a | ||
| Cnp1 | 0.67 ± 0.12 | 0.46 ± 0.04 | n.m. | n.m. | ||
| Cldn11 | 0.66 ± 0.13 | 0.35 ± 0.09 | n.m. | n.m. | ||
| Mag | 0.63 ± 0.10 | 0.41 ± 0.03 | n.m. | n.m. | ||
| UPR | Chop | 2.1 ± 0.40 | 1.9 ± 0.40 | 4.2 ± 1.0 a | 3.5 ± 1.0 a | |
| Atf3 | 1.5 ± 0.20 | 1.7 ± 0.30 | 13.8 ± 3.8 a | 7.5 ± 1.5 a | ||
| Atf4 | 1.3 ± 0.20 | 1.3 ± 0.10 | 1.4 ± 0.07 a | 1.5 ± 0.02 a | ||
| Noxa | n.d. | n.d. | 1.79 ± 0.18 a | 1.67 ± 0.41 a | ||
| Trib3 | 3.2 ± 0.40 | 3.0 ± 0.70 | 72 ± 19 a | 58 ± 10 a | ||
| 4eBP1 | 1.8 ± 0.01 | 1.8 ± 0.01 | 2.8 ± 0.4 a | 4.0 ± 0.5 a | ||
| BiP | 1.7 ± 0.37 | 1.7 ± 0.35 | 1.7 ± 0.4 a | 1.7 ± 0.2 a | ||
| Calreticulin | 1.3 ± 0.36 | 1.4 ± 0.46 | n.m. | 1.2 b | ||
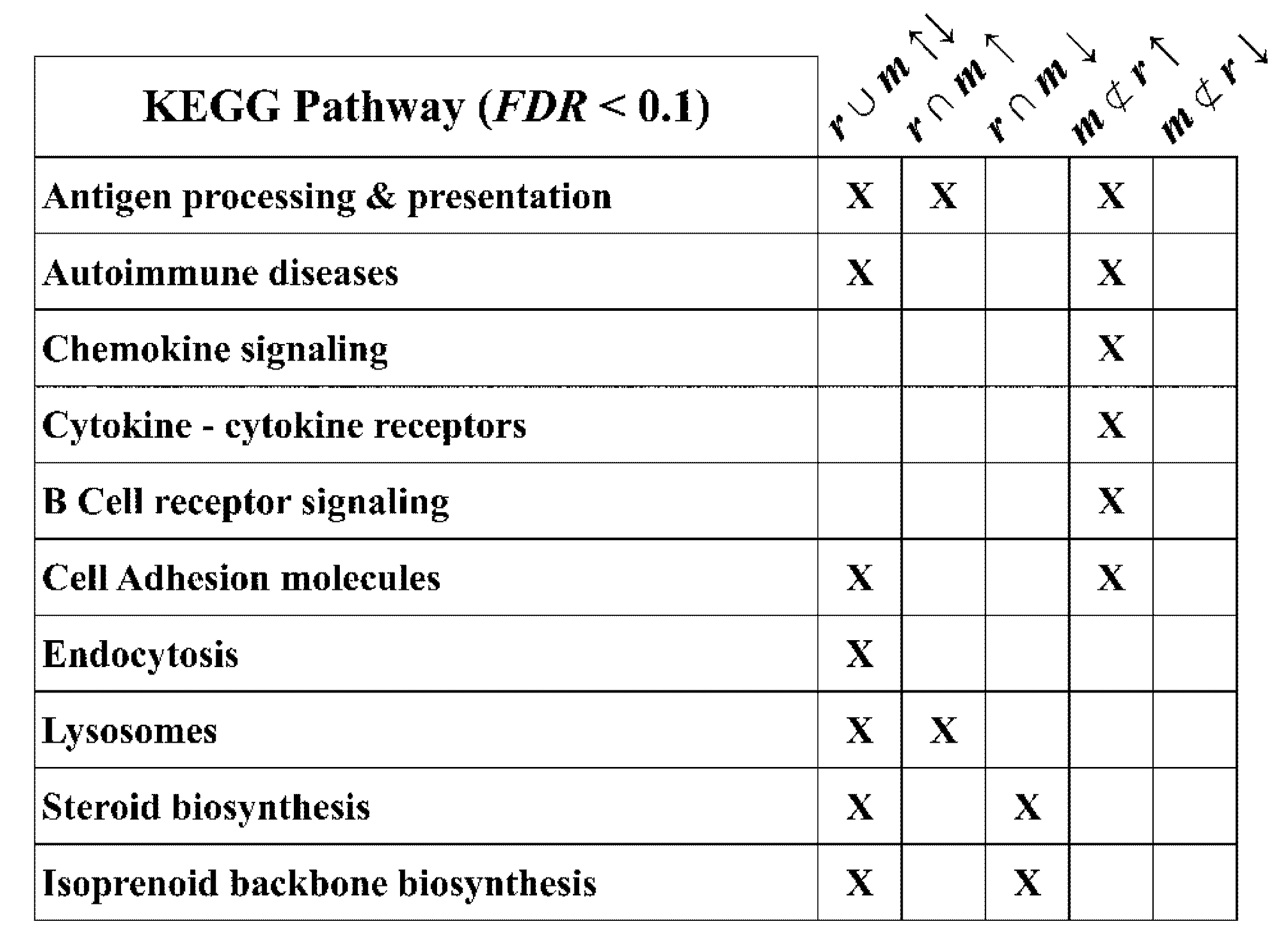
2.5. Optic Nerve Microarray Data Reveal Disease-Severity Dependent Interferon-Responsive Gene Induction in rsh and msd Mice
| Gene | Fold Change | Gene | Fold Change | ||
|---|---|---|---|---|---|
| rsh | msda | rsh | msda | ||
| H2-Ab1 | 1.21 ± 0.22 c | 1.53 ± 0.17 b | H2-T22 | 1.52 ± 0.16 a | 2.21 ± 0.56 b |
| H2-K1 | 1.67 ± 0.39 a | 2.69 ± 0.49 b | H2-T23 | 1.6 ± 0.31 a | 2.85 ± 0.27 b |
| H2-K2 | 1.37 ± 0.13 c | 2.17 ± 0.25 b | Tapbp1 | 1.27 ± 0.15 c | 1.62 ± 0.18 b |
| H2-L | 1.37 ± 0.26 c | 2.22 ± 0.15 b | Aif1 | 2.09 ± 0.55 a | 2.28 ± 0.40 |
| H2-Q2 | 1.53 ± 0.13 a | 2.75 ± 0.43 b | Psmb8 | 1.4 ± 0.09 c | 1.92 ± 0.28 b |
| H2-Q4 | 1.33 ± 0.17 c | 1.94 ± 0.34 b | Psmb9 | 1.28 ± 0.15 c | 1.58 ± 0.14 b |
| H2-Q7 | 1.49 ± 0.20 c | 2.67 ± 0.30 b | Psmb10 | 1.59 ± 0.37 a | 2.12 ± 0.31 b |
| H2-Q10 | 1.39 ± 0.21 c | 2.44 ± 0.18 b | Psme2 | 1.36 ± 0.22 c | 1.62 ± 0.35 b |
| Gene | Fold Change | Gene | Fold Change | ||
|---|---|---|---|---|---|
| rsh | msda | rsh | msda | ||
| Irf1 | 1.3 ± 0.26 c | 1.79 ± 0.14 b | Ccl19 | 1.13 ± 0.17 c | 1.77 ± 0.23 b |
| Irf8 | 1.64 ± 0.32 a | 1.87 ± 0.20 b | Cxcl1 | 1.25 ± 0.17 c | 1.86 ± 0.10 b |
| 2m | 1.95 ± 0.50 a | 2.77 ± 0.54 b | Cxcl10 | 3.95 ±1.51 a | 8.66 ± 2.92 b |
| Ifit1 | 1.52 ± 0.31 a | 2.47 ± 0.46 b | Lcn2 | 1.2 ± 0.04 | 3.01 ± 0.25 b |
| Ifit3 | 1.86 ± 0.41 a | 2.84 ± 0.49 b | Icam1 | 1.31 ± 0.24 c | 1.68 ± 0.17 b |
| Ifitm3 | 1.3 ± 0.14 | 1.5 ± 0.20 | Cd52 | 2.25 ± 0.39 a | 2.59 ± 0.84 |
| Ifitm7 | 1.28 ± 0.16 | 1.86 ± 0.45 b | Cd68 | 1.79 ± 0.26 a | 1.98 ± 0.28 |
| Isg15 | 1.38 ± 0.23 | 1.92 ± 0.13 b | Csf1r | 1.4 ± 0.21 c | 1.66 ± 0.18 b |
| Fcgr1 | 2.0 ± 0.26 a | 2.43 ± 0.11 b | Csf2r | 1.47 ± 0.35 c | 1.56 ± 0.41 |
| Fcgr2 | 1.3 ± 0.27 c | 1.68 ± 0.32 | Trem2 | 3.43 ± 0.62 a | 3.39 ± 0.92 |
| Fcgr3 | 1.59 ± 0.49 a | 1.84 ± 0.33 b | Tyrobp | 2.44 ± 0.69 a | 2.24 ± 0.5 |
| Fcrls | 2.03 ± 0.39 a | 2.38 ± 0.48 | Inpp5d | 1.4 ± 0.22 c | 1.51 ± 0.23 |
| Ccl2 | 1.52 ± 0.51 c | 2.69 ± 0.39 b | Stat1 | 1.38 ± 0.25 c | 1.90 ± 0.33 b |
| cl5 | 1.23 ± 0.17 c | 1.71 ± 0.21 b | |||
2.6. Steroid and Isoprenoid Pathways Are Downregulated in rsh and msd Mice
2.7. Mitochondrial and Oxidative Stress Response Pathways Are Not Broadly Transcriptionally Dysregulated in msd and rsh Mice
2.8. Is Immune Activation in PMD Likely to Precede or Follow Mutant PLP1 Expression and UPR Induction in Oligodendrocytes?
| Gene | Expression Fold-Change Compared to Controls | ||||
|---|---|---|---|---|---|
| Microarrays | Taqman PCR | ||||
| rsh | msd | rsh | msd | ||
| Nfe2l2 | 1.03 ± 0.19 | 1.15 ± 0.13 | 1.53 ± 0.12 b | 1.5 ± 0.08 b | |
| Tfb2m | 1.02 ± 0.12 | 0.99 ± 0.15 | n.m. | n.m. | |
| Tfam | 1.13 ± 0.03 | 1.14 ± 0.07 | n.m. | n.m. | |
| MafF | 1.11 ± 0.15 | 1.21 ± 0.16 | n.m. | n.m. | |
| Sesn2 | 2.85 ± 0.42 a | 2.56 ± 0.51 a | n.m. | n.m. | |
| Prdx6 | 1.1 ± 0.25 | 1.50 ± 0.28 a | n.m. | n.m. | |
| Gpx | 1.24 ± 0.24 | 1.57 ± 0.40 a | n.m. | n.m. | |
| RelA | 1.06 ± 0.14 | 1.03 ± 0.10 | 1.61 ± 0.05 b | 1.04 ± 0.10 | |
| Sod1 | 1.06 ± 0.23 | 1.15 ± 0.24 | n.m. | n.m. | |
| Sod2 | 1.02 ± 0.19 | 1.01 ± 0.16 | n.m. | n.m. | |
| Cat | 0.81 ± 0.17 | 0.91 ± 0.12 | n.m. | n.m. | |
2.9. A Genetically Defined Model to Examine Secondary Activation of the Immune System in Disease
2.10. Is UPR Pathology Associated with Other Autoimmune Responses in Different Tissues?
2.11. Uncoupling of Metabolic Stress from Oxidative Stress in rsh and msd Mice
3. Experimental Section
3.1. Animals
3.2. PMD Autopsy Specimens
3.3. Immunofluorescence Labeling
3.4. Immunofluorescence Image Processing
3.5. Cell Counts and Statistics
3.6. Mouse Tissue Dissections, Microarrays and Taqman PCR Analyses
3.7. Quantification of Microarray Expression Data
3.8. Bioinformatics Analyses of Microarray Expression Data
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar]
- Frank-Cannon, T.C.; Alto, L.T.; McAlpine, F.E.; Tansey, M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009, 4. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Merson, T.D.; Binder, M.D.; Kilpatrick, T.J. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromol. Med. 2010, 12, 99–132. [Google Scholar] [CrossRef]
- Napoli, I.; Neumann, H. Protective effects of microglia in multiple sclerosis. Exp. Neurol. 2009, 225, 24–28. [Google Scholar] [CrossRef]
- Van Noort, J.M.; van den Elsen, P.J.; van Horssen, J.; Geurts, J.J.; van der Valk, P.; Amor, S. Preactive multiple sclerosis lesions offer novel clues for neuroprotective therapeutic strategies. CNS Neurol. Disord. Drug Targets 2011, 10, 68–81. [Google Scholar] [CrossRef]
- Gow, A.; Sharma, R. The unfolded protein response in protein aggregating diseases. Neuromol. Med. 2003, 4, 73–94. [Google Scholar] [CrossRef]
- Gow, A.; Friedrich, V.L.; Lazzarini, R.A. Many naturally occurring mutations of myelin proteolipid protein impair its intracellular transport. J. Neurosci. Res. 1994, 37, 574–583. [Google Scholar] [CrossRef]
- Gow, A.; Lazzarini, R.A. A cellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nat. Genet. 1996, 13, 422–428. [Google Scholar] [CrossRef]
- Barrie, J.A.; Montague, P.; Karim, S.; Kirkham, D.; Nave, K.A.; Anderson, T.J.; Griffiths, I.R.; McLaughlin, M. Modulation of rumpshaker phenotype with wild-type PLP/DM20 suggests several pathogenic mechanisms. J. Neurosci. Res. 2010, 88, 2135–2145. [Google Scholar] [CrossRef]
- Matus, S.; Lisbona, F.; Torres, M.; Leon, C.; Thielen, P.; Hetz, C. The stress rheostat: An interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr. Mol. Med. 2008, 8, 157–172. [Google Scholar]
- Gow, A.; Wrabetz, L. CHOP and the endoplasmic reticulum stress response in myelinating glia. Curr. Opin. Neurobiol. 2009, 19, 1–6. [Google Scholar] [CrossRef]
- Lin, W.; Popko, B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat. Neurosci. 2009, 12, 379–385. [Google Scholar] [CrossRef]
- Katayama, T.; Imaizumi, K.; Manabe, T.; Hitomi, J.; Kudo, T.; Tohyama, M. Induction of neuronal death by ER stress in Alzheimer’s disease. J. Chem. Neuroanat. 2004, 28, 67–78. [Google Scholar] [CrossRef]
- Paschen, W.; Mengesdorf, T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 2005, 38, 409–415. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009, 6, 41–53. [Google Scholar] [CrossRef]
- Uehara, T. Accumulation of misfolded protein through nitrosative stress linked to neurodegenerative disorders. Antioxid. Redox Signal. 2007, 9, 597–601. [Google Scholar] [CrossRef]
- Wang, H.Q.; Takahashi, R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid. Redox Signal. 2007, 9, 553–561. [Google Scholar] [CrossRef]
- Southwood, C.M.; Garbern, J.; Jiang, W.; Gow, A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron 2002, 36, 585–596. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Lazzarini, R.A. Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. J. Cell Biol. 1998, 140, 925–934. [Google Scholar] [CrossRef]
- Gow, A. Protein Misfolding as a Disease Determinant. In Myelin Biology and Disorders; Lazzarini, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 877–885. [Google Scholar]
- Tatar, C.L.; Appikatla, S.; Bessert, D.A.; Paintlia, A.S.; Singh, I.; Skoff, R.P. Increased Plp1 gene expression leads to massive microglial cell activation and inflammation throughout the brain. ASN Neuro 2010, 2, e00043. [Google Scholar]
- Lin, W.; Kemper, A.; Dupree, J.L.; Harding, H.P.; Ron, D.; Popko, B. Interferon-γ inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain 2006, 129, 1306–1318. [Google Scholar] [CrossRef]
- Southwood, C.M.; Gow, A. Molecular mechanisms of disease stemming from mutations in the proteolipid protein gene. Microsc. Res. Tech. 2001, 52, 700–708. [Google Scholar]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004, 24, 10161–10168. [Google Scholar]
- Pahl, H.L.; Baeuerle, P.A. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996, 392, 129–136. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Wu, S.; Tan, M.; Hu, Y.; Wang, J.L.; Scheuner, D.; Kaufman, R.J. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBα synthesis. J. Biol. Chem. 2004, 279, 34898–34902. [Google Scholar]
- Zhang, K.; Shen, X.; Wu, J.; Sakaki, K.; Saunders, T.; Rutkowski, D.T.; Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 2006, 124, 587–599. [Google Scholar]
- Lin, W.; Bailey, S.L.; Ho, H.; Harding, H.P.; Ron, D.; Miller, S.D.; Popko, B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 2007, 117, 448–456. [Google Scholar]
- Leder, C.; Schwab, N.; Ip, C.W.; Kroner, A.; Nave, K.A.; Dornmair, K.; Martini, R.; Wiendl, H. Clonal expansions of pathogenic CD8+ effector cells in the CNS of myelin mutant mice. Mol. Cell. Neurosci. 2007, 36, 416–424. [Google Scholar]
- Lin, W.; Harding, H.P.; Ron, D.; Popko, B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J. Cell Biol. 2005, 169, 603–612. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Ramprasad, M.P.; Terpstra, V.; Kondratenko, N.; Quehenberger, O.; Steinberg, D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1996, 93, 14833–14838. [Google Scholar] [CrossRef]
- Deininger, M.H.; Pater, S.; Strik, H.; Meyermann, R. Macrophage/microglial cell subpopulations in glioblastoma multiforme relapses are differentially altered by radiochemotherapy. J. Neurooncol. 2001, 55, 141–147. [Google Scholar] [CrossRef]
- Caffo, M.; Caruso, G.; Germano, A.; Galatioto, S.; Meli, F.; Tomasello, F. CD68 and CR3/43 immunohistochemical expression in secretory meningiomas. Neurosurgery 2005, 57, 551–557. [Google Scholar]
- Al-Saktawi, K.; McLaughlin, M.; Klugmann, M.; Schneider, A.; Barrie, J.A.; McCulloch, M.C.; Montague, P.; Kirkham, D.; Nave, K.A.; Griffiths, I.R. Genetic background determines phenotypic severity of the Plp rumpshaker mutation. J. Neurosci. Res. 2003, 72, 12–24. [Google Scholar] [CrossRef]
- Fanarraga, M.L.; Sommer, I.U.; Griffiths, I.R.; Montague, P.; Groome, N.P.; Nave, K.A.; Schneider, A.; Brophy, P.J.; Kennedy, P.G. Oligodendrocyte development and differentiation in the rumpshaker mutation. Glia 1993, 9, 146–156. [Google Scholar] [CrossRef]
- Gardinier, M.V.; Macklin, W.B. Myelin proteolipid protein gene expression in jimpy and jimpy (msd) mice. J. Neurochem. 1988, 51, 360–369. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar]
- Amadou, C.; Kumanovics, A.; Jones, E.P.; Lambracht-Washington, D.; Yoshino, M.; Lindahl, K.F. The mouse major histocompatibility complex: Some assembly required. Immunol. Rev. 1999, 167, 211–221. [Google Scholar] [CrossRef]
- Kruger, E.; Kloetzel, P.M. Immunoproteasomes at the interface of innate and adaptive immune responses: Two faces of one enzyme. Curr.Opin. Immunol. 2012, 24, 77–83. [Google Scholar]
- Jang, E.; Lee, S.; Kim, J.H.; Kim, J.H.; Seo, J.W.; Lee, W.H.; Mori, K.; Nakao, K.; Suk, K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013, 27, 1176–1190. [Google Scholar] [CrossRef]
- Glass, W.G.; Hickey, M.J.; Hardison, J.L.; Liu, M.T.; Manning, J.E.; Lane, T.E. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 2004, 172, 4018–4025. [Google Scholar]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012, 380, 1829–1839. [Google Scholar]
- Ip, C.W.; Kroner, A.; Crocker, P.R.; Nave, K.A.; Martini, R. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol. Dis. 2007, 25, 105–111. [Google Scholar] [CrossRef]
- Ip, C.W.; Kroner, A.; Groh, J.; Huber, M.; Klein, D.; Spahn, I.; Diem, R.; Williams, S.K.; Nave, K.A.; Edgar, J.M.; et al. Neuroinflammation by cytotoxic T-lymphocytes impairs retrograde axonal transport in an oligodendrocyte mutant mouse. PLoS One 2012, 7, e42554. [Google Scholar]
- Mitchell, L.S.; Gillespie, S.C.; McAllister, F.; Fanarraga, M.L.; Kirkham, D.; Kelly, B.; Brophy, P.J.; Griffiths, I.R.; Montague, P.; Kennedy, P.G. Developmental expression of major myelin protein genes in the CNS of X-linked hypomyelinating mutant rumpshaker. J. Neurosci. Res. 1992, 33, 205–217. [Google Scholar] [CrossRef]
- Gibson, G.E.; Blass, J.P.; Beal, M.F.; Bunik, V. The α-ketoglutarate-dehydrogenase complex: A mediator between mitochondria and oxidative stress in neurodegeneration. Mol. Neurobiol. 2005, 31, 43–63. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Katsuoka, F.; Motohashi, H.; Engel, J.D.; Yamamoto, M. Nrf2 transcriptionally activates the mafG gene through an antioxidant response element. J. Biol. Chem. 2005, 280, 4483–4490. [Google Scholar]
- Sorce, S.; Krause, K.H.; Jaquet, V. Targeting NOX enzymes in the central nervous system: Therapeutic opportunities. Cell. Mol. Life Sci. 2012, 69, 2387–2407. [Google Scholar] [CrossRef]
- Joshi, G.; Johnson, J.A. The Nrf2-ARE pathway: A valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2012, 7, 218–229. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Melo, A.; Monteiro, L.; Lima, R.M.; Oliveira, D.M.; Cerqueira, M.D.; El-Bacha, R.S. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev. 2011, 2011, e467180. [Google Scholar]
- Ienco, E.C.; LoGerfo, A.; Carlesi, C.; Orsucci, D.; Ricci, G.; Mancuso, M.; Siciliano, G. Oxidative stress treatment for clinical trials in neurodegenerative diseases. J. Alzheimers Dis. 2011, 24, 111–126. [Google Scholar]
- Ghandour, M.; Skoff, R. Expression of galactocerebroside in developing normal and jimpy oligodendrocytes in situ. J. Neurocytol. 1988, 17, 485–498. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Wu, J.; Back, S.H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J.; et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Gorgun, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. Divergent roles of IRE1α and PERK in the unfolded protein response. Curr. Mol. Med. 2006, 6, 5–36. [Google Scholar] [CrossRef]
- Cunnea, P.; Mhaille, A.N.; McQuaid, S.; Farrell, M.; McMahon, J.; FitzGerald, U. Expression profiles of endoplasmic reticulum stress-related molecules in demyelinating lesions and multiple sclerosis. Mult. Scler. 2011, 17, 808–818. [Google Scholar]
- Gorman, M.P.; Golomb, M.R.; Walsh, L.E.; Hobson, G.M.; Garbern, J.Y.; Kinkel, R.P.; Darras, B.T.; Urion, D.K.; Eksioglu, Y.Z. Steroid-responsive neurologic relapses in a child with a proteolipid protein-1 mutation. Neurology 2007, 68, 1305–1307. [Google Scholar] [CrossRef]
- Warshawsky, I.; Rudick, R.A.; Staugaitis, S.M.; Natowicz, M.R. Primary progressive multiple sclerosis as a phenotype of a PLP1 gene mutation. Ann. Neurol. 2005, 58, 470–473. [Google Scholar] [CrossRef]
- Grundtner, R.; Dornmair, K.; Dahm, R.; Flugel, A.; Kawakami, N.; Zeitelhofer, M.; Schoderboeck, L.; Nosov, M.; Selzer, E.; Willheim, M.; et al. Transition from enhanced T cell infiltration to inflammation in the myelin-degenerative central nervous system. Neurobiol. Dis. 2007, 28, 261–275. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, H.K.; Kim, J.H.; Lee, S.Y.; Jou, I.; Joe, E.H. Uridine 5′-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J. Immunol. 2011, 186, 3701–3709. [Google Scholar] [CrossRef]
- Vela, J.M.; Dalmau, I.; Acarin, L.; Gonzalez, B.; Castellano, B. Microglial cell reaction in the gray and white matter in spinal cords from jimpy mice. An enzyme histochemical study at the light and electron microscope level. Brain Res. 1995, 694, 287–298. [Google Scholar] [CrossRef]
- Wolf, M.K.; Kardon, G.B.; Adcock, L.H.; Billings-Gagliardi, S. Hypomyelinated mutant mice. V. Relationship between jp and jpmsd re-examined on identical genetic backgrounds. Brain Res. 1983, 271, 121–129. [Google Scholar] [CrossRef]
- Colbert, R.A.; DeLay, M.L.; Klenk, E.I.; Layh-Schmitt, G. From HLA-B27 to spondyloarthritis: A journey through the ER. Immunol. Rev. 2010, 233, 181–202. [Google Scholar] [CrossRef]
- Nagaraju, K.; Casciola-Rosen, L.; Lundberg, I.; Rawat, R.; Cutting, S.; Thapliyal, R.; Chang, J.; Dwivedi, S.; Mitsak, M.; Chen, Y.W.; et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: Potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005, 52, 1824–1835. [Google Scholar] [CrossRef]
- Nagaraju, K.; Raben, N.; Loeffler, L.; Parker, T.; Rochon, P.J.; Lee, E.; Danning, C.; Wada, R.; Thompson, C.; Bahtiyar, G.; et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc. Natl. Acad. Sci. USA 2000, 97, 9209–9214. [Google Scholar] [CrossRef]
- Schneider, A.; Montague, P.; Griffiths, I.; Fanarraga, M.; Kennedy, P.; Brophy, P.; Nave, K.A. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature 1992, 358, 758–761. [Google Scholar] [CrossRef]
- Gencic, S.; Hudson, L.D. Conservative amino acid substitution in the myelin proteolipid protein of jimpymsd mice. J. Neurosci. 1990, 10, 117–124. [Google Scholar]
- Kobayashi, H.; Hoffman, E.P.; Marks, H.G. The rumpshaker mutation in spastic paraplegia. Nat. Genet. 1994, 7, 351–352. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nanba, E.; Zhang, H.; Sasaki, M.; Komaki, H.; Takeshita, K. jimpymsd mouse mutation and connatal Pelizaeus-Merzbacher disease. Am. J. Med. Genet. 1998, 75, 439–440. [Google Scholar] [CrossRef]
- Readhead, C.; Schneider, A.; Griffiths, I.R.; Nave, K.A. Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron 1994, 12, 583–595. [Google Scholar] [CrossRef]
- Sharma, R.; Jiang, H.; Zhong, L.; Tseng, J.; Gow, A. Minimal role for activating transcription factor 3 in the oligodendrocyte unfolded protein response in vivo. J. Neurochem. 2007, 102, 1703–1712. [Google Scholar] [CrossRef]
- Chirgwin, J.M.; Przybyla, A.E.; MacDonald, R.J.; Rutter, W.J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18, 5294–5299. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Southwood, C.M.; Fykkolodziej, B.; Dachet, F.; Gow, A. Potential for Cell-Mediated Immune Responses in Mouse Models of Pelizaeus-Merzbacher Disease. Brain Sci. 2013, 3, 1417-1444. https://doi.org/10.3390/brainsci3041417
Southwood CM, Fykkolodziej B, Dachet F, Gow A. Potential for Cell-Mediated Immune Responses in Mouse Models of Pelizaeus-Merzbacher Disease. Brain Sciences. 2013; 3(4):1417-1444. https://doi.org/10.3390/brainsci3041417
Chicago/Turabian StyleSouthwood, Cherie M., Bozena Fykkolodziej, Fabien Dachet, and Alexander Gow. 2013. "Potential for Cell-Mediated Immune Responses in Mouse Models of Pelizaeus-Merzbacher Disease" Brain Sciences 3, no. 4: 1417-1444. https://doi.org/10.3390/brainsci3041417
APA StyleSouthwood, C. M., Fykkolodziej, B., Dachet, F., & Gow, A. (2013). Potential for Cell-Mediated Immune Responses in Mouse Models of Pelizaeus-Merzbacher Disease. Brain Sciences, 3(4), 1417-1444. https://doi.org/10.3390/brainsci3041417