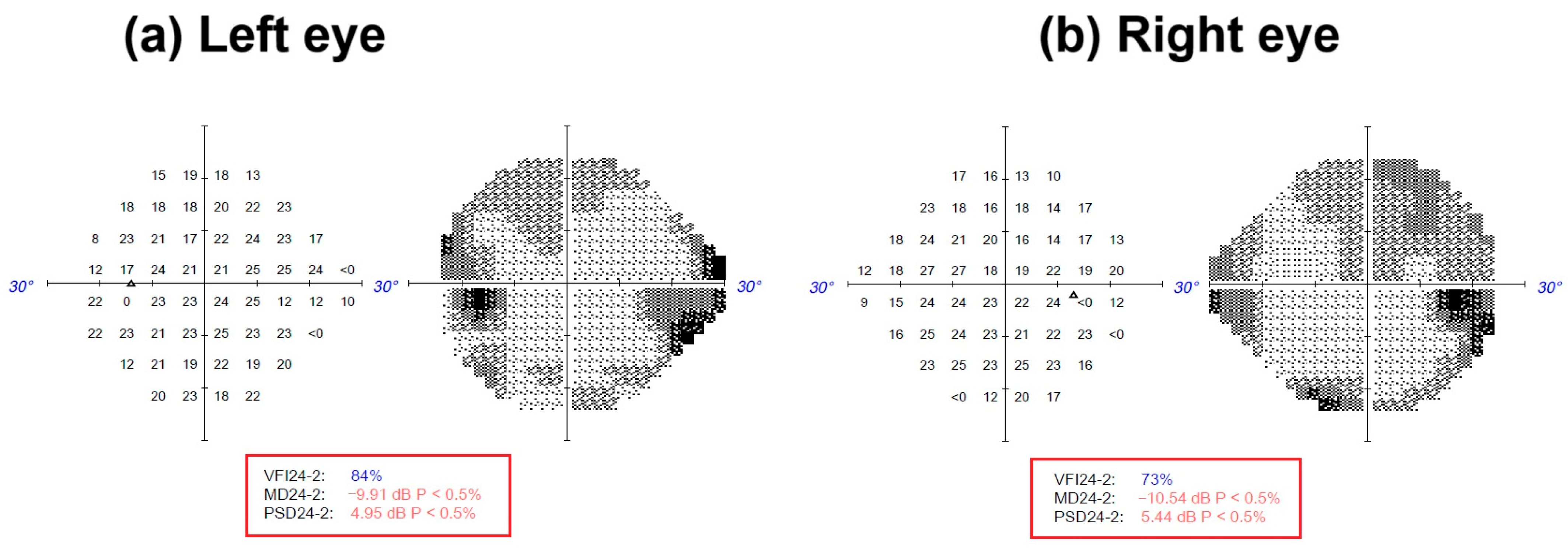
Figure 1.
Visual field index (VFI) perimetry of the patient was obtained by standard automated perimetry (SAP) with a ZEISS Humphrey Field Analyzer 3™ (ZEISS, Jena, Germany). The 24-2 visual field test divides the central 24° into 54 threshold points. A light stimulus dims or brightens until the patient detects it, mapping retinal sensitivity across the near-periphery [
5]. (
a)
Left eye: VFI24-2: 84%; MD24-2: −9.1 dB P < 0.5%; PSD24-2: 4.95 dB P < 0.5%. (
b)
Right eye: VFI24-2: 73%; MD24-2: −10.54 dB P < 0.5%; PSD24-2: 5.44 dB P < 0.5%. dB: decibel (indicates the light stimulus intensity that a patient can detect. This is a logarithmic scale, where higher dB values indicate greater sensitivity to light). MD: mean deviation. PSD: pattern standard deviation.
Figure 1.
Visual field index (VFI) perimetry of the patient was obtained by standard automated perimetry (SAP) with a ZEISS Humphrey Field Analyzer 3™ (ZEISS, Jena, Germany). The 24-2 visual field test divides the central 24° into 54 threshold points. A light stimulus dims or brightens until the patient detects it, mapping retinal sensitivity across the near-periphery [
5]. (
a)
Left eye: VFI24-2: 84%; MD24-2: −9.1 dB P < 0.5%; PSD24-2: 4.95 dB P < 0.5%. (
b)
Right eye: VFI24-2: 73%; MD24-2: −10.54 dB P < 0.5%; PSD24-2: 5.44 dB P < 0.5%. dB: decibel (indicates the light stimulus intensity that a patient can detect. This is a logarithmic scale, where higher dB values indicate greater sensitivity to light). MD: mean deviation. PSD: pattern standard deviation.
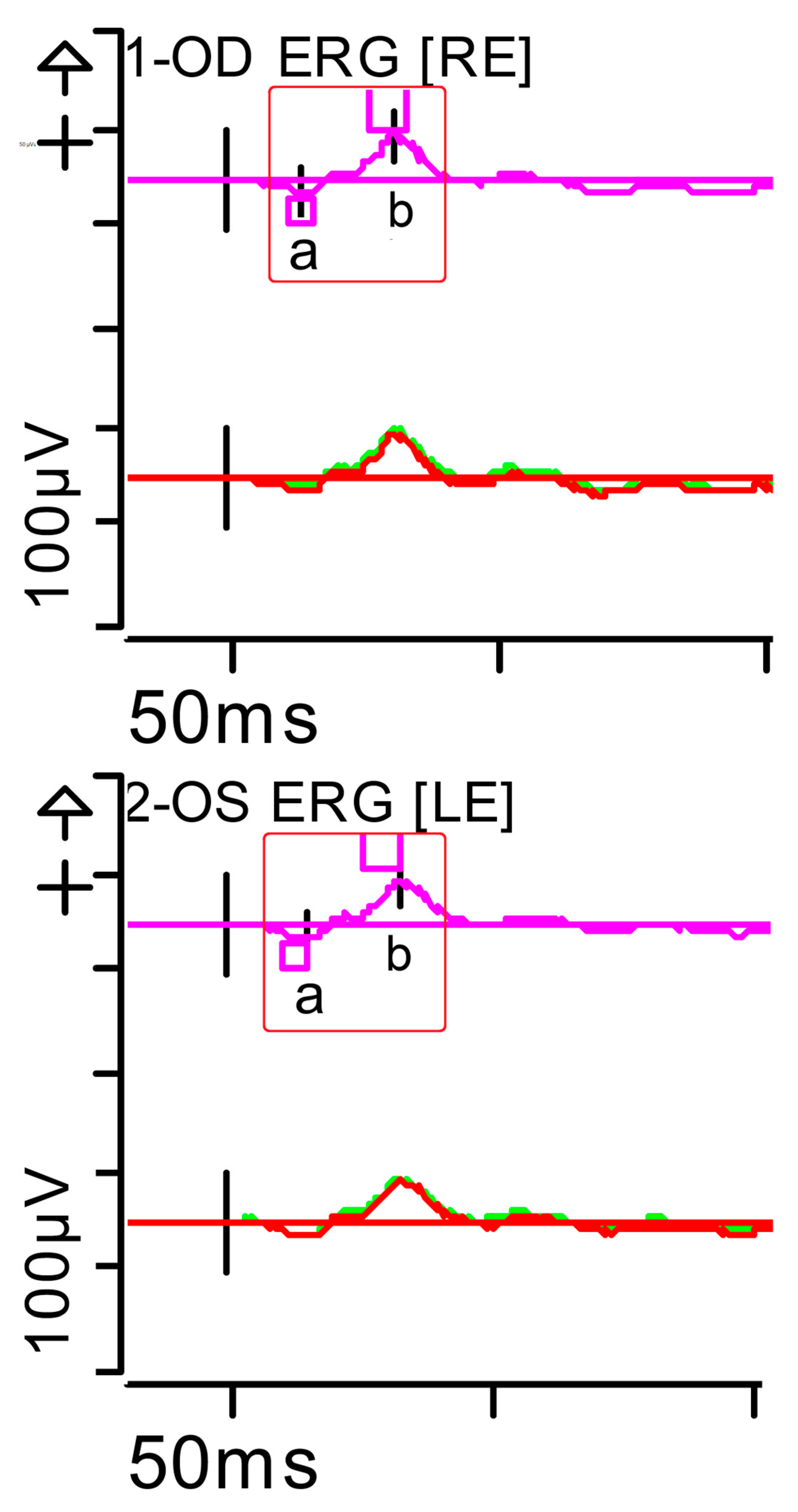
Figure 2.
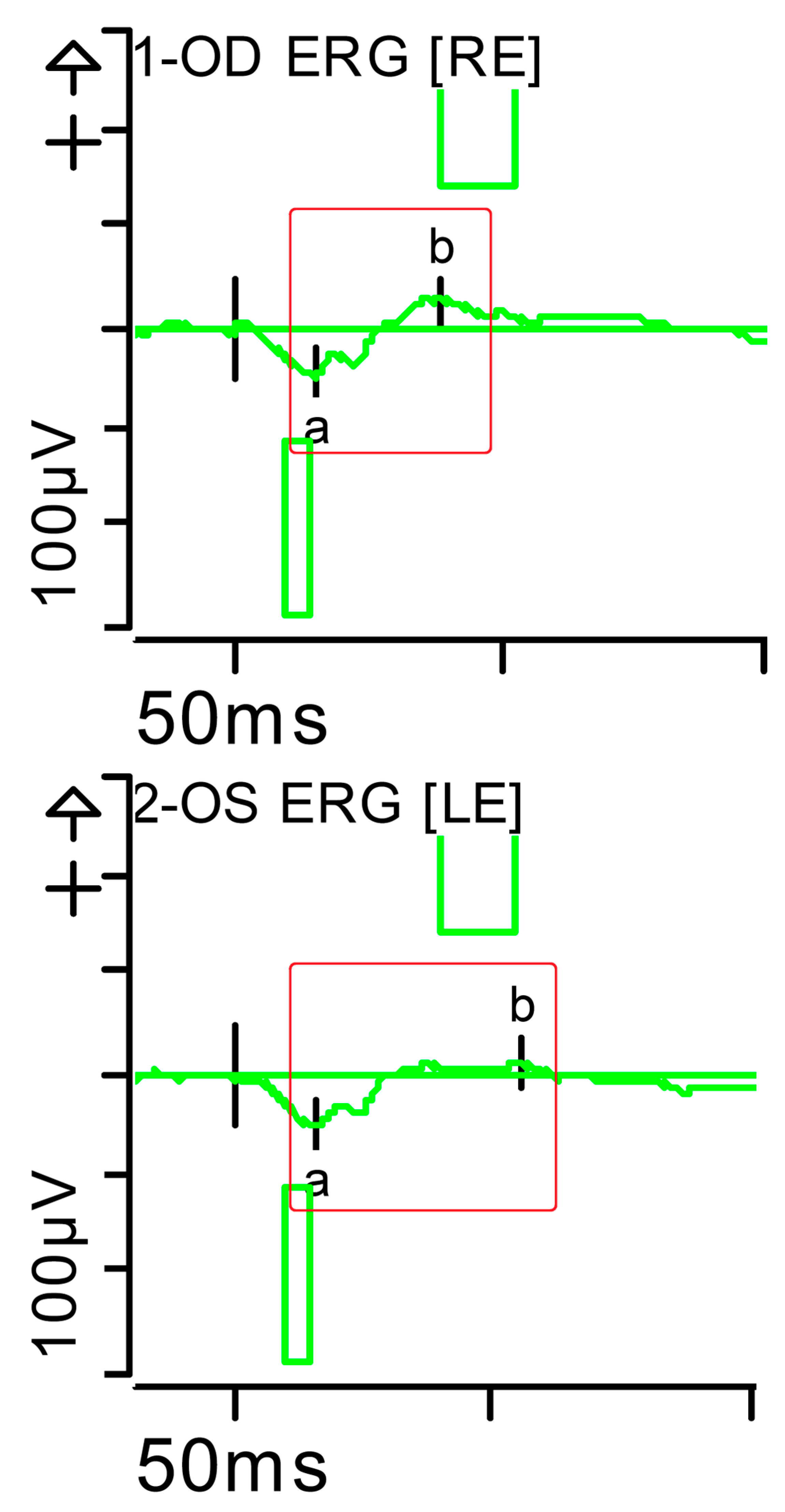
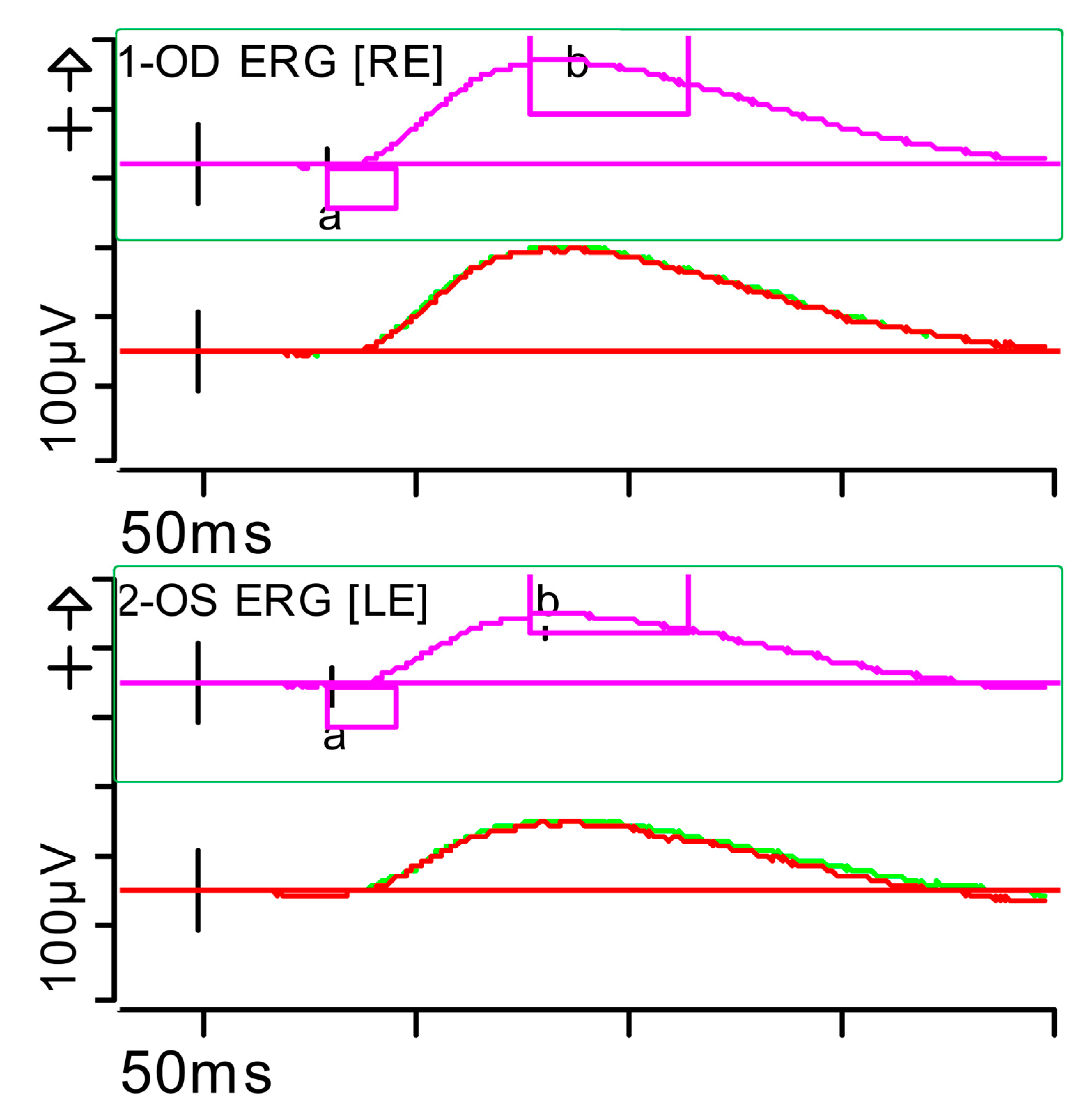
Full-field ERG at presentation. Cone response (light-adapted [LA] 3.0 candelas per square metre [cd·s·m−2]): peak latency (implicit time) of the a- and b-waves within normal limits, with amplitudes at the lower limits of normality (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: a: −12.09 µV (Norm: −29.4 ± 15.7), 15 ms (Norm: 14.1 ± 2.7); b: 59.37 µV (Norm: 118.3 ± 55.9), 32 ms (Norm: 30.5 ± 3.7). Markers 2-OS: a: −11.47 µV (Norm: −29.4 ± 15.7), 15.5 ms (Norm: 14.1 ± 2.7); b: 55.4 µV (Norm: 118.3 ± 55.9), 33.5 ms (Norm: 30.5 ± 3.7). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 2.
Full-field ERG at presentation. Cone response (light-adapted [LA] 3.0 candelas per square metre [cd·s·m−2]): peak latency (implicit time) of the a- and b-waves within normal limits, with amplitudes at the lower limits of normality (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: a: −12.09 µV (Norm: −29.4 ± 15.7), 15 ms (Norm: 14.1 ± 2.7); b: 59.37 µV (Norm: 118.3 ± 55.9), 32 ms (Norm: 30.5 ± 3.7). Markers 2-OS: a: −11.47 µV (Norm: −29.4 ± 15.7), 15.5 ms (Norm: 14.1 ± 2.7); b: 55.4 µV (Norm: 118.3 ± 55.9), 33.5 ms (Norm: 30.5 ± 3.7). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
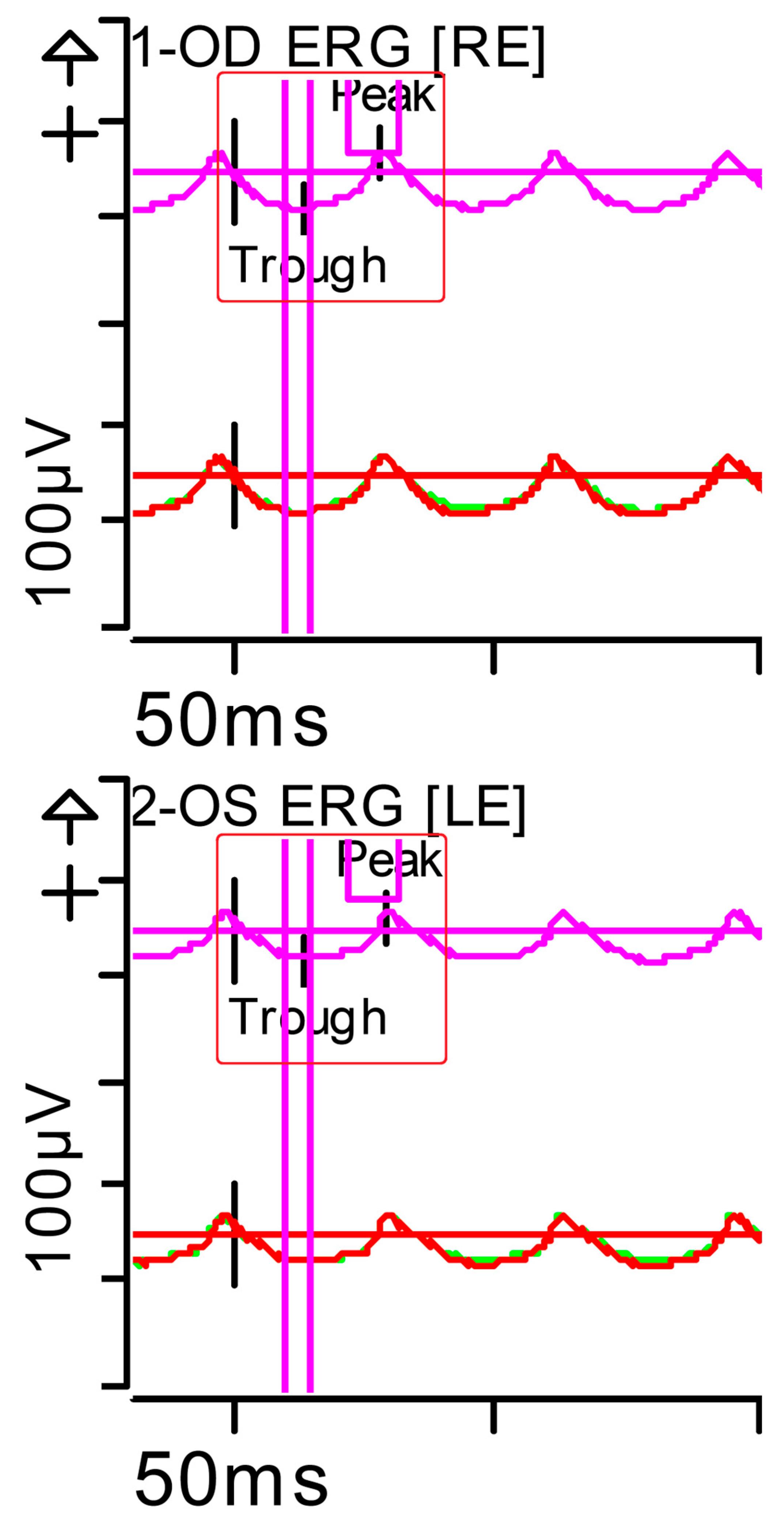
Figure 3.
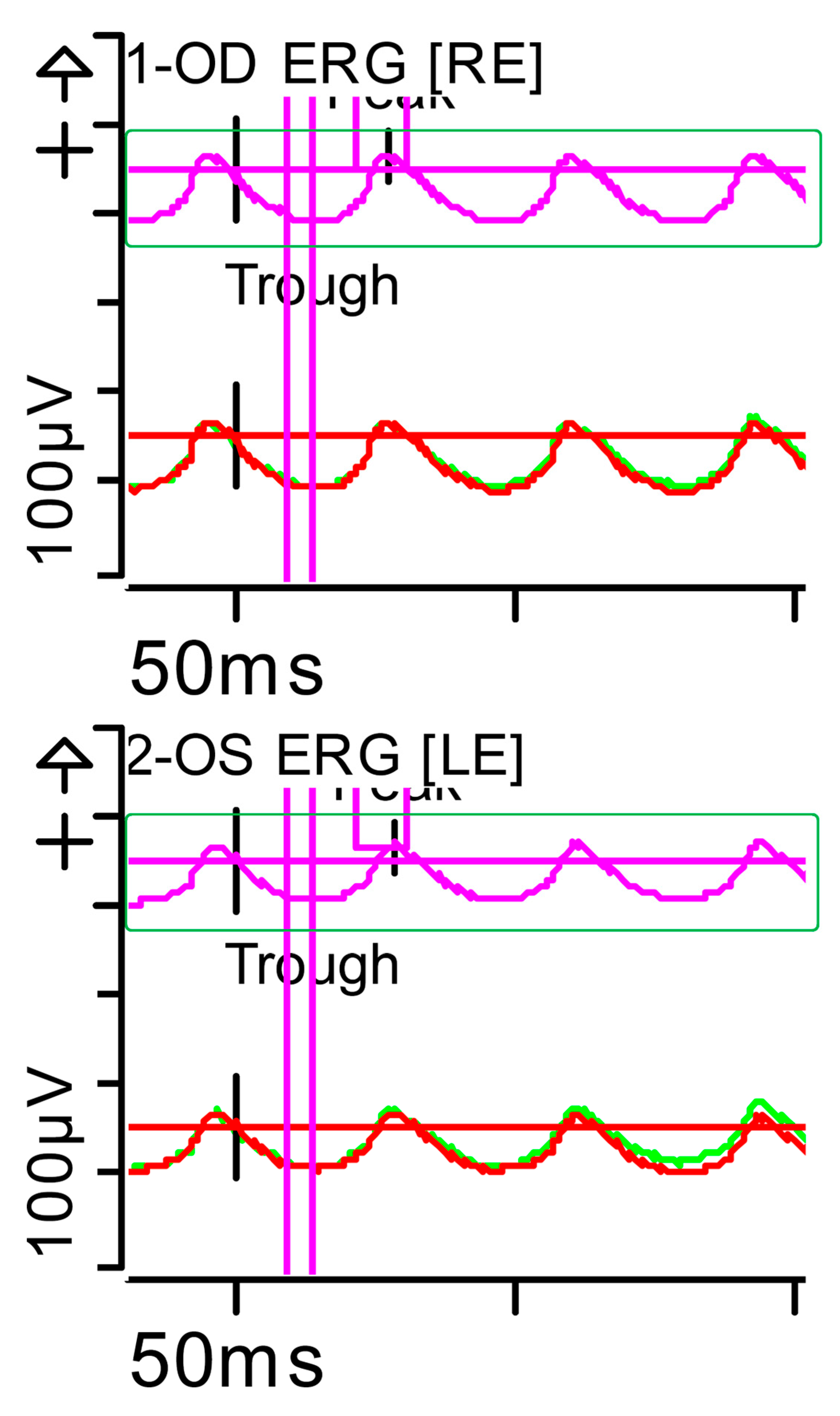
Full-field ERG at presentation. Flicker (light-adapted [LA] 30 Hz 3.0 candelas per square metre [cd·s·m−2]): peak and trough latencies within normal limits, with amplitudes at the lower limits of normality (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: Trough 13.5 ms (Norm: 12.5 ± 2.8); Peak 55.48 ms (Norm: 103.8 ± 47.4), 29 ms (Norm: 26.7 ± 4.9). Markers 2-OS: Trough 14.5 ms (Norm: 12.5 ± 2.8); Peak 44.79 ms (Norm: 103.8 ± 47.4), 30 ms (Norm: 26.7 ± 4.9). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). Bgnd: background; K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 3.
Full-field ERG at presentation. Flicker (light-adapted [LA] 30 Hz 3.0 candelas per square metre [cd·s·m−2]): peak and trough latencies within normal limits, with amplitudes at the lower limits of normality (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: Trough 13.5 ms (Norm: 12.5 ± 2.8); Peak 55.48 ms (Norm: 103.8 ± 47.4), 29 ms (Norm: 26.7 ± 4.9). Markers 2-OS: Trough 14.5 ms (Norm: 12.5 ± 2.8); Peak 44.79 ms (Norm: 103.8 ± 47.4), 30 ms (Norm: 26.7 ± 4.9). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). Bgnd: background; K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
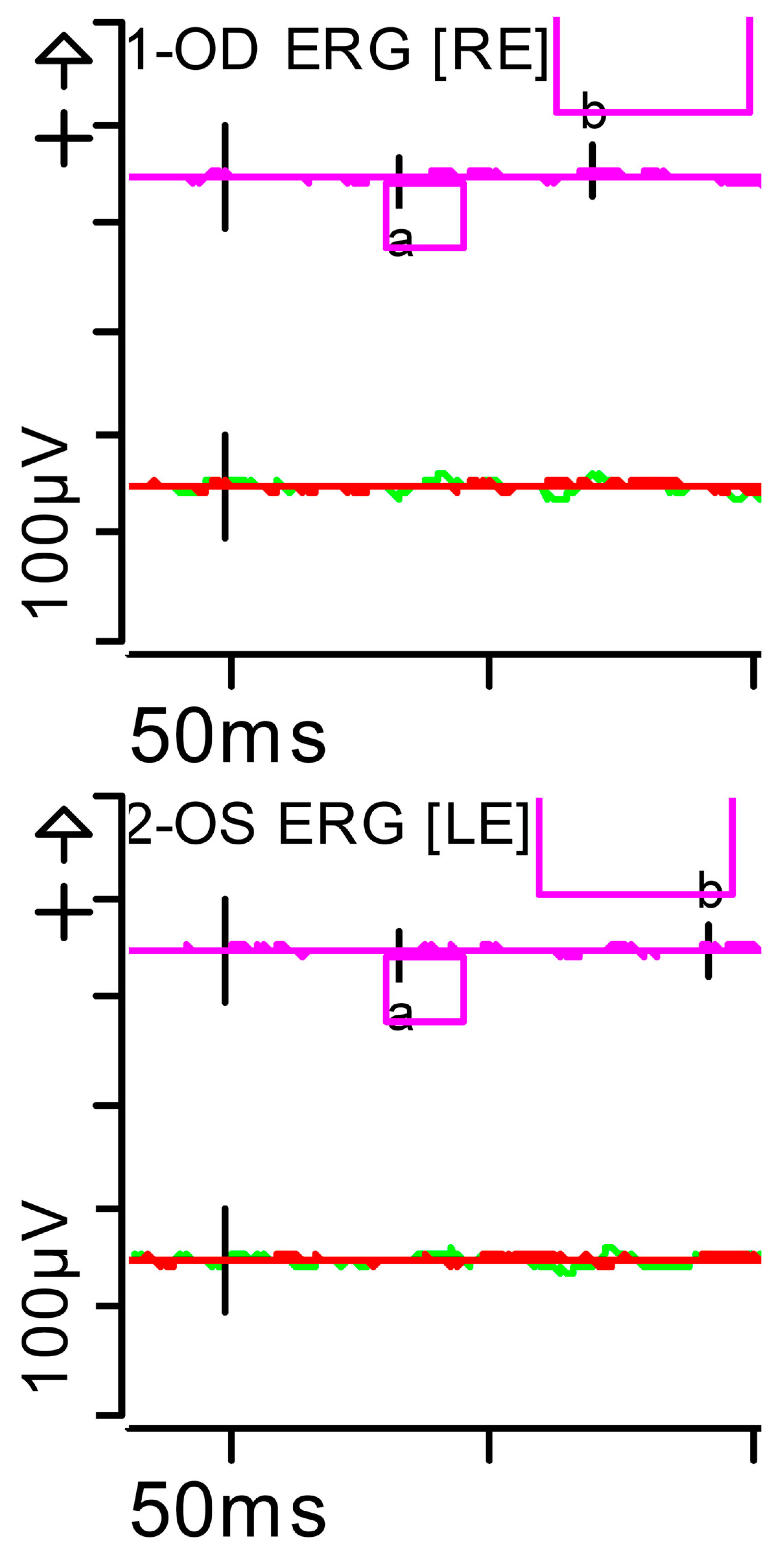
Figure 4.
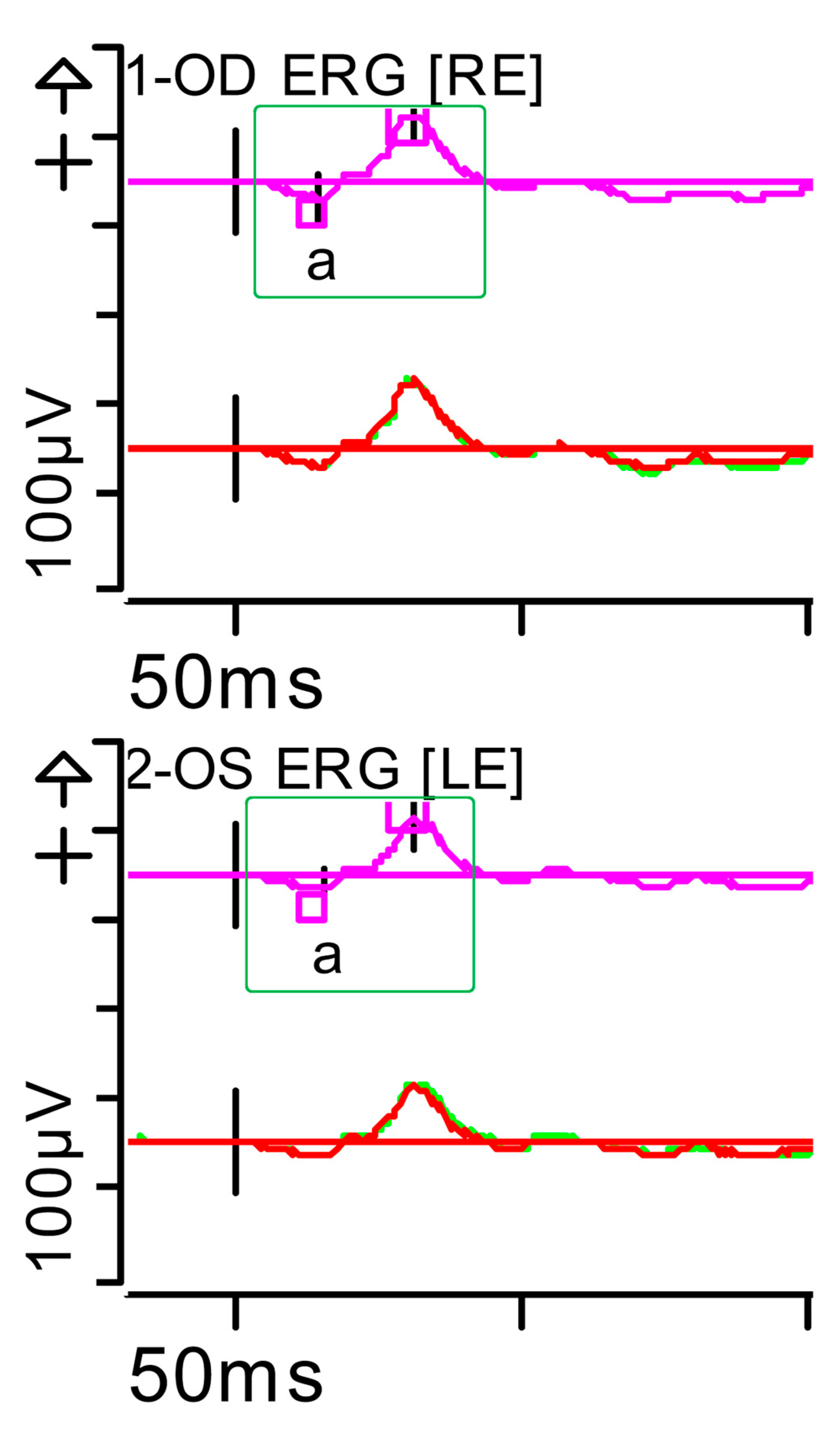
Full-field ERG at presentation. Rod response (dark-adapted [DA] 0.01 candelas per square metre [cd·s·m−2]): absent responses were noted. Step parameters: Flash. 0.01 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −4.201 µV (Norm: −31.5 ± 33.9), 34 ms (Norm: 39.1 ± 8.3); b: 10.83 µV (Norm: 208.9 ± 139.3), 70.5 ms (Norm: 97.7 ± 19.5). Markers 2-OS: a: −2.011 µV (Norm: −31.5 ± 33.9), 33.5 ms (Norm: 39.1 ± 8.3); b: 7.877 µV (Norm: 208.9 ± 139.3), 93.5 ms (Norm: 97.7 ± 19.5). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 4.
Full-field ERG at presentation. Rod response (dark-adapted [DA] 0.01 candelas per square metre [cd·s·m−2]): absent responses were noted. Step parameters: Flash. 0.01 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −4.201 µV (Norm: −31.5 ± 33.9), 34 ms (Norm: 39.1 ± 8.3); b: 10.83 µV (Norm: 208.9 ± 139.3), 70.5 ms (Norm: 97.7 ± 19.5). Markers 2-OS: a: −2.011 µV (Norm: −31.5 ± 33.9), 33.5 ms (Norm: 39.1 ± 8.3); b: 7.877 µV (Norm: 208.9 ± 139.3), 93.5 ms (Norm: 97.7 ± 19.5). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
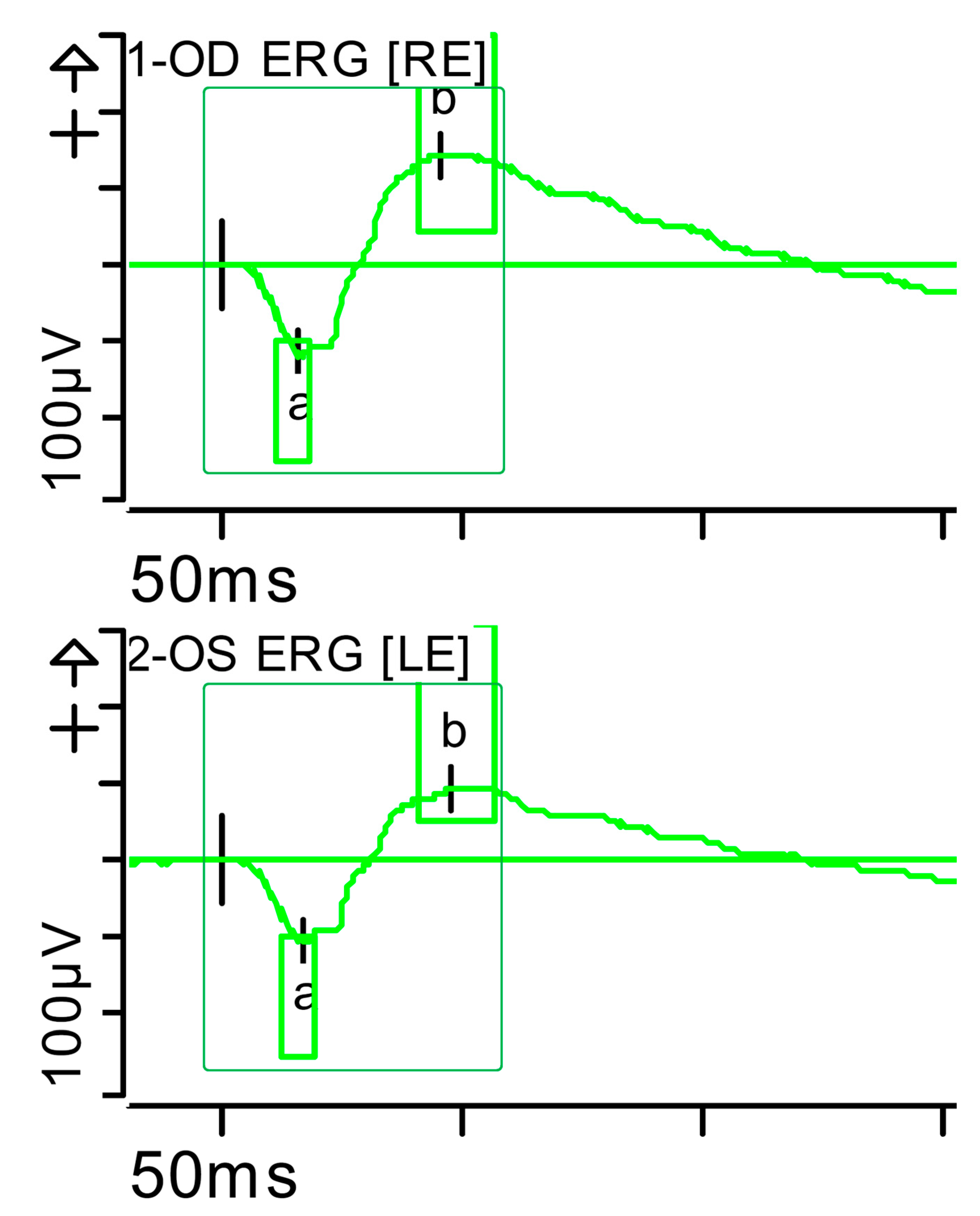
Figure 5.
Combined response (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2]): normal latencies, with severely reduced amplitudes of the a- and b-waves were noted (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −39.12 µV (Norm: −178.7 ± 80.7), 17.5 ms (Norm: 15.9 ± 4.4); b: 72.87 µV (Norm: 290.4 ± 131.2), 42.5 ms (Norm: 49.2 ± 8). Markers 2-OS: −34.09 µV (Norm: −178.7 ± 80.7), 18 ms (Norm: 15.9 ± 4.4); b: 60.51 µV (Norm: 290.4 ± 131.2), 46 ms (Norm: 49.2 ± 8). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 5.
Combined response (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2]): normal latencies, with severely reduced amplitudes of the a- and b-waves were noted (red rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −39.12 µV (Norm: −178.7 ± 80.7), 17.5 ms (Norm: 15.9 ± 4.4); b: 72.87 µV (Norm: 290.4 ± 131.2), 42.5 ms (Norm: 49.2 ± 8). Markers 2-OS: −34.09 µV (Norm: −178.7 ± 80.7), 18 ms (Norm: 15.9 ± 4.4); b: 60.51 µV (Norm: 290.4 ± 131.2), 46 ms (Norm: 49.2 ± 8). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
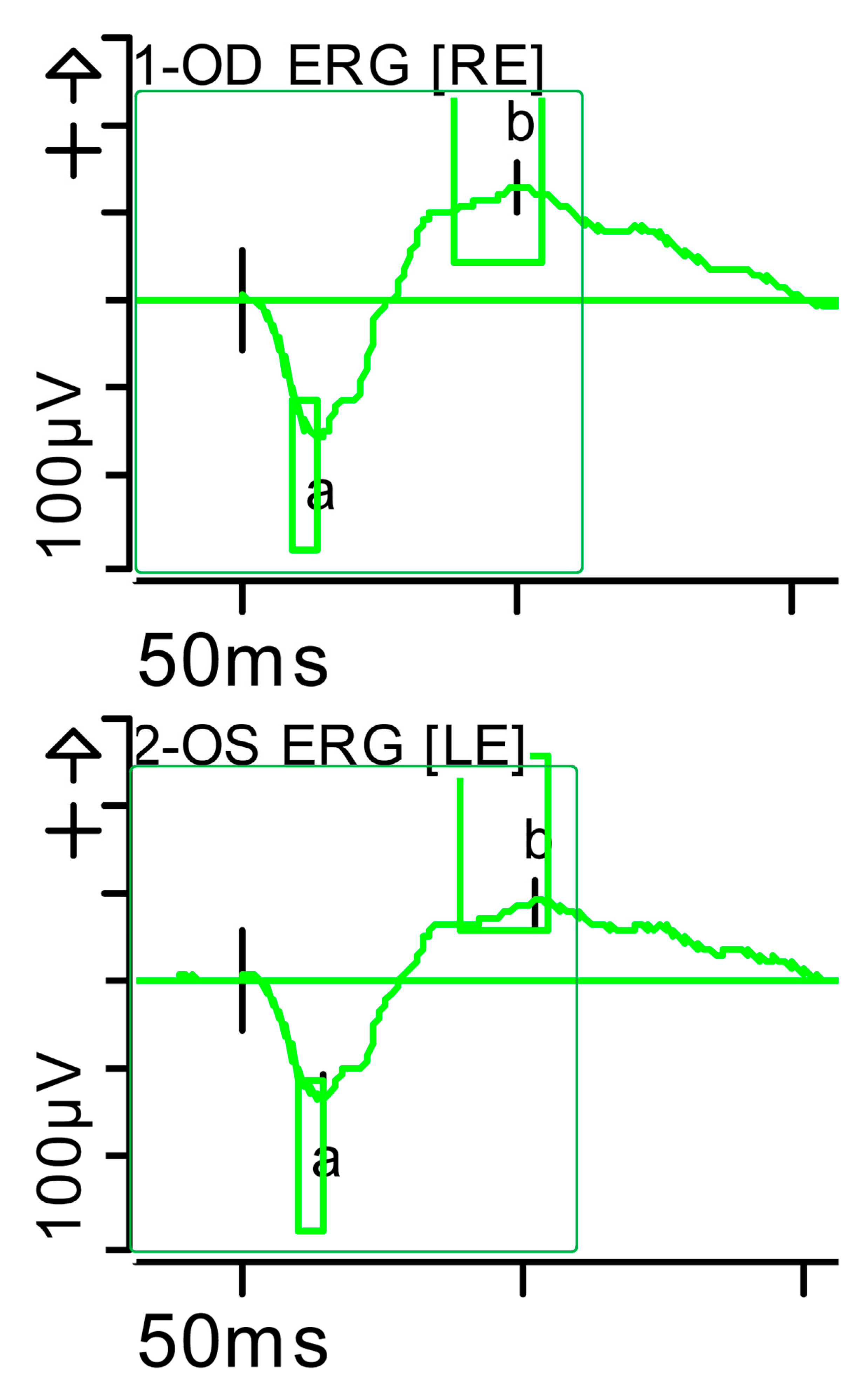
Figure 6.
Full-field ERG at presentation. Maximum response 10 (dark-adapted [DA] 10.0 candelas per square metre [cd·s·m−2]): normal latencies, with severely reduced amplitudes of the a- and b-waves were noted, especially on the left side (red rectangles). Step parameters: Flash. 10 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −47.3 µV (Norm: −200.5 ± 90.5), 16 ms (Norm: 12.5 ± 2.6); b: 76.33 µV (Norm: 297.3 ± 105.8), 40 ms (Norm: 47.2 ± 8.1). Markers 2-OS: a: −51.19 µV (Norm: −200.5 ± 90.5), 16.5 ms (Norm: 12.5 ± 2.6); b: 65.28 µV (Norm: 297.3 ± 105.8), 56 ms (Norm: 47.2 ± 8.1). K: Kelvin; LE: left eye; OD: oculus dexter; (P): photometric; OS: oculus sinister; RE: right eye.
Figure 6.
Full-field ERG at presentation. Maximum response 10 (dark-adapted [DA] 10.0 candelas per square metre [cd·s·m−2]): normal latencies, with severely reduced amplitudes of the a- and b-waves were noted, especially on the left side (red rectangles). Step parameters: Flash. 10 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −47.3 µV (Norm: −200.5 ± 90.5), 16 ms (Norm: 12.5 ± 2.6); b: 76.33 µV (Norm: 297.3 ± 105.8), 40 ms (Norm: 47.2 ± 8.1). Markers 2-OS: a: −51.19 µV (Norm: −200.5 ± 90.5), 16.5 ms (Norm: 12.5 ± 2.6); b: 65.28 µV (Norm: 297.3 ± 105.8), 56 ms (Norm: 47.2 ± 8.1). K: Kelvin; LE: left eye; OD: oculus dexter; (P): photometric; OS: oculus sinister; RE: right eye.
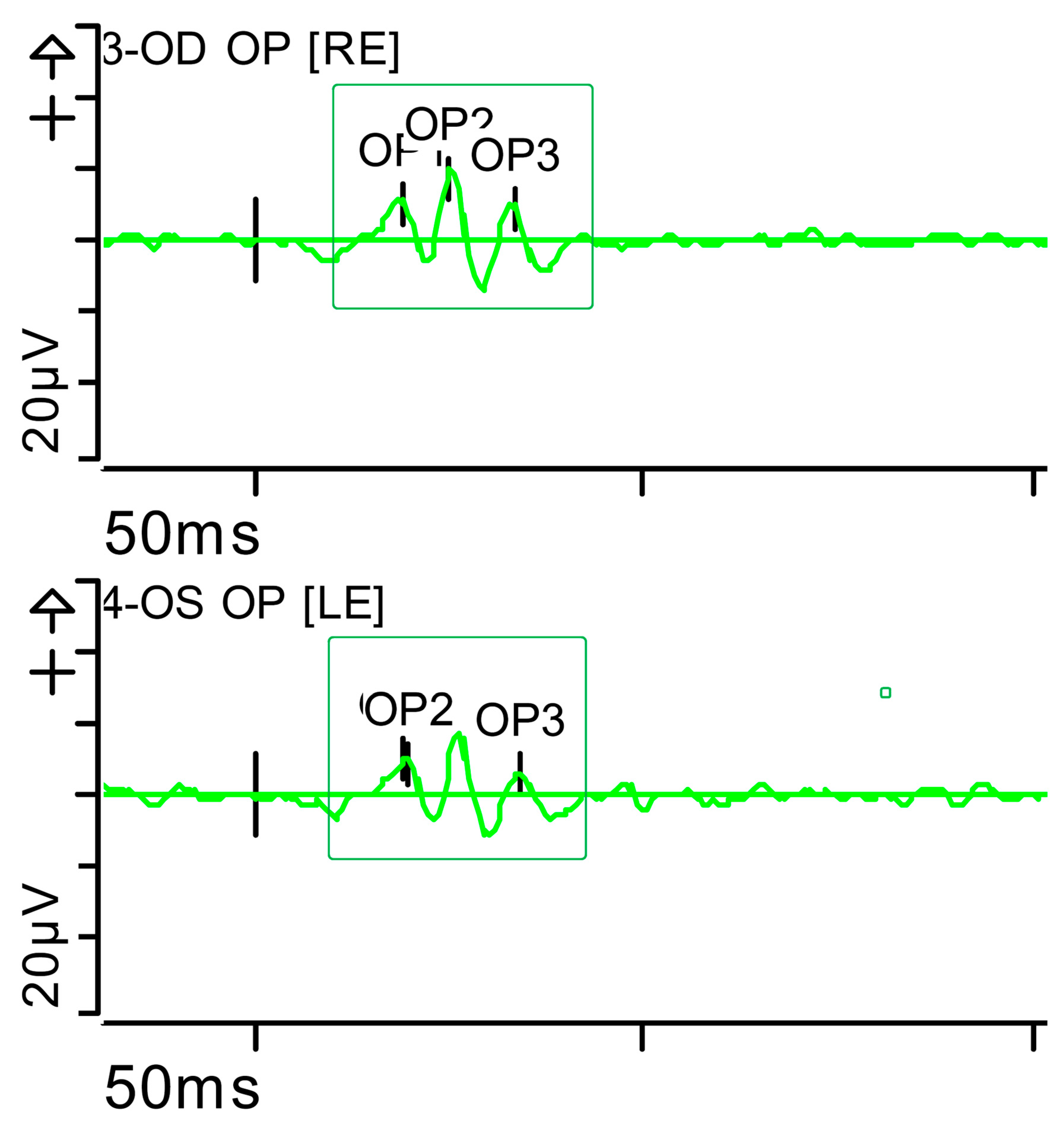
Figure 7.
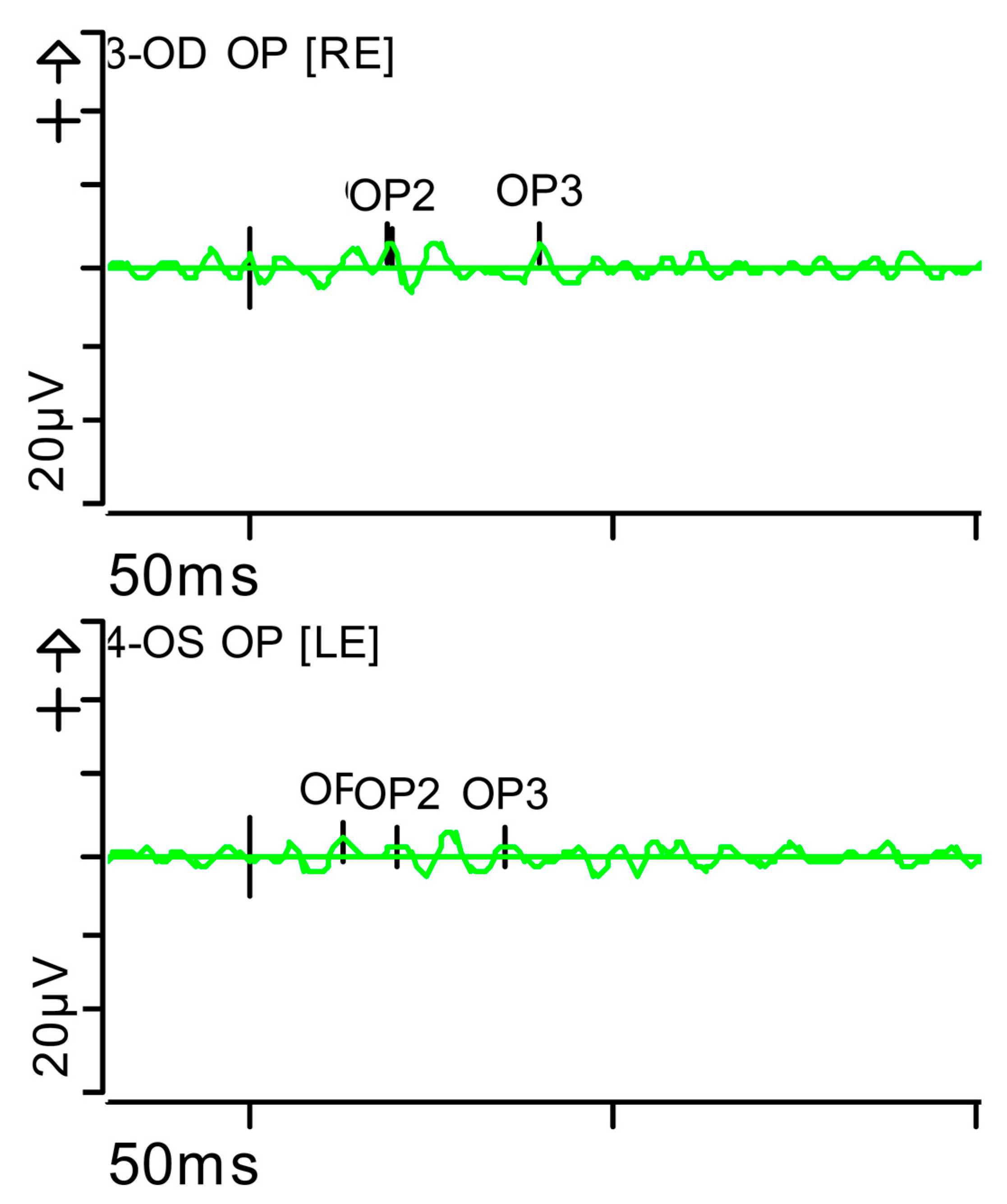
Full-field ERG at presentation. Oscillatory potentials (OP) (dark-adapted [DA] candelas per square metre 3.0 cd·s·m−2]): very poorly configured on both sides, hardly discernible. Oscillatory potentials: inner plexiform layer (amacrine cells). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 7.
Full-field ERG at presentation. Oscillatory potentials (OP) (dark-adapted [DA] candelas per square metre 3.0 cd·s·m−2]): very poorly configured on both sides, hardly discernible. Oscillatory potentials: inner plexiform layer (amacrine cells). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 8.
Full-field ERG at month 5. Cone response (light-adapted [LA] 3.0 candelas per square metre [cd·s·m−2]: peak latency (implicit time) of the a- and b-waves within normal limits, as well as their amplitudes (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: a: −19.98 µV (Norm: −29.4 ± 15.7), 15.5 ms (Norm: 14.1 ± 2.7); b: 93.96 µV (Norm: 118.3 ± 55.9), 31.5 ms (Norm: 30.5 ± 3.7). Markers 2-OS: a: −15.32 µV (Norm: −29.4 ± 15.7), 16 ms (Norm: 14.1 ± 2.7); b: 76.96 µV (Norm: 118.3 ± 55.9), 31.5 ms (Norm: 30.5 ± 3.7). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 8.
Full-field ERG at month 5. Cone response (light-adapted [LA] 3.0 candelas per square metre [cd·s·m−2]: peak latency (implicit time) of the a- and b-waves within normal limits, as well as their amplitudes (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: a: −19.98 µV (Norm: −29.4 ± 15.7), 15.5 ms (Norm: 14.1 ± 2.7); b: 93.96 µV (Norm: 118.3 ± 55.9), 31.5 ms (Norm: 30.5 ± 3.7). Markers 2-OS: a: −15.32 µV (Norm: −29.4 ± 15.7), 16 ms (Norm: 14.1 ± 2.7); b: 76.96 µV (Norm: 118.3 ± 55.9), 31.5 ms (Norm: 30.5 ± 3.7). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 9.
Full-field ERG at month 5. Flicker (light-adapted [LA] 30 Hz 3.0 candelas per square metre [cd·s·m−2]: symmetrical responses, with normal peak latency (implicit time), morphology, and peak amplitude bilaterally (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: Trough 14 ms (Norm: 12.5 ± 2.8); Peak 73.48 ms (Norm: 103.8 ± 47.4), 27.5 ms (Norm: 26.7 ± 4.9). Markers 2-OS: Trough 14.5 ms (Norm: 12.5 ± 2.8); Peak 63.34 ms (Norm: 103.8 ± 47.4), 28.5 ms (Norm: 26.7 ± 4.9). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). Bgnd: background; K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 9.
Full-field ERG at month 5. Flicker (light-adapted [LA] 30 Hz 3.0 candelas per square metre [cd·s·m−2]: symmetrical responses, with normal peak latency (implicit time), morphology, and peak amplitude bilaterally (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Background (Bgnd) 30 (P) cd·s·m−2. White—6.500K. Markers 1-OD: Trough 14 ms (Norm: 12.5 ± 2.8); Peak 73.48 ms (Norm: 103.8 ± 47.4), 27.5 ms (Norm: 26.7 ± 4.9). Markers 2-OS: Trough 14.5 ms (Norm: 12.5 ± 2.8); Peak 63.34 ms (Norm: 103.8 ± 47.4), 28.5 ms (Norm: 26.7 ± 4.9). Each division on the Y-axis represents 100 microvolts (100 μV) and each division on the X-axis represents 50 milliseconds (50 ms). Bgnd: background; K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 10.
Full-field ERG at month 5. Rod response (dark-adapted [DA] 0.01 candelas per square metre [cd·s·m−2]): normal b-wave peak latency (implicit time) as well as their amplitudes (slight inter-side asymmetry, amplitude on the left side 32% lower than the right, not significant) (green rectangles). Step parameters: Flash. 0.01 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −2.87 µV (Norm: −31.5 ± 33.9), 30.5 ms (Norm: 39.1 ± 8.3); b: 150.5 µV (Norm: 208.9 ± 139.3), 89 ms (Norm: 97.7 ± 19.5). Markers 2-OS: a: −3.661 µV (Norm: −31.5 ± 33.9), 31.5 ms (Norm: 39.1 ± 8.3); b: 102.2 µV (Norm: 208.9 ± 139.3), 81.5 ms (Norm: 97.7 ± 19.5). Each division on the Y-axis represents 100 microvolts (100 μV) and on the X-axis 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 10.
Full-field ERG at month 5. Rod response (dark-adapted [DA] 0.01 candelas per square metre [cd·s·m−2]): normal b-wave peak latency (implicit time) as well as their amplitudes (slight inter-side asymmetry, amplitude on the left side 32% lower than the right, not significant) (green rectangles). Step parameters: Flash. 0.01 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −2.87 µV (Norm: −31.5 ± 33.9), 30.5 ms (Norm: 39.1 ± 8.3); b: 150.5 µV (Norm: 208.9 ± 139.3), 89 ms (Norm: 97.7 ± 19.5). Markers 2-OS: a: −3.661 µV (Norm: −31.5 ± 33.9), 31.5 ms (Norm: 39.1 ± 8.3); b: 102.2 µV (Norm: 208.9 ± 139.3), 81.5 ms (Norm: 97.7 ± 19.5). Each division on the Y-axis represents 100 microvolts (100 μV) and on the X-axis 50 milliseconds (50 ms). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 11.
Full-field ERG at month 5. Combined response (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2]): normal peak latency (implicit time) of the a- and b-waves, as well as their amplitudes (slight interocular asymmetry, amplitude on the left side 22% lower than the right, not significant) (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −117 µV (Norm: −178.7 ± 80.7), 17 ms (Norm: 15.9 ± 4.4); b: 259.7 µV (Norm: 290.4 ± 131.2), 46.5 ms (Norm: 49.2 ± 8). Markers 2-OS: −108.02 µV (Norm: −178.7 ± 80.7), 18 ms (Norm: 15.9 ± 4.4); b: 201 µV (Norm: 290.4 ± 131.2), 48.5 ms (Norm: 49.2 ± 8). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 11.
Full-field ERG at month 5. Combined response (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2]): normal peak latency (implicit time) of the a- and b-waves, as well as their amplitudes (slight interocular asymmetry, amplitude on the left side 22% lower than the right, not significant) (green rectangles). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −117 µV (Norm: −178.7 ± 80.7), 17 ms (Norm: 15.9 ± 4.4); b: 259.7 µV (Norm: 290.4 ± 131.2), 46.5 ms (Norm: 49.2 ± 8). Markers 2-OS: −108.02 µV (Norm: −178.7 ± 80.7), 18 ms (Norm: 15.9 ± 4.4); b: 201 µV (Norm: 290.4 ± 131.2), 48.5 ms (Norm: 49.2 ± 8). K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 12.
Full-field ERG at month 5. Maximum response 10 (dark-adapted [DA] 10.0 0 candelas per square metre [cd·s·m−2]: normal peak latency (implicit time) of the a- and b-waves, as well as their amplitudes (slight interocular asymmetry, amplitude on the left side 20% lower than the right, not significant) (green rectangles). Step parameters: Flash. 10 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −153.4 µV (Norm: −200.5 ± 90.5), 14.5 ms (Norm: 12.5 ± 2.6); b: 281.2 µV (Norm: 297.3 ± 105.8), 51 ms (Norm: 47.2 ± 8.1). Markers 2-OS: a: −136.5 µV (Norm: −200.5 ± 90.5), 15 ms (Norm: 12.5 ± 2.6); b: 226.7 µV (Norm: 297.3 ± 105.8), 53 ms (Norm: 47.2 ± 8.1). K: Kelvin; LE: left eye; OD: oculus dexter; (P): photometric; OS: oculus sinister; RE: right eye.
Figure 12.
Full-field ERG at month 5. Maximum response 10 (dark-adapted [DA] 10.0 0 candelas per square metre [cd·s·m−2]: normal peak latency (implicit time) of the a- and b-waves, as well as their amplitudes (slight interocular asymmetry, amplitude on the left side 20% lower than the right, not significant) (green rectangles). Step parameters: Flash. 10 (P)cd·s·m−2. White—6.500K. Markers 1-OD: a: −153.4 µV (Norm: −200.5 ± 90.5), 14.5 ms (Norm: 12.5 ± 2.6); b: 281.2 µV (Norm: 297.3 ± 105.8), 51 ms (Norm: 47.2 ± 8.1). Markers 2-OS: a: −136.5 µV (Norm: −200.5 ± 90.5), 15 ms (Norm: 12.5 ± 2.6); b: 226.7 µV (Norm: 297.3 ± 105.8), 53 ms (Norm: 47.2 ± 8.1). K: Kelvin; LE: left eye; OD: oculus dexter; (P): photometric; OS: oculus sinister; RE: right eye.
Figure 13.
Full-field ERG at month 5. Oscillatory potentials (OP) (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2] : well-configured bilaterally, with a normal number of them (green rectangles). oscillatory potentials: inner plexiform layer (amacrine cells). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.
Figure 13.
Full-field ERG at month 5. Oscillatory potentials (OP) (dark-adapted [DA] 3.0 candelas per square metre [cd·s·m−2] : well-configured bilaterally, with a normal number of them (green rectangles). oscillatory potentials: inner plexiform layer (amacrine cells). Step parameters: Flash. 3 (P)cd·s·m−2. White—6.500K. K: Kelvin; LE: left eye; OD: oculus dexter; OS: oculus sinister; (P): photometric; RE: right eye.