Statistical Parametric Mapping and Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD): Principles and Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Selection Process
2.2. Fundamentals of VBM and VSRAD
2.3. Applications of VSRAD
2.4. Z-Score and Quantitative Analysis of VSRAD
- Severity of VOI atrophy: This index represents the mean Z-score within the VOI, with higher values indicating more severe atrophy. A Z-score of 0–1 indicates minimal or no atrophy; 1–2 indicates mild atrophy; 2–3 indicates moderate atrophy, and >3 indicates severe atrophy;
- Extent of VOI atrophy: This refers to the percentage of the VOI with a Z-score ≥ 2. Proportions of 0–30%, 30–50%, and >50% are indicative of localized, moderate, and widespread atrophy, respectively;
- Extent of GM atrophy: This index represents the proportion of the entire GM volume with a Z-score ≥ 2. A value exceeding 10% suggests significant global GM atrophy;
- Ratio of VOI to GM atrophy: This ratio reflects the extent of atrophy within the VOI relative to the entire brain. Higher values indicate greater selectivity for atrophy within the VOI. Specifically, ratios of 0–5, 5–10, and >10 indicate no, moderate, and high selectivity, respectively.
2.5. Development of VSRAD
2.6. Use of 3T RI
2.7. Early-Onset Dementia
2.8. VSRAD and Artifact Images
- Visual assessment by two radiologists;
- Quantitative image quality metrics, including the peak signal/noise ratio and structural similarity index measure;
- Changes in Z-scores obtained through VSRAD analysis.
2.9. VSRAD in Clinical Practice
3. Diagnostic Accuracy of the VSRAD
3.1. Diagnostic Accuracy for Alzheimer’s Disease
3.2. Diagnostic Performance of VSRAD for Dementia with Lewy Bodies
3.2.1. Atrophy in the Entorhinal Cortex Atrophy
3.2.2. Atrophy in the Dorsal Brainstem
3.2.3. Evaluation of Dorsal Brainstem Atrophy Using the VSRAD
- The case is classified as AD when the severity of VOI atrophy is ≥2.185;
- The case is classified as DLB when the severity of VOI atrophy is <2.185, the ratio of GM atrophy in the dorsal brainstem to that in the medial temporal lobe is ≥0.195, and the ratio of white matter atrophy in the dorsal brainstem to that in the medial temporal lobe is ≥0.195;
- All other patients were classified as having AD.
4. Comparison Between the VSRAD and Cerebral Blood Flow SPECT
4.1. Supporting Dementia Diagnosis Using eZIS and the VSRAD
4.1.1. Comparison with Cerebral Blood Flow
4.1.2. Comparison of Combined Use of the VSRAD and eZIS with Characteristic Findings of Dementia with Lewy Bodies
4.2. Characteristic Findings of MCI and AD and Their Comparison Using the VSRAD
- Severity: defined as the sum of positive Z-scores on the hypoperfused side within the disease-specific ROI divided by the number of voxels showing positive Z-scores in the same region (normal ≤ 1.19);
- Extent: calculated as the percentage of voxels with Z ≥ 2 on the hypoperfused side relative to the total number of voxels in the ROI (normal ≤ 14.2%);
- Ratio: the extent of hypoperfusion in the disease-specific ROI divided by the extent of whole-brain hypoperfusion (normal ≤ 2.22) [28].
5. Comparison Between the VSRAD and Arterial Spin Labeling (ASL)
6. Combination VSRAD and Magnetic Resonance Spectroscopy (MRS)
7. Applications of the VSRAD in Various Diseases and Research Contexts
7.1. Conversion from MCI to AD
7.2. Comparison with Neuropsychological Assessments
7.3. Comparison with Executive Function Disorders and VSRAD
7.4. Evaluation of Brain Atrophy in Diabetes Mellitus
7.4.1. Quantitative Assessment Using the VSRAD in Patients with Diabetes
7.4.2. Relationship Between Visceral Fat and Hippocampal Atrophy in Patients with Diabetes
7.4.3. Relationship Between Homocysteine Levels and Hippocampal Atrophy in Patients with Diabetes
7.4.4. The Relationship Between Inflammation and Hippocampal Atrophy in Patients with Diabetes
7.4.5. Relationship Between MMSE and VSRAD Scores in Elderly Patients with Diabetes
7.5. Association Between Oral Health and VSRAD Scores
7.6. Association Between Olfactory Dysfunction and VSRAD Findings
7.7. Association Between Driving Reaction and Medial Temporal Lobe Atrophy in Patients with MCI and AD
- Simple reaction task: Participants were instructed to press the accelerator pedal when a green light appeared. This task assessed reaction time and variability;
- Choice reaction task: Participants were required to respond in accordance with the light color: brake and then accelerate for red, release and then press the accelerator for yellow, and continue pressing the accelerator for green. This task evaluated patients’ reaction time, its variability, and the number of errors that they made;
- Divided-attention complex task: This task involved responding to both traffic lights and directional arrows displayed on the screen, which required the simultaneous use of both hands and the right foot. The instructions included pressing either the right or left button, depending on the direction of the arrow, or refraining from pressing if no arrow was shown;
- In all tasks, CT was used to measure the operation time, variability, and error count. The AD group showed a significantly higher number of errors than the MCI group. The MMSE scores were negatively correlated with reaction time in the complex task (r = −0.3680) and error count in the divided-attention complex task (r = −0.4354) [46].
7.8. Investigation of Gut Microbiota, Cognitive Function, and VSRAD Findings
7.9. Depression and VSRAD Findings
7.10. Semantic Dementia and VSRAD Findings
7.11. Alcohol Consumption and VSRAD Findings
7.12. Vitamin B12 Deficiency and VSRAD
7.13. Effectiveness of Non-Pharmacological Interventions Involving Physical Exercise in the Primary and Secondary Prevention of Dementia: VBM Reports
7.14. VSRAD Findings in Patients with HIV (Human Immunodeficiency Virus)
7.15. VSRAD Findings in Patients Undergoing Hemodialysis
7.16. Finger Function and VSRAD Finding
7.17. Eye Movement and VSRAD Findings
7.18. Alcohol and VSRAD Finding
8. VSRAD and Artificial Intelligence
Artificial Intelligence and VSRAD
9. Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| CDR | Clinical Dementia Rating |
| CT | Computed tomography |
| DARTEL | Diffeomorphic anatomical registration through exponentiated lie algebra |
| DICOM | Digital Imaging and Communications in Medicine |
| DLB | Dementia with Lewy bodies |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion-weighted imaging |
| fMRI | Functional MRI |
| GM | Gray matter |
| HDS-R | Hasegawa Dementia Scale |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic resonance imaging |
| ROI | Regions of interest |
| SPECT | Single-photon emission computed tomography |
| SPM | Statistical parametric mapping |
| VBM | Voxel-based morphometry |
| VOI | Volume of interest |
| VSRAD | Voxel-based specific regional analysis system for Alzheimer’s disease |
References
- Lonbaridi, G.; Cresciolo, G.; Cavedo, E.; Lucentedorte, E.; Casazza, G.; Bellatorre, A.G.; Lista, C.; Costantino, G.; Frisoni, G.; Virgili, G.; et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer’s disease in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2020, 2, CD009628. [Google Scholar] [CrossRef]
- Matsuda, H. Voxel-based morphometry of brain MRI in normal aging and Alzheimer’s disease. Aging Dis. 2013, 4, 29–37. [Google Scholar] [PubMed]
- Aoki, S.; Kasai, K. Sugu Dekiru VBM: Neuroimaging Analysis of Psychiatric and Neurological Disorders Using SPM12, with DVD; DVD-Tusk: Tokyo, Japan, 2017. [Google Scholar]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Available online: https://www.liberworks.co.jp/know/know_dicom.html (accessed on 15 September 2025).
- Lövdén, M.; Wenger, E.; Mårtensson, J.; Lindenberger, U.; Båckman, L. Structural brain plasticity in adult learning and development. Neurosci. Biobehav. Rev. 2013, 37, 2296–2310. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kojima, S.; Nagasaka, K.; Ohno, K.; Sakurai, N.; Kodama, N.; Otsuru, N.; Onishi, H. Gray matter volume variability in young healthy adults: Influence of gender difference and brain-derived neurotrophic factor genotype. Cereb. Cortex 2022, 32, 2635–2643. [Google Scholar] [CrossRef]
- Matsuda, H. Recent application of VSRAD® to clinical psychiatry. Jpn. J. Gen. Hosp. Psychiatry 2016, 28, 210–217. (In Japanese) [Google Scholar]
- Matsuda, H. Interpretation of structural MRI in Alzheimers disease. Jpn. J. Od. Imaging Diagn. 2018, 9, 889–896. [Google Scholar]
- Matsuda, H. MRI diagnosis of Alzheimer’s disease. In Neuroimaging of Dementia; Medical View Co, Ltd.: Tokyo, Japan, 2016; pp. 38–49. (In Japanese) [Google Scholar]
- Matsuda, H. Principles and types of statistical image analysis: VSRAD. In Visual Learning of Imaging Diagnosis for Dementia, 2nd ed.; Nagai Shoten Co., Ltd.: Osaka, Japan, 2013; pp. 110–118. (In Japanese) [Google Scholar]
- Matsuda, H. Overview of VSRAD. In Clinical and Imaging Features of Dementia You Should Know; Kanehara & Co., Ltd.: Tokyo, Japan, 2010; pp. 1354–1360. (In Japanese) [Google Scholar]
- Matsuda, H. MRI of Alzheimer’s disease. In Neuroimaging Diagnosis of Dementia for Radiologists; Shujunsha: Tokyo, Japan, 2018; pp. 889–896. (In Japanese) [Google Scholar]
- Sone, D.; Imabayashi, E.; Maikysa, N.; Ogawa, M.; Sato, N.; Matsuda, H.; Japanese Alzheimer’s Disease Neuroimaging Initiative. Voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD) on a 3-tesla normal database: Diagnostic accuracy in two independent cohorts with early. J. Alzheimers Dis. 2018, 9, 755–760. [Google Scholar] [CrossRef]
- Shibuya, Y.; Kawakatsu, S.; Hayashi, H.; Kobayashi, R.; Suzuki, A.; Sato, C.; Otani, K. Comparison of entorhinal cortex atrophy between early onset and late-onset Alzheimer’s disease using the VSRAD, a specific and sensitive voxel-based morphometry. Int. J. Geriatr. Psychiatry 2013, 28, 372–376. [Google Scholar] [CrossRef]
- Yoshida, N.; Kageyama, H.; Akai, H.; Yasaka, K.; Sugawara, H.; Okada, Y.; Kunimatsu, A. Motion correction in MR image for analysis of VSRAD using generative adversarial network. PLoS ONE 2022, 17, e0274576. [Google Scholar] [CrossRef]
- Hirata, Y.; Matsuda, H.; Nemoto, K.; Ohnishi, T.; Hirao, K.; Yamashita, F.; Asada, T.; Iwabuchi, S.; Samejima, H. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci. Lett. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Matsuda, H.; Mizumura, S.; Nemoto, K.; Yamashita, F.; Imabayashi, E.; Sato, N.; Asada, T. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomical registration through exponentiated lie algebra improves diagnosis of probable Alzheimer disease. AJNR Am. J. Neuroradiol. 2012, 33, 1109–1114. [Google Scholar] [CrossRef]
- Li, F.; Takechi, H.; Saito, R.; Ayaki, T.; Kokuryu, A.; Kuzuya, A.; Takahashi, R. A comparative study: Visual rating scores and the voxel-based specific regional analysis system for Alzheimer’s disease on magnetic resonance imaging among subjects with Alzheimer’s disease, mild cognitive impairment, and normal cognition. Psychogeriatrics 2019, 19, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kawakatsu, S.; Suzuki, A.; Shibuya, Y.; Kobayashi, R.; Sato., C.; Otani., K. Application of the VSRAD, a specific and sensitive voxel-based morphometry, to comparison of entorhinal cortex atrophy between dementia with Lewy bodies and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2012, 34, 328–331. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Imabayashi, E.; Matsuda, H.; Sakakibara, R.; Inaoka, T.; Terada, H. Discrimination of dementia with Lewy bodies from Alzheimer’s disease using voxel-based morphometry of white matter by statistical parametric mapping 8 plus diffeomorphic anatomic registration through exponentiated Lie algebra. Neuroradiology 2013, 55, 559–566. [Google Scholar] [CrossRef]
- Matsuda, H.; Yokoyama, K.; Sato, N.; Ito, K.; Nemoto, K.; Oba, H.; Hanyu, H.; Kanetaka, H.; Mizumura, S.; Kitamura, S.; et al. Differentiation between dementia with Lewy bodies and Alzheimer’s disease using voxel-based morphometry of structural MRI: A multicenter StudyNeuropsychiatr. Neuropsychiatr. Dis. Treat. 2019, 15, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ono, N.; Takana, K. Correlation between Alzheimer’s disease and dementia with Lewy body scores using VSRAD Advance. Adv. Alzheimers Dis. 2021, 10, 33–45. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, S.; Jibiki, I.; Watanabe, K.; Kubota, T. Correlations between Z-scores of VSRAD and regional cerebral blood flow of SPECT in patients with Alzheimer’s disease and mild cognitive impairment. Psychiatry Clin. Neurosci. 2010, 64, 284–292. [Google Scholar] [CrossRef]
- Honda, G.; Nagamachi, S.; Takahashi, M.; Higuma, Y.; Tani, T.; Hida, K.; Yoshimitsu, K.; Ogomori, K.; Tsuboi, Y. The usefulness of combined analysis using CIScore and VSRAD parameters for differentiating dementia with Lewy bodies from Alzheimer’s disease. Jpn. J. Radiol. 2024, 42, 1206–1212. [Google Scholar] [CrossRef]
- Imabayashi, E.; Soma, T.; Sone, D.; Tsukamoto, T.; Kimura, Y.; Sato, N.; Murata, M.; Matsuda, H. Validation of the cingulate island sign with optimized ratios for discriminating dementia with Lewy bodies from Alzheimer’s disease using brain-perfusion SPECT. Ann. Nucl. Med. 2017, 31, 536–543. [Google Scholar] [CrossRef]
- Tokumitsu, K.; Yasui-Furukori, N.; Takeuchi, J.; Yachimori, K.; Sugawara, N.; Terayama, Y.; Tanaka, N.; Naraoka, T.; Shimoda, K. The Combination of MMSE with VSRAD and eZIS has greater accuracy for discriminating mild cognitive impairment from early Alzheimer’s disease than MMSE alone. PLoS ONE 2021, 16, e0247427. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Mizumura, S.; Nagao, T.; Ota, T.; Iizuka, T.; Nemoto, K.; Takemura, N.; Arai, H.; Homma, A. Automated discrimination between very early Alzheimer’s disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. AJNR Am. J. Neuroradiol. 2007, 28, 731–736. [Google Scholar]
- Matsusue, E.; Inoue, C.; Shimoda, M.; Nakamura, T.; Matsumoto, S.; Matsumoto, K.; Tanino, T.; Nakamura, K.; Fujii, S. Utility of combining multiple parameters of 123I-IMP SPECT and voxel-based morphometry MRI using a multiparametric scoring system to differentiate dementia with Lewy bodies from Alzheimer’s disease. Acta Radiol. 2024, 65, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Ohno, N.; Kitahara, Y.; Niioka, H.; Tanaka, K.; Ueda, H.; Tsujii, K.; Sato, M. Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease and Arterial Spin Labeling in Brain Magnetic Resonance Imaging: A Comparative Study. Curr. Med. Imaging 2025, 21, e15734056337828. [Google Scholar] [CrossRef]
- Waragai, M.; Hata, S.; Suzuki, T.; Ishii, R.; Fujii, C.; Tokuda, T.; Arai, H.; Ohrui, T.; Higuchi, S.; Yoshida, M.; et al. Utility of SPM8 plus DARTEL (VSRAD) combined with magnetic resonance spectroscopy as adjunct techniques for screening and predicting dementia due to Alzheimer’s disease in clinical practice. J. Alzheimers Dis. 2014, 44, 1207–1222. [Google Scholar] [CrossRef]
- Tokuchi, R.; Hishikawa, N.; Kurata, T.; Sato, K.K.S.; Yamashita, T.; Deguchi, K.; Abe, K. Clinical and demographic predictors of mild cognitive impairment for converting to Alzheimer’s disease and reverting to normal cognition. J. Neurol. Sci. 2014, 15, 288–292. [Google Scholar] [CrossRef]
- Li, X.; Jiao, J.; Shimizu, S.; Jibiki, I.; Watanabe, K.I.; Kubota, T. Correlations between atrophy of the entorhinal cortex and cognitive function in patients with Alzheimer’s disease and mild cognitive impairment. Psychiatry Clin. Neurosci. 2012, 66, 587–593. [Google Scholar] [CrossRef]
- Hashimoto, M.; Araki, Y.; Takashima, Y.; Nogami, K.; Uchino, A.; Yuzuriha, T.; Yao, H. Hippocampal atrophy and memory dysfunction associated with physical inactivity in community-dwelling elderly subjects: The Sefuri study. Brain Behav. 2017, 7, e00620. [Google Scholar] [CrossRef]
- Oshikubo, G.; Akahane, A.; Unno, A.; Watanabe, Y.; Ikebuchi, E.; Tochigi, M.; Hayashi, N. Utility of VSRAD for diagnosing Alzheimer’s disease in patients screened for dementia. J. Int. Med. Res. 2020, 48, 300060520917270. [Google Scholar] [CrossRef]
- Yamashita, K.; Kuwashiro, T.; Ishikawa, K.; Furuya, K.; Harada, S.; Shin, S.; Wada, N.; Hirakawa, C.; Okada, Y.; Noguchi, T. Identification of predictors for Mini-Mental State Examination and revised Hasegawa’s Dementia Scale scores using MR-based brain morphometry. Eur. J. Radiol. Open 2021, 24, 100359. [Google Scholar] [CrossRef] [PubMed]
- Togawa, R.; Hashimoto, H.; Nakanishi, A.; Kawarada, Y.; Muramatsu, T.; Matsuda, Y.; Kataoka, K.; Shimada, A.; Uchida, K.; Yoshida, A.; et al. The Relationship Between Medial Temporal Lobe Atrophy and Cognitive Impairment in Patients with Dementia With Lewy Bodies. J. Geriatr. Psychiatry Neurol. 2015, 28, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Shinagawa, S.; Ochiai, Y.; Aoki, R.; Kasahara, H.; Nukariya, K.; Nakayama, K. Association between executive dysfunction and hippocampal volume in Alzheimer’s disease. Int. Psychogeriatr. 2011, 23, 764–771. [Google Scholar] [CrossRef]
- Kamiyama, K.; Wada, A.; Sugihara, M.; Kurioka, S.; Hayashi, K.; Hayashi, T.; Yoshisako, T.; Yamamoto, N.; Tsuchie, Y.; Yamaguchi, S.; et al. Potential hippocampal region atrophy in diabetes mellitus type 2: A voxel-based morphometry VSRAD study. Jpn. J. Radiol. 2010, 28, 266–272. [Google Scholar] [CrossRef]
- Anan, F.; Masaki, T.; Shimomura, T.; Fujiki, M.; Eshima, N.; Saikawa, T.; Yoshimatsu, H. Abdominal visceral fat accumulation is associated with hippocampus volume in non-dementia patients with type 2 diabetes mellitus. Neuroimage 2010, 49, 57–62. [Google Scholar] [CrossRef]
- Shimomura, T.; Anan, F.; Masaki, T.; Umeno, Y.; Eshima, N.; Saikawa, T.; Yoshimatsu, H.; Fujiki, M.; Kobayashi, H. Homocysteine levels associated with hippocampus volume in type 2 diabetes patients. Eur. J. Clin. Investig. 2011, 41, 751–758. [Google Scholar] [CrossRef]
- Anan, F.; Masaki, T.; Shimomura, T.; Fujiki, M.; Umeno, Y.; Eshima, N.; Saikawa, T.; Yoshimatsu, H. High-sensitivity C-reactive protein is associated with hippocampus volume in nondementia patients with type 2 diabetes mellitus. Metabolism 2011, 60, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ueba, Y.; Murakami, T.; Yamamoto, T.; Kuroe, A.; Yamasaki, M.; Kaneda, D.; Otani, D.; Kiyobayashi, S.; Ikeda, K.; Yabe, D.; et al. Voxel-based specific regional analysis system for Alzheimer’s disease utility as a screening tool for unrecognized cognitive dysfunction of elderly patients in diabetes outpatient clinics: Multicenter retrospective exploratory study. J. Diabetes Investig. 2022, 13, 177–184. [Google Scholar] [CrossRef]
- Egashira, R.; Umezaki, Y.; Mizutani, S.; Obata, T.; Yamaguchi, M.; Tamai, K.; Yoshida, M.; Makino, M.; Naito, T. Relationship between cerebral atrophy and number of present teeth in elderly individuals with cognitive decline. Exp. Gerontol. 2021, 144, 111189. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shoji, Y.; Yanagimoto, H.; Morita, K.; Kodama, H.; Tsuruhisa, Y.; Ookawa, J. A characteristic of olfactory function in four types of dementia and non-dementia subjects using smell identification test1. Psychogeriatrics 2024, 24, 25–34. [Google Scholar] [CrossRef]
- Wakita, H.; Takahashi, Y.; Masuzugawa, S.; Miyasaka, H.; Sonoda, S.; Shindo, A.; Tomimoto, H. Alterations in driving ability and their relationship with morphometric magnetic resonance imaging indicators in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Psychogeriatrics 2024, 24, 830–837. [Google Scholar] [CrossRef]
- Asaoka, D.; Xiao, J.; Takeda, T.; Yanagisawa, N.; Yamazaki, T.; Matsubara, Y.; Sugiyama, H.; Endo, N.; Higa, M.; Kasanuki, K.; et al. Effect of probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: Results of a 24-week randomized, double-blind, placebo-controlled trial. J. Alzheimers Dis. 2022, 88, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Kimura, M.; Yokota, M.; Okubo, Y. Comparison of regional gray matter volume abnormalities in Alzheimer’s disease and late life depression with hippocampal atrophy using VSRAD analysis: A voxel-based morphometry study. Psychiatry Res. 2015, 232, 71–75. [Google Scholar] [CrossRef]
- Niida, R.; Niida, A.; Motomura, M.; Uechi, A. Diagnosis of depression by MRI scans with the use of VSRAD—A promising auxiliary means of diagnosis: A report of 10 years research. Int. J. Gen. Med. 2011, 4, 377–387. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hayashi, H.; Kawakatsu, S.; Shibuya, Y.; Morioka, D.; Ohba, M.; Yoshioka, M.; Sakamoto, K.; Kanoto, M.; Otani, K. Comparing medial temporal atrophy between early-onset semantic dementia and early-onset Alzheimer’s disease using voxel-based morphometry: A multicenter MRI Study. Curr. Alzheimer Res. 2022, 19, 503–510. [Google Scholar] [CrossRef]
- Suzuki, Y.; Oishi, M.; Ogawa, K.; Mizutani, T. Atrophy of the parahippocampal gyrus and regional cerebral blood flow in the limbic system in chronic alcoholic patients. Alcohol 2010, 44, 439–445. [Google Scholar] [CrossRef]
- Ueno, A.; Hamano, T.; Nagata, M.; Yamaguchi, T.; Endo, Y.; Enomoto, S.; Kimura, H.; Ikawa, M.; Amamura, O.; Yamanaka, D.; et al. Association of vitamin B12 deficiency in a dementia cohort with hippocampal atrophy on MRI. J. Prev. Alzheimers Dis. 2025, 12, 100265. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Societas Neurologica Japonica. Guideline for Dementing Disorder; Igaku-Shoin Limited: Tokyo, Japan, 2017. [Google Scholar]
- Shatil, E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 2013, 5, 8. [Google Scholar] [CrossRef]
- Tay, L.; Lim, W.S.; Chan, M.; Ali, N.; Chong, M.S. A combined cognitive stimulation and physical exercise programme (MINDVital) in early dementia: Differential effects on single- and dual-task gait performance. Gerontology 2016, 62, 604–610. [Google Scholar] [CrossRef]
- Satoh, M.; Ogawa, J.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. The effects of physical exercise with music on cognitive function of elderly people: Mihama-Kiho project. PLoS ONE 2014, 9, e95230. [Google Scholar] [CrossRef] [PubMed]
- Tabei, K.I.; Satoh, M.; Ogawa, J.I.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. Physical exercise with music reduces gray and white matter loss in the frontal cortex of elderly people: The Mihama-kiho scan project. Front. Aging Neurosci. 2017, 9, 174. [Google Scholar] [CrossRef]
- Satoh, M.; Ogawa, J.I.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. Physical exercise with music maintains activities of daily living in patients with dementia: Mihama-kiho project Part 21. J. Alzheimers Dis. 2017, 57, 85–96. [Google Scholar] [CrossRef]
- Tabei, K.I.; Satoh, M.; Ogawa, J.I.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. Cognitive function and brain atrophy predict non-pharmacological efficacy in dementia: The Mihama-kiho scan Project2. Front. Aging Neurosci. 2018, 10, 87. [Google Scholar] [CrossRef]
- Satoh, M.; Ogawa, J.I.; Tokita, T.; Matsumoto, Y.; Nakao, K.; Tabei, K.I.; Kato, N.; Tomimoto, H. The effects of a 5-year physical exercise intervention with music in community- dwelling normal elderly people: The Mihama-kiho follow-up project. J. Alzheimers Dis. 2020, 78, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Koga, I.; Kitazawa, T.; Oba, H.; Furui, S.; Matsuda, H.; Ota, Y. Magnetic resonance imaging changes in Asian people living with HIV. Infect. Dis. 2021, 53, 89–93. [Google Scholar] [CrossRef]
- Maesato, K.; Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Moriya, H.; Hidaka, S.; Kobaashi, S. Correlation of hippocampal atrophy with hyperhomocysteinemia in hemodialysis patients: An exploratory pilot study. PLoS ONE 2017, 12, e0175102. [Google Scholar] [CrossRef]
- Sugioka, J.; Suzumura, S.; Kuno, K.; Kizuka, S.; Sakurai, H.; Kanada, Y.; Mizuguchi, T.; Kondo, I. Relationship between finger movement characteristics and brain voxel-based morphometry. PLoS ONE 2022, 17, e0269351. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Morita, K.; Ishii, Y.; Shouji, Y.; Uchimura, N. Characteristics of exploratory eye movements in elderly people: Possibility of early diagnosis of dementia. Psychogeriatrics 2010, 10, 124–130. [Google Scholar] [CrossRef]
- Fujita, K.; Katsuki, M.; Takasu, A.; Kitajima, A.; Shimazu, T.; Marui, Y. Development of an artificial intelligence-based diagnostic model for Alzheimer’s disease. Aging Med. 2022, 25, 167–173. [Google Scholar] [CrossRef]
- Wakisaka, Y.; Furuta, A.; Tanizaki, Y.; Kiyohara, Y.; Iida, M.; Iwaki, T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: The Hisayama study. Acta Neuropathol. 2003, 106, 374–382. [Google Scholar] [CrossRef]
- Sakurai, K.; Kaneda, D.; Morimoto, S.; Uchida, Y.; Inui, S.; Kimura, Y.; Kato, T.; Ito, K.; Hashizume, Y. Asymmetric cerebral peduncle atrophy: A simple diagnostic clue for distinguishing frontotemporal lobar degeneration from Alzheimer’s disease. J. Alzheimers Dis. 2023, 95, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Rabinovici, G.D.; Gatsonis, C.; Apgar, C.; Chaudhary, K.; Gareen, I.; Hanna, L.; Hendrix, J.; Hillner, B.E.; Olson, C.; Lesman-Segev, O.H.; et al. Association of Amyloid Positron Emission Tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 2019, 321, 1286–1294. [Google Scholar] [CrossRef] [PubMed]

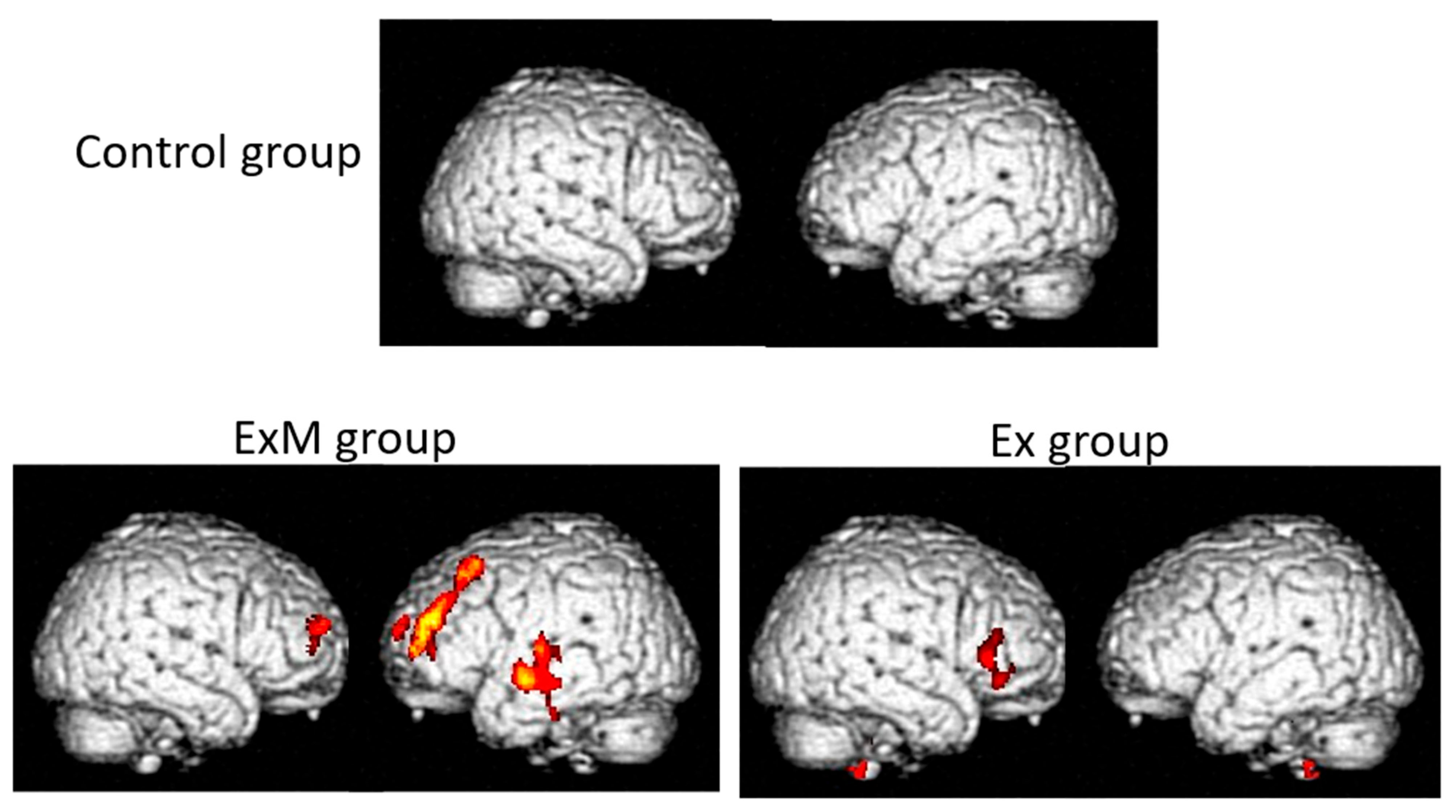
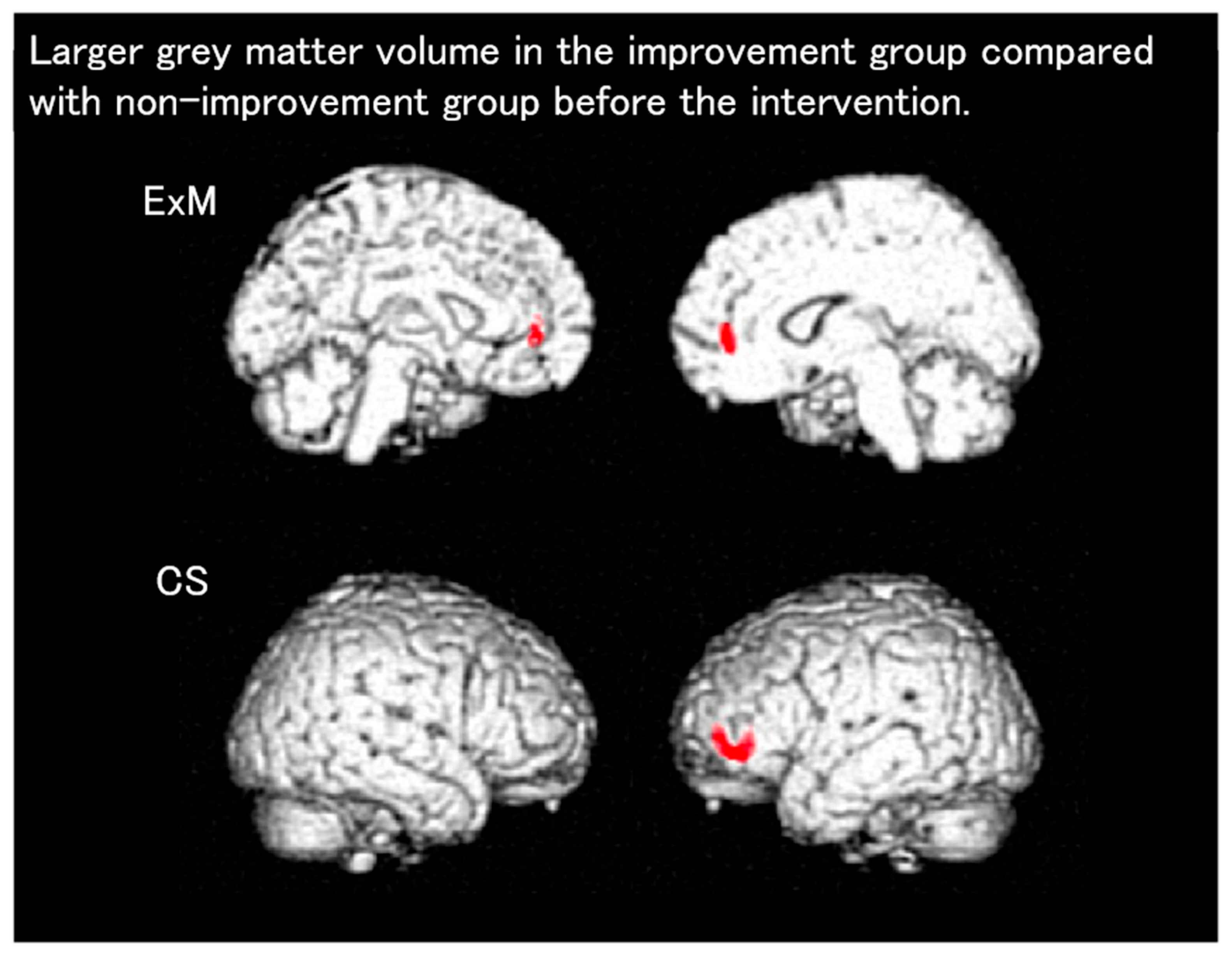
| Disease/Condition | Key VSRAD Findings | Limitations |
|---|---|---|
| mer’s disease (AD) | High diagnostic accuracy (>90%); hippocampal/parahippocampal atrophy correlates with severity | Underestimates early-onset AD; limited generalizability outside Japan |
| Mild cognitive impairment (MCI) | Higher Z-scores in converters to AD; correlation with MMSE/memory tests | Predictive value moderate; overlap with aging |
| Dementia with Lewy bodies (DLB) | Lower entorhinal cortex Z-scores vs. AD; dorsal brainstem indices useful | Sensitivity/specificity moderate; overlap with AD |
| Diabetes mellitus | Hippocampal and whole-brain GM atrophy; linked with visceral fat, homocysteine, hs-CRP | Cross-sectional; causality unclear |
| Oral health | Fewer teeth/reduced masticatory function → more GM atrophy | Small sample; cross-sectional |
| Olfactory dysfunction | Hippocampal Z-scores correlated with impaired smell identification | Confounding factors not fully addressed |
| Depression | Specific atrophy in subgenual ACC; distinct from AD | Small samples; partial overlap with AD |
| Semantic dementia | Z-score cut-off differentiates from AD (sens. 87%, spec. 85%) | Needs larger validation |
| Alcoholism | Parahippocampal atrophy in chronic alcoholics | Confounding by nutrition, comorbidities |
| Vitamin B12 deficiency | Higher prevalence of deficiency in patients with Z ≥ 2 | Observational only |
| HIV infection | Greater GM atrophy in younger HIV patients | Not disease-specific |
| Hemodialysis | Hippocampal atrophy correlates with homocysteine/age | Pilot study |
| Interventions (exercise, probiotics, AI) | Exercise+music preserved GM; probiotics slowed atrophy; AI models improved AUC | Heterogeneous methods; limited long-term data |
| VSRAD =The Voxel-Based Specific Regional Analysis System for Alzheimer’s disease; MCI = mild cognitive impairment; | ||
| DLB = dementia with Lewy bodies; AD = Alzheimer’s disease; PSP = progressive supranuclear palsy; DB = database. | ||
| AD = Alzheimer’s disease; MCI = Mild cognitive impairment; DLB = Dementia with Lewy bodies; DM: Diabetes mellitus; | ||
| GM = Gray matter; MMSE = Mini-Mental State Examination; ACC = Anterior cingulate cortex; ACC = Anterior cingulate cortex; | ||
| MDD = Major depressive disorder; LOD = Late-onset depression; SD = Semantic dementia; HIV = Human immunodeficiency virus; | ||
| HD = Hemodialysis; AI = Artificial intelligence; AUC: Area under the curve. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, S.; Yoshida, N.; Sakurai, N.; Okada, Y.; Ohno, N.; Satoh, M.; Takeshita, K.; Ishida, M.; Saito, K. Statistical Parametric Mapping and Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD): Principles and Clinical Applications. Brain Sci. 2025, 15, 999. https://doi.org/10.3390/brainsci15090999
Yamamoto S, Yoshida N, Sakurai N, Okada Y, Ohno N, Satoh M, Takeshita K, Ishida M, Saito K. Statistical Parametric Mapping and Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD): Principles and Clinical Applications. Brain Sciences. 2025; 15(9):999. https://doi.org/10.3390/brainsci15090999
Chicago/Turabian StyleYamamoto, Shinji, Nobukiyo Yoshida, Noriko Sakurai, Yukinori Okada, Norikazu Ohno, Masayuki Satoh, Koji Takeshita, Masanori Ishida, and Kazuhiro Saito. 2025. "Statistical Parametric Mapping and Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD): Principles and Clinical Applications" Brain Sciences 15, no. 9: 999. https://doi.org/10.3390/brainsci15090999
APA StyleYamamoto, S., Yoshida, N., Sakurai, N., Okada, Y., Ohno, N., Satoh, M., Takeshita, K., Ishida, M., & Saito, K. (2025). Statistical Parametric Mapping and Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD): Principles and Clinical Applications. Brain Sciences, 15(9), 999. https://doi.org/10.3390/brainsci15090999






