Immunological Targets in Generalized Myasthenia Gravis Treatment: Where Are We Going Now?
Abstract
1. Introduction
2. Classification of gMG
2.1. EOMG
2.2. TAMG
2.3. LOMG
2.4. MuSK-MG
2.5. LRP4-MG
2.6. Seronegative MG
2.7. Similarities and Differences Among gMG Subtypes
2.7.1. Immunoglobulin Structure
2.7.2. Antibody Classes
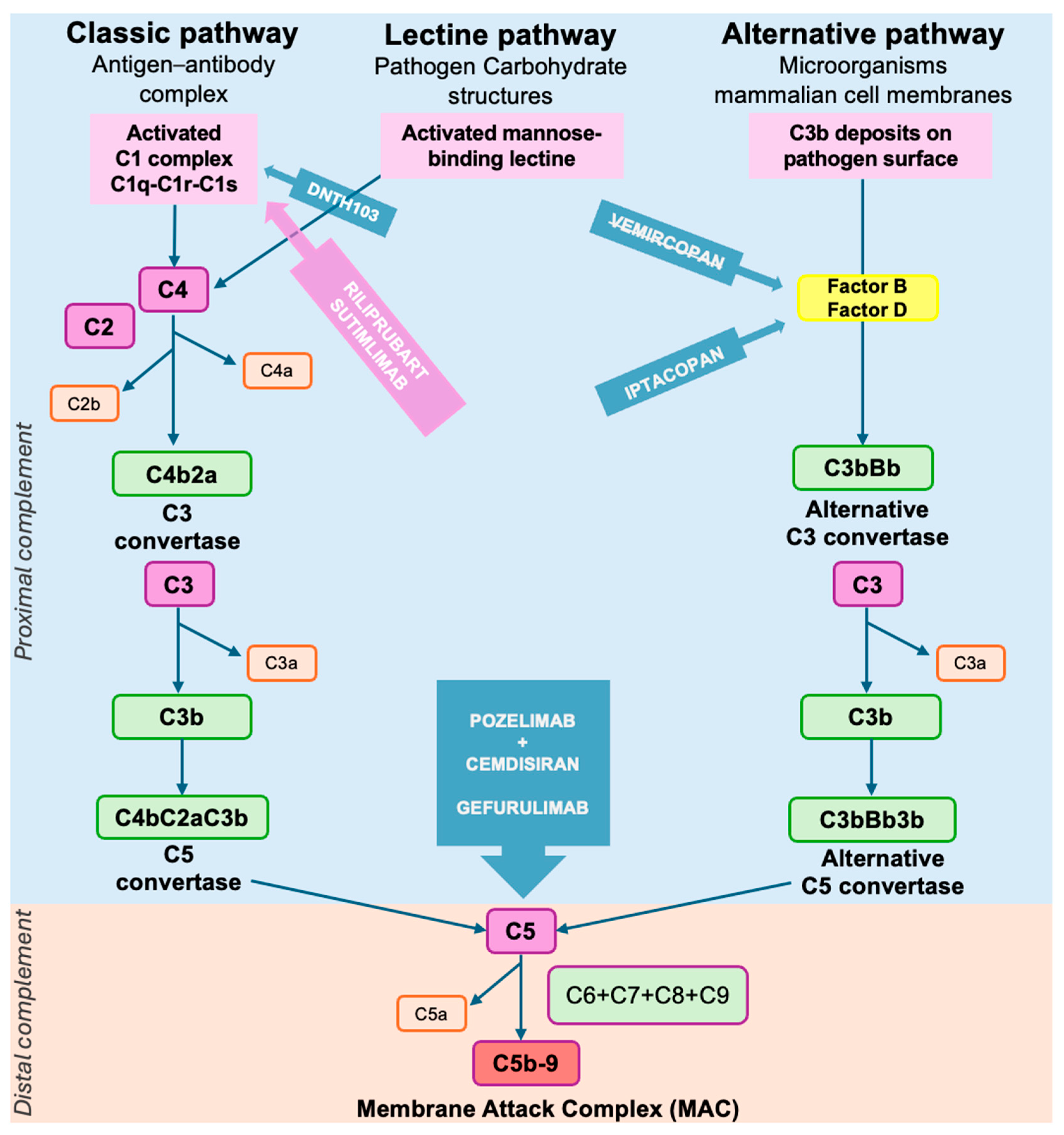
2.7.3. Complement Cascade Activation
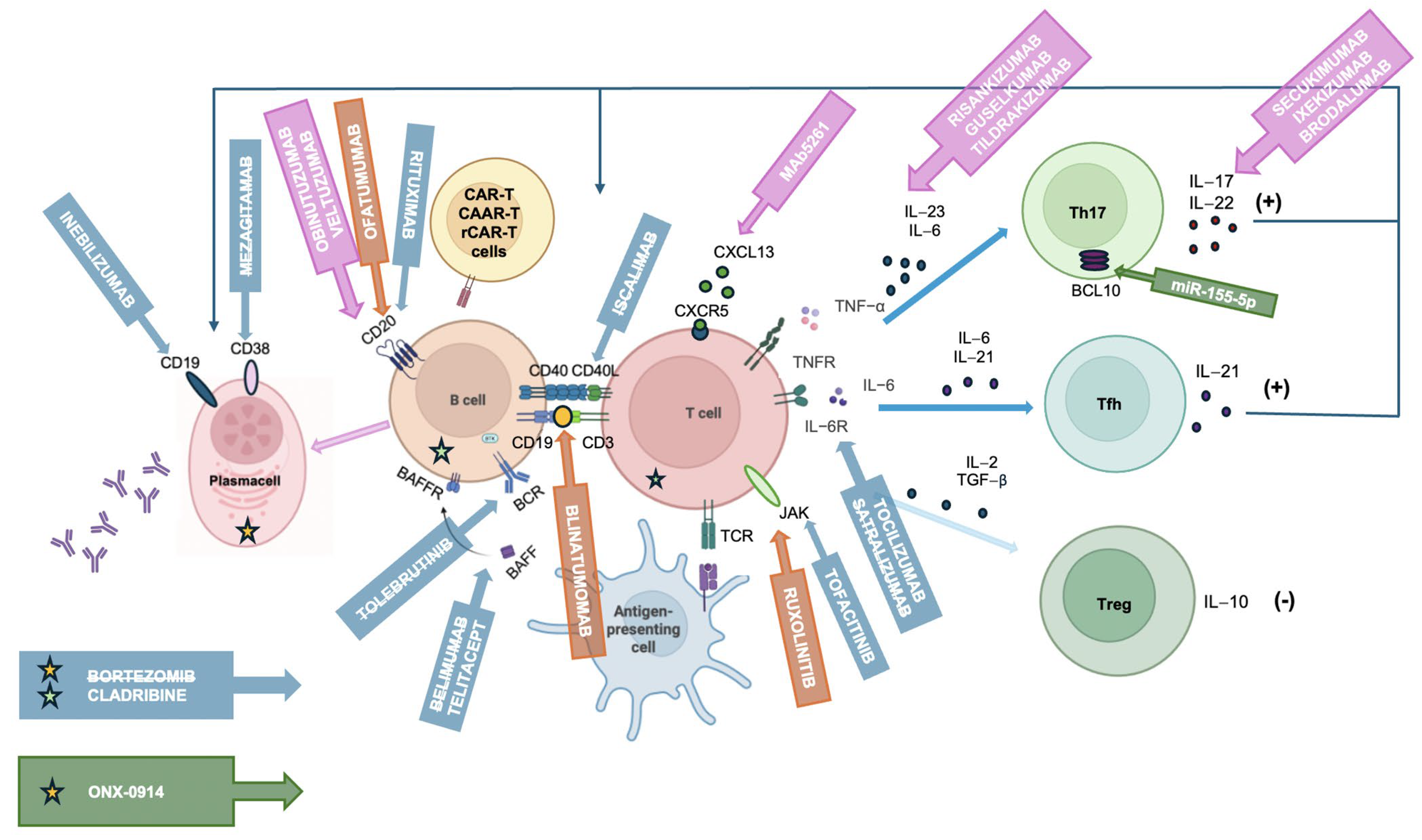
3. Biological Targets
3.1. T Cell- and Cytokine-Targeted Therapies
3.2. B Cell-Targeted Therapies
3.3. Other B and T Cell Depletion Therapies
3.4. Gene Therapies
3.5. Anti-FcRn Drugs
3.6. Complement Inhibitors
| Target Group | Drug | Mechanism/ Target | Subtype of MG | Clinical Trial Status/ Approval | Key Highlights |
|---|---|---|---|---|---|
| T cell- and cytokine-targeted therapies | Tofacitinib | JAK1/3 | AChR+ MG | NCT04431895 [99] ongoing | Pilot study: no data available. |
| Ruxolitinib | JAK1/2 | MuSK+ MG | - | Case report: MuSK-MG clinical remission [100]. | |
| Etanercept | TNF-α | AChR+ MG | - | Pilot study: mixed results depending on cytokine profile [101]. | |
| Satralizumab | IL-6R | AChR+ MG MuSK+ MG LRP4+ MG | NCT04963270 [111] terminated | Phase III trial: Satralizumab was well tolerated and resulted in small improvements in patient-reported and clinician-reported outcomes compared with placebo at week 24 in AChR+ MG patients [112]. | |
| Tocilizumab | IL-6R | AChR+ MG | NCT05067348 [115] ongoing | Case series: benefit in refractory/VLOMG [113,114]. Phase II trial: ongoing. | |
| miR-155-5p | BCL10 | - | Preclinical evidence: inhibits Treg activation, immunosuppressive capacity [116]. | ||
| B cell-targeted therapies | Rituximab | CD20 | AChR+ MG MuSK+ MG | NCT05868837 [126] ongoing NCT06342544 [127] ongoing | Highly effective in MuSK+ [117,118]. Mixed results in AChR+ [118,119,120,121,122,123,124]: Rituximab could be more effective if administered at disease onset [119]. Currently, two phase III trials are ongoing [126,127]. |
| Inebilizumab | CD19 | AChR+ MG MuSK+ MG | NCT04524273 [128] ongoing | Phase III trial: effective in both AChR+ and MuSK+, stronger data in AChR+ cohort [129]. | |
| Ofatumumab | CD20 | AChR+ MG | - | Pilot study: improvement in clinical scales at 1 and 3 months [130]. | |
| Mezagitamab (TAK-079) | CD38 | AChR+ MG MuSK+ MG | NCT04159805 [131] completed | Phase II trial: negative results posted [131]. | |
| Iscalimab | CD40 | AChR+ MG MuSK+ MG | NCT02565576 [131] completed | Phase II trial: primary endpoint not met [132]. | |
| Belimumab | BAFF | AChR+ MG MuSK+ MG | NCT01480596 [140] completed | Phase II trial: any significant difference in clinical scores [141]. | |
| Telitacicept | BAFF/APRIL | AChR+ MG MuSK+ MG | NCT06456580 [147] ongoing | Phase II and III in China: positive results [145,146]. Global phase III: ongoing [147]. | |
| Tolebrutinib | BTK | AChR+ MG MuSK+ MG Seronegative MG | NCT05132569 [148] terminated | Phase III trial: terminated early by the sponsor for strategic reasons [149]. | |
| Remibrutinib | BTK | AChR+ MG MuSK+ MG Seronegative MG | NCT06744920 [155] | Phase III trial: ongoing | |
| Plasma cell-directed therapies | Bortezomib | Proteasome | AChR+ MG | NCT02102594 [138] terminated | Phase II trial: terminated early due to recruitment difficulties [137,138]. |
| ONX-0914 | Immunoproteasome | - | Preclinical evidence: reduced autoantibody affinity and the percentage of Th17 cells; inhibited the secretion of IL-17 [139]. | ||
| Other depletion/immune reset | Cladribine | Purine analog, lymphocyte depletion and immune reset | AChR+ MG MuSK+ MG LRP4+ MG | NCT06463587 [156] ongoing | Pilot study: clinical improvement [154]. Phase III trial: ongoing [156]. |
| HSCT | Immune ablation/reconstitution | AChR+ MG | - | Case series: long-term remission; high toxicity [169]. | |
| Cellular therapies | CAR-T | CD19 | AChR+ MG | NCT06193889 [165] ongoing NCT06359041 [166] ongoing | Case report in MG [164]. Phase II and Phase I/II: ongoing. |
| CAAR-T (MuSK) | MuSK auto-reactive B cells | MuSK+ MG | NCT05451212 [160] ongoing | Preclinical evidence, phase I ongoing [160,161]. | |
| rCAR-T (Descartes-08) | BCMA | AChR+ MG MuSK+ MG Seronegative MG | NCT04146051 [163] ongoing | Phase I/II trial: safe and sustained clinical benefit [162]. Phase IIb: ongoing [163]. | |
| Bispecific antibodies | Blinatumomab (CD19 × CD3) | T cell engager | AChR+ MG | - | Two cases of refractory MG: good response [168]. |
| FcRn antagonists | Efgartigimod | FcRn | AChR+ MG MuSK+ MG Seronegative MG | Approved | In-label for AChR+ MG [10]. |
| Rozanolixizumab | FcRn | AChR+ MG MuSK+ MG | Approved | In-label for AChR+ MG and MuSK+ MG [24]. | |
| Batoclimab | FcRn | AChR+ MG MuSK+ MG | NCT05403541 [181] ongoing | Phase III trial: significant clinical improvement [183]. | |
| Nipocalimab | FcRn | AChR+ MG MuSK+ MG | NCT04951622 [182] ongoing | Phase III trial: significant clinical improvement [184]. | |
| Complement inhibitors | Eculizumab | C5 | AChR+ MG | Approved | In-label for AChR+ gMG [9]. |
| Ravulizumab | C5 | AChR+ MG | Approved | In-label for AChR+ gMG [23]. | |
| Zilucoplan | C5 | AChR+ MG | Approved | In-label for AChR+ gMG [25]. | |
| DNTH103 | C1s | AChR+ MG | NCT06282159 [193] ongoing | Phase II trial: ongoing. | |
| Iptacopan | Factor B | AChR+ MG | NCT06517758 [197] ongoing | Phase III trial: ongoing. | |
| Vemircopan | Factor D | AChR+ MG | NCT05218096 [195] terminated | Phase III trial: terminated early by the sponsor. | |
| Cemdisiran + Pozelimab | C5 | AChR+ MG | NCT05070858 [198] ongoing | Phase III trial: ongoing. | |
| Gefurulimab | C5 | AChR+ MG | NCT05556096 [199] ongoing | Phase III trial: ongoing. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCallion, J.; Borsi, A.; Noel, W.; Lee, J.; Karmous, W.; Sattler, S.; Boggia, G.; Hardy, E.; Mitchell, C.; Mitchell, S.; et al. Systematic review of the patient burden of generalised myasthenia gravis in Europe, the Middle East, and Africa. BMC Neurol. 2024, 24, 61. [Google Scholar] [CrossRef]
- Grob, D.; Brunner, N.; Namba, T.; Pagala, M. Lifetime course of myasthenia gravis. Muscle Nerve 2008, 37, 141–149. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M. International consensus guidance for management of myasthenia gravis. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef]
- Wolfe, G.; Herbelin, L.; Nations, S.; Foster, B.; Bryan, W.; Barohn, R. Myasthenia gravis activities of daily living profile. Neurology 1999, 52, 1487. [Google Scholar] [CrossRef] [PubMed]
- Barohn, R.J.; McINTIRE, D.; Herbelin, L.; Wolfe, G.I.; Nations, S.; Bryan, W.W. Reliability Testing of the Quantitative Myasthenia Gravis Scorea. Ann. N. Y. Acad. Sci. 1998, 841, 769–772. [Google Scholar] [CrossRef]
- Burns, T.M.; Conaway, M.; Sanders, D.B. The MG Composite: A valid and reliable outcome measure for myasthenia gravis. Neurology 2010, 74, 1434–1440. [Google Scholar] [CrossRef]
- Burns, T.M.; Sadjadi, R.; Utsugisawa, K.; Gwathmey, K.G.; Joshi, A.; Jones, S.; Bril, V.; Barnett, C.; Guptill, J.T.; Sanders, D.B.; et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve 2016, 54, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Bril, V. Pharmacotherapy of Generalized Myasthenia Gravis with Special Emphasis on Newer Biologicals. Drugs 2022, 82, 865–887. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986. [Google Scholar] [CrossRef]
- Howard, J.F.; Bril, V.; Vu, T.; Karam, C.; Peric, S.; Margania, T.; Murai, H.; Bilinska, M.; Shakarishvili, R.; Smilowski, M.; et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): A multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021, 20, 526–536. [Google Scholar] [CrossRef]
- Reyes-Leiva, D.; Carbayo, Á.; Vesperinas-Castro, A.; Rojas-García, R.; Querol, L.; Turon-Sans, J.; Pla-Junca, F.; Olivé, M.; Gallardo, E.; Pujades-Rodriguez, M.; et al. Persistent symptoms, exacerbations and drug side effects despite treatment in myasthenia gravis. Eur. J. Neurol. 2025, 32, e16463. [Google Scholar] [CrossRef]
- Binks, S.N.M.; Morse, I.M.; Ashraghi, M.; Vincent, A.; Waters, P.; Leite, M.I. Myasthenia gravis in 2025: Five new things and four hopes for the future. J. Neurol. 2025, 272, 226. [Google Scholar] [CrossRef]
- Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002, 2, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis—Autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.-C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019, 18, 259–268. [Google Scholar] [CrossRef]
- Zisimopoulou, P.; Evangelakou, P.; Tzartos, J.; Lazaridis, K.; Zouvelou, V.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 2014, 52, 139–145. [Google Scholar] [CrossRef]
- Evoli, A.; Palace, J.; Spagni, G.; Cheli, M.; Ruiter, A.; Verschuuren, J.; Maggi, L.; Niks, E.; Ramdas, S.; Natera-de Benito, D.; et al. 275th ENMC international workshop: Seronegative myasthenia gravis: An update paradigm for diagnosis and management, 9–11 February 2024, Hoofddorp, the Netherlands. Neuromuscul. Disord. 2024, 44, 104468. [Google Scholar] [CrossRef]
- Murai, H.; Utsugisawa, K.; Motomura, M.; Imai, T.; Uzawa, A.; Suzuki, S. The Japanese clinical guidelines 2022 for myasthenia gravis and Lambert–Eaton myasthenic syndrome. Clin. Exp. Neuroim 2023, 14, 19–27. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Andersen, H.; Andersen, L.K.; Boldingh, M.; Laakso, S.; Leopoldsdottir, M.O.; Madsen, S.; Piehl, F.; Popperud, T.H.; Punga, A.R.; et al. Generalized myasthenia gravis with acetylcholine receptor antibodies: A guidance for treatment. Eur. J. Neurol. 2024, 31, e16229. [Google Scholar] [CrossRef]
- Wiendl, H.; Abicht, A.; Chan, A.; Della Marina, A.; Hagenacker, T.; Hekmat, K.; Hoffmann, S.; Hoffmann, H.-S.; Jander, S.; Keller, C.; et al. Guideline for the management of myasthenic syndromes. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231213240. [Google Scholar] [CrossRef]
- Gerischer, L.; Doksani, P.; Hoffmann, S.; Meisel, A. New and Emerging Biological Therapies for Myasthenia Gravis: A Focussed Review for Clinical Decision-Making. BioDrugs 2025, 39, 185–213. [Google Scholar] [CrossRef]
- Vu, T.; Meisel, A.; Mantegazza, R.; Annane, D.; Katsuno, M.; Aguzzi, R.; Enayetallah, A.; Beasley, K.N.; Rampal, N.; Howard, J.F. Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis. NEJM Evid. 2022, 1, 5. [Google Scholar] [CrossRef]
- Bril, V.; Drużdż, A.; Grosskreutz, J.; Habib, A.A.; Mantegazza, R.; Sacconi, S.; Utsugisawa, K.; Vissing, J.; Vu, T.; Boehnlein, M.; et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): A randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023, 22, 383–394. [Google Scholar] [CrossRef]
- Howard, J.F.; Bresch, S.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; Maniaol, A.; Mantegazza, R.; et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023, 22, 395–406. [Google Scholar] [CrossRef]
- Rossini, E.; Di Stefano, V.; Iorio, R.; Habetswallner, F.; Maestri, M.; Vinciguerra, C.; Pennisi, E.M.; Di Martino, G.; Rini, N.; Falso, S.; et al. Ravulizumab for generalized Myasthenia Gravis: A multicenter real-life experience. J. Neurol. 2025, 272, 396. [Google Scholar] [CrossRef]
- Jin, L.; Zou, Z.; Wang, Q.; Zeng, W.; Jiang, Q.; Chen, J.; Shi, J.; Yu, Y.; Hong, D.; Zeng, Q.; et al. Patterns and predictors of therapeutic response to efgartigimod in acetylcholine receptor-antibody generalized myasthenia gravis subtypes. Ther. Adv. Neurol. Disord. 2025, 18, 17562864251319656. [Google Scholar] [CrossRef] [PubMed]
- Dragin, N.; Bismuth, J.; Cizeron-Clairac, G.; Biferi, M.G.; Berthault, C.; Serraf, A.; Nottin, R.; Klatzmann, D.; Cumano, A.; Barkats, M.; et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Investig. 2016, 126, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Zito, A.; Davies, M.N.; Tsai, P.-C.; Roberts, S.; Andres-Ejarque, R.; Nardone, S.; Bell, J.T.; Wong, C.C.Y.; Small, K.S. Heritability of skewed X-inactivation in female twins is tissue-specific and associated with age. Nat. Commun. 2019, 10, 5339. [Google Scholar] [CrossRef] [PubMed]
- Roxanis, I. Thymic myoid cells and germinal center formation in myasthenia gravis; possible roles in pathogenesis. J. Neuroimmunol. 2002, 125, 185–197. [Google Scholar] [CrossRef]
- Berrih-Aknin, S.; Le Panse, R. Myasthenia gravis: A comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 2014, 52, 90–100. [Google Scholar] [CrossRef]
- Payet, C.A.; You, A.; Fayet, O.-M.; Dragin, N.; Berrih-Aknin, S.; Le Panse, R. Myasthenia Gravis: An Acquired Interferonopathy? Cells 2022, 11, 1218. [Google Scholar] [CrossRef]
- Bach, J.-F. Infections and autoimmune diseases. J. Autoimmun. 2005, 25, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Shekhar, S.; Levey, D.F.; Straub, P.; Kraft, J.; Panagiotaropoulou, G.M.; Heilbron, K.; Awasthi, S.; Meleka Hanna, R.; Hoffmann, S.; et al. Genome-wide meta-analysis of myasthenia gravis uncovers new loci and provides insights into polygenic prediction. Nat. Commun. 2024, 15, 9839. [Google Scholar] [CrossRef] [PubMed]
- Tereshko, Y.; Gigli, G.L.; Pez, S.; De Pellegrin, A.; Valente, M. New-onset Myasthenia Gravis after SARS-CoV-2 infection: Case report and literature review. J. Neurol. 2023, 270, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, V.; Chang, Y.-M.; Pham, A.; Luo, J.; Garden, O.A. A Systematic Review of the Potential Implication of Infectious Agents in Myasthenia Gravis. Front. Neurol. 2021, 12, 618021. [Google Scholar] [CrossRef]
- Cavalcante, P.; Marcuzzo, S.; Franzi, S.; Galbardi, B.; Maggi, L.; Motta, T.; Ghislandi, R.; Buzzi, A.; Spinelli, L.; Novellino, L.; et al. Epstein-Barr virus in tumor-infiltrating B cells of myasthenia gravis thymoma: An innocent bystander or an autoimmunity mediator? Oncotarget 2017, 8, 95432–95449. [Google Scholar] [CrossRef]
- Cavalcante, P.; Galbardi, B.; Franzi, S.; Marcuzzo, S.; Barzago, C.; Bonanno, S.; Camera, G.; Maggi, L.; Kapetis, D.; Andreetta, F.; et al. Increased expression of Toll-like receptors 7 and 9 in myasthenia gravis thymus characterized by active Epstein–Barr virus infection. Immunobiology 2016, 221, 516–527. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Qin, L.; Wang, T.; Bai, O. Insights into the mechanisms of Th17 differentiation and the Yin-Yang of Th17 cells in human diseases. Mol. Immunol. 2021, 134, 109–117. [Google Scholar] [CrossRef]
- Gradolatto, A.; Nazzal, D.; Truffault, F.; Bismuth, J.; Fadel, E.; Foti, M.; Berrih-Aknin, S. Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: Roles of IL-17 and TNF-α. J. Autoimmun. 2014, 52, 53–63. [Google Scholar] [CrossRef]
- Cufi, P.; Dragin, N.; Ruhlmann, N.; Weiss, J.M.; Fadel, E.; Serraf, A.; Berrih-Aknin, S.; Le Panse, R. Central role of interferon-beta in thymic events leading to myasthenia gravis. J. Autoimmun. 2014, 52, 44–52. [Google Scholar] [CrossRef]
- Le Panse, R.; Cizeron-Clairac, G.; Bismuth, J.; Berrih-Aknin, S. Microarrays Reveal Distinct Gene Signatures in the Thymus of Seropositive and Seronegative Myasthenia Gravis Patients and the Role of CC Chemokine Ligand 21 in Thymic Hyperplasia. J. Immunol. 2006, 177, 7868–7879. [Google Scholar] [CrossRef]
- Le Panse, R.; Bismuth, J.; Cizeron-Clairac, G.; Weiss, J.M.; Cufi, P.; Dartevelle, P.; De Rosbo, N.K.; Berrih-Aknin, S. Thymic remodeling associated with hyperplasia in myasthenia gravis. Autoimmunity 2010, 43, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Clauder, A.-K.; Manz, R.A. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef]
- Pillai, S.; Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009, 9, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Thangarajh, M.; Masterman, T.; Helgeland, L.; Rot, U.; Jonsson, M.V.; Eide, G.E.; Pirskanen, R.; Hillert, J.; Jonsson, R. The thymus is a source of B-cell-survival factors–APRIL and BAFF–in myasthenia gravis. J. Neuroimmunol. 2006, 178, 161–166. [Google Scholar] [CrossRef]
- Zografou, C.; Vakrakou, A.G.; Stathopoulos, P. Short- and Long-Lived Autoantibody-Secreting Cells in Autoimmune Neurological Disorders. Front. Immunol. 2021, 12, 686466. [Google Scholar] [CrossRef] [PubMed]
- Huda, R. New Approaches to Targeting B Cells for Myasthenia Gravis Therapy. Front. Immunol. 2020, 11, 240. [Google Scholar] [CrossRef]
- Khodadadi, L.; Cheng, Q.; Radbruch, A.; Hiepe, F. The Maintenance of Memory Plasma Cells. Front. Immunol. 2019, 10, 721. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Cascalho, M.; Noelle, R.J. Short-lived and Long-lived Bone Marrow Plasma Cells Are Derived from a Novel Precursor Population. J. Exp. Med. 2002, 195, 737–745. [Google Scholar] [CrossRef]
- Schönbeck, S.; Padberg, F.; Hohlfeld, R.; Wekerle, H. Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J. Clin. Investig. 1992, 90, 245–250. [Google Scholar] [CrossRef]
- Marx, A.; Willcox, N.; Leite, M.I.; Chuang, W.-Y.; Schalke, B.; Nix, W.; Ströbel, P. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Ströbel, P.; Chuang, W.; Chuvpilo, S.; Zettl, A.; Katzenberger, T.; Kalbacher, H.; Rieckmann, P.; Nix, W.; Schalke, B.; Gold, R.; et al. Common Cellular and Diverse Genetic Basis of Thymoma-associated Myasthenia Gravis: Role of MHC Class II and AIRE Genes and Genetic Polymorphisms. Ann. N. Y. Acad. Sci. 2008, 1132, 143–156. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Palombi, M.; Fierabracci, A. The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity. Autoimmun. Rev. 2013, 12, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Holbro, A.; Jauch, A.; Lardinois, D.; Tzankov, A.; Dirnhofer, S.; Hess, C. High prevalence of infections and autoimmunity in patients with thymoma. Hum. Immunol. 2012, 73, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.G.; Marx, A.; Plomp, J.J.; Le Panse, R.; Phillips, W.D. Advances in the understanding of disease mechanisms of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 163–175. [Google Scholar] [CrossRef]
- Yasumizu, Y.; Ohkura, N.; Murata, H.; Kinoshita, M.; Funaki, S.; Nojima, S.; Kido, K.; Kohara, M.; Motooka, D.; Okuzaki, D.; et al. Myasthenia gravis-specific aberrant neuromuscular gene expression by medullary thymic epithelial cells in thymoma. Nat. Commun. 2022, 13, 4230. [Google Scholar] [CrossRef]
- Yasumizu, Y.; Kinoshita, M.; Zhang, M.J.; Motooka, D.; Suzuki, K.; Nojima, S.; Koizumi, N.; Okuzaki, D.; Funaki, S.; Shintani, Y.; et al. Spatial transcriptomics elucidates medulla niche supporting germinal center response in myasthenia gravis-associated thymoma. Cell Rep. 2024, 43, 114677. [Google Scholar] [CrossRef]
- Nagvekar, N.; Moody, A.M.; Moss, P.; Roxanis, I.; Curnow, J.; Beeson, D.; Pantic, N.; Newsom-Davis, J.; Vincent, A.; Willcox, N. A pathogenetic role for the thymoma in myasthenia gravis. Autosensitization of IL-4- producing T cell clones recognizing extracellular acetylcholine receptor epitopes presented by minority class II isotypes. J. Clin. Investig. 1998, 101, 2268–2277. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.-S.; Wang, Y.-G.; Lu, C.; Li, J.; Zhang, P. Imbalance of Th17 and Tregs in thymoma may be a pathological mechanism of myasthenia gravis. Mol. Immunol. 2021, 133, 67–76. [Google Scholar] [CrossRef]
- Ströbel, P.; Bauer, A.; Puppe, B.; Kraushaar, T.; Krein, A.; Toyka, K.; Gold, R.; Semik, M.; Kiefer, R.; Nix, W.; et al. Tumor Recurrence and Survival in Patients Treated for Thymomas and Thymic Squamous Cell Carcinomas: A Retrospective Analysis. JCO 2004, 22, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ströbel, P.; Helmreich, M.; Menioudakis, G.; Lewin, S.R.; Rüdiger, T.; Bauer, A.; Hoffacker, V.; Gold, R.; Nix, W.; Schalke, B.; et al. Paraneoplastic myasthenia gravis correlates with generation of mature naive CD4+ T cells in thymomas. Blood 2002, 100, 159–166. [Google Scholar] [CrossRef]
- Lefeuvre, C.M.j.; Payet, C.A.; Fayet, O.-M.; Maillard, S.; Truffault, F.; Bondet, V.; Duffy, D.; De Montpreville, V.; Ghigna, M.-R.; Fadel, E.; et al. Risk factors associated with myasthenia gravis in thymoma patients: The potential role of thymic germinal centers. J. Autoimmun. 2020, 106, 102337. [Google Scholar] [CrossRef]
- Cebi, M.; Cakar, A.; Erdogdu, E.; Durmus-Tekce, H.; Yegen, G.; Ozkan, B.; Parman, Y.; Saruhan-Direskeneli, G. Thymoma patients with or without myasthenia gravis have increased Th17 cells, IL-17 production and ICOS expression. J. Neuroimmunol. 2023, 381, 578129. [Google Scholar] [CrossRef]
- Villegas, J.A.; Bayer, A.C.; Ider, K.; Bismuth, J.; Truffault, F.; Roussin, R.; Santelmo, N.; Le Panse, R.; Berrih-Aknin, S.; Dragin, N. Il-23/Th17 cell pathway: A promising target to alleviate thymic inflammation maintenance in myasthenia gravis. J. Autoimmun. 2019, 98, 59–73. [Google Scholar] [CrossRef]
- Romi, F.; Hong, Y.; Gilhus, N.E. Pathophysiology and immunological profile of myasthenia gravis and its subgroups. Curr. Opin. Immunol. 2017, 49, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Teleshova, N.; Matusevicius, D.; Kivisäkk, P.; Mustafa, M.; Pirskanen, R.; Link, H. Altered expression of costimulatory molecules in myasthenia gravis. Muscle Nerve 2000, 23, 946–953. [Google Scholar] [CrossRef]
- Ots, H.D.; Tracz, J.A.; Vinokuroff, K.E.; Musto, A.E. CD40–CD40L in Neurological Disease. Int. J. Mol. Sci. 2022, 23, 4115. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; López De Munain, A.; et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Handunnetthi, L.; Knezevic, B.; Kasela, S.; Burnham, K.L.; Milani, L.; Irani, S.R.; Fang, H.; Knight, J.C. Genomic Insights into Myasthenia Gravis Identify Distinct Immunological Mechanisms in Early and Late Onset Disease. Ann. Neurol. 2021, 90, 455–463. [Google Scholar] [CrossRef]
- Yi, J.S.; Guptill, J.T.; Stathopoulos, P.; Nowak, R.J.; O’Connor, K.C. B cells in the pathophysiology of myasthenia gravis. Muscle Nerve 2018, 57, 172–184. [Google Scholar] [CrossRef]
- Uzawa, A.; Kanai, T.; Kawaguchi, N.; Oda, F.; Himuro, K.; Kuwabara, S. Changes in inflammatory cytokine networks in myasthenia gravis. Sci. Rep. 2016, 6, 25886. [Google Scholar] [CrossRef] [PubMed]
- Guptill, J.T.; Sanders, D.B.; Evoli, A. Anti-musk antibody myasthenia gravis: Clinical findings and response to treatment in two large cohorts. Muscle Nerve 2011, 44, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, H.-F.; Skeie, G.O.; Romi, F.; Hao, H.-J.; Zhang, X.; Gao, X.; Owe, J.F.; Gilhus, N.E. Autoantibody profile and clinical characteristics in a cohort of Chinese adult myasthenia gravis patients. J. Neuroimmunol. 2016, 298, 51–57. [Google Scholar] [CrossRef]
- Yilmaz, V.; Oflazer, P.; Aysal, F.; Durmus, H.; Poulas, K.; Yentur, S.P.; Gulsen-Parman, Y.; Tzartos, S.; Marx, A.; Tuzun, E.; et al. Differential Cytokine Changes in Patients with Myasthenia Gravis with Antibodies against AChR and MuSK. PLoS ONE 2015, 10, e0123546. [Google Scholar] [CrossRef]
- Uzawa, A.; Kuwabara, S.; Suzuki, S.; Imai, T.; Murai, H.; Ozawa, Y.; Yasuda, M.; Nagane, Y.; Utsugisawa, K. Roles of cytokines and T cells in the pathogenesis of myasthenia gravis. Clin. Exp. Immunol. 2021, 203, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, P.; Kumar, A.; Heiden, J.A.V.; Pascual-Goñi, E.; Nowak, R.J.; O’Connor, K.C. Mechanisms underlying B cell immune dysregulation and autoantibody production in MuSK myasthenia gravis. Ann. N. Y. Acad. Sci. 2018, 1412, 154–165. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Karachaliou, E.; Chroni, E.; Zouvelou, V.; Tzanetakos, D.; Salakou, S.; Papadopoulou, M.; Tzartos, S.; Voumvourakis, K.; Kilidireas, C.; et al. Immunotherapies in MuSK-positive Myasthenia Gravis; an IgG4 antibody-mediated disease. Front. Immunol. 2023, 14, 1212757. [Google Scholar] [CrossRef]
- Guptill, J.T.; Yi, J.S.; Sanders, D.B.; Guidon, A.C.; Juel, V.C.; Massey, J.M.; Howard, J.F.; Scuderi, F.; Bartoccioni, E.; Evoli, A.; et al. Characterization of B cells in muscle-specific kinase antibody myasthenia gravis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e77. [Google Scholar] [CrossRef]
- Li, M.; Han, J.; Zhang, Y.; Lv, J.; Zhang, J.; Zhao, X.; Ren, L.; Fang, H.; Yang, J.; Zhang, Y.; et al. Clinical analysis of Chinese anti-low-density-lipoprotein-receptor-associated protein 4 antibodies in patients with myasthenia gravis. Eur. J. Neurol. 2019, 26, 1296. [Google Scholar] [CrossRef]
- Marino, M.; Scuderi, F.; Samengo, D.; Saltelli, G.; Maiuri, M.T.; Shen, C.; Mei, L.; Sabatelli, M.; Pani, G.; Antonini, G.; et al. Flow Cytofluorimetric Analysis of Anti-LRP4 (LDL Receptor-Related Protein 4) Autoantibodies in Italian Patients with Myasthenia Gravis. PLoS ONE 2015, 10, e0135378. [Google Scholar] [CrossRef]
- Evoli, A. Myasthenia gravis: New developments in research and treatment. Curr. Opin. Neurol. 2017, 30, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Rivner, M.H.; Quarles, B.M.; Pan, J.; Yu, Z.; Howard, J.F.; Corse, A.; Dimachkie, M.M.; Jackson, C.; Vu, T.; Small, G.; et al. Clinical features of LRP4/agrin-antibody–positive myasthenia gravis: A multicenter study. Muscle Nerve 2020, 62, 333–343. [Google Scholar] [CrossRef]
- Koneczny, I.; Rennspiess, D.; Marcuse, F.; Dankerlui, N.; Abdul Hamid, M.; Mané-Damas, M.; Maessen, J.; Van Schil, P.; Saxena, A.; Zisimopoulou, P.; et al. Characterization of the thymus in Lrp4 myasthenia gravis: Four cases. Autoimmun. Rev. 2019, 18, 50–55. [Google Scholar] [CrossRef]
- Shen, C.; Lu, Y.; Zhang, B.; Figueiredo, D.; Bean, J.; Jung, J.; Wu, H.; Barik, A.; Yin, D.-M.; Xiong, W.-C.; et al. Antibodies against low-density lipoprotein receptor–related protein 4 induce myasthenia gravis. J. Clin. Investig. 2013, 123, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Lehnerer, S.; Stegherr, R.; Grittner, U.; Stein, M.; Gerischer, L.; Stascheit, F.; Herdick, M.; Doksani, P.; Meisel, A.; Hoffmann, S. The burden of disease in seronegative myasthenia gravis: A patient-centered perspective. Front. Immunol. 2025, 16, 1555075. [Google Scholar] [CrossRef]
- Maccora, S.; Vinciguerra, C.; Messina, C.; Bevilacqua, L.; Rini, N.; Barone, P.; Brighina, F.; Di Stefano, V. Double seronegative myasthenia gravis and mimics: A retrospective cross-sectional study by two tertiary centers in the Southern Italy. J. Neurol. 2025, 272, 433. [Google Scholar] [CrossRef]
- Vinciguerra, C.; Bevilacqua, L.; Lupica, A.; Ginanneschi, F.; Piscosquito, G.; Rini, N.; Rossi, A.; Barone, P.; Brighina, F.; Di Stefano, V. Diagnosis and Management of Seronegative Myasthenia Gravis: Lights and Shadows. Brain Sci. 2023, 13, 1286. [Google Scholar] [CrossRef]
- Okuzono, Y.; Miyakawa, S.; Itou, T.; Sagara, M.; Iwata, M.; Ishizuchi, K.; Sekiguchi, K.; Motegi, H.; Oyama, M.; Warude, D.; et al. B-cell immune dysregulation with low soluble CD22 levels in refractory seronegative myasthenia gravis. Front. Immunol. 2024, 15, 1382320. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef]
- Mantegazza, R.; Vanoli, F.; Frangiamore, R.; Cavalcante, P. Complement Inhibition for the Treatment of Myasthenia Gravis. Immunotargets Ther. 2020, 9, 317–331. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef]
- Howard, J.F. Myasthenia gravis: The role of complement at the neuromuscular junction. Ann. N. Y. Acad. Sci. 2018, 1412, 113–128. [Google Scholar] [CrossRef]
- Ma, Q.; Ran, H.; Li, Y.; Lu, Y.; Liu, X.; Huang, H.; Yang, W.; Yu, L.; Chen, P.; Huang, X.; et al. Circulating Th1/17 cells serve as a biomarker of disease severity and a target for early intervention in AChR-MG patients. Clin. Immunol. 2020, 218, 108492. [Google Scholar] [CrossRef]
- Aguilo-Seara, G.; Xie, Y.; Sheehan, J.; Kusner, L.L.; Kaminski, H.J. Ablation of IL-17 expression moderates experimental autoimmune myasthenia gravis disease severity. Cytokine 2017, 96, 279–285. [Google Scholar] [CrossRef]
- Villegas, J.A.; Van Wassenhove, J.; Merrheim, J.; Matta, K.; Hamadache, S.; Flaugère, C.; Pothin, P.; Truffault, F.; Hascoët, S.; Santelmo, N.; et al. Blocking interleukin-23 ameliorates neuromuscular and thymic defects in myasthenia gravis. J. Neuroinflamm. 2023, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, Q.; Yu, L.; Huang, H.; Liu, X.; Chen, P.; Ran, H.; Liu, W. JAK2 inhibitor ameliorates the progression of experimental autoimmune myasthenia gravis and balances Th17/Treg cells via regulating the JAK2/STAT3-AKT/mTOR signaling pathway. Int. Immunopharmacol. 2023, 115, 109693. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of Tofacitinib in Patients with Refractory Myasthenia Gravis: A Pilot Study. Available online: https://clinicaltrials.gov/search?cond=NCT04431895 (accessed on 7 September 2025).
- Alboini, P.E.; Evoli, A.; Damato, V.; Iorio, R.; Bartoccioni, E. Remission of myasthenia gravis with MuSK antibodies during ruxolitinib treatment: MuSK-MG Recovery after Ruxolitinib. Muscle Nerve 2017, 55, E12–E13. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, E.; Meriggioli, M.; Rowin, J.; Yang, H.; Christadoss, P. Myasthenia gravis patients with low plasma IL-6 and IFN-γ benefit from etanercept treatment. J. Autoimmun. 2005, 24, 261–268. [Google Scholar] [CrossRef]
- Pelechas, E.; Memi, T.; Markatseli, T.E.; Voulgari, P.V.; Drosos, A.A. Adalimumab-induced myasthenia gravis: Case-based review. Rheumatol. Int. 2020, 40, 1891–1894. [Google Scholar] [CrossRef]
- Baik, S.J.; Kim, T.H.; Kim, H.I.; Rhie, J.Y. Myasthenia Crisis Induced by Pegylated-Interferon in Patient with Chronic Hepatitis C: A Case Report. Medicine 2016, 95, e3782. [Google Scholar] [CrossRef]
- Congeni, J.P.; Kirkpatrick, R.B. Pegylated Interferon Induced Myasthenia Crisis—A Case Report. J. Clin. Neuromuscul. Dis. 2013, 14, 123–125. [Google Scholar] [CrossRef]
- Meager, A.; Wadhwa, M.; Dilger, P.; Bird, C.; Thorpe, R.; Newsom-Davis, J.; Willcox, N. Anti-cytokine autoantibodies in autoimmunity: Preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin. Exp. Immunol. 2003, 132, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Meraouna, A.; Cizeron-Clairac, G.; Panse, R.L.; Bismuth, J.; Truffault, F.; Tallaksen, C.; Berrih-Aknin, S. The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood 2006, 108, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Klimatcheva, E.; Pandina, T.; Reilly, C.; Torno, S.; Bussler, H.; Scrivens, M.; Jonason, A.; Mallow, C.; Doherty, M.; Paris, M.; et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015, 16, 6. [Google Scholar] [CrossRef]
- Uzawa, A.; Akamine, H.; Kojima, Y.; Ozawa, Y.; Yasuda, M.; Onishi, Y.; Sawai, S.; Kawasaki, K.; Asano, H.; Ohyama, S.; et al. High levels of serum interleukin-6 are associated with disease activity in myasthenia gravis. J. Neuroimmunol. 2021, 358. [Google Scholar] [CrossRef]
- Miyake, S.; Serizawa, K.; Onishi, S.; Katsura, Y.; Baba, M.; Kurasawa, M.; Tomizawa-Shinohara, H.; Yorozu, K.; Matsumoto, Y.; Noguchi-Sasaki, M. IL-6 receptor antibody treatment improves muscle weakness in experimental autoimmune myasthenia gravis mouse model. Front. Neurol. 2024, 15, 1356300. [Google Scholar] [CrossRef]
- Aricha, R.; Mizrachi, K.; Fuchs, S.; Souroujon, M.C. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J. Autoimmun. 2011, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Satralizumab in Patients with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT04963270 (accessed on 7 September 2025).
- Habib, A.A.; Zhao, C.; Aban, I.; França, M.C.; José, J.G.; Hörste, G.M.; Klimiec-Moskal, E.; Pulley, M.T.; Tavolini, D.; Krumova, P.; et al. Safety and efficacy of satralizumab in patients with generalised myasthenia gravis (LUMINESCE): A randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2025, 24, 117–127. [Google Scholar] [CrossRef]
- Yang, T.-T.; Wang, Z.-Y.; Fan, Z.-X.; Yuan, B.-Y.; Ma, L.; Lu, J.-F.; Liu, P.-J.; He, Y.; Liu, G.-Z. A Pilot Study on Tocilizumab in Very-Late-Onset Myasthenia Gravis. JIR 2023, 16, 5835–5843. [Google Scholar] [CrossRef]
- Ruan, Z.; Tang, Y.; Gao, T.; Li, C.; Guo, R.; Sun, C.; Huang, X.; Li, Z.; Chang, T. Efficacy and safety of tocilizumab in patients with refractory generalized myasthenia gravis. CNS Neurosci. Ther. 2024, 30, e14793. [Google Scholar] [CrossRef]
- Efficacy and Safety of Tocilizumab in the Treatment of Generalized Myasthenia Gravis (tMG). Available online: https://clinicaltrials.gov/study/NCT05067348?intr=NCT05067348&rank=1 (accessed on 7 September 2025).
- Sun, J.; Sun, M.; Li, X.; Xie, Q.; Zhang, W.; Wang, M. MicroRNA-155-5p affects regulatory T cell activation and immunosuppressive function by targeting BCL10 in myasthenia gravis. Exp. Ther. Med. 2023, 27, 6. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Kumar, A.; Nowak, R.J.; O’Connor, K.C. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2017, 2, e94263. [Google Scholar] [CrossRef]
- Marino, M.; Basile, U.; Spagni, G.; Napodano, C.; Iorio, R.; Gulli, F.; Todi, L.; Provenzano, C.; Bartoccioni, E.; Evoli, A. Long-Lasting Rituximab-Induced Reduction of Specific—But Not Total—IgG4 in MuSK-Positive Myasthenia Gravis. Front. Immunol. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Chayanopparat, S.; Banyatcharoen, P.; Jitprapaikulsan, J.; Uawithya, E.; Apiraksattayakul, N.; Viarasilpa, V. Efficacy and safety of rituximab in anti-MuSK myasthenia Gravis: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 7219. [Google Scholar] [CrossRef]
- Piehl, F.; Eriksson-Dufva, A.; Budzianowska, A.; Feresiadou, A.; Hansson, W.; Hietala, M.A.; Håkansson, I.; Johansson, R.; Jons, D.; Kmezic, I.; et al. Efficacy and Safety of Rituximab for New-Onset Generalized Myasthenia Gravis: The RINOMAX Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1105. [Google Scholar] [CrossRef]
- Du, Y.; Li, C.; Hao, Y.; Zhao, C.; Yan, Q.; Yao, D.; Li, L.; Zhang, W. Individualized regimen of low-dose rituximab monotherapy for new-onset AChR-positive generalized myasthenia gravis. J. Neurol. 2022, 269, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Brauner, S.; Eriksson-Dufva, A.; Hietala, M.A.; Frisell, T.; Press, R.; Piehl, F. Comparison Between Rituximab Treatment for New-Onset Generalized Myasthenia Gravis and Refractory Generalized Myasthenia Gravis. JAMA Neurol. 2020, 77, 974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Z.; Jia, D.; Xue, H.; Pan, J.; Zhang, M.; Shi, K.; Shi, F.-D.; Zhang, C. Low-dose rituximab treatment for new-onset generalized myasthenia gravis. J. Neuroimmunol. 2021, 354, 577528. [Google Scholar] [CrossRef]
- Dodd, K.C.; Clay, F.J.; Forbes, A.-M.; Handley, J.; Keh, R.Y.S.; Miller, J.A.; Storms, K.; White, L.M.; Lilleker, J.B.; Sussman, J. Rituximab for myasthenia gravis. Cochrane Database Syst. Rev. 2025, 2025, CD014574. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.J.; Coffey, C.S.; Goldstein, J.M.; Dimachkie, M.M.; Benatar, M.; Kissel, J.T.; Wolfe, G.I.; Burns, T.M.; Freimer, M.L.; Nations, S.; et al. Phase 2 Trial of Rituximab in Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: The BeatMG Study. Neurology 2022, 98, e376–e389. [Google Scholar] [CrossRef]
- Rituximab EfFicacy in MyasthEnia Gravis (REFINE) (REFINE). Available online: https://clinicaltrials.gov/study/NCT05868837?intr=NCT05868837&rank=1 (accessed on 7 September 2025).
- Immediate Corticosteroid Therapy and Rituximab to Prevent Generalization in Ocular Myasthenia: A PROBE Multicenter Open-label Randomized Controlled Trial. (IMCOMG). Available online: https://clinicaltrials.gov/study/NCT06342544?intr=NCT06342544&rank=1 (accessed on 7 September 2025).
- Myasthenia Gravis Inebilizumab Trial (MINT). Available online: https://clinicaltrials.gov/study/NCT04524273 (accessed on 7 September 2025).
- Nowak, R.J.; Benatar, M.; Ciafaloni, E.; Howard, J.F.; Leite, M.I.; Utsugisawa, K.; Vissing, J.; Rojavin, M.; Li, Q.; Tang, F.; et al. A Phase 3 Trial of Inebilizumab in Generalized Myasthenia Gravis. N. Engl. J. Med. 2025, 392, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Z.; Liu, Z. Therapeutic effect of ofatumumab in patients with myasthenia gravis: Immunoregulation of follicular T helper cells and T helper type 17 cells. Front. Neurol. 2023, 14, 1278250. [Google Scholar] [CrossRef]
- A Study of TAK-079 in People with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT04159805?intr=NCT04159805&rank=1 (accessed on 7 September 2025).
- GomezMancilla, B.; Meriggioli, M.N.; Genge, A.; Roubenoff, R.; Espié, P.; Dupuy, C.; Hartmann, N.; Pezous, N.; Kinhikar, A.; Tichy, M.; et al. Efficacy and safety of iscalimab, a novel anti-CD40 monoclonal antibody, in moderate-to-severe myasthenia gravis: A phase 2 randomized study. J. Clin. Neurosci. 2024, 119, 76–84. [Google Scholar] [CrossRef]
- Ristov, J.; Espie, P.; Ulrich, P.; Sickert, D.; Flandre, T.; Dimitrova, M.; Müller-Ristig, D.; Weider, D.; Robert, G.; Schmutz, P.; et al. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am. J. Transplant. 2018, 18, 2895–2904. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, H.; Zhang, M.; Xu, H.; Yue, L.-T.; Zhang, X.-X.; Wang, S.; Wang, C.-C.; Li, Y.-B.; Dou, Y.-C.; et al. Exosomes derived from atorvastatin-modified bone marrow dendritic cells ameliorate experimental autoimmune myasthenia gravis by up-regulated levels of IDO/Treg and partly dependent on FasL/Fas pathway. J. Neuroinflamm. 2016, 13, 8. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, R.-T.; Du, T.; Yang, C.-L.; Liu, Y.-D.; Ge, M.-R.; Zhang, M.; Li, X.-L.; Li, H.; Dou, Y.-C.; et al. Exosomes derived from statin-modified bone marrow dendritic cells increase thymus-derived natural regulatory T cells in experimental autoimmune myasthenia gravis. J. Neuroinflamm. 2019, 16, 202. [Google Scholar] [CrossRef]
- Gomez, A.M.; Vrolix, K.; Martínez-Martínez, P.; Molenaar, P.C.; Phernambucq, M.; Van Der Esch, E.; Duimel, H.; Verheyen, F.; Voll, R.E.; Manz, R.A.; et al. Proteasome Inhibition with Bortezomib Depletes Plasma Cells and Autoantibodies in Experimental Autoimmune Myasthenia Gravis. J. Immunol. 2011, 186, 2503–2513. [Google Scholar] [CrossRef]
- Therapy of Antibody-mediated Autoimmune Diseases by Bortezomib (TAVAB) (TAVAB). Available online: https://clinicaltrials.gov/study/NCT02102594?intr=NCT02102594&rank=1&tab=history&a=13#version-content-panel (accessed on 7 September 2025).
- Kohler, S.; Märschenz, S.; Grittner, U.; Alexander, T.; Hiepe, F.; Meisel, A. Bortezomib in antibody-mediated autoimmune diseases (TAVAB): Study protocol for a unicentric, non-randomised, non-placebo controlled trial. BMJ Open 2019, 9, e024523. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-T.; Zhang, P.; Yang, C.-L.; Pang, Y.; Zhang, M.; Zhang, N.; Yue, L.-T.; Li, X.-L.; Li, H.; Duan, R.-S. ONX-0914, a selective inhibitor of immunoproteasome, ameliorates experimental autoimmune myasthenia gravis by modulating humoral response. J. Neuroimmunol. 2017, 311, 71–78. [Google Scholar] [CrossRef]
- The Evaluation of Belimumab in Myasthenia Gravis (MG). Available online: https://clinicaltrials.gov/study/NCT01480596 (accessed on 7 September 2025).
- Hewett, K.; Sanders, D.B.; Grove, R.A.; Broderick, C.L.; Rudo, T.J.; Bassiri, A.; Zvartau-Hind, M.; Bril, V.; On behalf of the BEL115123 Study Group; BEL115123 Study Group; et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology 2018, 90, e1425–e1434. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Wang, F.; Fang, L. Case Report: Telitacicept in severe myasthenia gravis: A case study with multiple autoantibodies. Front. Immunol. 2023, 14, 1270011. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Gui, M.; Bu, B.; Li, Z. Effectiveness and safety of telitacicept for refractory generalized myasthenia gravis: A retrospective study. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241251476. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Lu, J.; Song, J.; Luo, S.; Zhou, L.; Quan, C.; Xi, J.; Zhao, C. Effect of low-dose rituximab treatment on T- and B-cell lymphocyte imbalance in refractory myasthenia gravis. J. Neuroimmunol. 2019, 332, 216–223. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, M.; Xu, X.; Zhang, M.; Xu, Z.; Li, Z.; Qin, X.; Li, Z.; Zhao, C.; Zhou, H.; et al. A multicenter, randomized, open-label, phase 2 clinical study of telitacicept in adult patients with generalized myasthenia gravis. Eur. J. Neurol. 2024, 31, e16322. [Google Scholar] [CrossRef] [PubMed]
- Study of Telitacicept in Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT05737160?cond=NCT05737160&rank=1 (accessed on 7 September 2025).
- A Study of Telitacicept for the Treatment of Generalized Myasthenia Gravis (RemeMG). Available online: https://clinicaltrials.gov/study/NCT06456580?cond=NCT06456580&rank=1 (accessed on 7 September 2025).
- Efficacy and Safety of Tolebrutinib (SAR442168) Tablets in Adult Participants with Generalized Myasthenia Gravis (URSA). Available online: https://clinicaltrials.gov/study/NCT05132569?cond=NCT05132569&rank=1 (accessed on 7 September 2025).
- Strong Sales Performance and Double Digit EPS Growth Marking the Achievement of the 2022 Profitability Milestone. Available online: https://www.sanofi.com/en/media-room/press-releases/2023/2023-02-03-06-30-00-2601072 (accessed on 7 September 2025).
- Reich, D.S.; Arnold, D.L.; Vermersch, P.; Bar-Or, A.; Fox, R.J.; Matta, A.; Turner, T.; Wallström, E.; Zhang, X.; Mareš, M.; et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021, 20, 729–738. [Google Scholar] [CrossRef]
- Fissolo, N.; Calvo-Barreiro, L.; Eixarch, H.; Boschert, U.; Espejo, C.; Montalban, X.; Comabella, M. Immunomodulatory Effects Associated with Cladribine Treatment. Cells 2021, 10, 3488. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef]
- Rejdak, K.; Szklener, S.; Korchut, A.; Baranowski, D. Cladribine in myasthenia gravis: A pilot open-label study. Eur. J. Neurol. 2020, 27, 586–589. [Google Scholar] [CrossRef]
- A Study to Investigate the Efficacy, Safety and Tolerability of Remibrutinib Versus Placebo in Adult Patients with Generalized Myasthenia Gravis (RELIEVE). Available online: https://clinicaltrials.gov/study/NCT06744920 (accessed on 7 September 2025).
- Efficacy and Safety of a New Formulation of Oral Cladribine Compared with Placebo in Participants with Generalized Myasthenia Gravis (MyClad). Available online: https://clinicaltrials.gov/study/NCT06463587?cond=NCT06463587&rank=1 (accessed on 7 September 2025).
- Miliotou, A.N.; Papadopoulou, L.C. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Blache, U.; Tretbar, S.; Koehl, U.; Mougiakakos, D.; Fricke, S. CAR T cells for treating autoimmune diseases. RMD Open 2023, 9, e002907. [Google Scholar] [CrossRef]
- Heidenreich, F.; Vincent, A.; Roberts, A.; Newsom-Davis, J. Epitopes on Human Acetylcholine Receptor Defined by Monoclonal Antibodies and Myasthenia Gravis Sera. Autoimmunity 1988, 1, 285–297. [Google Scholar] [CrossRef]
- Open-Label Study to Evaluate the Safety of Various Dosing Regimens of MuSK-CAART for MuSK Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT05451212?term=NCT05451212&rank=1 (accessed on 7 September 2025).
- Oh, S.; Mao, X.; Manfredo-Vieira, S.; Lee, J.; Patel, D.; Choi, E.J.; Alvarado, A.; Cottman-Thomas, E.; Maseda, D.; Tsao, P.Y.; et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat. Biotechnol. 2023, 41, 1229–1238. [Google Scholar] [CrossRef]
- Granit, V.; Benatar, M.; Kurtoglu, M.; Miljković, M.D.; Chahin, N.; Sahagian, G.; Feinberg, M.H.; Slansky, A.; Vu, T.; Jewell, C.M.; et al. Safety and Efficacy of Autologous RNA Chimeric Antigen Receptor T-cell (rCAR-T) Therapy in Myasthenia Gravis: A prospective, multicenter, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 2023, 22, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Descartes-08 CAR-T Cells in Generalized Myasthenia Gravis (MG). Available online: https://clinicaltrials.gov/study/NCT04146051?intr=NCT04146051&rank=1 (accessed on 7 September 2025).
- Haghikia, A.; Hegelmaier, T.; Wolleschak, D.; Böttcher, M.; Desel, C.; Borie, D.; Motte, J.; Schett, G.; Schroers, R.; Gold, R.; et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 2023, 22, 1104–1105. [Google Scholar] [CrossRef]
- KYSA-6: A Study of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy, in Subjects with Refractory Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT06193889?cond=NCT06193889&rank=1 (accessed on 7 September 2025).
- RESET-MG: A Study to Evaluate the Safety and Efficacy of CABA-201 in Participants with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT06359041?cond=NCT06359041&rank=1 (accessed on 7 September 2025).
- Wang, J.; Hu, Y.; Huang, H. Current development of chimeric antigen receptor T-cell therapy. Stem Cell Investig. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Ruck, T.; Huntemann, N.; Öztürk, M.; Schreiber, S.; Lichtenberg, S.; Masanneck, L.; Nelke, C.; Ben Moussa, H.; Ulrych, T.; Seifert, M.; et al. CD19xCD3 T-cell engager blinatumomab effective in refractory generalized myasthenic syndromes. Mol. Ther. 2025, 33, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Myasthenia Gravis Treated with Autologous Hematopoietic Stem Cell Transplantation|Stem Cell Transplantation|JAMA Neurology|JAMA Network. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2506781 (accessed on 24 June 2025).
- Tan, S.; Liu, J.; Chen, L.; Li, R.; Li, J. miR-125a-5p regulates Treg function by targeting Foxp3 in experimental autoimmune myasthenia gravis mice. Immunol. Lett. 2025, 276, 107050. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Xin, N.; Dou, C.; Fu, L.; Zhang, X.; Chen, J.; Zhang, Y.; Geng, D.; Xiao, C.; et al. Identification of Novel MicroRNA Signatures Linked to Experimental Autoimmune Myasthenia Gravis Pathogenesis: Down-Regulated miR-145 Promotes Pathogenetic Th17 Cell Response. J. Neuroimmune Pharmacol. 2013, 8, 1287–1302. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, G.; Liu, Q.; Hu, J.; Yan, M.; Yang, B.; Yang, H.; Zhou, W.; Li, J. Silencing miR-146a influences B cells and ameliorates experimental autoimmune myasthenia gravis. Immunology 2015, 144, 56–67. [Google Scholar] [CrossRef]
- Sabre, L.; Punga, T.; Punga, A.R. Circulating miRNAs as Potential Biomarkers in Myasthenia Gravis: Tools for Personalized Medicine. Front. Immunol. 2020, 11, 213. [Google Scholar] [CrossRef]
- Angelini, C.; Martignago, S.; Bisciglia, M. New treatments for myasthenia: A focus on antisense oligonucleotides. Drug Des. Dev. Ther. 2013, 7, 13–17. [Google Scholar] [CrossRef]
- Blotnick, E.; Hamra-Amitai, Y.; Wald, C.; Brenner, T.; Anglister, L. Changes in acetylcholinesterase in experimental autoimmune myasthenia gravis and in response to treatment with a specific antisense. Eur. J. Neurosci. 2012, 36, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Huda, R.; Tüzün, E.; Christadoss, P. Complement C2 siRNA mediated therapy of myasthenia gravis in mice. J. Autoimmun. 2013, 42, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Kusner, L.L.; Yucius, K.; Sengupta, M.; Sprague, A.G.; Desai, D.; Nguyen, T.; Charisse, K.; Kuchimanchi, S.; Kallanthottathil, R.; Fitzgerald, K.; et al. Investigational RNAi Therapeutic Targeting C5 Is Efficacious in Pre-clinical Models of Myasthenia Gravis. Mol. Ther. Methods Clin. Dev. 2019, 13, 484–492. [Google Scholar] [CrossRef]
- Ibtehaj, N.; Bahauddin, A.; Ivannikov, M.; Rytting, E.; Jamaluddin, M.; Liang, Y.; Sun, J.; Haller, S.L.; Wu, X.; Huda, R. B cell-specific mAb–siRNA conjugates improve experimental myasthenia. J. Autoimmun. 2023, 135, 102983. [Google Scholar] [CrossRef]
- Kuo, T.T.; Baker, K.; Yoshida, M.; Qiao, S.-W.; Aveson, V.G.; Lencer, W.I.; Blumberg, R.S. Neonatal Fc Receptor: From Immunity to Therapeutics. J. Clin. Immunol. 2010, 30, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Cines, D.B.; Blumberg, R.S. Neonatal Fc Receptor—Biology and Therapeutics. N. Engl. J. Med. 2025, 392, 1621–1635. [Google Scholar] [CrossRef]
- Phase 3 Study to Assess the Efficacy and Safety of Batoclimab as Induction and Maintenance Therapy in Adult Participants with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT05403541?term=NCT03863080%20NCT04346888%20NCT05039190%20NCT05332210%20NCT05403541&rank=1 (accessed on 7 September 2025).
- A Study of Nipocalimab Administered to Adults with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT04951622 (accessed on 7 September 2025).
- Yan, C.; Yue, Y.; Guan, Y.; Bu, B.; Ke, Q.; Duan, R.; Deng, H.; Xue, Q.; Jiang, H.; Xiao, F.; et al. Batoclimab vs. Placebo for Generalized Myasthenia Gravis: A Randomized Clinical Trial. JAMA Neurol. 2024, 81, 336–345. [Google Scholar] [CrossRef]
- Antozzi, C.; Vu, T.; Ramchandren, S.; Nowak, R.J.; Farmakidis, C.; Bril, V.; Bleecker, J.D.; Yang, H.; Minks, E.; Park, J.-S.; et al. Safety and efficacy of nipocalimab in adults with generalised myasthenia gravis (Vivacity-MG3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2025, 24, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Chu, T.; Tan, X.; Wang, S.; Qu, R.; Chen, Z.; Wang, Z. The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: A systematic review and meta-analysis. J. Neurol. 2024, 271, 2298–2308. [Google Scholar] [CrossRef]
- Akhtar, M.; Akhtar, M.; Farooqi, H.A.; Maryam, A.; Muzammil, A.; Hanif, U.; Athar, Z.; Hassan, S.M.; Khan, Z. Efficacy and safety of FcRn inhibitors in patients with Myasthenia gravis: An updated systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2025, 254, 108910. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jiang, Q.; Zeng, W.; Wang, Q.; Zou, Z.; Yu, Y.; Hong, D.; Zeng, Q.; Tan, S.; Zhang, Z.; et al. Efgartigimod for generalized myasthenia gravis: A multicenter real-world cohort study in China. Ann. Clin. Transl. Neurol. 2024, 11, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F.; Bresch, S.; Farmakidis, C.; Freimer, M.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; et al. Long-term safety and efficacy of zilucoplan in patients with generalized myasthenia gravis: Interim analysis of the RAISE-XT open-label extension study. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241243186. [Google Scholar] [CrossRef]
- Tüzün, E.; Li, J.; Saini, S.S.; Yang, H.; Christadoss, P. Pros and cons of treating murine myasthenia gravis with anti-C1q antibody. J. Neuroimmunol. 2007, 182, 167–176. [Google Scholar] [CrossRef]
- Röth, A.; Barcellini, W.; D’Sa, S.; Miyakawa, Y.; Broome, C.M.; Michel, M.; Kuter, D.J.; Jilma, B.; Tvedt, T.H.A.; Fruebis, J.; et al. Sutimlimab in Cold Agglutinin Disease. N. Engl. J. Med. 2021, 384, 1323–1334. [Google Scholar] [CrossRef]
- D’Sa, S.; Vos, J.M.I.; Barcellini, W.; Wardęcki, M.; Perrin, L.; Barker, G.; Zilberstein, M.; Storek, M.; Chow, T.; Röth, A. Safety, tolerability, and activity of the active C1s antibody riliprubart in cold agglutinin disease: A phase 1b study. Blood 2024, 143, 713–720. [Google Scholar] [CrossRef]
- A Study to Test the Effects and Safety of Riliprubart in People with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) for Which the Usual Treatments Do Not Work (MOBILIZE). Available online: https://clinicaltrials.gov/study/NCT06290128?cond=NCT06290128&rank=1 (accessed on 7 September 2025).
- A Phase 2 Study to Evaluate DNTH103 in Adults with Generalized Myasthenia Gravis (MAGIC) (MAGIC). Available online: https://clinicaltrials.gov/study/NCT06282159?cond=NCT06282159&rank=1 (accessed on 7 September 2025).
- Barratt, J.; Weitz, I. Complement Factor D as a Strategic Target for Regulating the Alternative Complement Pathway. Front. Immunol. 2021, 12, 712572. [Google Scholar] [CrossRef]
- Study of ALXN2050 in Adult Participants with Generalized Myasthenia Gravis. Available online: https://www.clinicaltrials.gov/study/NCT05218096 (accessed on 7 September 2025).
- Syed, S.; Khan, R.; Khurram, F.; Khan, F.H.; Safi, D.; Safi, S.U.D. Treatment of eculizumab refractory paroxysmal nocturnal hemoglobinuria: A systematic review about current treatment options and future direction. SAGE Open Med. 2023, 11, 20503121231181267. [Google Scholar] [CrossRef] [PubMed]
- A Phase III Study to Investigate Efficacy, Safety and Tolerability of Iptacopan Compared With Placebo in Participants Aged 18 to 75 Years with gMG. Available online: https://clinicaltrials.gov/study/NCT06517758?term=NCT06517758&rank=1 (accessed on 7 September 2025).
- A Study to Test How Safe Pozelimab and Cemdisiran Combination Therapy and Cemdisiran Alone Are and How Well They Work in Adult Patients with Generalized Myasthenia Gravis (NIMBLE). Available online: https://clinicaltrials.gov/study/NCT05070858?term=NCT05070858&rank=1 (accessed on 7 September 2025).
- Safety and Efficacy of ALXN1720 in Adults with Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/study/NCT05556096?cond=NCT05556096&rank=1 (accessed on 7 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, E.; Leonardi, L.; Morino, S.; Antonini, G.; Fionda, L. Immunological Targets in Generalized Myasthenia Gravis Treatment: Where Are We Going Now? Brain Sci. 2025, 15, 978. https://doi.org/10.3390/brainsci15090978
Rossini E, Leonardi L, Morino S, Antonini G, Fionda L. Immunological Targets in Generalized Myasthenia Gravis Treatment: Where Are We Going Now? Brain Sciences. 2025; 15(9):978. https://doi.org/10.3390/brainsci15090978
Chicago/Turabian StyleRossini, Elena, Luca Leonardi, Stefania Morino, Giovanni Antonini, and Laura Fionda. 2025. "Immunological Targets in Generalized Myasthenia Gravis Treatment: Where Are We Going Now?" Brain Sciences 15, no. 9: 978. https://doi.org/10.3390/brainsci15090978
APA StyleRossini, E., Leonardi, L., Morino, S., Antonini, G., & Fionda, L. (2025). Immunological Targets in Generalized Myasthenia Gravis Treatment: Where Are We Going Now? Brain Sciences, 15(9), 978. https://doi.org/10.3390/brainsci15090978






