In the Blink of an Eye: Investigating the Role of Awareness in Fear Responding by Measuring the Latency of Startle Potentiation
Abstract
:1. Introduction
1.1. Background
1.2. Consciousness
1.2.1. Automatic Processing and Awareness
1.2.2. Problems Related to Measuring Awareness
1.3. Fear
1.3.1. Fear in the Brain
1.3.1.1. The Amygdala
1.3.1.2. The Pathways to the Amygdala
1.3.2. Pre-Conscious Fear
1.4. The Startle Reflex
1.4.1. Startle Reflex Modification
1.4.2. Startle as a Measure of Fear: Startle Reflex Potentiation
1.4.3. Measuring the Latency of the Fear Reaction
1.5. Classical Conditioning
1.5.1. Awareness in Conditioning
1.5.2. Delay and Trace Conditioning
1.5.3. Temporal Specificity of Fear Conditioning
1.6. The General Idea
2. Investigating the Role of Awareness by Measuring the Latency of Startle Potentiation
2.1. Methods
2.2. Study 1: Conditioned Facilitation of the Unconditioned Reflex After Classical Eyeblink Conditioning [8]
2.2.1. Structure
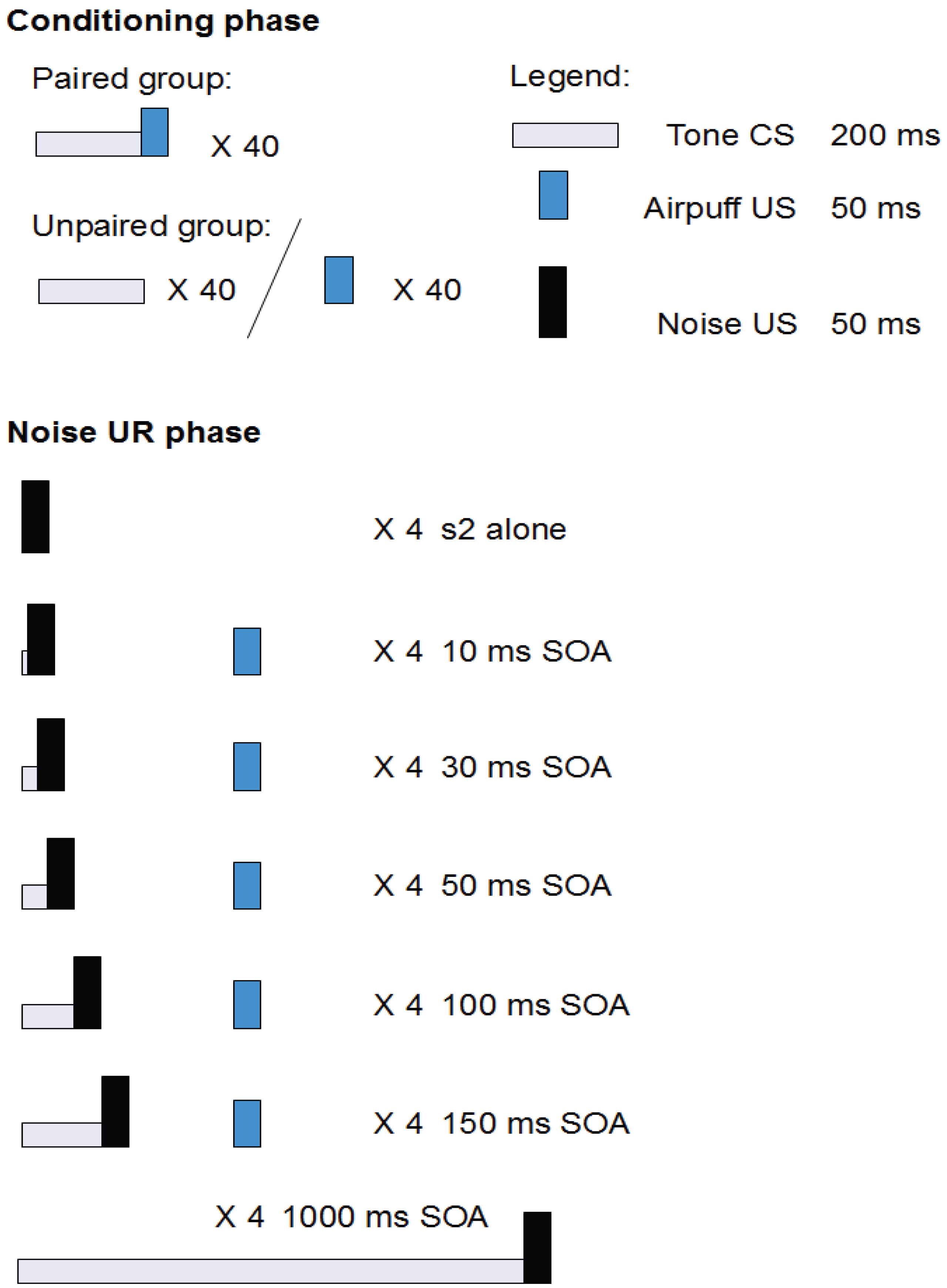
2.2.2. Aim and Background
2.2.3. Results
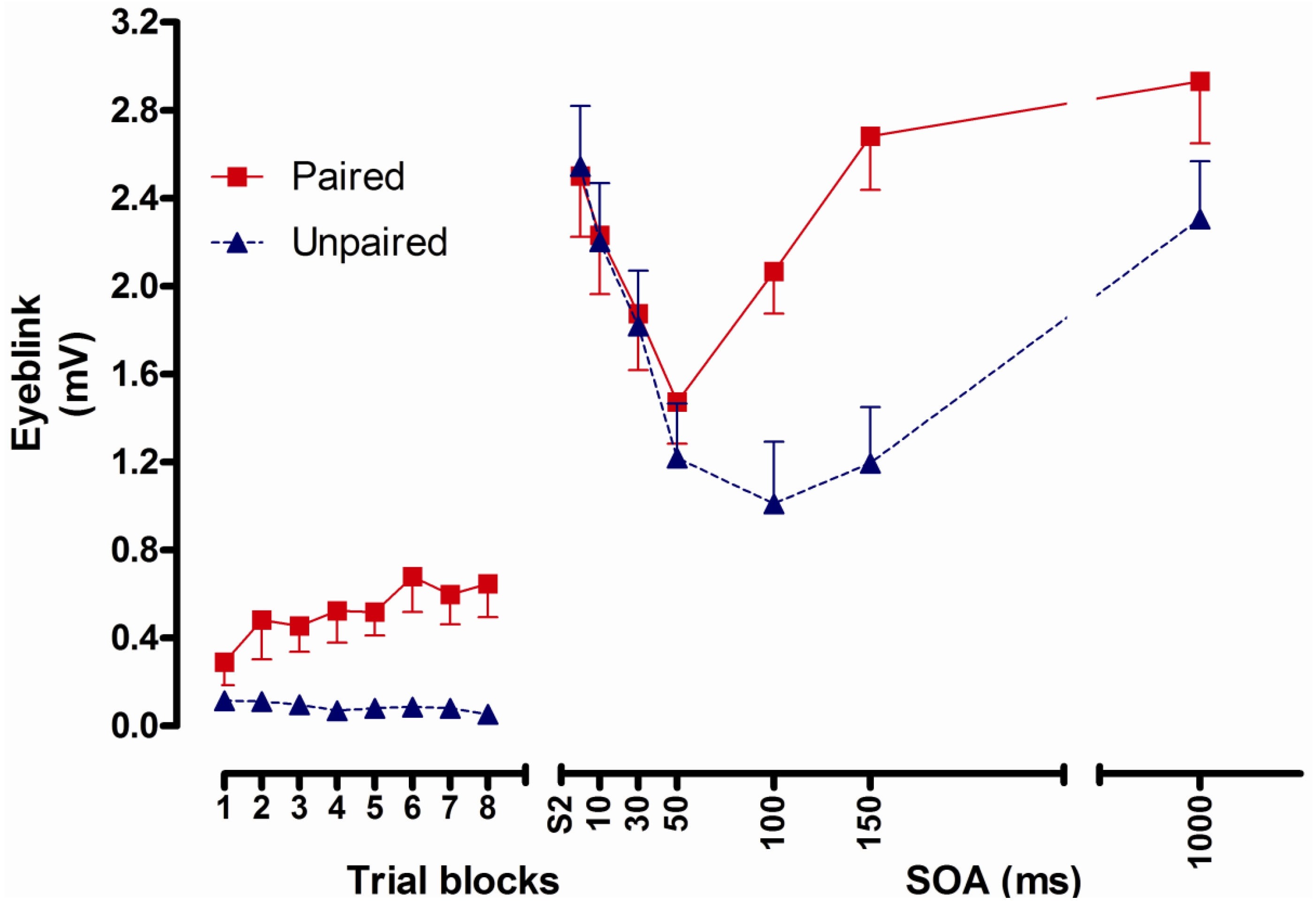
2.2.4. Conclusions
2.3. Study 2: Fear Potentiated Startle at Short Intervals Following Conditioned Stimulus Onset During Delay but Not Trace Conditioning [10]
2.3.1. Structure
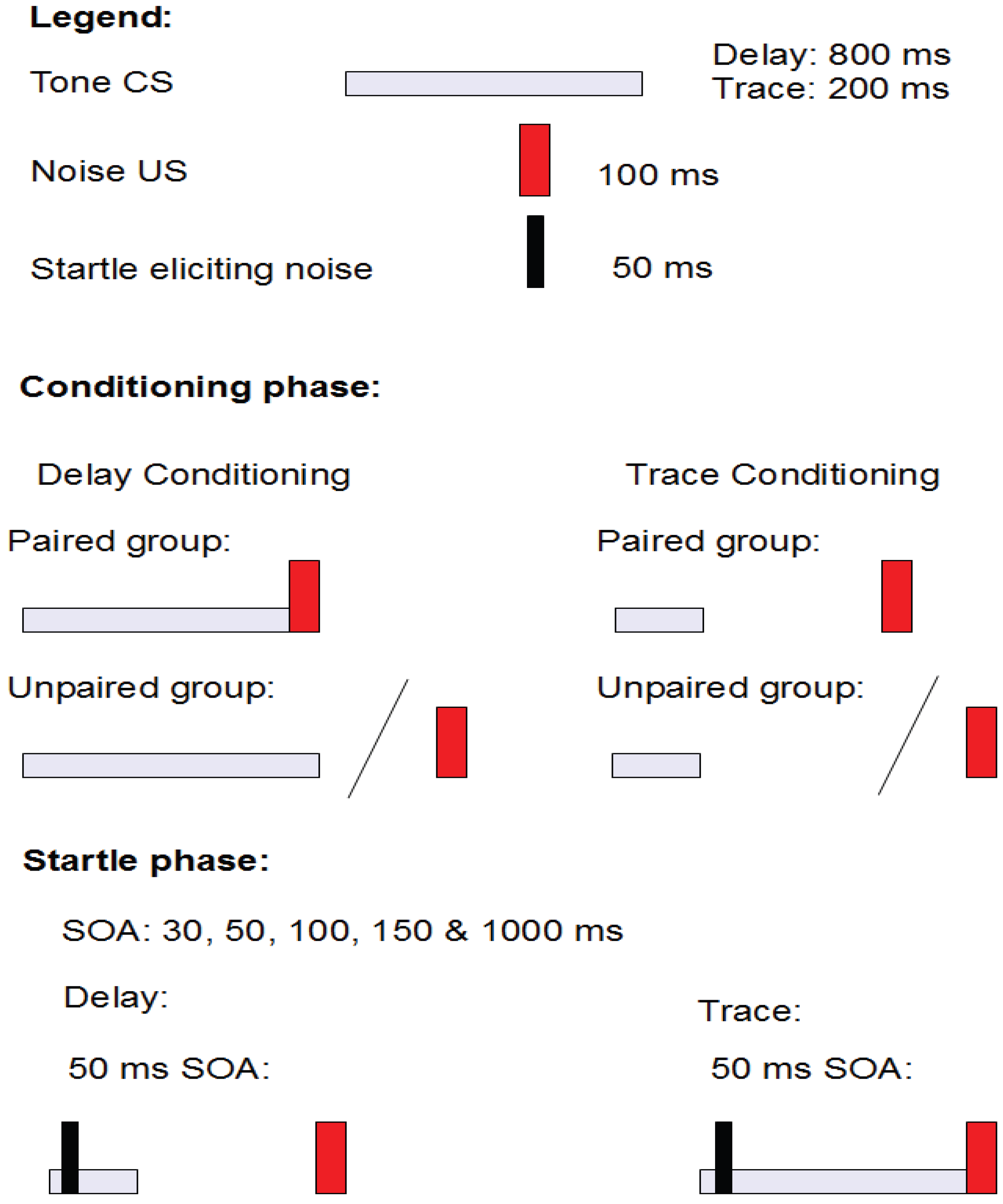
2.3.2. Aim and Background
2.3.3. Results
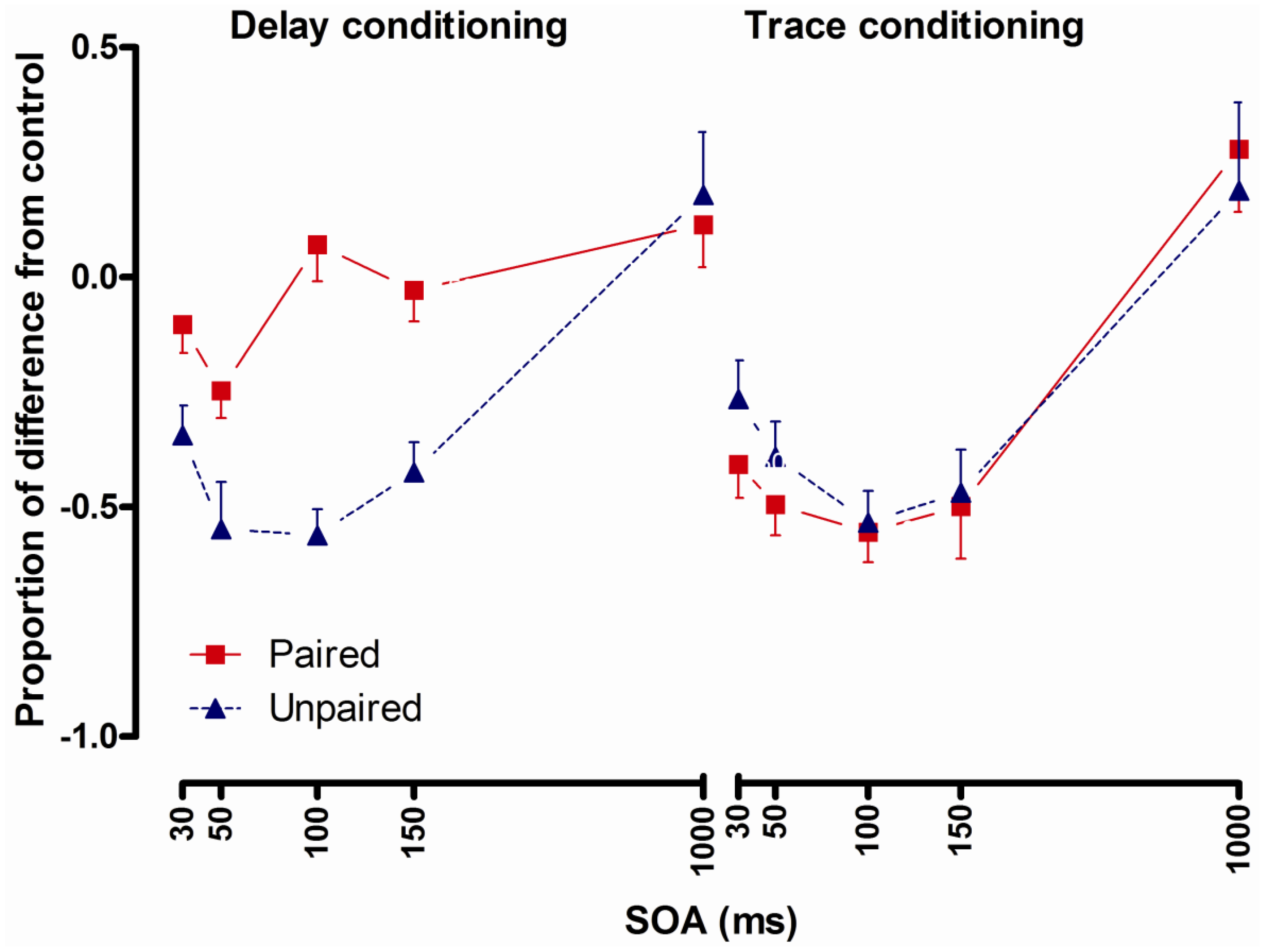
2.3.4. Conclusions
2.4. Study 3: How Fast Is Fear? Automatic and Controlled Processing in Conditioned Fear [9]
2.4.1. Structure
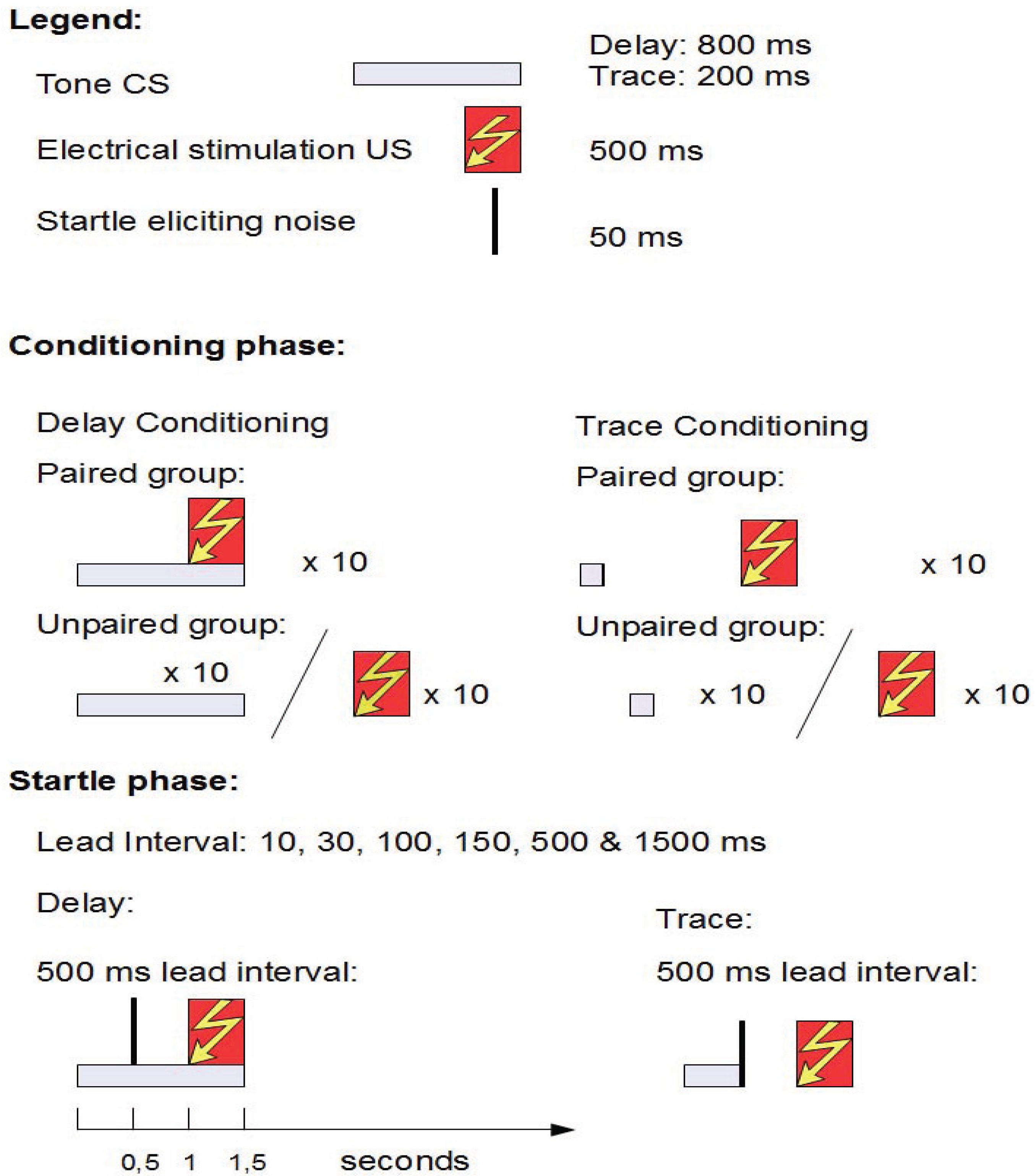
2.4.2. Aim and Background
2.4.3. Results
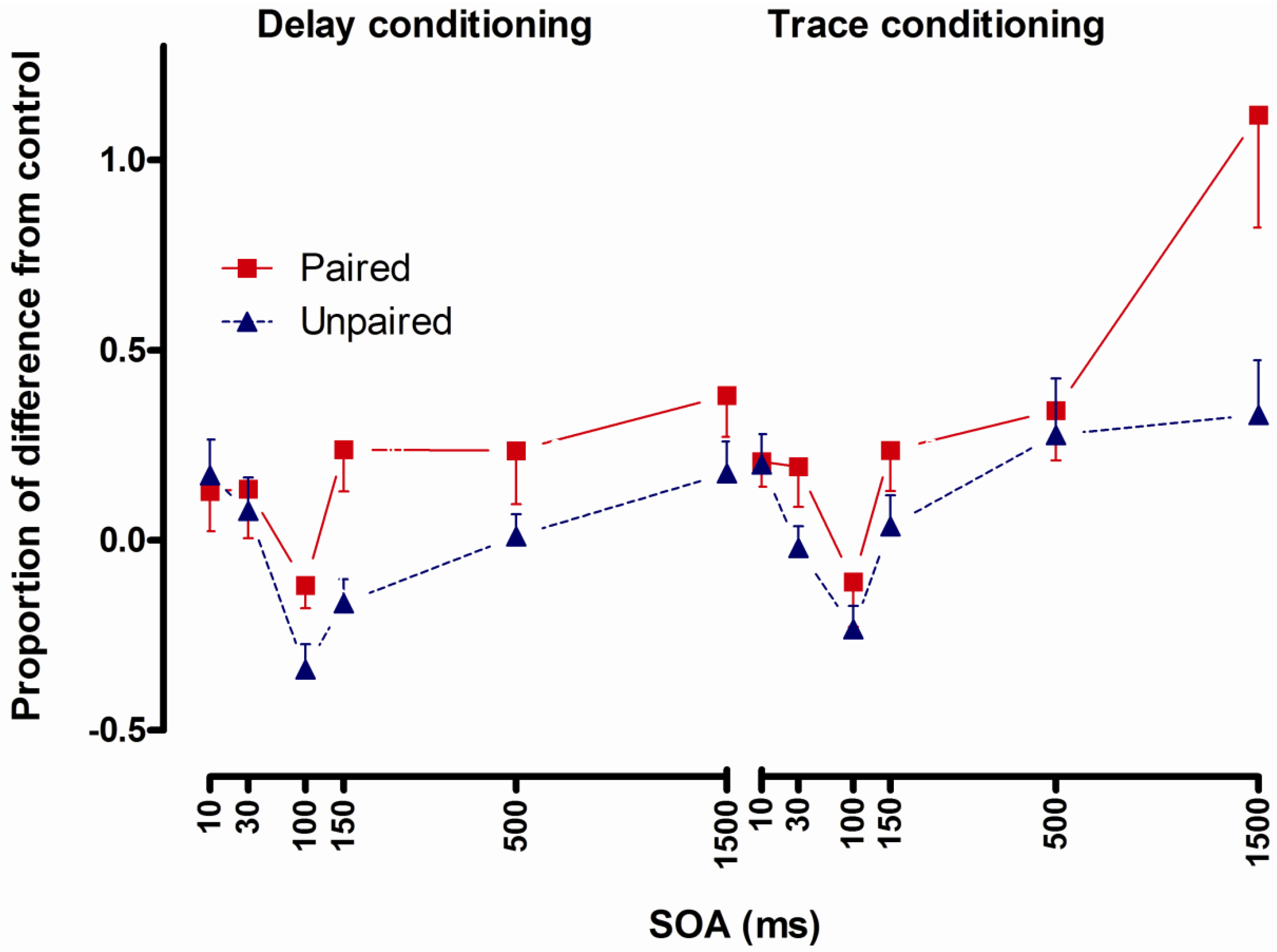
2.4.4. Conclusions
3. General Discussion
4. Conclusions
- (i)
- (ii)
- Conditioned UR potentiation following delay conditioning has a latency of more than 50 and no more than 100 ms. Correlational analyses indicate that this UR potentiation is a function of processes underlying CR formation [8].
- (iii)
- Potentiated startle at short latencies (100 ms and less) after CS presentation following delay conditioning [8,9,10] and startle potentiation only at 1500 ms after CS presentation following trace conditioning [9] indicate that startle potentiation at short intervals could be a method to investigate pre-conscious learning effects.
- (iv)
References
- Clark, R.E.; Squire, L.R. Classical conditioning and brain systems: The role of awareness. Science 1998, 280, 77–81. [Google Scholar] [CrossRef]
- Clark, R.E.; Squire, L.R. Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychol. Sci. 1999, 10, 14–18. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Shanks, D.R. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. J. Exp. Psychol. Anim. Behav. Process. 2002, 28, 3–26. [Google Scholar] [CrossRef]
- Smith, C.N.; Clark, R.E.; Manns, J.R.; Squire, L.R. Acquisition of differential delay eyeblink classical conditioning is independent of awareness. Behav. Neurosci. 2005, 119, 78–86. [Google Scholar] [CrossRef]
- Manns, J.R.; Clark, R.E.; Squire, L.R. Single-cue delay eyeblink conditioning is unrelated to awareness. Cogn. Affect. Behav. Neurosci. 2001, 1, 192–198. [Google Scholar] [CrossRef]
- Manns, J.R.; Clark, R.E.; Squire, L.R. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus 2000, 10, 181–186. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Shanks, D.R. Automatic and eyeblink conditioning are closely related to contingency awareness: Reply to Wiens and Öhman (2002) and Manns et al. (2002). J. Exp. Psychol. Anim. Behav. Process. 2002, 28, 38–42. [Google Scholar] [CrossRef]
- Åsli, O.; Flaten, M.A. Conditioned facilitation of the unconditioned reflex after classical eyeblink conditioning. Int. J. Psychophysiol. 2008, 67, 17–22. [Google Scholar] [CrossRef]
- Åsli, O.; Flaten, M.A. How fast is fear? Automatic and controlled processing in conditioned fear. J. Psychophysiol. 2012, 1, 20–28. [Google Scholar] [CrossRef]
- Åsli, O.; Kulvedrøsten, S.; Solbakken, L.E.; Flaten, M.A. Fear potentiated startle at short intervals following conditioned stimulus onset during delay but not trace conditioning. Psychophysiology 2009, 46, 880–888. [Google Scholar] [CrossRef]
- Chaplin, J. Dictionary of Psychology, 2nd ed.; Dell Publishing: New York, NY, USA, 1985; p. 499. [Google Scholar]
- Gazzaniga, M.S.; Ivry, R.B.; Mangun, G.R.; Steven, M.S. Cognitive Neuroscience: The Biology of the Mind., 3rd ed.; Norton: New York, NY, USA, 2009. [Google Scholar]
- Moors, A.; de Houwer, J. Automaticity: A theoretical and conceptual analysis. Psychol. Bull. 2006, 132, 297–326. [Google Scholar] [CrossRef]
- Pessoa, L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin. Neurobiol. 2005, 15, 188–196. [Google Scholar] [CrossRef]
- Wiens, S. Unawareness is more than a chance event: Comment on Lovibond and Shanks. J. Exp. Psychol. Anim. Behav. Process. 2002, 28, 27–31. [Google Scholar] [CrossRef]
- James, W. What Is an Emotion? [1884]. In Collected Essays and Reviews; Russel & Russel: New York, NY, USA, 1969; pp. 244–275. [Google Scholar]
- LeDoux, J.E. The Emotional Brain; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Öhman, A.; Mineka, S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learing. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef]
- Öhman, A.; Soares, J.J.F. “Unconscious anxiety”: Phobic responses to masked stimuli. J. Abnorm. Psychol. 1994, 103, 231–240. [Google Scholar] [CrossRef]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage; Appelton and Company: New York, NY, USA, 1915. [Google Scholar]
- Morris, J.S.; Buchel, C.; Dolan, R.J. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage 2001, 13, 1044–1052. [Google Scholar] [CrossRef]
- Morris, J.S.; Öhman, A.; Dolan, R.J. Conscious and unconscious emotional learning in the human amygdala. Nature 1998, 393, 467–470. [Google Scholar] [CrossRef]
- Wik, G.; Fredrikson, M.; Fischer, H. Evidence of altered cerebral blood-flow relationships in acute phobia. Int. J. Neurosci. 1997, 91, 253–263. [Google Scholar] [CrossRef]
- Phillips, M.L.; Young, A.W.; Scott, S.K.; Calder, A.J.; Andrew, C.; Giampietro, V.; Williams, S.C.R.; Bullmore, E.T.; Brammer, M.; Gray, J.A. Neural responses to facial and vocal expressions of fear and disgust. Proc. R. Soc. Lond. Ser. B 1998, 265, 1809–1817. [Google Scholar] [CrossRef]
- Hariri, A.R.; Bookheimer, S.Y.; Mazziotta, J.C. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 2000, 11, 43–48. [Google Scholar] [CrossRef]
- Birbaumer, N.; Grodd, W.; Diedrich, O.; Klose, U.; Erb, M.; Lotze, M.; Schneider, F.; Weiss, U.; Flor, H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 1998, 9, 1223–1226. [Google Scholar] [CrossRef]
- Morris, J.S.; Friston, K.J.; Buchel, C.; Frith, C.D.; Young, A.W.; Calder, A.J.; Dolan, R.J. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998, 121, 47–57. [Google Scholar] [CrossRef]
- Phillips, M.L.; Young, A.W.; Senior, C.; Brammer, M.; Andrew, C.; Calder, A.J.; Bullmore, E.T.; Perrett, D.I.; Rowland, D.; Williams, S.C.R.; et al. A specific neural substrate for perceiving facial expressions of disgust. Nature 1997, 389, 495–498. [Google Scholar] [CrossRef]
- Adolphs, R.; Tranel, D.; Damasio, H.; Damasio, A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994, 372, 669–672. [Google Scholar] [CrossRef]
- Niehoff, D.L.; Whitehouse, P.J. Multiple benzodiazepine receptors: Autoradiographic localization in normal human amygdala. Brain Res. 1983, 276, 237–245. [Google Scholar] [CrossRef]
- Bechara, A.; Tranel, D.; Damasio, H.; Adolphs, R.; Rockland, C.; Damasio, A.R. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 1995, 269, 1115–1118. [Google Scholar] [CrossRef]
- Labar, K.S.; LeDoux, J.E.; Spencer, D.D.; Phelps, E.A. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 1995, 15, 6846–6855. [Google Scholar]
- Antoniadis, E.A.; Winslow, J.T.; Davis, M.; Amaral, D.G. Role of the primate amygdala in fear-potentiated startle: Effects of chronic lesions in the rhesus monkey. J. Neurosci. 2007, 27, 7386–7396. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Cicchetti, P.; Xagoraris, A.; Romanski, L.M. The lateral amygdaloid nucleus—Sensory interface of the amygdala in fear conditioning. J. Neurosci. 1990, 10, 1062–1069. [Google Scholar]
- Choi, J.S.; Brown, T.H. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J. Neurosci. 2003, 23, 8713–8721. [Google Scholar]
- LeDoux, J.E.; Sakaguchi, A.; Reis, D.J. Subcortical efferent projections of the medial geniculate-nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 1984, 4, 683–698. [Google Scholar]
- Romanski, L.M.; LeDoux, J.E. Information cascade from primary auditory-cortex to the amygdala—Corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb. Cortex 1993, 3, 515–532. [Google Scholar] [CrossRef]
- Öhman, A.; Hamm, A.O.; Hugdahl, K. Cognition and the Autonomic Nervous System: Orienting, Anticipation, and Conditioning. In Handbook of Psychophysiology, 2nd ed.; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 533–575. [Google Scholar]
- Hamm, A.O.; Weike, A.I.; Schupp, H.T.; Treig, T.; Dressel, A.; Kessler, C. Affective blindsight: Intact fear conditioning to a visual cue in a cortically blind patient. Brain 2003, 126, 267–275. [Google Scholar] [CrossRef]
- Knight, D.C.; Nguyen, H.T.; Bandettini, P.A. The role of awareness in delay and trace fear conditioning in humans. Cogn. Affect. Behav. Neurosci. 2006, 6, 157–162. [Google Scholar] [CrossRef]
- Knight, D.C.; Nguyen, H.T.; Bandettini, P.A. Expression of conditional fear with and without awareness. Proc. Natl. Acad. Sci. USA 2003, 100, 15280–15283. [Google Scholar] [CrossRef]
- Blumenthal, T.D.; Goode, C.T. The startle eyeblink response to low intensity acoustic stimuli. Psychophysiology 1991, 28, 296–306. [Google Scholar] [CrossRef]
- Flaten, M.A.; Blumenthal, T.D. A parametric study of the separate contributions of the tactile and acoustic components of airpuffs to the blink reflex. Biol. Psychol. 1998, 48, 227–234. [Google Scholar] [CrossRef]
- Berg, W.K.; Balaban, M.T. Startle Elicitation: Stimulus Parameters, Recording Techniqes, and Quantification. In Startle Modification: Implications for Neuroscience, Cognitive Science, and Clinical Science; Dawson, M.E., Schell, A.M., Böhmelt, A.H., Eds.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Silverstein, L.D.; Graham, F.K.; Calloway, J.M. Preconditioning and excitability of the human orbicularis oculi reflex as a function of state. Electroencephalogr. Clin. Neurophysiol. 1980, 48, 406–417. [Google Scholar] [CrossRef]
- Graham, F.K. More or less startling effects of weak pre-stimulation. Psychophysiology 1975, 12, 238–248. [Google Scholar] [CrossRef]
- Elden, A.; Flaten, M.A. The relationship of automatic and controlled processing to prepulse inhibition. J. Psychophysiol. 2002, 16, 46–55. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Wible, B.L. Role of weak signals in acoustic startle. J. Acoust. Soc. Am. 1970, 47, 489–497. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Wible, B.L. Temporal parameters in startle facilitation by steady background signals. J. Acoust. Soc. Am. 1969, 45, 7–12. [Google Scholar] [CrossRef]
- Norris, C.M.; Blumenthal, T.D. A relationship between inhibition of the acoustic startle response and the protection of prepulse processing. Psychobiology 1996, 24, 160–168. [Google Scholar]
- Elden, A.; Flaten, M.A. Similar effects of attention directed to acoustic and tactile stimuli on prepulse inhibition of acoustic startle. Scand. J. Psychol. 2003, 44, 363–372. [Google Scholar] [CrossRef]
- Flaten, M.A.; Elden, A. Caffeine and prepulse inhibition of the acoustic startle reflex. Psychopharmacology 1999, 147, 322–330. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.; Cuthbert, B.N. Emotion, attention, and the startle reflex. Psychol. Rev. 1990, 97, 377–395. [Google Scholar] [CrossRef]
- Hamm, A.O.; Vaitl, D. Affective learning: Awareness and aversion. Psychophysiology 1996, 33, 698–710. [Google Scholar] [CrossRef]
- Filion, D.L.; Poje, A.B. Selective and nonselective attention effects on prepulse inhibition of startle: A comparison of task and no-task protocols. Biol. Psychol. 2003, 64, 283–296. [Google Scholar] [CrossRef]
- Schell, A.M.; Wynn, J.K.; Dawson, M.E.; Sinaii, N.; Niebala, C.B. Automatic and controlled attentional processes in startle eyeblink modification: Effects of habituation of the prepulse. Psychophysiology 2000, 37, 409–417. [Google Scholar]
- Thorne, G.L.; Dawson, M.E.; Schell, A.M. Attention and prepulse inhibition: The effects of task-relevant, irrelevant, and no-task conditions. Int. J. Psychophysiol. 2005, 56, 121–128. [Google Scholar] [CrossRef]
- Esteves, F.; Parra, C.; Dimberg, U.; Ohman, A. Nonconscious associative learning—Pavlovian conditioning of skin-conductance responses to masked fear-relevant facial stimuli. Psychophysiology 1994, 31, 375–385. [Google Scholar] [CrossRef]
- Clark, R.E.; Manns, J.R.; Squire, L.R. Classical conditioning, awareness, and brain systems. Trends Cogn. Sci. 2002, 6, 524–531. [Google Scholar] [CrossRef]
- Clark, R.E.; Manns, J.R.; Squire, L.R. Trace and delay eyeblink conditioning: Contrasting phenomena of declarative and nondeclarative memory. Psychol. Sci. 2001, 12, 304–308. [Google Scholar]
- Clark, R.E.; Zhang, A.A.; Lavond, D.G. Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behav. Neurosci. 1992, 106, 879–888. [Google Scholar] [CrossRef]
- Krupa, D.; Thompson, J.K.; Thompson, R.F. Localization of a memory trace in the mammalian brain. Science 1993, 260, 989–991. [Google Scholar]
- Lavond, D.G.; Kim, J.J.; Thompson, R.F. Mammalian brain substrates of aversive classical conditioning. Annu. Rev. Psychol. 1993, 44, 317–342. [Google Scholar] [CrossRef]
- Berger, T.W.; Orr, W.B. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav. Brain Res. 1983, 8, 49–68. [Google Scholar] [CrossRef]
- Schmaltz, L.; Theios, J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus). J. Comp. Physiol. Psychol. 1972, 79, 328–333. [Google Scholar] [CrossRef]
- Solomon, P.R.; Vander Schaaf, E.R.; Thompson, R.F.; Weisz, D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986, 100, 729–744. [Google Scholar] [CrossRef]
- Gabrieli, J.D.E.; Carrillo, M.C.; Cermak, L.S.; McGlinchey-Berroth, R.; Gluck, M.A.; Disterhoft, J.F. Intact delay-eyeblink classical-conditioning in amnesia. Behav. Neurosci. 1995, 109, 819–827. [Google Scholar] [CrossRef]
- Davis, M.; Schlesinger, L.S.; Sorenson, C.A. Temporal specificity of fear conditioning: Effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J. Exp. Psychol. Anim. Behav. Process. 1989, 15, 295–310. [Google Scholar] [CrossRef]
- Bradley, M.; Lang, P.J. Measuring emotion—The self-assessment mannequin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Flaten, M.A. Startle reflex facilitation as a function of classical eyeblink conditioning in humans. Psychophysiology 1993, 30, 581–588. [Google Scholar] [CrossRef]
- Flaten, M.A.; Hugdahl, K. Does classical conditioning generate sensitization of the neural pathway of the conditional stimulus (CS+)? Psychobiology 1991, 19, 51–57. [Google Scholar]
- Weisz, D.J.; McInerney, J. An associative process maintains reflex facilitation of the unconditioned nictitating membrane response during the early stages of training. Behav. Neurosci. 1990, 104, 21–27. [Google Scholar] [CrossRef]
- Wikgren, J.; Ruusuvirta, T.; Korhonen, T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus). Behav. Neurosci. 2002, 116, 1052–1058. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Saunders: Philadelphia, PA, USA, 2006; pp. 555–571. [Google Scholar]
- Clark, R.E.; Squire, L.R. The importance of awareness for eyeblink conditioning is conditional: Theoretical comment on Bellebaum and Daum. Behav. Neurosci. 2004, 118, 1466–1468. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Farb, C.R.; Romanski, L.M. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci. Lett. 1991, 134, 139–144. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotional memory systems in the brain. Behav. Brain Res. 1993, 58, 69–79. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Liu, J.C.J.; Weidemann, G.; Mitchell, C.J. Awareness is necessary for differential trace and delay eyeblink conditioning in humans. Biol. Psychol. 2011, 87, 393–400. [Google Scholar] [CrossRef]
- Mitchell, C.J.; de Houwer, J.; Lovibond, P.F. The propositional nature of human associative learning. Behav. Brain Sci. 2009, 32, 183–198. [Google Scholar] [CrossRef]
- Tabbert, K.; Merz, C.J.; Klucken, T.; Schweckendiek, J.; Vaitl, D.; Wolf, O.T.; Stark, R. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc. Cogn. Affect. Neurosci. 2011, 6, 495–506. [Google Scholar] [CrossRef]
- Merz, C.J.; Tabbert, K.; Schweckendiek, J.; Klucken, T.; Vaitl, D.; Stark, R.; Wolf, O.T. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 2010, 35, 33–46. [Google Scholar] [CrossRef]
- Tabbert, K.; Stark, R.; Kirsch, P.; Vaitl, D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage 2006, 32, 761–770. [Google Scholar] [CrossRef]
- Weike, A.I.; Schupp, H.T.; Hamm, A.O. In Dubio Pro Defensio: Initial activation of conditioned fear is not cue specific. Behav. Neurosci. 2008, 122, 685–696. [Google Scholar] [CrossRef]
- Bouton, M.E.; Moody, E.W. Memory processes in classical conditioning. Neurosci. Biobehav. Rev. 2004, 28, 663–674. [Google Scholar] [CrossRef]
- LaBar, K.S.; LeDoux, J.E. Partial disruption of fear conditioning in rats with unilateral amygdala damage: Correspondence with unilateral temporal lobectomy in humans. Behav. Neurosci. 1996, 110, 991–997. [Google Scholar] [CrossRef]
- Helmstetter, F.J.; Bellgowan, P.S. Hypoalgesia in response to sensitization during acute noise stress. Behav. Neurosci. 1994, 108, 177–185. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Åsli, O.; Flaten, M.A. In the Blink of an Eye: Investigating the Role of Awareness in Fear Responding by Measuring the Latency of Startle Potentiation. Brain Sci. 2012, 2, 61-84. https://doi.org/10.3390/brainsci2010061
Åsli O, Flaten MA. In the Blink of an Eye: Investigating the Role of Awareness in Fear Responding by Measuring the Latency of Startle Potentiation. Brain Sciences. 2012; 2(1):61-84. https://doi.org/10.3390/brainsci2010061
Chicago/Turabian StyleÅsli, Ole, and Magne A. Flaten. 2012. "In the Blink of an Eye: Investigating the Role of Awareness in Fear Responding by Measuring the Latency of Startle Potentiation" Brain Sciences 2, no. 1: 61-84. https://doi.org/10.3390/brainsci2010061
APA StyleÅsli, O., & Flaten, M. A. (2012). In the Blink of an Eye: Investigating the Role of Awareness in Fear Responding by Measuring the Latency of Startle Potentiation. Brain Sciences, 2(1), 61-84. https://doi.org/10.3390/brainsci2010061



