Beyond Hot Flashes: The Role of Estrogen Receptors in Menopausal Mental Health and Cognitive Decline
Abstract
1. Introduction
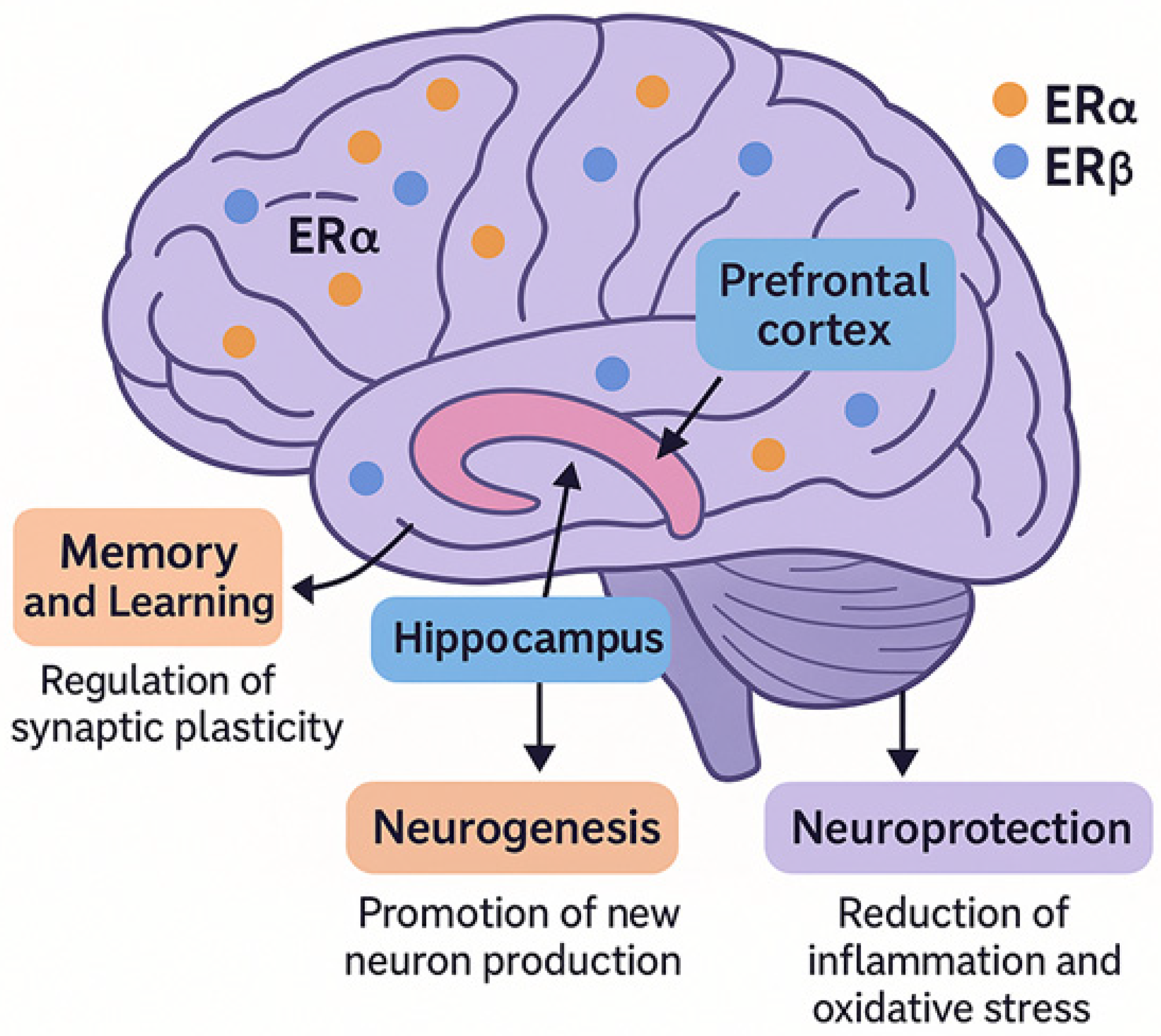
2. Estrogen Receptors in the Brain
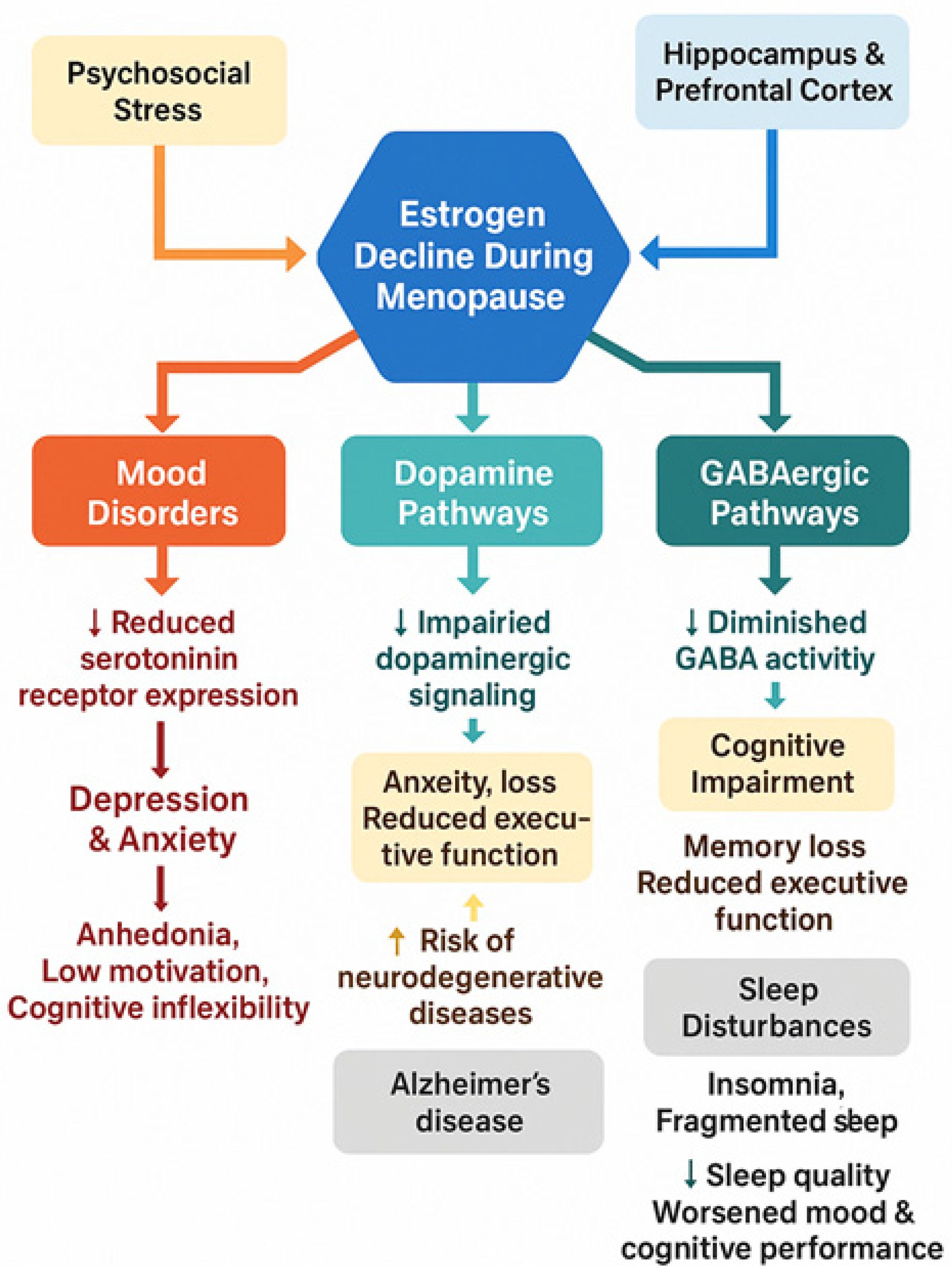
3. Estrogen and Menopausal Mental Health
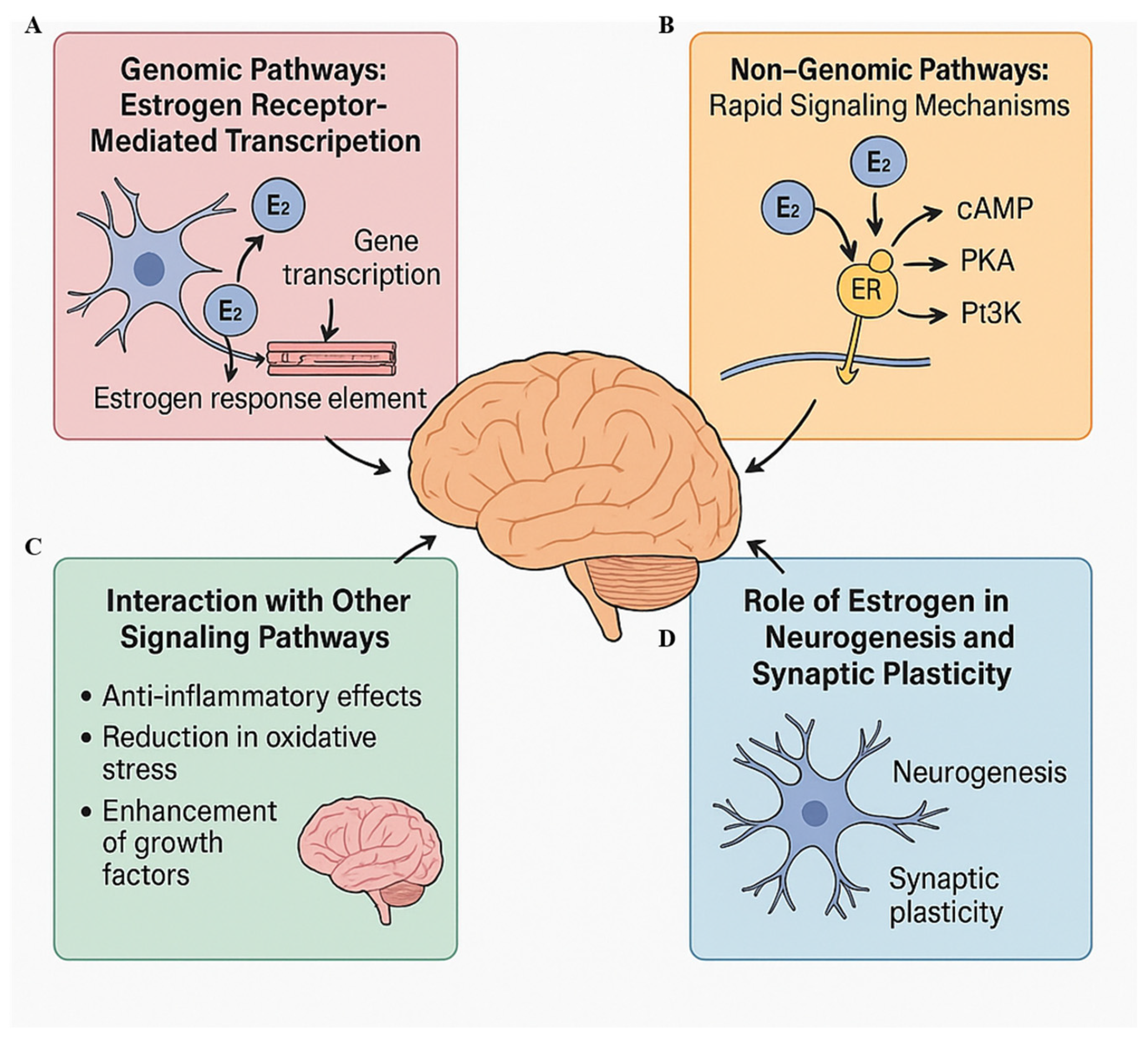
4. Mechanisms of Estrogen Action on the Brain
4.1. Genomic Pathways: Estrogen Receptor-Mediated Transcription
4.2. Non-Genomic Pathways: Rapid Signaling Mechanisms
4.3. Interaction with Other Signaling Pathways
4.4. Role of Estrogen in Neurogenesis and Synaptic Plasticity
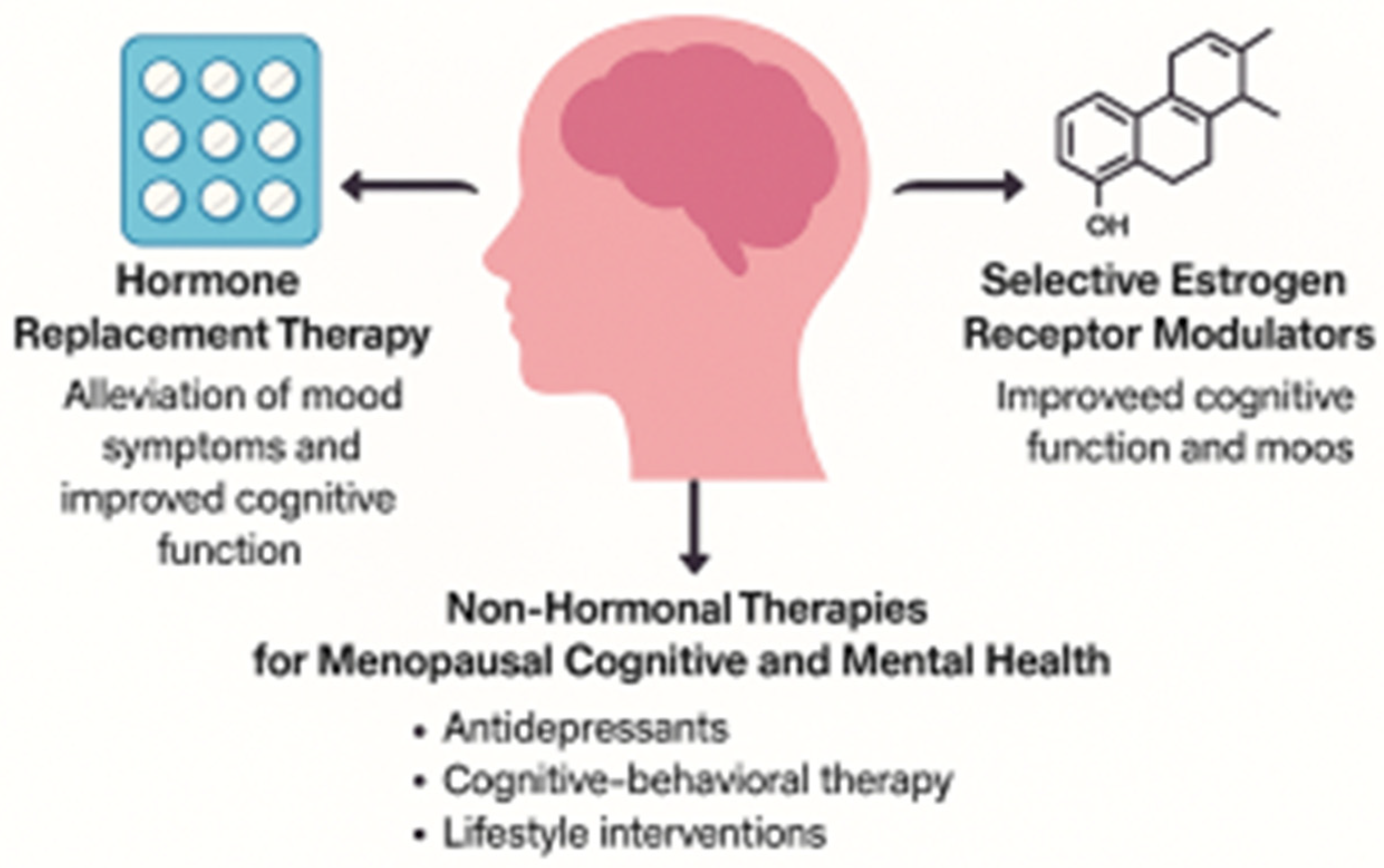
5. Clinical Implications and Current Therapies
5.1. Hormone Replacement Therapy (HRT)
5.2. Selective Estrogen Receptor Modulators (SERMs)
5.3. Non-Hormonal Therapies and Lifestyle Interventions
6. Challenges and Gaps in Research
6.1. Safety Concerns and Risks
6.2. Personalization of Treatment
6.3. Social and Cultural Factors
6.4. Unanswered Questions and Research Gaps
7. Future Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERs | Estrogen receptors |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| HRT | Hormone replacement therapy |
| GABA | Gamma-aminobutyric acid |
| GDA | Generalized anxiety disorder |
| EREs | Estrogen response elements |
| BDNF | Brain-derived neurotrophic factor |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein kinase A |
| PI3K | Phosphatidylinositol-3-kinase |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| WHI | Women’s health initiative |
| SERMs | Selective estrogen receptor modulators |
| CBT | Cognitive-behavioral therapy |
| SSRIs | Selective serotonin reuptake inhibitors |
| SNRIs | Serotonin-norepinephrine reuptake inhibitors |
| VTE | Venous thromboembolism |
| DVT | Deep vein thrombosis |
References
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef]
- Caldwell, J.Z.K.; Isenberg, N. The aging brain: Risk factors and interventions for long term brain health in women. Curr. Opin. Obstet. Gynecol. 2023, 35, 169–175. [Google Scholar] [CrossRef]
- Iqbal, J.; Huang, G.D.; Xue, Y.X.; Yang, M.; Jia, X.J. Role of estrogen in sex differences in memory, emotion and neuropsychiatric disorders. Mol. Biol. Rep. 2024, 51, 415. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, M.K.; Hurd, Y.L. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog. Neurobiol. 2001, 64, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Baek, D.C.; Kang, J.Y.; Lee, J.S.; Lee, E.J.; Son, C.G. Linking alterations in estrogen receptor expression to memory deficits and depressive behavior in an ovariectomy mouse model. Sci. Rep. 2024, 14, 6854. [Google Scholar] [CrossRef]
- Meng, Q.; Chao, Y.; Zhang, S.; Ding, X.; Feng, H.; Zhang, C.; Liu, B.; Zhu, W.; Li, Y.; Zhang, Q.; et al. Attenuation of estrogen and its receptors in the post-menopausal stage exacerbates dyslipidemia and leads to cognitive impairment. Mol. Brain 2023, 16, 80. [Google Scholar] [CrossRef]
- Hwang, W.J.; Lee, T.Y.; Kim, N.S.; Kwon, J.S. The Role of Estrogen Receptors and Their Signaling across Psychiatric Disorders. Int. J. Mol. Sci. 2020, 22, 373. [Google Scholar] [CrossRef]
- Sato, K.; Takayama, K.I.; Inoue, S. Expression and function of estrogen receptors and estrogen-related receptors in the brain and their association with Alzheimer’s disease. Front. Endocrinol. 2023, 14, 1220150. [Google Scholar] [CrossRef]
- González, M.; Cabrera-Socorro, A.; Pérez-García, C.G.; Fraser, J.D.; López, F.J.; Alonso, R.; Meyer, G. Distribution patterns of estrogen receptor α and β in the human cortex and hippocampus during development and adulthood. Cereb. Cortex 2007, 17, 1455–1465. [Google Scholar] [CrossRef]
- Tanapat, P.; Hastings, N.B.; Reeves, A.J.; Gould, E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 1999, 19, 5792–5801. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Numpang, B.; Ke, X.; Yu, X.; Callaway, C.; McKnight, R.; Joss-Moore, L.; Lane, R. Fetal growth restriction alters hippocampal 17-beta estradiol and estrogen receptor alpha levels in the newborn male rat. Syst. Biol. Reprod. Med. 2013, 59, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.M. Gonadal hormone modulation of neurogenesis in the dentate gyrus of the adult hippocampus. Front. Neuroendocrinol. 2008, 29, 412–423. [Google Scholar]
- Mazzucco, C.A.; Lieblich, S.E.; Bingham, B.I.; Williamson, M.A.; Viau, V.; Galea, L.A.M. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience 2006, 141, 1793–1800. [Google Scholar] [CrossRef]
- Woolley, C.S. Estrogen-mediated structural and functional synaptic plasticity in the female brain. Horm. Behav. 2007, 51, 2–7. [Google Scholar]
- Flannery, J.C.; Tirrell, P.S.; Baumgartner, N.E.; Daniel, J.M. Neuroestrogens, the hippocampus, and female cognitive aging. Horm. Behav. 2025, 170, 105710. [Google Scholar] [CrossRef]
- Nerattini, M.; Jett, S.; Andy, C.; Carlton, C.; Zarate, C.; Boneu, C.; Battista, M.; Pahlajani, S.; Loeb-Zeitlin, S.; Havryulik, Y.; et al. Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer’s disease and dementia. Front. Aging Neurosci. 2023, 15, 1260427. [Google Scholar] [CrossRef]
- Shansky, R.M. Estrogen, stress, and the prefrontal cortex: Towards a balanced view. Horm. Behav. 2009, 55, 50–59. [Google Scholar]
- Bethea, C.L.; Lu, N.Z.; Gundlah, C.; Streicher, J.M. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocrinol. 2002, 23, 41–100. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Nerattini, M.; Matthews, D.C.; Jett, S.; Andy, C.; Williams, S.; Yepez, C.B.; Zarate, C.; Carlton, C.; Fauci, F.; et al. In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci. Rep. 2024, 14, 12680. [Google Scholar] [CrossRef] [PubMed]
- Shanmugan, S.; Epperson, C.N. Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 2014, 35, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, D.R.; Schmidt, P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019, 44, 111–128. [Google Scholar] [CrossRef]
- Österlund, M.K.; Overstreet, D.H.; Hurd, Y.L. The estrogen receptor β agonist diarylpropionitrile increases serotonin transporter binding in the rat brain. Brain Res. 1999, 849, 35–43. [Google Scholar]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Tang, C. Estrogen-immuno-neuromodulation disorders in menopausal depression. J. Neuroinflamm. 2024, 21, 159. [Google Scholar] [CrossRef]
- Yoest, K.E.; Cummings, J.A.; Becker, J.B. Estradiol, Dopamine and Motivation. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 83–89. [Google Scholar] [CrossRef]
- Eck, S.R.; Bangasser, D.A. The effects of early life stress on motivated behaviors: A role for gonadal hormones. Neurosci. Biobehav. Rev. 2020, 119, 86–100. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, Y.M. Effects of Menopause on Physical Activity and Dopamine Signaling in Women. Iran. J. Public Health 2022, 51, 2372–2374. [Google Scholar] [CrossRef]
- Raz, L.; Khan, M.M.; Mahesh, V.B.; Vadlamudi, R.K.; Brann, D.W. Rapid estrogen signaling in the brain. Neurosignals 2008, 16, 140–153. [Google Scholar] [CrossRef]
- Ogiue-Ikeda, M.; Tanabe, N.; Mukai, H.; Hojo, Y.; Murakami, G.; Tsurugizawa, T.; Takata, N.; Kimoto, T.; Kawato, S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res. Rev. 2008, 57, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Lin, C.H.; Lane, H.Y. From Menopause to Neurodegeneration—Molecular Basis and Potential Therapy. Int. J. Mol. Sci. 2021, 22, 8654. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Tóth, K.; Likó, I.; Széles, J.; Mahó, S.; Molnár, B.; Liposits, Z. Estrogens regulate neuroinflammatory genes via estrogen receptors α and β in the frontal cortex of middle-aged female rats. J. Neuroinflamm. 2011, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Razmara, A.; Duckles, S.P.; Krause, D.N.; Procaccio, V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007, 1176, 71–81. [Google Scholar] [CrossRef]
- Tecalco-Cruz, A.C.; López-Canovas, L.; Azuara-Liceaga, E. Estrogen signaling via estrogen receptor alpha and its implications for neurodegeneration associated with Alzheimer’s disease in aging women. Metab. Brain Dis. 2023, 38, 783–793. [Google Scholar] [CrossRef]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef]
- Soares, C.N. Menopause and Mood: The Role of Estrogen in Midlife Depression and Beyond. Psychiatr. Clin. North Am. 2023, 46, 463–473. [Google Scholar] [CrossRef]
- Hogervorst, E.; Craig, J.; O’Donnell, E. Cognition and mental health in menopause: A review. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 69–84. [Google Scholar] [CrossRef]
- Leng, G.; Leng, R.I. Oxytocin: A citation network analysis of 10 000 papers. J. Neuroendocrinol. 2021, 33, e13014. [Google Scholar] [CrossRef]
- Kulkarni, J.; Gavrilidis, E.; Hudaib, A.R.; Bleeker, C.; Worsley, R.; Gurvich, C. Development and Validation of a New Rating Scale for Perimenopausal Depression—The Meno-D. Transl. Psychiatry 2018, 8, 123. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Ben Dor, R.; Martinez, P.E.; Guerrieri, G.M.; Harsh, V.L.; Thompson, K.; Koziol, D.E.; Nieman, L.K.; Rubinow, D.R. Effects of Estradiol Withdrawal on Mood in Women with Past Perimenopausal Depression: A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 714–726. [Google Scholar] [CrossRef]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Kravitz, H.M.; Matthews, K.; Youk, A.; Brown, C.; Feng, W. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol. Med. 2009, 39, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatry 2006, 63, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 1999, 64, 803–812. [Google Scholar] [CrossRef]
- Yoest, K.E.; Quigley, J.A.; Becker, J.B. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav. 2018, 104, 119–129. [Google Scholar] [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef]
- Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Kravitz, H.M.; Chang, Y.; Randolph, J.F.; Avis, N.E.; Gold, E.B.; Matthews, K.A. Does Risk for Anxiety Increase During the Menopausal Transition? Study of Women’s Health Across the Nation (SWAN). Menopause 2013, 20, 488–495. [Google Scholar] [CrossRef]
- Hantsoo, L.; Epperson, C.N. Anxiety Disorders Among Women: A Female Lifespan Approach. Focus 2017, 15, 162–172. [Google Scholar] [CrossRef]
- Maran, M.; Friederici, A.D.; Zaccarella, E. Syntax through the looking glass: A review on two-word linguistic processing across behavioral, neuroimaging and neurostimulation studies. Neurosci. Biobehav. Rev. 2022, 142, 104881. [Google Scholar] [CrossRef]
- Deng, W.; Li, R. Juxtamembrane contribution to transmembrane signaling. Biopolymers. 2015, 104, 317–322. [Google Scholar] [CrossRef]
- Alblooshi, S.; Taylor, M.; Gill, N. Does menopause elevate the risk for developing depression and anxiety? Results from a systematic review. Australas. Psychiatry 2023, 31, 165–173. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Zheng, D.; Liu, L. Anxiety disorder in menopausal women and the intervention efficacy of mindfulness-based stress reduction. Am. J. Transl. Res. 2023, 15, 2016–2024. [Google Scholar]
- Soares, C.N.; Frey, B.N. Challenges and Opportunities to Manage Depression and Anxiety in Midlife Women. Psychiatr. Clin. North Am. 2010, 33, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.; Rao, T.S.S. Psychological Changes at Menopause: Anxiety, Mood Swings, and Sexual Health in the Biopsychosocial Context. J. Psychosex. Health 2025, 7, 11–14. [Google Scholar] [CrossRef]
- Uçan Yamaç, S.; Bakir, N. Aging anxiety levels of women in menopause and associated factors. Rev. Assoc. Med. Bras. 2025, 71, e20240573. [Google Scholar] [CrossRef]
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and cognitive impairment: A narrative review of current knowledge. World J. Psychiatry 2021, 11, 412–428. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Duffy, K.A.; Page, C.E.; Novick, A.M. Cognitive Problems in Perimenopause: A Review of Recent Evidence. Curr. Psychiatry Rep. 2023, 25, 501–511. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Sohail, R.; Jaffer, S.R.; Siddique, S.; Kaya, B.; Atowoju, I.; Imran, A.; Wright, W.; Pamulapati, S.; Choudhry, F.; et al. The Role of Estrogen Therapy as a Protective Factor for Alzheimer’s Disease and Dementia in Postmenopausal Women: A Comprehensive Review of the Literature. Cureus 2023, 15, e43053. [Google Scholar] [CrossRef] [PubMed]
- Mervosh, N.; Devi, G. Estrogen, menopause, and Alzheimer’s disease: Understanding the link to cognitive decline in women. Front. Mol. Biosci. 2025, 12, 1634302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, L. Association between sleep duration and depression in menopausal women: A population-based study. Front. Endocrinol. 2024, 15, 1301775. [Google Scholar] [CrossRef]
- Brown, A.M.C.; Gervais, N.J. Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan. Endocrinology 2020, 161, bqaa128. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Sahab-Negah, S.; Hajali, V.; Moradi, H.R.; Gorji, A. The Impact of Estradiol on Neurogenesis and Cognitive Functions in Alzheimer’s Disease. Cell Mol. Neurobiol. 2020, 40, 283–299. [Google Scholar] [CrossRef]
- Deb, P.; Chini, A.; Guha, P.; Rishi, A.; Bhan, A.; Brady, B.; Perrotti, L.I.; Mandal, S.S. Dynamic regulation of BDNF gene expression by estradiol and lncRNA HOTAIR. Gene 2024, 897, 148055. [Google Scholar] [CrossRef]
- Gordon, J.L.; Rubinow, D.R.; Eisenlohr-Moul, T.A.; Xia, K.; Schmidt, P.J.; Girdler, S.S. Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 149–157. [Google Scholar] [CrossRef]
- Kulkarni, J.; Mu, E.; Li, Q.; Malicka, M.; Gavrilidis, E.; de Castella, A.; Gurvich, C. Bazedoxifene plus Conjugated Estrogen to Treat Menopausal Depression—A Pilot Study. J. Pharmacol. Exp. Ther. 2025, 392, 103527. [Google Scholar] [CrossRef]
- Krolick, K.N.; Zhu, Q.; Shi, H. Effects of Estrogens on Central Nervous System Neurotransmission: Implications for Sex Differences in Mental Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 160, 105–171. [Google Scholar]
- Frick, K.M.; Tuscher, J.J.; Koss, W.A.; Kim, J.; Taxier, L.R. Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiol. Behav. 2018, 187, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Yu, D.; Zhang, J.H.; Chen, G.J. Cooperation of Genomic and Rapid Nongenomic Actions of Estrogens in Synaptic Plasticity. Mol. Neurobiol. 2017, 54, 4113–4126. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, N.S.; Redman, W.T.; Karpinska, M.; Jacobs, E.G.; Goard, M.J. The estrous cycle modulates hippocampal spine dynamics, dendritic processing, and spatial coding. Neuron 2025, 113, 2297–2309.e7. [Google Scholar] [CrossRef] [PubMed]
- Maccora, S.; Bolognini, N.; Mannina, C.; Torrente, A.; Agnello, L.; Lo Sasso, B.; Ciaccio, M.; Sireci, G.; Brighina, F. The Effects of Estradiol Levels on Crossmodal Perception: A Study on the Sound-Induced Flash Illusion in Healthy and Menstrually Related Migraine Individuals. Neurol. Sci. 2023, 44, 2863–2870. [Google Scholar] [CrossRef]
- Lymer, J.M.; Sheppard, P.A.S.; Kuun, T.; Blackman, A.; Jani, N.; Mahbub, S.; Choleris, E. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 2018, 89, 30–38. [Google Scholar] [CrossRef]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef]
- Kumar, S.; Lata, K.; Mukhopadhyay, S.; Mukherjee, T.K. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: A perspective. Biochim. Biophys. Acta. 2010, 1800, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Pan, J.; Yuan, M.; Lang, Y.; Wei, X.; Zhang, C. The interaction of BDNF with estrogen in the development of hypertension and obesity, particularly during menopause. Front. Endocrinol. 2024, 15, 1384159. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, W.; Gao, F.; Ma, H.; Yuan, T.; Liu, Z.; Liu, H.; Hu, J.; Bai, J.; Zhang, X.; et al. Brain-Derived Estrogen Regulates Neurogenesis, Learning and Memory with Aging in Female Rats. Biology 2023, 12, 760. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Zhao, F.; Dong, M.; Xie, W.; Liu, Q.; Yang, W.; Cui, R. Role of estrogen in treatment of female depression. Aging 2024, 16, 3021–3042. [Google Scholar] [CrossRef]
- Davis, S.R.; Pinkerton, J.; Santoro, N.; Simoncini, T. Menopause—Biology, consequences, supportive care, and therapeutic options. Cell 2023, 186, 4038–4058. [Google Scholar] [CrossRef]
- Mu, E.; Chiu, L.; Kulkarni, J. Using estrogen and progesterone to treat premenstrual dysphoric disorder, postnatal depression and menopausal depression. Front. Pharmacol. 2025, 16, 1528544. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J. Menopausal Hormone Replacement Therapy and Reduction of All-Cause Mortality and Cardiovascular Disease: It Is About Time and Timing. Cancer J. 2022, 28, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Wei, W.; Xu, Y.; Li, D.; Yin, B.; Gu, W. Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis. Open Life Sci. 2023, 18, 20220759. [Google Scholar] [CrossRef]
- Wu, M.; Li, M.; Yuan, J.; Liang, S.; Chen, Z.; Ye, M.; Ryan, P.M.; Clark, C.; Tan, S.C.; Rahmani, J.; et al. Postmenopausal Hormone Therapy and Alzheimer’s Disease, Dementia, and Parkinson’s Disease: A Systematic Review and Time-Response Meta-Analysis. Pharmacol. Res. 2020, 155, 104693. [Google Scholar] [CrossRef]
- Farkas, S.; Szabó, A.; Hegyi, A.E.; Török, B.; Fazekas, C.L.; Ernszt, D.; Kovács, T.; Zelena, D. Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions. Biomedicines 2022, 10, 861. [Google Scholar] [CrossRef]
- Veenman, L. Raloxifene as Treatment for Various Types of Brain Injuries and Neurodegenerative Diseases: A Good Start. Int. J. Mol. Sci. 2020, 21, 7586. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Thomas, S. Use of SERMs for treatment in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2014, 142, 142–154. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.; Albert, K.; Astur, R.; Johnson, J.; Naylor, M.; Dumas, J. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsycho-pharmacology 2013, 38, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Kouremenos, K.; Tull, D.; Maggi, A.; Schroeder, A.; Gibbons, A.; Kulkarni, J.; Sun-dram, S.; Du, X. Bazedoxifene—A promising brain active SERM that crosses the blood brain barrier and enhances spatial memory. Psychoneuroendocrinology 2020, 121, 104830. [Google Scholar] [CrossRef] [PubMed]
- Martinkovich, S.; Shah, D.; Planey, S.L.; Arnott, J.A. Selective estrogen receptor modulators: Tissue specificity and clinical utility. Clin. Interv. Aging 2014, 9, 1437–1452. [Google Scholar] [CrossRef]
- Mills, Z.B.; Faull, R.L.M.; Kwakowsky, A. Is Hormone Replacement Therapy a Risk Factor or a Therapeutic Option for Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3205. [Google Scholar] [CrossRef]
- Stubbs, C.; Mattingly, L.; Crawford, S.A.; Wickersham, E.A.; Brockhaus, J.L.; McCarthy, L.H. Do SSRIs and SNRIs reduce the frequency and/or severity of hot flashes in menopausal women. J. Okla. State Med. Assoc. 2017, 110, 272–274. [Google Scholar]
- Garg, R.; Munshi, A. Menopause and Mental Health. J. Midlife Health 2025, 16, 119–123. [Google Scholar] [CrossRef]
- Hunter, M.S.; Chilcot, J. Is cognitive behaviour therapy an effective option for women who have troublesome menopausal symptoms? Br. J. Health Psychol. 2021, 26, 697–708. [Google Scholar] [CrossRef]
- Garg, R.; Munshi, A. Sleep and Brain Function at Menopause. J. Midlife Health 2024, 15, 221–224. [Google Scholar] [CrossRef]
- Hossain, M.N.; Lee, J.; Choi, H.; Kwak, Y.S.; Kim, J. The impact of exercise on depression: How moving makes your brain and body feel better. Phys. Act. Nutr. 2024, 28, 43–51. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Venier, M. The Controversial History of Hormone Replacement Therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef]
- Canonico, M.; Oger, E.; Conard, J.; Meyer, G.; Lévesque, H.; Trillot, N.; Barrellier, M.T.; Wahl, D.; Emmerich, J.; Scarabin, P.Y.; et al. Obesity and risk of venous thromboembolism among postmenopausal women: Differential impact of hormone therapy by route of estrogen administration. The ESTHER Study. J. Thromb. Haemost. 2006, 4, 1259–1265. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Hornberger, M.; Ritchie, C.W.; Minihane, A.M. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: Results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res. Ther. 2023, 15, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.M.; Lee, J.; Ahn, E.-M.; Bae, J. Beyond Hot Flashes: The Role of Estrogen Receptors in Menopausal Mental Health and Cognitive Decline. Brain Sci. 2025, 15, 1003. https://doi.org/10.3390/brainsci15091003
Cho JM, Lee J, Ahn E-M, Bae J. Beyond Hot Flashes: The Role of Estrogen Receptors in Menopausal Mental Health and Cognitive Decline. Brain Sciences. 2025; 15(9):1003. https://doi.org/10.3390/brainsci15091003
Chicago/Turabian StyleCho, Jung Min, Jihye Lee, Eun-Mi Ahn, and Jaehoon Bae. 2025. "Beyond Hot Flashes: The Role of Estrogen Receptors in Menopausal Mental Health and Cognitive Decline" Brain Sciences 15, no. 9: 1003. https://doi.org/10.3390/brainsci15091003
APA StyleCho, J. M., Lee, J., Ahn, E.-M., & Bae, J. (2025). Beyond Hot Flashes: The Role of Estrogen Receptors in Menopausal Mental Health and Cognitive Decline. Brain Sciences, 15(9), 1003. https://doi.org/10.3390/brainsci15091003





