Therapeutic Botulinum Neurotoxin Ameliorates Motor Deficits and Anxiety, Accompanied by Dopaminergic Neuroprotection and Diminished Microglia Burden in the MPTP-Induced Mouse Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Drug Administration

2.2. Open Field Test
2.3. Rotarod Test
2.4. Pole Test
2.5. Light and Dark Box
2.6. Elevated Plus Maze
2.7. Perfusion of Experimental Mice
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. BoNT Improved Locomotion and Reduced Anxiety-Related Behavior in MPTP-Induced Experimental Mice in the Open Field Test
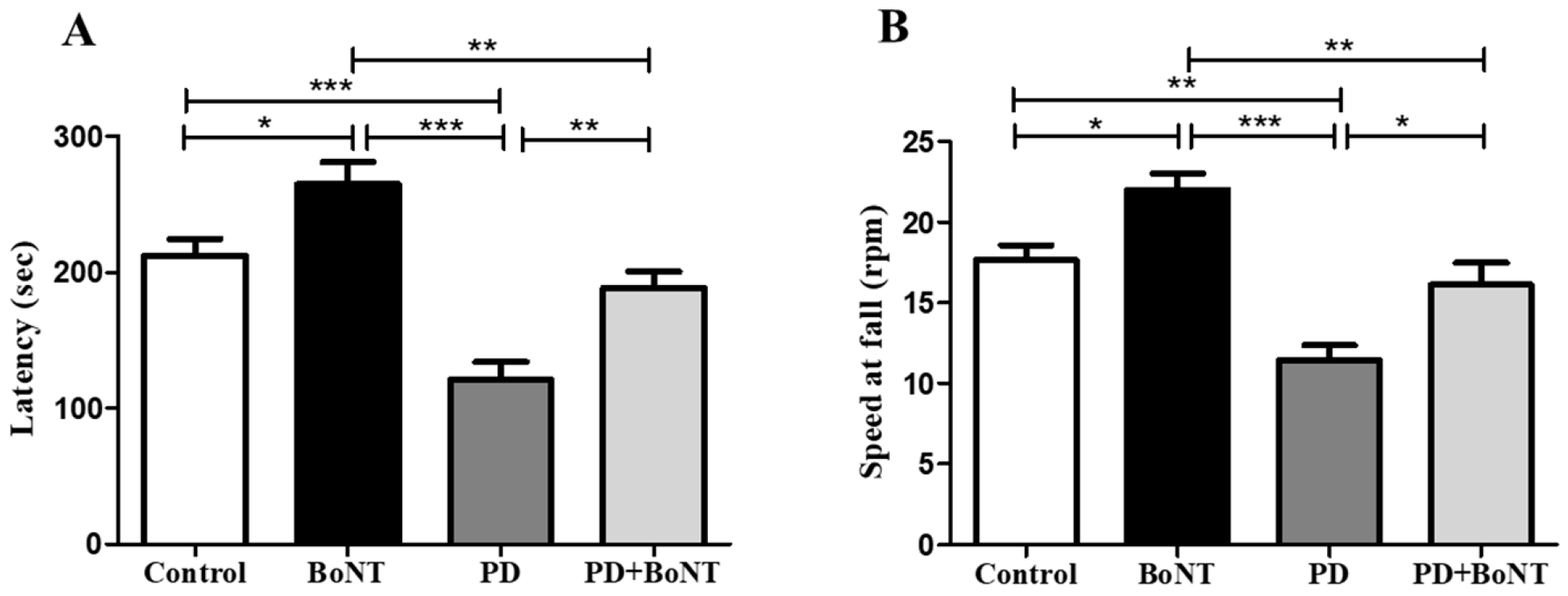
3.2. BoNT Improves Motor Coordination and Balance in MPTP-Induced Experimental Mice in the Rotarod Test
3.3. BoNT Restores Motor Functions in MPTP-Induced Experimental Mice in the Pole Test
3.4. BoNT Diminished Anxiogenic Symptoms in MPTP-Induced Experimental Mice in the Light and Dark Box Test
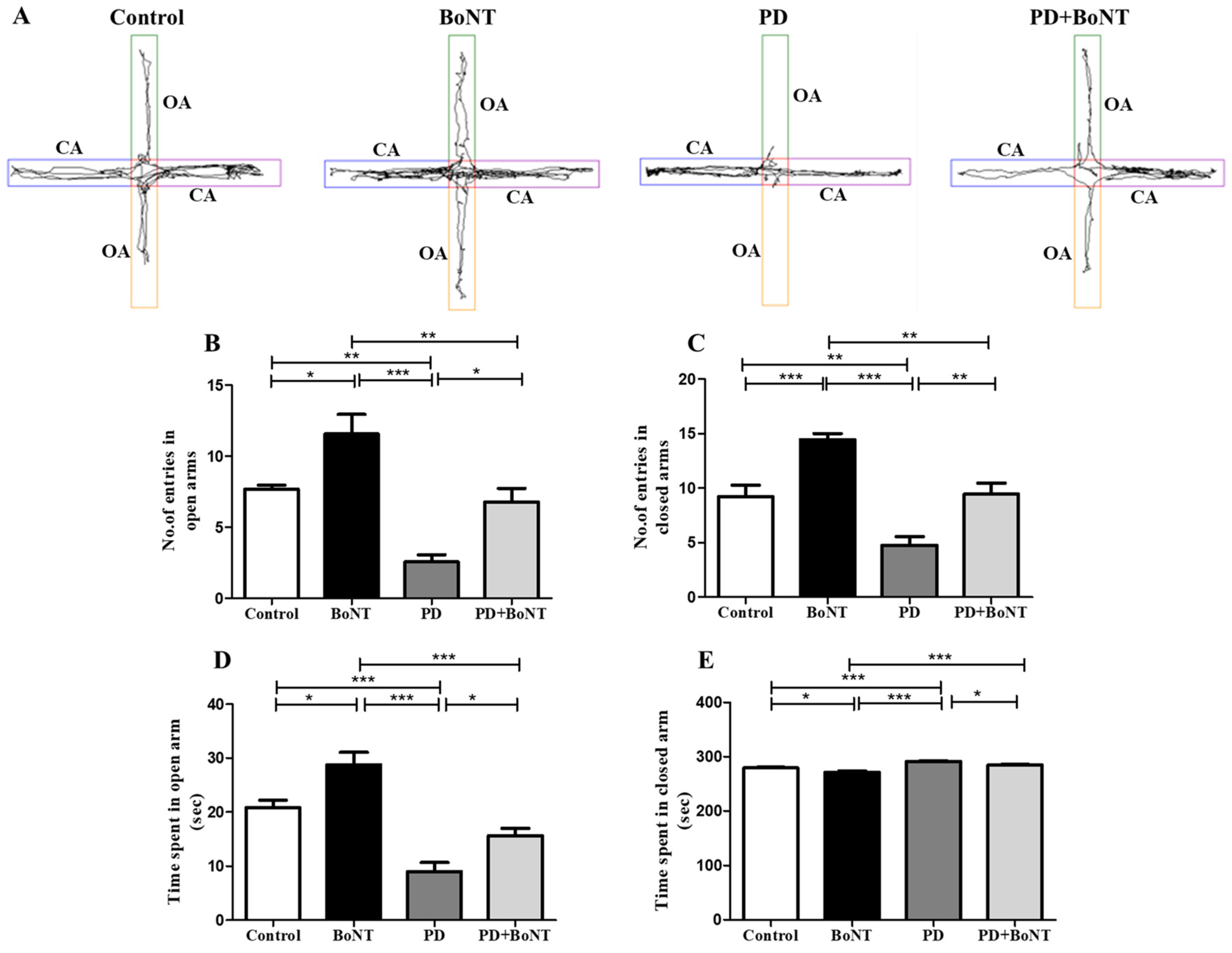
3.5. BoNT Decreased Acrophobic-Related Anxiety-like Behavior in MPTP-Induced Experimental Mice in the Elevated Plus Maze Test
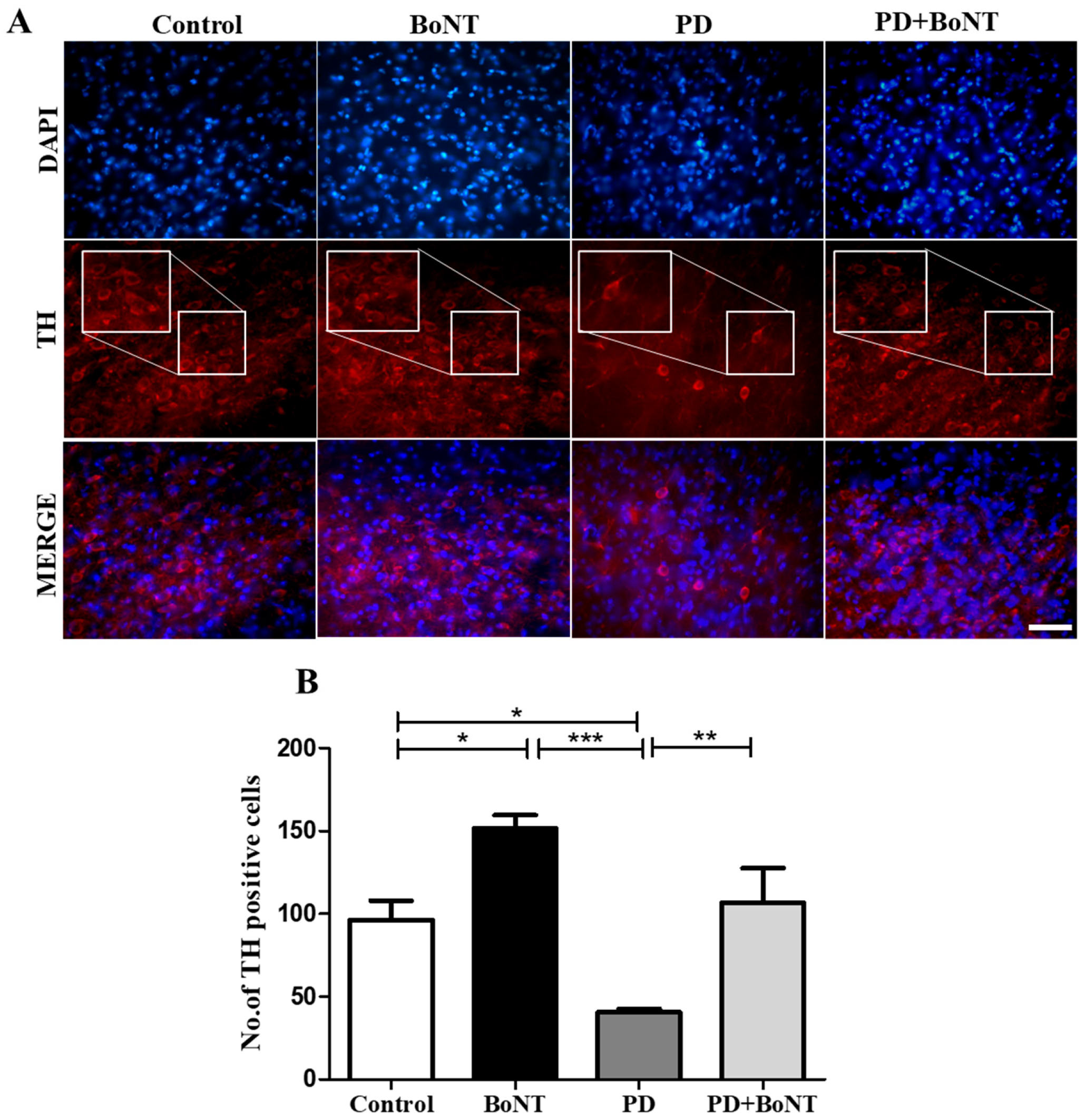
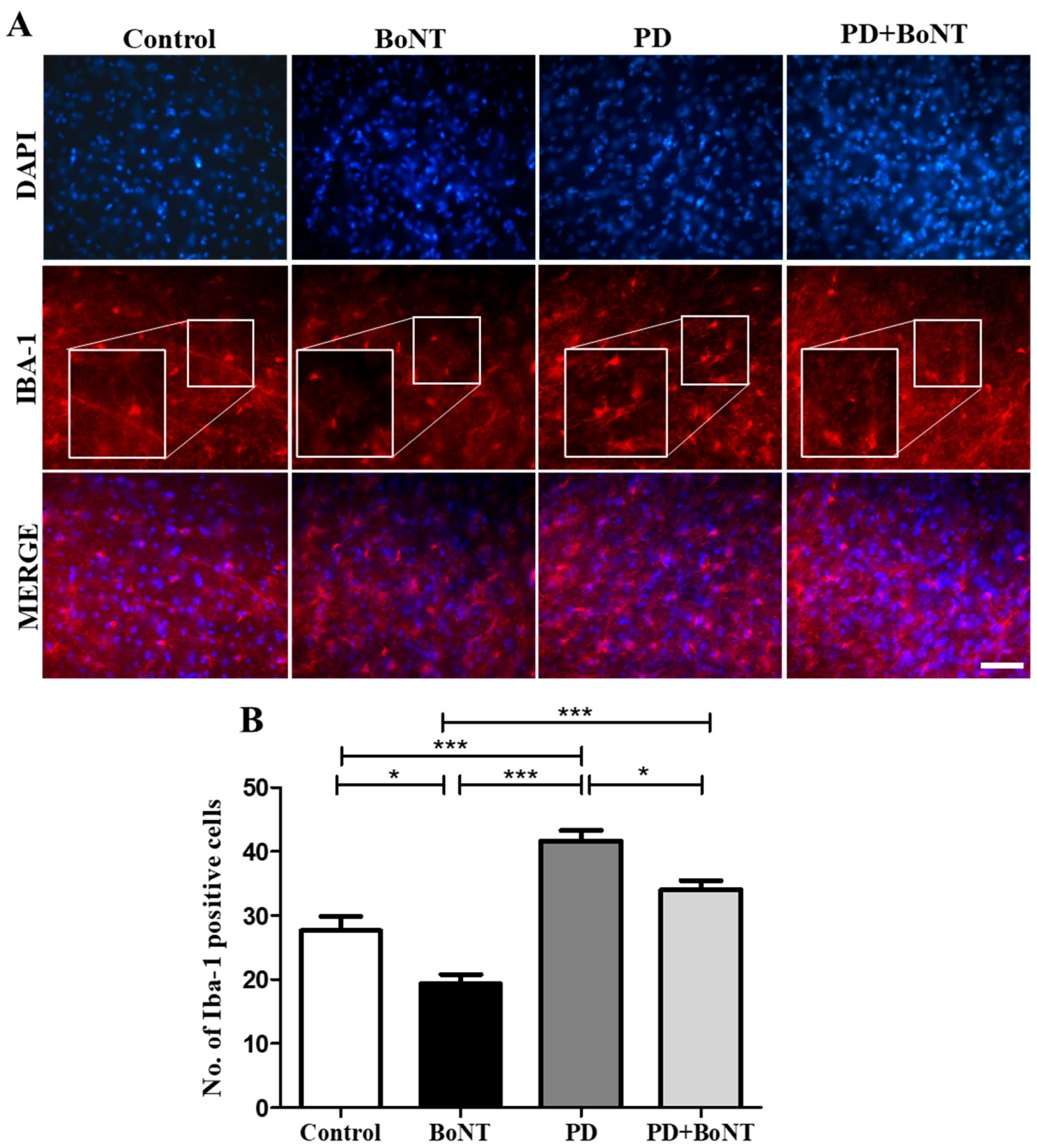
3.6. BoNT Protects Dopaminergic Neurons and Reduces the Number of Microglia in the Substantia Nigra in MPTP-Induced Experimental Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for Prevalence of Parkinson’s Disease and Its Driving Factors in 195 Countries and Territories to 2050: Modelling Study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired Dopamine Metabolism in Parkinson’s Disease Pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-Motor Symptoms of Parkinson’s Disease: Dopaminergic Pathophysiology and Treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Rethinavel, H.S.; Ravichandran, S.; Radhakrishnan, R.K.; Kandasamy, M. COVID-19 and Parkinson’s Disease: Defects in Neurogenesis as the Potential Cause of Olfactory System Impairments and Anosmia. J. Chem. Neuroanat. 2021, 115, 101965. [Google Scholar] [CrossRef]
- Blundell, E.K.; Grover, L.E.; Stott, J.; Schrag, A. The Experience of Anxiety for People with Parkinson’s Disease. npj Parkinsons Dis. 2023, 9, 75. [Google Scholar] [CrossRef]
- Pontone, G.M.; Perepezko, K.M.; Hinkle, J.T.; Gallo, J.J.; Grill, S.; Leoutsakos, J.-M.; Mills, K.A.; Weiss, H.D.; Mari, Z. “Anxious Fluctuators” a Subgroup of Parkinson’s Disease with High Anxiety and Problematic on-off Fluctuations. Parkinsonism Relat. Disord. 2022, 105, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Marsh, L. Anxiety in Parkinson’s Disease: Identification and Management. Ther. Adv. Neurol. Disord. 2014, 7, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.K.; Cronin-Golomb, A. Impact of Anxiety on Quality of Life in Parkinson′s Disease. Park. Dis. 2012, 2012, 640707. [Google Scholar] [CrossRef]
- Kano, O.; Ikeda, K.; Cridebring, D.; Takazawa, T.; Yoshii, Y.; Iwasaki, Y. Neurobiology of Depression and Anxiety in Parkinson′s Disease. Park. Dis. 2011, 2011, 143547. [Google Scholar] [CrossRef]
- Weiss, F.; Labrador-Garrido, A.; Dzamko, N.; Halliday, G. Immune Responses in the Parkinson’s Disease Brain. Neurobiol. Dis. 2022, 168, 105700. [Google Scholar] [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M. Perspectives for the Use of Therapeutic Botulinum Toxin as a Multifaceted Candidate Drug to Attenuate COVID-19. Med. Drug Discov. 2020, 6, 100042. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum Toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef]
- Grippe, T.; Chen, R. Botulinum Toxin in the Management of Parkinsonian Disorders. Toxicon 2023, 232, 107209. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Palea, S. Embracing the Versatility of Botulinum Neurotoxins in Conventional and New Therapeutic Applications. Toxins 2024, 16, 261. [Google Scholar] [CrossRef]
- Koizumi, H.; Goto, S.; Okita, S.; Morigaki, R.; Akaike, N.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kaji, R. Spinal Central Effects of Peripherally Applied Botulinum Neurotoxin A in Comparison between Its Subtypes A1 and A2. Front. Neurol. 2014, 5, 98. [Google Scholar] [CrossRef]
- Luvisetto, S. Botulinum Neurotoxins in Central Nervous System: An Overview from Animal Models to Human Therapy. Toxins 2021, 13, 751. [Google Scholar] [CrossRef]
- Borodic, G.E.; Acquadro, M.; Johnson, E.A. Botulinum Toxin Therapy for Pain and Inflammatory Disorders: Mechanisms and Therapeutic Effects. Expert Opin. Investig. Drugs 2001, 10, 1531–1544. [Google Scholar] [CrossRef]
- Fedoce, A.d.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The Role of Oxidative Stress in Anxiety Disorder: Cause or Consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Cerri, C.; Maya Vetencourt, J.F.; Caleo, M. Acute Neuroprotection by the Synaptic Blocker Botulinum Neurotoxin E in a Rat Model of Focal Cerebral Ischaemia. Neuroscience 2010, 169, 395–401. [Google Scholar] [CrossRef]
- Perna, G.; Iannone, G.; Alciati, A.; Caldirola, D. Are Anxiety Disorders Associated with Accelerated Aging? A Focus on Neuroprogression. Neural Plast. 2016, 2016, 8457612. [Google Scholar] [CrossRef]
- Yesudhas, A.; Radhakrishnan, R.K.; Sukesh, A.; Ravichandran, S.; Manickam, N.; Kandasamy, M. BOTOX® Counteracts the Innate Anxiety-Related Behaviours in Correlation with Increased Activities of Key Antioxidant Enzymes in the Hippocampus of Ageing Experimental Mice. Biochem. Biophys. Res. Commun. 2021, 569, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Misugi, K.; Goshima, Y.; Misu, Y. Evaluation of a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Treated C57 Black Mouse Model for Parkinsonism. Brain Res. 1990, 515, 57–63. [Google Scholar] [CrossRef]
- Joseph, J.H.M.; Kandasamy, M. Restoration of Memory Along with Neurogenic Enhancement by Therapeutic Botulinum Neurotoxin in a Preclinical Model of Parkinson’s Disease. Am. J. Alzheimers Dis. Other Demen. 2025, 40, 15333175251346292. [Google Scholar] [CrossRef]
- Selvaraj, D.B.; Panneerselvam, A.; Vergil Andrews, J.F.; Kandasamy, M. Cysteamine HCl Administration Impedes Motor and Olfactory Functions, Accompanied by a Reduced Number of Dopaminergic Neurons, in Experimental Mice: A Preclinical Mimetic Relevant to Parkinson’s Disease. Brain Sci. 2024, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s Disease and Its Potential as Therapeutic Target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- León-Rodríguez, A.; Fernández-Arjona, M.D.M.; Grondona, J.M.; Pedraza, C.; López-Ávalos, M.D. Anxiety-like Behavior and Microglial Activation in the Amygdala after Acute Neuroinflammation Induced by Microbial Neuraminidase. Sci. Rep. 2022, 12, 11581. [Google Scholar] [CrossRef]
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.D.; Lucassen, P.J.; van Dam, A.-M. Microglial Phenotypes and Toll-like Receptor 2 in the Substantia Nigra and Hippocampus of Incidental Lewy Body Disease Cases and Parkinson’s Disease Patients. Acta Neuropathol. Commun. 2014, 2, 90. [Google Scholar] [CrossRef]
- Peterson, L.J.; Flood, P.M. Oxidative Stress and Microglial Cells in Parkinson′s Disease. Mediat. Inflamm. 2012, 2012, 401264. [Google Scholar] [CrossRef]
- Cicchetti, F.; Brownell, A.L.; Williams, K.; Chen, Y.I.; Livni, E.; Isacson, O. Neuroinflammation of the Nigrostriatal Pathway during Progressive 6-OHDA Dopamine Degeneration in Rats Monitored by Immunohistochemistry and PET Imaging. Eur. J. Neurosci. 2002, 15, 991–998. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial Activation and Dopamine Terminal Loss in Early Parkinson’s Disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Wang, Y.-L.; Liu, C.-Y.; Zhou, Y.-T.; Zhang, X.-F. A Time-Course Study of Microglial Activation and Dopaminergic Neuron Loss in the Substantia Nigra of Mice with Paraquat-Induced Parkinson’s Disease. Food Chem. Toxicol. 2022, 164, 113018. [Google Scholar] [CrossRef]
- Zhang, W.; Phillips, K.; Wielgus, A.R.; Liu, J.; Albertini, A.; Zucca, F.A.; Faust, R.; Qian, S.Y.; Miller, D.S.; Chignell, C.F.; et al. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson’s Disease. Neurotox. Res. 2011, 19, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Deng, I.; Corrigan, F.; Zhai, G.; Zhou, X.-F.; Bobrovskaya, L. Lipopolysaccharide Animal Models of Parkinson’s Disease: Recent Progress and Relevance to Clinical Disease. Brain Behav. Immun.-Health 2020, 4, 100060. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat Neurotoxicity Is Distinct from That of MPTP and Rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef]
- Wu, X.-F.; Block, M.L.; Zhang, W.; Qin, L.; Wilson, B.; Zhang, W.-Q.; Veronesi, B.; Hong, J.-S. The Role of Microglia in Paraquat-Induced Dopaminergic Neurotoxicity. Antioxid. Redox Signal. 2005, 7, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.-X.; Chen, G.-H.; Hu, L. Dopaminergic System Alteration in Anxiety and Compulsive Disorders: A Systematic Review of Neuroimaging Studies. Front. Neurosci. 2020, 14, 608520. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and Its Comorbidity with Other Clinical Disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef]
- Yang, R.; Ye, S.; Zhang, S.; Huang, H.; Zhang, Y.; Yang, Y.; Xie, S.; He, L.; Yang, Y.; Shi, J. Serotonin and Dopamine Depletion in Distinct Brain Regions May Cause Anxiety in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Mice as a Model of Early Parkinson’s Disease. Neuroreport 2023, 34, 551–559. [Google Scholar] [CrossRef]
- Tadaiesky, M.T.; Dombrowski, P.A.; Figueiredo, C.P.; Cargnin-Ferreira, E.; Da Cunha, C.; Takahashi, R.N. Emotional, Cognitive and Neurochemical Alterations in a Premotor Stage Model of Parkinson’s Disease. Neuroscience 2008, 156, 830–840. [Google Scholar] [CrossRef]
- Vieira, J.C.F.; Bassani, T.B.; Santiago, R.M.; de O Guaita, G.; Zanoveli, J.M.; da Cunha, C.; Vital, M.A.B.F. Anxiety-like Behavior Induced by 6-OHDA Animal Model of Parkinson’s Disease May Be Related to a Dysregulation of Neurotransmitter Systems in Brain Areas Related to Anxiety. Behav. Brain Res. 2019, 371, 111981. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, N.N.W.; Sellbach, A.; Matheson, S.; O’Sullivan, J.D.; Silburn, P.A.; Byrne, G.J.; Marsh, R.; Mellick, G.D. Anxiety Disorders in Parkinson’s Disease: Prevalence and Risk Factors. Mov. Disord. 2010, 25, 838–845. [Google Scholar] [CrossRef]
- Nègre-Pagès, L.; Grandjean, H.; Lapeyre-Mestre, M.; Montastruc, J.L.; Fourrier, A.; Lépine, J.P.; Rascol, O. DoPaMiP Study Group Anxious and Depressive Symptoms in Parkinson’s Disease: The French Cross-Sectionnal DoPaMiP Study. Mov. Disord. 2010, 25, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Li, Q.; Huo, A.-R.; Shen, T.-T.; Cao, J.-Q.; Liu, C.-F.; Liu, T.; Luo, W.-F.; Cong, Q.-F. Botulinum Neurotoxin A Ameliorates Depressive-like Behavior in a Reserpine-Induced Parkinson’s Disease Mouse Model via Suppressing Hippocampal Microglial Engulfment and Neuroinflammation. Acta Pharmacol. Sin. 2023, 44, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weihe, E.; Depboylu, C.; Schütz, B.; Schäfer, M.K.-H.; Eiden, L.E. Three Types of Tyrosine Hydroxylase-Positive CNS Neurons Distinguished by Dopa Decarboxylase and VMAT2 Co-Expression. Cell. Mol. Neurobiol. 2006, 26, 657–676. [Google Scholar] [CrossRef]
- Haavik, J.; Toska, K. Tyrosine Hydroxylase and Parkinson’s Disease. Mol. Neurobiol. 1998, 16, 285–309. [Google Scholar] [CrossRef]
- Ibragić, S.; Matak, I.; Dračić, A.; Smajlović, A.; Muminović, M.; Proft, F.; Sofić, E.; Lacković, Z.; Riederer, P. Effects of Botulinum Toxin Type A Facial Injection on Monoamines and Their Metabolites in Sensory, Limbic and Motor Brain Regions in Rats. Neurosci. Lett. 2016, 617, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-Distance Retrograde Effects of Botulinum Neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef]
- Hussain, L.S.; Reddy, V.; Maani, C.V. Physiology, Noradrenergic Synapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Khalil, B.; Rosani, A.; Warrington, S.J. Physiology, Catecholamines. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Naoi, M.; Wu, Y.; Maruyama, W.; Shamoto-Nagai, M. Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. Int. J. Mol. Sci. 2025, 26, 2916. [Google Scholar] [CrossRef]
- Yao, L.; Chen, R.; Zheng, Z.; Hatami, M.; Koc, S.; Wang, X.; Bai, Y.; Yao, C.; Lu, G.; Skutella, T. Translational Evaluation of Metabolic Risk Factors Impacting DBS Efficacy for PD-Related Sleep and Depressive Disorders: Preclinical, Prospective and Cohort Studies. Int. J. Surg. 2025, 111, 543–566. [Google Scholar] [CrossRef]
- Kandasamy, M. Theta Rhythm as a Real-Time Quantitative Marker for Non-Invasive Analysis of Adult Neurogenesis in the Intact Brain. J. Biol. Methods 2025, 0, e99010061. [Google Scholar] [CrossRef]
- Joseph, J.H.M.; Irakkam, M.P.B.D.; Kandasamy, M. The Proneurogenic and Microglial Modulatory Properties of Botulinum Toxin in the Hippocampus of Aging Experimental Mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- He, Y.; Han, Y.; Liao, X.; Zou, M.; Wang, Y. Biology of Cyclooxygenase-2: An Application in Depression Therapeutics. Front. Psychiatry 2022, 13, 1037588. [Google Scholar] [CrossRef]
- O’Connor, J.L.; Fountos, D.M.; Firouzan, B.; Azizi, F.; Ghasemi, R.; Kashfi, K. The Role of Gasotransmitters in Parkinson’s Disease: Interplay of Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide. Neurotherapeutics 2025, e00710. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Yokoyama, R.; Shibata, T.; Dawson, V.L.; Dawson, T.M. Role of Neuronal Nitric Oxide in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Induced Dopaminergic Neurotoxicity. Proc. Natl. Acad. Sci. USA 1996, 93, 4565–4571. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.-H.; Lee, K.-J.; Choi, M.-M.; Kim, Y.H.; Rhie, G.-E.; Yoo, C.-K.; Cha, K.; Shin, N.-R. Botulinum Neurotoxin Type A Induces TLR2-Mediated Inflammatory Responses in Macrophages. PLoS ONE 2015, 10, e0120840. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.J.; Yeo, I.J.; Jeon, S.H.; Lim, J.H.; Yoo, S.S.; Son, D.J.; Jang, S.-S.; Lee, H.; Shin, S.-J.; Han, S.B.; et al. Botulinum Toxin A Ameliorates Neuroinflammation in the MPTP and 6-OHDA-Induced Parkinson’s Disease Models. Biomol. Ther. 2022, 30, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Seo, H.-R.; Choi, M.; Kim, H.-Y.; Jin, S.-K.; Lee, M.; Baek, K.-H. Regulation of Botulinum Neurotoxin Half-life through the Ubiquitin-proteasome System. Mol. Med. Rep. 2025, 32, 268. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, J.H.M.; Babu Deva Irakkam, M.P.; Kandasamy, M. Therapeutic Botulinum Neurotoxin Ameliorates Motor Deficits and Anxiety, Accompanied by Dopaminergic Neuroprotection and Diminished Microglia Burden in the MPTP-Induced Mouse Model of Parkinson’s Disease. Brain Sci. 2025, 15, 916. https://doi.org/10.3390/brainsci15090916
Joseph JHM, Babu Deva Irakkam MP, Kandasamy M. Therapeutic Botulinum Neurotoxin Ameliorates Motor Deficits and Anxiety, Accompanied by Dopaminergic Neuroprotection and Diminished Microglia Burden in the MPTP-Induced Mouse Model of Parkinson’s Disease. Brain Sciences. 2025; 15(9):916. https://doi.org/10.3390/brainsci15090916
Chicago/Turabian StyleJoseph, Jerly Helan Mary, Mercy Priyadharshini Babu Deva Irakkam, and Mahesh Kandasamy. 2025. "Therapeutic Botulinum Neurotoxin Ameliorates Motor Deficits and Anxiety, Accompanied by Dopaminergic Neuroprotection and Diminished Microglia Burden in the MPTP-Induced Mouse Model of Parkinson’s Disease" Brain Sciences 15, no. 9: 916. https://doi.org/10.3390/brainsci15090916
APA StyleJoseph, J. H. M., Babu Deva Irakkam, M. P., & Kandasamy, M. (2025). Therapeutic Botulinum Neurotoxin Ameliorates Motor Deficits and Anxiety, Accompanied by Dopaminergic Neuroprotection and Diminished Microglia Burden in the MPTP-Induced Mouse Model of Parkinson’s Disease. Brain Sciences, 15(9), 916. https://doi.org/10.3390/brainsci15090916






