Acute Vertigo, Dizziness and Imbalance in the Emergency Department—Beyond Stroke and Acute Unilateral Vestibulopathy—A Narrative Review
Abstract
1. Introduction
| Benign * or Less Urgent Causes | Dangerous * and More Urgent Causes | ||
|---|---|---|---|
| Neurological Causes | Non-Neurological Causes | Neurological Causes | Non-Neurological Causes |
Common Causes (>1% of AVS)
| Common Causes
| Common Causes (>1% of AVS)
| Common Causes
|
2. Methods
3. Non-Neurological Causes of AVS
3.1. Pharmacologic Intoxication, Drug Withdrawal and Psychiatric Disorders
3.2. Environmental Toxins
3.3. Endocrine Disorders
3.4. Electrolyte Disturbances
3.5. Orthostatic Hypotension
3.6. Nutritional Disturbances
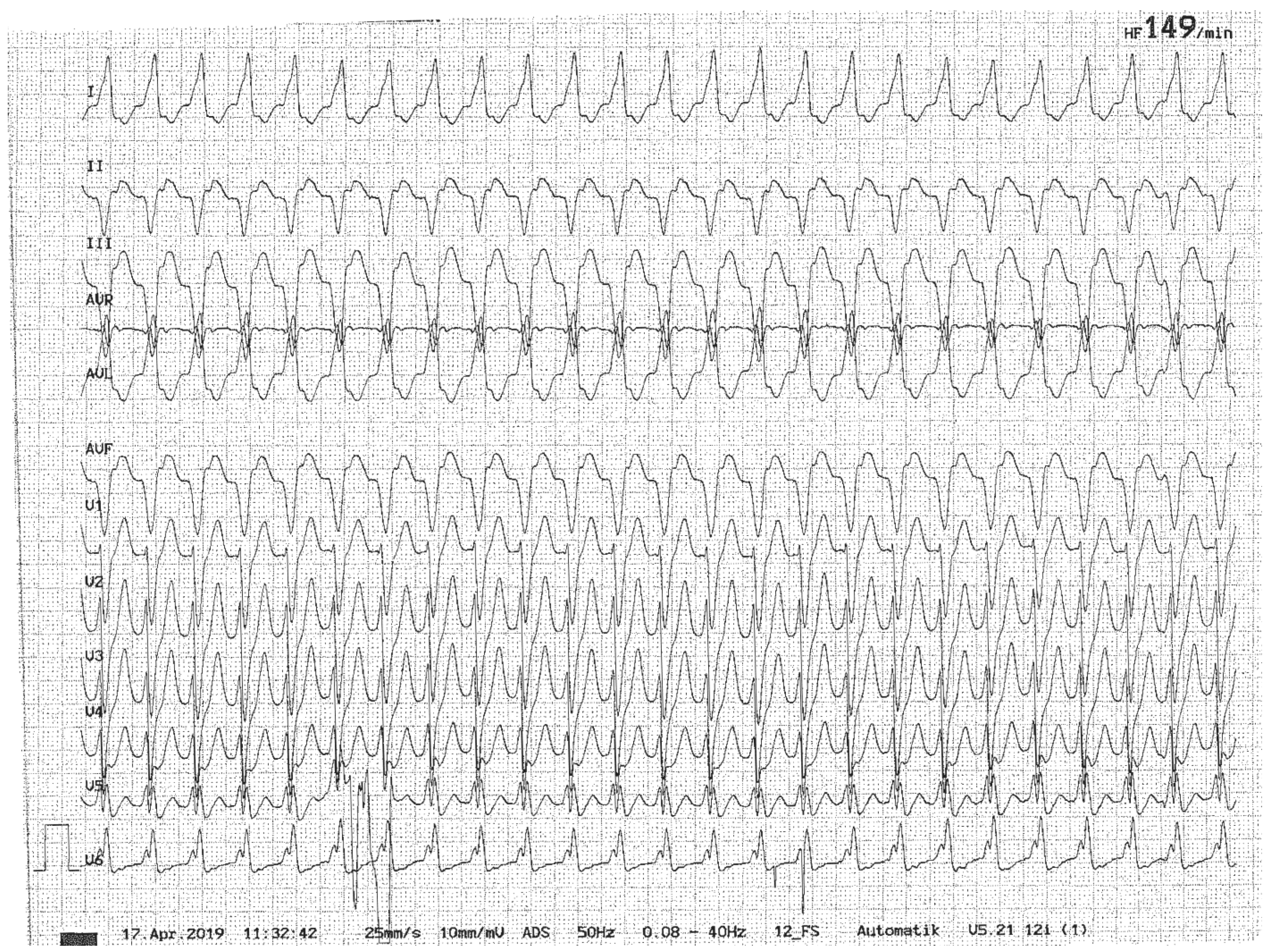
3.7. Cardiac Disorders
3.8. Rheologic Disorders and Respiratory Disorders
4. Non-Stroke Neurologic Conditions
4.1. Demyelinating Disease Including Multiple Sclerosis
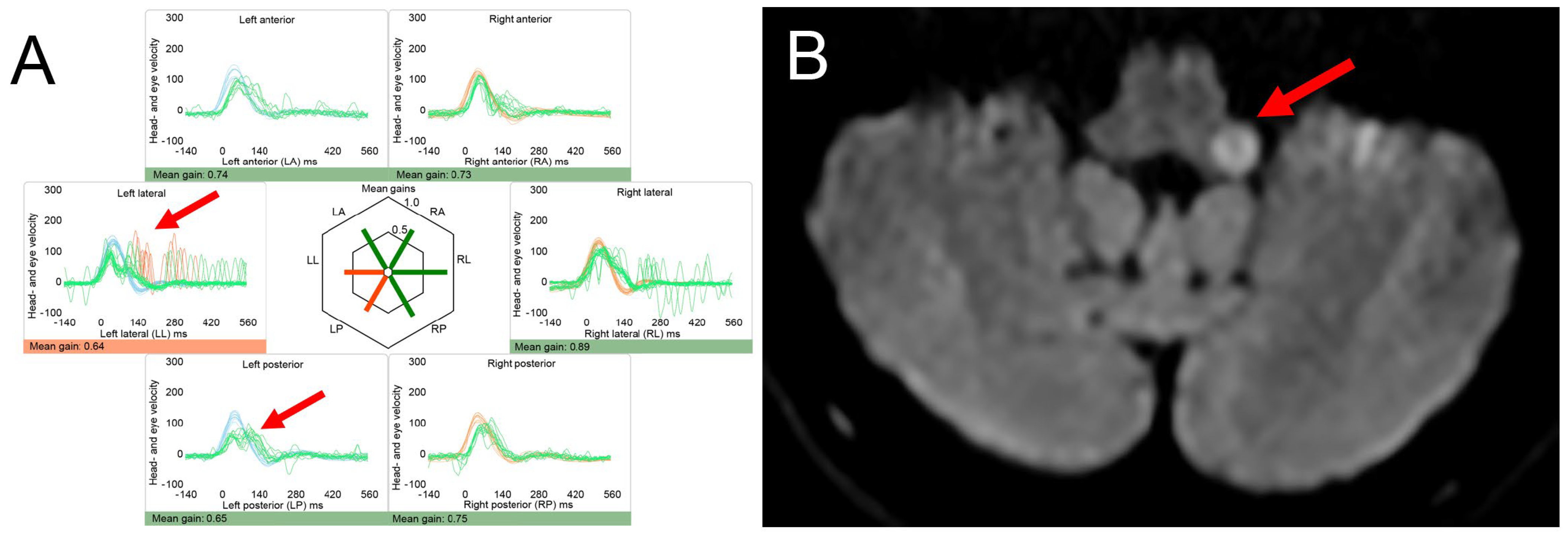
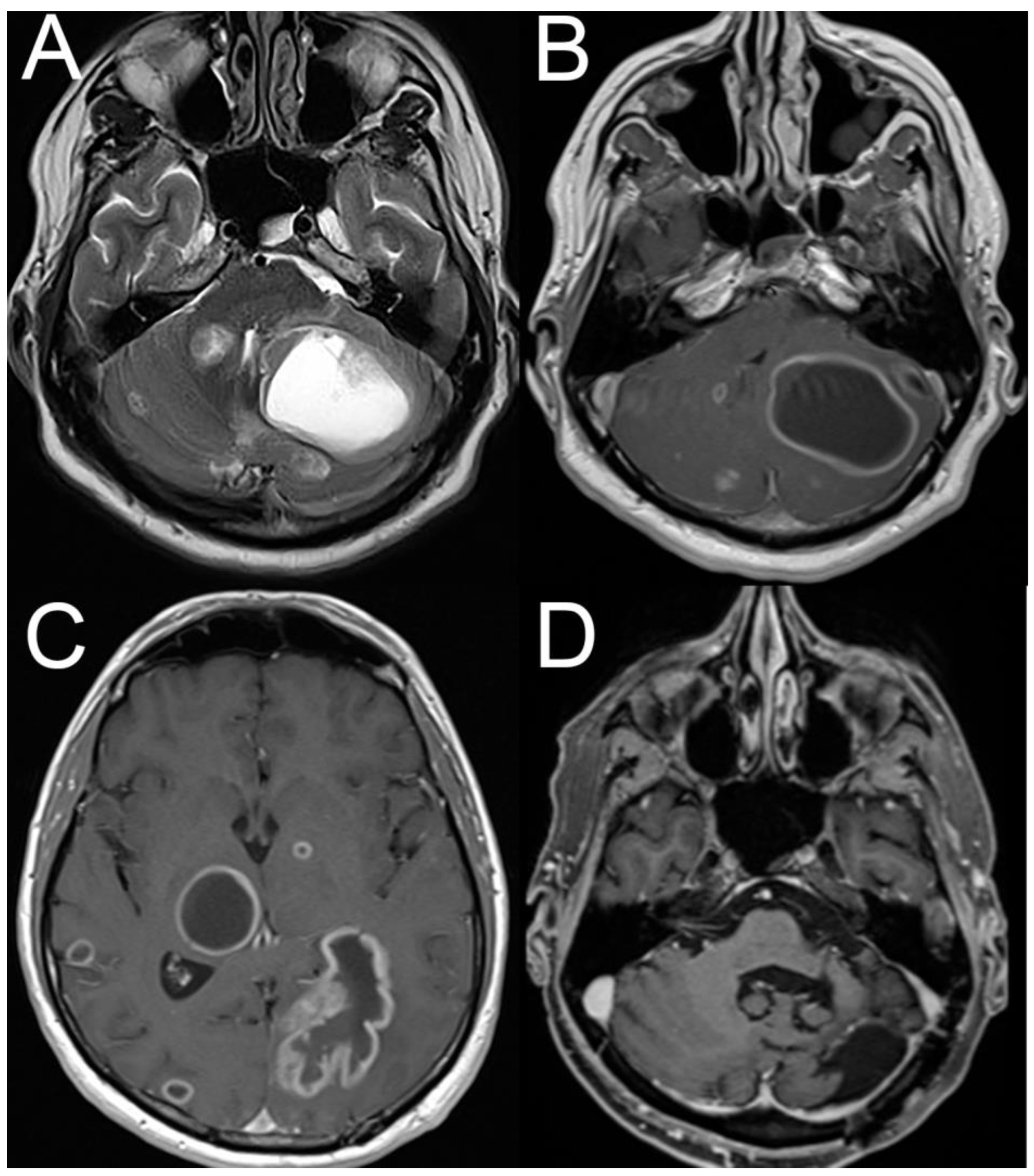
4.2. Posterior Fossa Tumors and (Other) Causes of Increased Intracranial Pressure
4.3. Vestibular Migraine
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Acute imbalance syndrome |
| AUVP | Acute unilateral vestibulopathy |
| AVS | Acute vestibular syndrome |
| BPPV | Benign paroxysmal positional vertigo |
| CNS | Central nervous system |
| DWI | Diffusion weighted imaging |
| ECG | Electrocardiogram |
| ED | Emergency department |
| EVS | Episodic vestibular syndrome |
| HINTS | Head-Impulse, Nystagmus, Test of Skew |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| OH | Orthostatic hypotension |
| VM | Vestibular migraine |
| WE | Wernicke encephalopathy |
References
- Newman-Toker, D.E.; Hsieh, Y.H.; Camargo, C.A., Jr.; Pelletier, A.J.; Butchy, G.T.; Edlow, J.A. Spectrum of dizziness visits to US emergency departments: Cross-sectional analysis from a nationally representative sample. Mayo Clin. Proc. 2008, 83, 765–775. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Cannon, L.M.; Stofferahn, M.E.; Rothman, R.E.; Hsieh, Y.H.; Zee, D.S. Imprecision in patient reports of dizziness symptom quality: A cross-sectional study conducted in an acute care setting. Mayo Clin. Proc. 2007, 82, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Goeldlin, M.; Gaschen, J.; Kammer, C.; Comolli, L.; Bernasconi, C.A.; Spiegel, R.; Bassetti, C.L.; Exadaktylos, A.K.; Lehmann, B.; Mantokoudis, G.; et al. Frequency, aetiology, and impact of vestibular symptoms in the emergency department: A neglected red flag. J. Neurol. 2019, 266, 3076–3086. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Edlow, J.A. TiTrATE: A Novel, Evidence-Based Approach to Diagnosing Acute Dizziness and Vertigo. Neurol. Clin. 2015, 33, 577–599. [Google Scholar] [CrossRef]
- Newman-Toker, D.E. Missed stroke in acute vertigo and dizziness: It is time for action, not debate. Ann. Neurol. 2016, 79, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Meurer, W.J.; Low, P.A.; Staab, J.P. Medical and Psychiatric Causes of Episodic Vestibular Symptoms. Neurol. Clin. 2015, 33, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, A.A.; Berkowitz, A.L.; Robinson, K.A.; Hsieh, Y.H.; Newman-Toker, D.E. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. Can. Med Assoc. J. 2011, 183, E571–E592. [Google Scholar] [CrossRef]
- Lee, S.U.; Tarnutzer, A.A. Usefulness of Nystagmus Patterns in Distinguishing Peripheral From Central Acute Vestibular Syndromes at the Bedside: A Critical Review. J. Clin. Neurol. 2025, 21, 161–172. [Google Scholar] [CrossRef]
- Kattah, J.C.; Talkad, A.V.; Wang, D.Z.; Hsieh, Y.H.; Newman-Toker, D.E. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009, 40, 3504–3510. [Google Scholar] [CrossRef]
- Edlow, J.A.; Carpenter, C.; Akhter, M.; Khoujah, D.; Marcolini, E.; Meurer, W.J.; Morrill, D.; Naples, J.G.; Ohle, R.; Omron, R.; et al. Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): Acute dizziness and vertigo in the emergency department. Acad. Emerg. Med. 2023, 30, 442–486. [Google Scholar] [CrossRef]
- Edlow, J.A.; Newman-Toker, D.E. Medical and Nonstroke Neurologic Causes of Acute, Continuous Vestibular Symptoms. Neurol. Clin. 2015, 33, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.; Edlow, J.A. Complete heart block complicating the head impulse test. Arch. Neurol. 2010, 67, 1272–1274. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Chung, E.J.; Yi, H.A.; Chung, I.S.; Lee, S.R.; Shin, J.Y. Infarction in the territory of anterior inferior cerebellar artery: Spectrum of audiovestibular loss. Stroke 2009, 40, 3745–3751. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chao, P.Z.; Lee, H.C. Sudden sensorineural hearing loss increases the risk of stroke: A 5-year follow-up study. Stroke 2008, 39, 2744–2748. [Google Scholar] [CrossRef]
- Bisdorff, A.; Von Brevern, M.; Lempert, T.; Newman-Toker, D.E. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J. Vestib. Res. 2009, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maddox, P.T.; Saunders, J.; Chandrasekhar, S.S. Sudden hearing loss from PDE-5 inhibitors: A possible cellular stress etiology. Laryngoscope 2009, 119, 1586–1589. [Google Scholar] [CrossRef]
- Seymour, J.F. Carbamazepine overdose. Features of 33 cases. Drug Saf. 1993, 8, 81–88. [Google Scholar] [CrossRef]
- Alekseeva, N.; McGee, J.; Kelley, R.E.; Maghzi, A.H.; Gonzalez-Toledo, E.; Minagar, A. Toxic-metabolic, nutritional, and medicinal-induced disorders of cerebellum. Neurol. Clin. 2014, 32, 901–911. [Google Scholar] [CrossRef]
- Arbusow, V.; Strupp, M.; Brandt, T. Amiodarone-induced severe prolonged head-positional vertigo and vomiting. Neurology 1998, 51, 917. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Gold, D.; Wang, Z.; Robinson, K.A.; Kattah, J.C.; Mantokoudis, G.; Saber Tehrani, A.S.; Zee, D.S.; Edlow, J.A.; Newman-Toker, D.E. Impact of Clinician Training Background and Stroke Location on Bedside Diagnostic Test Accuracy in the Acute Vestibular Syndrome—A Meta-Analysis. Ann. Neurol. 2023, 94, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kattah, J.C. Clinical Characteristics and Etiology of Bilateral Vestibular Loss in a Cohort from Central Illinois. Front. Neurol. 2018, 9, 46. [Google Scholar] [CrossRef]
- Fetter, M.; Haslwanter, T.; Bork, M.; Dichgans, J. New insights into positional alcohol nystagmus using three-dimensional eye-movement analysis. Ann. Neurol. 1999, 45, 216–223. [Google Scholar] [CrossRef]
- Romano, F.; Tarnutzer, A.A.; Straumann, D.; Ramat, S.; Bertolini, G. Gaze-evoked nystagmus induced by alcohol intoxication. J. Physiol. 2017, 595, 2161–2173. [Google Scholar] [CrossRef]
- Chiao, A.; Hughes, M.L.; Karimuddanahalli Premkumar, P.; Zoucha, K. The Effects of Substance Misuse on Auditory and Vestibular Function: A Systematic Review. Ear Hear. 2024, 45, 276–296. [Google Scholar] [CrossRef]
- Rivetti, S.; Romano, A.; Mastrangelo, S.; Attina, G.; Maurizi, P.; Ruggiero, A. Aminoglycosides-Related Ototoxicity: Mechanisms, Risk Factors, and Prevention in Pediatric Patients. Pharmaceuticals 2023, 16, 1353. [Google Scholar] [CrossRef]
- Lu, C.M.; James, S.H.; Lien, Y.H. Acute massive gentamicin intoxication in a patient with end-stage renal disease. Am. J. Kidney Dis. 1996, 28, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, A.A.; Bockisch, C.J.; Buffone, E.; Weiler, S.; Bachmann, L.M.; Weber, K.P. Disease-specific sparing of the anterior semicircular canals in bilateral vestibulopathy. Clin. Neurophysiol. 2016, 127, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Kulkantrakorn, K.; Chunhachatrachai, P.; Kulkantrakorn, W. Nitrous oxide abuse and associated neurological diseases. BMC Neurol. 2024, 24, 461. [Google Scholar] [CrossRef]
- Maixner, S.M.; Greden, J.F. Extended antidepressant maintenance and discontinuation syndromes. Depress. Anxiety 1998, 8 (Suppl. 1), 43–53. [Google Scholar] [CrossRef][Green Version]
- Henssler, J.; Heinz, A.; Brandt, L.; Bschor, T. Antidepressant Withdrawal and Rebound Phenomena. Dtsch. Arztebl. Int. 2019, 116, 355–361. [Google Scholar] [CrossRef]
- Fava, G.A.; Gatti, A.; Belaise, C.; Guidi, J.; Offidani, E. Withdrawal Symptoms after Selective Serotonin Reuptake Inhibitor Discontinuation: A Systematic Review. Psychother. Psychosom. 2015, 84, 72–81. [Google Scholar] [CrossRef]
- Staab, J.P. Psychiatric Considerations in the Management of Dizzy Patients. Adv. Otorhinolaryngol. 2019, 82, 170–179. [Google Scholar] [CrossRef]
- Kerber, K.A.; Meurer, W.J.; West, B.T.; Fendrick, A.M. Dizziness presentations in U.S. emergency departments, 1995-2004. Acad. Emerg. Med. 2008, 15, 744–750. [Google Scholar] [CrossRef]
- Sass, J.B.; Raichel, D. Human acute poisoning incidents associated with neonicotinoid pesticides in the U.S. Incident Data System (IDS) database from 2018-2022—Frequency and severity show public health risks, regulatory failures. Environ. Health 2024, 23, 102. [Google Scholar] [CrossRef]
- Nanagas, K.A.; Penfound, S.J.; Kao, L.W. Carbon Monoxide Toxicity. Emerg. Med. Clin. N. Am. 2022, 40, 283–312. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.; Weaver, L.K. Carbon monoxide poisoning mimicking arterial gas embolism in a commercial diver. Undersea Hyperb. Med. 2012, 39, 687–690. [Google Scholar]
- Carnovale, C.; Battini, V.; Mazhar, F.; Mosini, G.; Gringeri, M.; Vicenzi, A.; Clementi, E.; Radice, S. Are dizziness-related symptoms signals for suboptimal treatment of hypothyroidism? New insights from the FDA adverse event reporting system (FAERS) database. Eur. J. Clin. Pharmacol. 2020, 76, 733–734. [Google Scholar] [CrossRef]
- Dave, A.; Ludlow, J.; Malaty, J. Thyrotoxicosis: An under-recognised aetiology. BMJ Case Rep. 2015, 2015, bcr2014208119. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, H.F.; Tan, S.W.; Su, M.C.; Ng, K.W.; Jiang, C.F. Severely sustained vomiting as the main symptom in a man with thyrotoxicosis. J. Chin. Med. Assoc. 2003, 66, 311–314. [Google Scholar] [PubMed]
- Moaty, A.S.; Ewida, S.A.; Kabel Abd El Mageed, H. audio vestibular assessment in hypothyroidism. Menoufia Med. J. 2025, 38, 104–108. [Google Scholar]
- Wiggli, B.; Kapitza, S.; Ahlhelm, F.; Tarnutzer, A.A. Early recognition of thiamine deficiency: Ocular motor deficits in a patient with nutritional deprivation due to persistent antibiotic-related nausea. Infection 2020, 48, 137–140. [Google Scholar] [CrossRef]
- Ohmori, N.; Tushima, T.; Sekine, Y.; Sato, K.; Shibagaki, Y.; Ijuchi, S.; Akano, K. Gestational thyrotoxicosis with acute Wernicke encephalopathy: A case report. Endocr. J. 1999, 46, 787–793. [Google Scholar] [CrossRef]
- Currier, W.D. Dizziness related to hypoglycemia: The role of adrenal steroids and nutrition. Laryngoscope 1971, 81, 18–35. [Google Scholar] [CrossRef]
- Binder, C.; Bendtson, I. Endocrine emergencies. Hypoglycaemia. Baillieres Clin. Endocrinol. Metab. 1992, 6, 23–39. [Google Scholar] [CrossRef]
- Jaap, A.J.; Jones, G.C.; McCrimmon, R.J.; Deary, I.J.; Frier, B.M. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet. Med. 1998, 15, 398–401. [Google Scholar] [CrossRef]
- Rodriguez-Hurtado, D.; Camones-Huerta, J.; Nunez Mochizaki, C. A delayed diagnosis of hyperthyroidism in a patient with persistent vomiting in the presence of Chiari type 1 malformation. Diagnosis 2025, 12, 131–135. [Google Scholar] [CrossRef]
- Lok, U.; Hatipoglu, S.; Gulacti, U.; Arpaci, A.; Aktas, N.; Borta, T. The role of thyroid and parathyroid metabolism disorders in the etiology of sudden onset dizziness. Med. Sci. Monit. 2014, 20, 2689–2694. [Google Scholar] [CrossRef]
- Jahangiri, A.; Wagner, J.; Tran, M.T.; Miller, L.M.; Tom, M.W.; Kunwar, S.; Blevins, L., Jr.; Aghi, M.K. Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J. Neurosurg. 2013, 119, 1478–1483. [Google Scholar] [CrossRef]
- Arieff, A.I.; Guisado, R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976, 10, 104–116. [Google Scholar] [CrossRef]
- Bezzeccheri, A.; Di Giovanni, G.; Belli, M.; Mollace, R.; Barone, L.; Macrini, M.; Di Landro, A.; Muscoli, S. The Impact of Gitelman Syndrome on Cardiovascular Disease: From Physiopathology to Clinical Management. Rev. Cardiovasc. Med. 2022, 23, 289. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.B.; Kwak, Y.H.; Hwang, S.S.; Oh, W.S.; Bae, J.H. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: A retrospective cross-sectional study. J. Crit. Care 2013, 28, 1112.e7–1112.e13. [Google Scholar] [CrossRef]
- Farkas, J.D.; Long, B.; Koyfman, A.; Menson, K. BRASH Syndrome: Bradycardia, Renal Failure, AV Blockade, Shock, and Hyperkalemia. J. Emerg. Med. 2020, 59, 216–223. [Google Scholar] [CrossRef]
- Grubina, R.; Klocke, D.L. 47-year-old woman with dizziness, weakness, and confusion. Mayo Clin. Proc. 2011, 86, e1–e4. [Google Scholar] [CrossRef]
- Shah, J.; Fadah, K.; Lopes, J.M.; Abedin, M. Unveiling the Link: Hypocalcemia-Induced Unstable Sustained Ventricular Tachycardia in Nonischemic Cardiomyopathy. Cardiol. Res. 2024, 15, 314–317. [Google Scholar] [CrossRef]
- Vosnakidis, A.; Polymeropoulos, K.; Zarogoulidis, P.; Zarifis, I. Atrioventricular nodal dysfunction secondary to hyperparathyroidism. J. Thorac. Dis. 2013, 5, E90–E92. [Google Scholar] [CrossRef]
- Ray, S.; Park, K.W. Movement Disorders and Other Neurologic Impairment Associated With Hypomagnesemia: A Systematic Review. Neurol. Clin. Pract. 2023, 13, e200202. [Google Scholar] [CrossRef]
- Bartolini, E.; Sodini, R.; Nardini, C. Acute-Onset Vertical Nystagmus and Limb Tremors in Chronic Renal Failure. J. Emerg. Med. 2019, 56, e13–e15. [Google Scholar] [CrossRef]
- Viola, P.; Marcelli, V.; Sculco, D.; Pisani, D.; Caglioti, A.; Ricciardiello, F.; Scarpa, A.; Astorina, A.; Tortoriello, G.; Gallelli, L.; et al. Vestibular Disorders after Kidney Transplantation: Focus on the Pathophysiological Mechanisms Underlying the Vertical Nystagmus Associated with Tacrolimus-Related Hypomagnesamia. Int. J. Environ. Res. Public Health 2022, 19, 2260. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, J.D.; Kim, M.J.; Choi, B.Y.; Choi, Y.R.; Cho, B.M.; Kim, J.S.; Choi, K.D. Vertigo and nystagmus in orthostatic hypotension. Eur. J. Neurol. 2015, 22, 648–655. [Google Scholar] [CrossRef]
- Whitman, G.T.; Yates, B.J. Orthostatic intolerance in acute vestibular neuritis. Mayo Clin. Proc. 2015, 90, 308–309. [Google Scholar] [CrossRef]
- Singh, B.; Arora, S. Acute presentation of dizziness in vitamin B12 deficient old patient of cardiac disease: A case report. Clin. Chim. Acta 2010, 411, 2104–2106. [Google Scholar] [CrossRef]
- Pandey, S.; Holla, V.V.; Rizvi, I.; Qavi, A.; Shukla, R. Can vitamin B12 deficiency manifest with acute posterolateral or posterior cord syndrome? Spinal Cord. Ser. Cases 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Kattah, J.C.; Dhanani, S.S.; Pula, J.H.; Mantokoudis, G.; Saber Tehrani, A.S.; Newman-Toker, D.E. Vestibular signs of thiamine deficiency during the early phase of suspected Wernicke encephalopathy. Neurol. Clin. Pract. 2013, 3, 460–468. [Google Scholar] [CrossRef]
- Kattah, J.C. The Spectrum of Vestibular and Ocular Motor Abnormalities in Thiamine Deficiency. Curr. Neurol. Neurosci. Rep. 2017, 17, 40. [Google Scholar] [CrossRef]
- Victor, M.; Adams, R.D.; Collins, G.H. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp. Neurol. Ser. 1971, 7, 1–206. [Google Scholar]
- Kattah, J.C.; McClelland, C.; Zee, D.S. Vertical nystagmus in Wernicke’s encephalopathy: Pathogenesis and role of central processing of information from the otoliths. J. Neurol. 2019, 266, 139–145. [Google Scholar] [CrossRef]
- Sechi, G.; Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet. Neurol. 2007, 6, 442–455. [Google Scholar] [CrossRef]
- Harper, C.G.; Giles, M.; Finlay-Jones, R. Clinical signs in the Wernicke-Korsakoff complex: A retrospective analysis of 131 cases diagnosed at necropsy. J. Neurol. Neurosurg. Psychiatry 1986, 49, 341–345. [Google Scholar] [CrossRef]
- Galvin, R.; Brathen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418. [Google Scholar] [CrossRef]
- Tanphaichitr, V. Thiamin. In Modern Nutrition in Health and Disease, 9th ed.; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1999; pp. 381–389. [Google Scholar]
- Newman-Toker, D.E.; Dy, F.J.; Stanton, V.A.; Zee, D.S.; Calkins, H.; Robinson, K.A. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J. Gen. Intern. Med. 2008, 23, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Cheung, C.S.; Mak, P.S.; Manley, K.V.; Lam, J.M.; Tsang, A.Y.; Chan, H.M.; Rainer, T.H.; Graham, C.A. Predictors of important neurological causes of dizziness among patients presenting to the emergency department. Emerg. Med. J. 2010, 27, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hernandez, R.; Macias-Rodriguez, D.H.; Martin-Sanchez, V.; Cordero-Civantos, C.; Santa Cruz-Ruiz, S.; Batuecas-Caletrio, A. Vertigo as the first sign of chronic myeloid leukemia: A case report and literature review. Case Rep. Otolaryngol. 2013, 2013, 505636. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; McMurray, E.; Robinson, O. Stroke as the presenting feature of new onset diabetes in a young man. BMJ Case Rep. 2014, 2014, bcr2014204251. [Google Scholar] [CrossRef]
- Nam, H.W.; Yoo, D.; Lee, S.U.; Choi, J.Y.; Yu, S.; Kim, J.S. Pearls & Oysters: Labyrinthine Infarction Mimicking Vestibular Neuritis. Neurology 2021, 97, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Gempp, E.; Louge, P.; Soulier, B.; Alla, P. Cerebellar infarction presenting as inner ear decompression sickness following scuba diving: A case report. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 313–315. [Google Scholar] [CrossRef]
- Klingmann, C. Inner ear decompression sickness in compressed-air diving. Undersea Hyperb. Med. 2012, 39, 589–594. [Google Scholar]
- Kammeyer, R.; Devnani, R.; Mehta, R. Cerebral fat embolism syndrome mimicking thrombotic thrombocytopenic purpura in a patient with hemoglobin SC disease. Am. J. Hematol. 2016, 91, 539–542. [Google Scholar] [CrossRef]
- Ljunggren, M.; Persson, J.; Salzer, J. Dizziness and the Acute Vestibular Syndrome at the Emergency Department: A Population-Based Descriptive Study. Eur. Neurol. 2018, 79, 5–12. [Google Scholar] [CrossRef]
- Gatterer, H.; Villafuerte, F.C.; Ulrich, S.; Bhandari, S.S.; Keyes, L.E.; Burtscher, M. Altitude illnesses. Nat. Rev. Dis. Primers 2024, 10, 43. [Google Scholar] [CrossRef]
- Lee, E.-J. Oculomotor manifestations in inflammatory central nervous system demyelinating diseases: A narrative review. Res. Vestib. Sci. 2025, 24, 27–36. [Google Scholar] [CrossRef]
- Lee, S.U.; Kim, H.J.; Choi, J.H.; Choi, J.Y.; Kim, J.S. Comparison of Ocular Motor Findings Between Neuromyelitis Optica Spectrum Disorder and Multiple Sclerosis Involving the Brainstem and Cerebellum. Cerebellum 2019, 18, 511–518. [Google Scholar] [CrossRef]
- Olbert, E.; Brunner, C.; Alhani, N.; Nasel, C.; Struhal, W. MOG antibody associated disease (MOGAD) presenting with extensive brain stem encephalitis: A case report. eNeurologicalSci 2022, 29, 100432. [Google Scholar] [CrossRef]
- Patti, F.; Vila, C. Symptoms, Prevalence and Impact of Multiple Sclerosis in Younger Patients: A Multinational Survey. Neuroepidemiology 2014, 42, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Swingler, R.J.; Compston, D.A. The morbidity of multiple sclerosis. Q. J. Med. 1992, 83, 325–337. [Google Scholar] [PubMed]
- Magnano, I.; Pes, G.M.; Pilurzi, G.; Cabboi, M.P.; Ginatempo, F.; Giaconi, E.; Tolu, E.; Achene, A.; Salis, A.; Rothwell, J.C.; et al. Exploring brainstem function in multiple sclerosis by combining brainstem reflexes, evoked potentials, clinical and MRI investigations. Clin. Neurophysiol. 2014, 125, 2286–2296. [Google Scholar] [CrossRef]
- Weier, K.; Penner, I.K.; Magon, S.; Amann, M.; Naegelin, Y.; Andelova, M.; Derfuss, T.; Stippich, C.; Radue, E.W.; Kappos, L.; et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS ONE 2014, 9, e86916. [Google Scholar] [CrossRef]
- Martinez, C.; Wang, Z.; Zalazar, G.; Carmona, S.; Kattah, J.; Tarnutzer, A.A. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of a Graded Gait and Truncal Instability Rating in Acutely Dizzy and Ataxic Patients. Cerebellum 2024, 23, 2244–2256. [Google Scholar] [CrossRef]
- Frohman, E.M. Multiple sclerosis. Med. Clin. N. Am. 2003, 87, 867–897. [Google Scholar] [CrossRef]
- Pula, J.H.; Newman-Toker, D.E.; Kattah, J.C. Multiple sclerosis as a cause of the acute vestibular syndrome. J. Neurol. 2013, 260, 1649–1654. [Google Scholar] [CrossRef]
- Anagnostou, E.; Mandellos, D.; Limbitaki, G.; Papadimitriou, A.; Anastasopoulos, D. Positional nystagmus and vertigo due to a solitary brachium conjunctivum plaque. J. Neurol. Neurosurg. Psychiatry 2006, 77, 790–792. [Google Scholar] [CrossRef]
- Anagnostou, E.; Varaki, K.; Anastasopoulos, D. A minute demyelinating lesion causing acute positional vertigo. J. Neurol. Sci. 2008, 266, 187–189. [Google Scholar] [CrossRef]
- Lemos, J.; Strupp, M. Central positional nystagmus: An update. J. Neurol. 2022, 269, 1851–1860. [Google Scholar] [CrossRef]
- Serra, A.; Chisari, C.G.; Matta, M. Eye Movement Abnormalities in Multiple Sclerosis: Pathogenesis, Modeling, and Treatment. Front. Neurol. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Zhang, H.; Dewey, R.B.; Hawker, K.S.; Racke, M.K.; Frohman, T.C. Vertigo in MS: Utility of positional and particle repositioning maneuvers. Neurology 2000, 55, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Antonio, L.A.; Garcia Castanon, I.; Aguilar-Amat Prior, M.J.; Puertas, I.; Gonzalez Suarez, I.; Oreja Guevara, C. Non-inflammatory causes of emergency consultation in patients with multiple sclerosis. Neurologia 2021, 36, 403–411. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Huang, H. Clinical characteristics and prognostic factors of adult brainstem gliomas: A retrospective analysis of histologically-proven 40 cases. Medicine 2024, 103, e37910. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.H. Vestibular Schwannoma Presenting as Acute Vertigo Mimicking Vestibular Neuritis. Case Rep. Neurol. 2022, 14, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Avalos, L.N.; Morshed, R.A.; Goldschmidt, E. Hemorrhagic vestibular schwannoma: A case example of vestibular apoplexy syndrome. Illustrative case. J. Neurosurg. Case Lessons 2022, 3, 1–4. [Google Scholar] [CrossRef]
- Kapitza, S.; Pangalu, A.; Horstmann, G.A.; van Eck, A.T.; Regli, L.; Tarnutzer, A.A. Acute necrosis after Gamma Knife surgery in vestibular schwannoma leading to multiple cranial nerve palsies. J. Clin. Neurosci. 2016, 30, 141–142. [Google Scholar] [CrossRef]
- Fadul, C.; Misulis, K.E.; Wiley, R.G. Cerebellar metastases: Diagnostic and management considerations. J. Clin. Oncol. 1987, 5, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, M.; Lemos, J.; Lee, S.U.; Choi, J.Y.; Tarnutzer, A.A. Transient Response to Liberation Maneuvers in Central Positional Nystagmus Due to Cerebral Metastases Mimicking Benign Paroxysmal Positional Vertigo- A Case Report. Cerebellum 2025, 24, 96. [Google Scholar] [CrossRef]
- Harriott, A.M.; Karakaya, F.; Ayata, C. Headache after ischemic stroke: A systematic review and meta-analysis. Neurology 2020, 94, e75–e86. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Sharma, P.; Robinson, K.A.; Arnan, M.; Tsui, M.; Ladha, K.; Newman-Toker, D.E. Clinical characteristics of symptomatic vertebral artery dissection: A systematic review. Neurologist 2012, 18, 245–254. [Google Scholar] [CrossRef]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Kheradmand, A.; et al. Vestibular migraine: Diagnostic criteria1. J. Vestib. Res. 2022, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Sohn, S.I.; Lee, H. Cerebral venous thrombosis mimicking acute unilateral vestibulopathy. Neurol. Sci. 2008, 29, 41–43. [Google Scholar] [CrossRef]
- Jones, J.S.; Nevai, J.; Freeman, M.P.; McNinch, D.E. Emergency department presentation of idiopathic intracranial hypertension. Am. J. Emerg. Med. 1999, 17, 517–521. [Google Scholar] [CrossRef]
- Fernando, E.Z.; Jamora, R.D.G.; Torio, E.F.; Mariano, M.M.; Cuanang, J.R.; de Guzman, V.E. Acute Subdural Hemorrhage as the Initial Presentation of Intracranial Hypotension Following Cervical Chiropractic Manipulation: A Case Report and Systematic Review. Neurohospitalist 2022, 12, 57–62. [Google Scholar] [CrossRef]
- Couch, J.R. Spontaneous intracranial hypotension: The syndrome and its complications. Curr. Treat. Options Neurol. 2008, 10, 3–11. [Google Scholar] [CrossRef]
- Rocha, M.F.; Sacks, B.; Al-Lamki, A.; Koohi, N.; Kaski, D. Acute vestibular migraine: A ghost diagnosis in patients with acute vertigo. J. Neurol. 2023, 270, 6155–6158. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, A.A.; Koohi, N.; Lee, S.U.; Kaski, D. Diagnostic Errors in the Acutely Dizzy Patient-Lessons Learned. Brain Sci. 2025, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Calic, Z.; Nham, B.; Taylor, R.L.; Young, A.S.; Bradshaw, A.P.; McGarvie, L.M.; Colebatch, J.G.; Cordato, D.; Cappelen-Smith, C.; Welgampola, M.S. Vestibular migraine presenting with acute peripheral vestibulopathy: Clinical, oculographic and vestibular test profiles. Cephalalgia Rep. 2020, 3, 2515816320958175. [Google Scholar] [CrossRef]
- Young, A.S.; Nham, B.; Bradshaw, A.P.; Calic, Z.; Pogson, J.M.; D’Souza, M.; Halmagyi, G.M.; Welgampola, M.S. Clinical, oculographic, and vestibular test characteristics of vestibular migraine. Cephalalgia 2021, 41, 1039–1052. [Google Scholar] [CrossRef]
- Klarendic, M.; Joffily, L.; Rodrigues, F.A.; Gomes, T.A.; Edlow, J.; Koohi, N.; Kaski, D. The dizzy patient: Duration from symptom onset to specialist review. J. Neurol. 2024, 271, 7024–7025. [Google Scholar] [CrossRef]
- Sharif, S.; Kotwal, S.; Edlow, J.A. Differentiating Vestibular Migraine and Posterior Circulation Transient Ischemic Attack in the Emergency Department: An Expert Practice Review. J. Emerg. Med. 2025, 68, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A.; Bellolio, F. Recognizing Posterior Circulation Transient Ischemic Attacks Presenting as Episodic Isolated Dizziness. Ann. Emerg. Med. 2024, 84, 428–438. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-U.; Edlow, J.A.; Tarnutzer, A.A. Acute Vertigo, Dizziness and Imbalance in the Emergency Department—Beyond Stroke and Acute Unilateral Vestibulopathy—A Narrative Review. Brain Sci. 2025, 15, 995. https://doi.org/10.3390/brainsci15090995
Lee S-U, Edlow JA, Tarnutzer AA. Acute Vertigo, Dizziness and Imbalance in the Emergency Department—Beyond Stroke and Acute Unilateral Vestibulopathy—A Narrative Review. Brain Sciences. 2025; 15(9):995. https://doi.org/10.3390/brainsci15090995
Chicago/Turabian StyleLee, Sun-Uk, Jonathan A. Edlow, and Alexander A. Tarnutzer. 2025. "Acute Vertigo, Dizziness and Imbalance in the Emergency Department—Beyond Stroke and Acute Unilateral Vestibulopathy—A Narrative Review" Brain Sciences 15, no. 9: 995. https://doi.org/10.3390/brainsci15090995
APA StyleLee, S.-U., Edlow, J. A., & Tarnutzer, A. A. (2025). Acute Vertigo, Dizziness and Imbalance in the Emergency Department—Beyond Stroke and Acute Unilateral Vestibulopathy—A Narrative Review. Brain Sciences, 15(9), 995. https://doi.org/10.3390/brainsci15090995







