Advancing Medulloblastoma Therapy in Pediatrics: Integrative Molecular Classification and Emerging Treatments
Abstract
1. Introduction
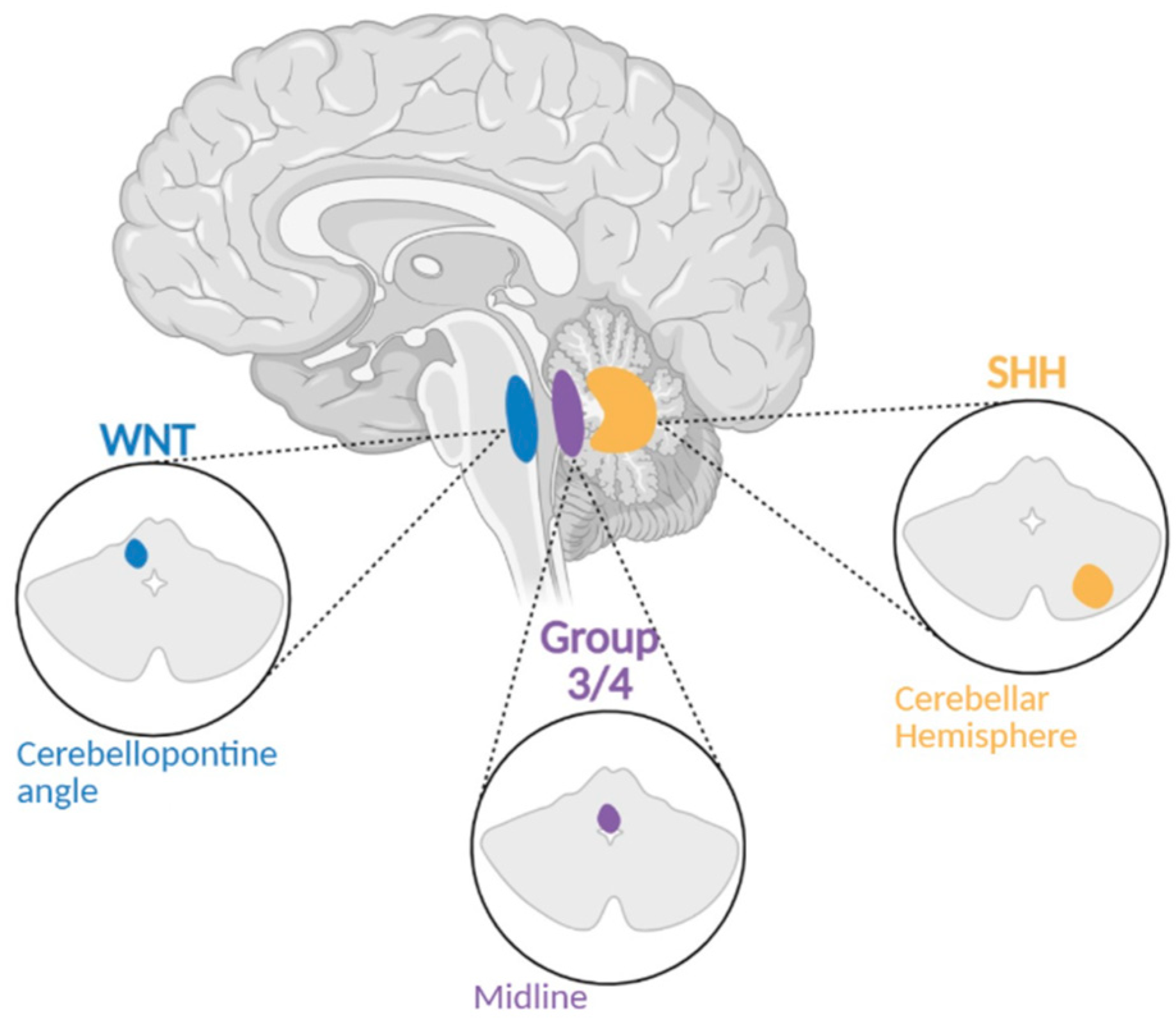
Classification of MB Subgroups
2. Subgroups of MB Expanded
- WNT: ~10% present with metastatic disease, but prognosis remains excellent.
- SHH: Metastatic rates are variable; worse outcomes are observed, particularly with TP53 mutations.
- Group 3: ~50% of cases present with metastasis.
- Group 4: ~30% of patients present with metastatic disease.
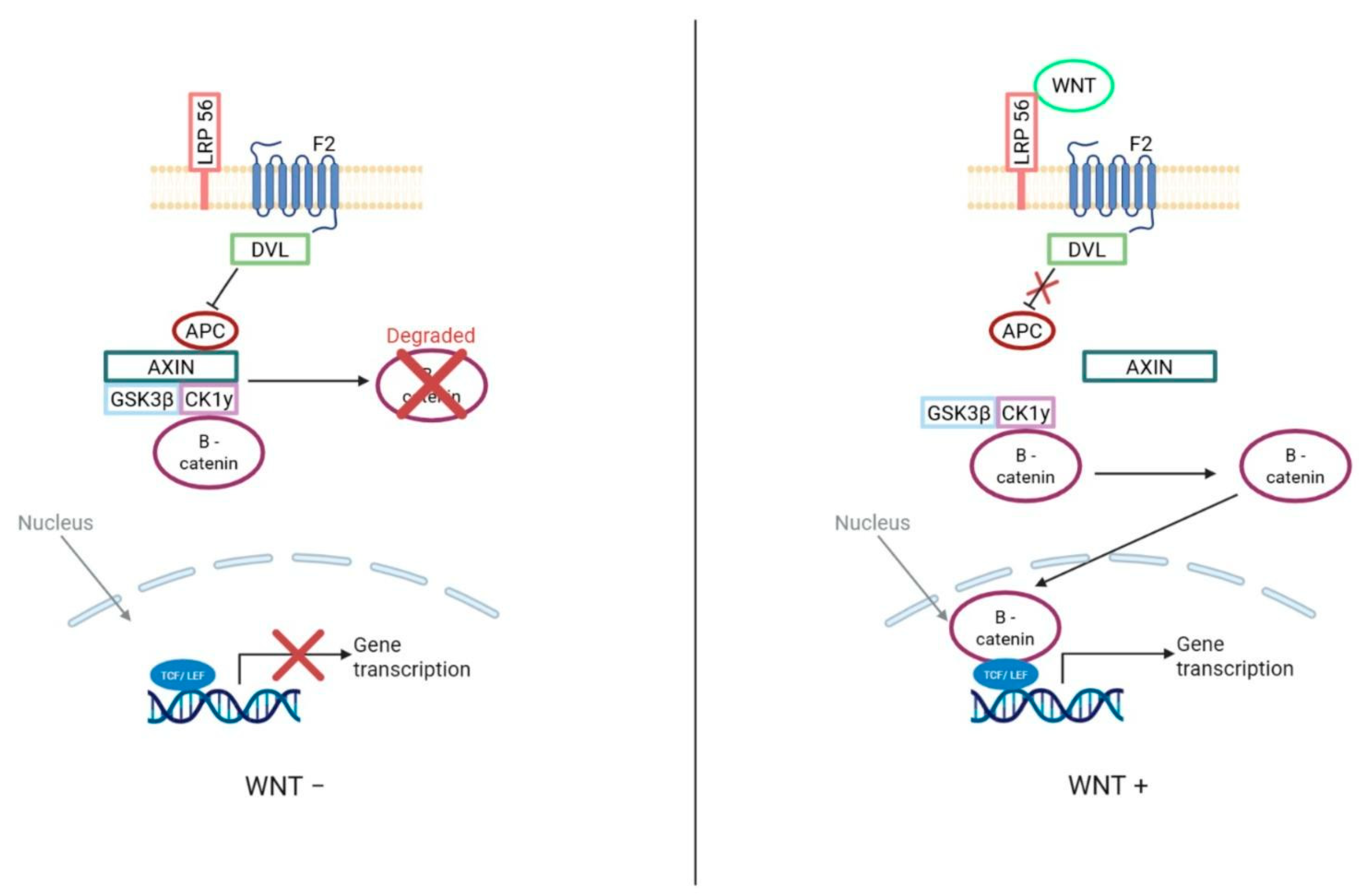
2.1. Wnt Subgroup
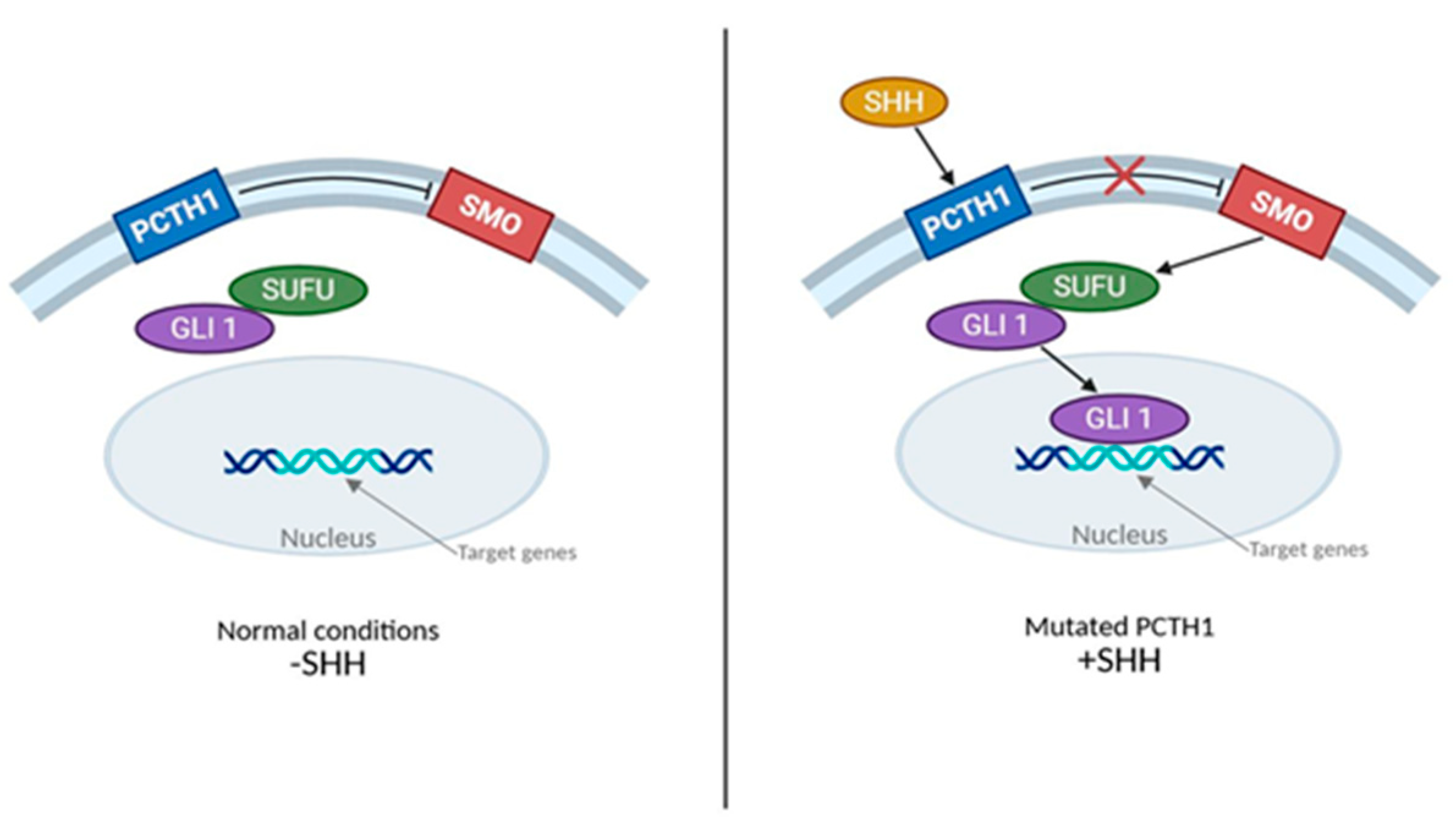
2.2. SHH Subgroup
2.3. Group 3
2.4. Group 4
3. Standard Treatment: Surgery, Radiation Therapy, and Targeted Therapies
3.1. Surgical Interventions
3.2. Chemotherapy and Radiation Treatments
- a.
- ACNS0331
- b.
- ACNS0332
- c.
- ACNS0334
- d.
- Head Start III
- e.
- Head Start IV
- f.
- SJMB03
3.3. Molecularly Targeted Therapies: SHH Subgroup, WNT Subgroup, Non-SHH/WNT Subgroup
3.3.1. SHH
3.3.2. WNT
3.3.3. Non-WNT/SHH
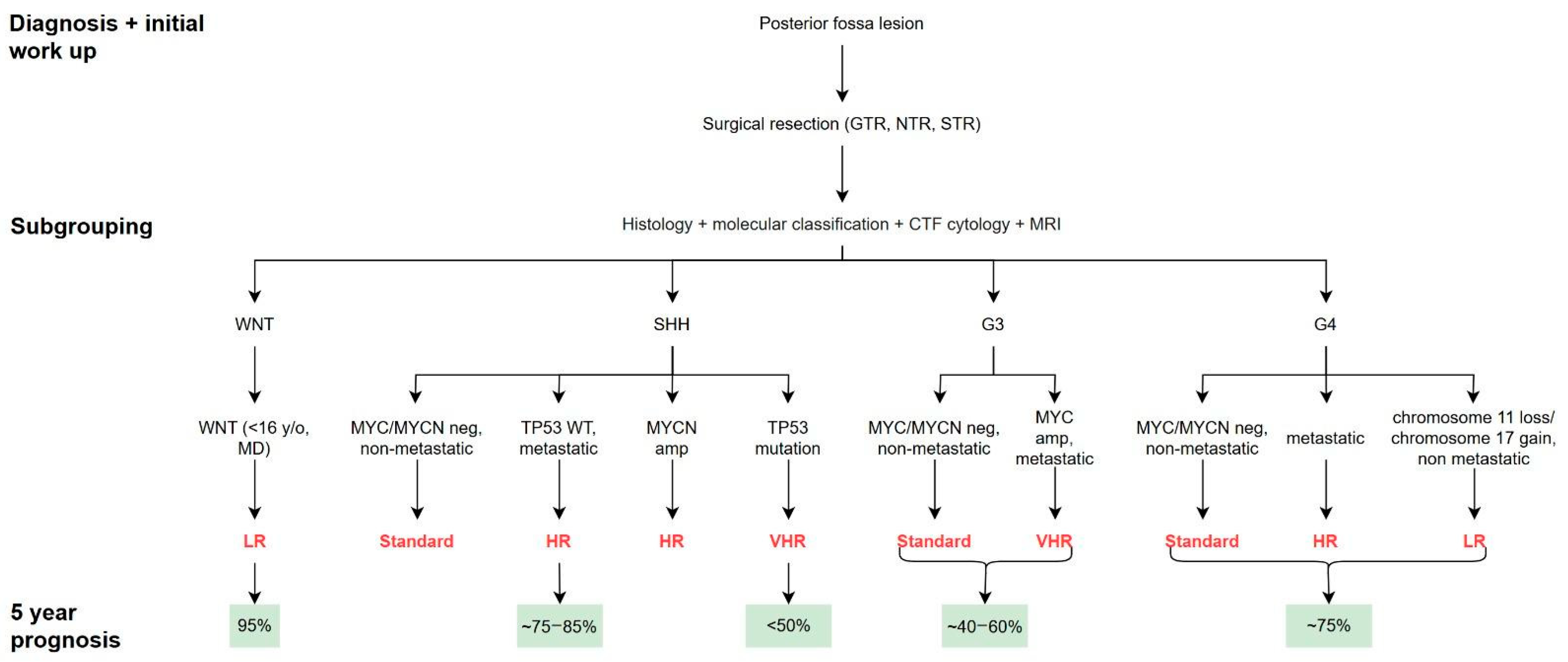
4. Outcomes Based on Molecular Subtypes
4.1. WNT Subgroup
4.2. SHH Subgroup
4.3. Group 3 MB
4.4. Group 4 MB
5. The Future of Medulloblastoma Treatment
Global Disparities and Translational Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahapatra, S.; Amsbaugh, M.J. Medulloblastoma; In: StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. PMID2861 3723.
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.J. Medulloblastoma: Signalling a change in treatment. Lancet Oncol. 2004, 5, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.J.; Gupta, H.; Ward, A.; Li, Y.Y.; Doan, D.; Al-Ibraheemi, A.; Alexandrescu, S.; Bandopadhayay, P.; Shusterman, S.; Mullen, E.A.; et al. Molecular profiling of 888 pediatric tumors informs future precision trials and data-sharing initiatives in pediatric cancer. Nat. Commun. 2024, 15, 5837. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef]
- Bourdeaut, F.; Delattre, O. Genetic predisposition to medulloblastomas: Just follow the tumour genome. Lancet Oncol. 2018, 19, 722–723. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Liu, B.; Parsons, R.E.; Papadopoulos, N.; Jen, J.; Powell, S.M.; Krush, A.J.; Berk, T.; Cohen, Z.; Tetu, B.; et al. The molecular basis of Turcot’s syndrome. N. Engl. J. Med. 1995, 332, 839–847. [Google Scholar] [CrossRef]
- Zurawel, R.H.; Chiappa, S.A.; Allen, C.; Raffel, C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998, 58, 896–899. [Google Scholar]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Cho, Y.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Swarup, A.; Bolger, T.A. The Role of the RNA Helicase DDX3X in Medulloblastoma Progression. Biomolecules 2024, 14, 803. [Google Scholar] [CrossRef]
- Cho, Y.J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Giannini, C.; Furtner, J.; Pajtler, K.W.; Asioli, S.; Guzman, R.; Seidel, C.; Gatto, L.; Hau, P. Adult Medulloblastoma: Updates on Current Management and Future Perspectives. Cancers 2022, 14, 3708. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.A. Pathology, diagnostics, and classification of medulloblastoma. Brain Pathol. 2020, 30, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Faria, C.C.; Perreault, S.; Cho, Y.J.; Shih, D.J.; Luu, B.; Dubuc, A.M.; Northcott, P.A.; et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013, 14, 1200–1207. [Google Scholar] [CrossRef]
- Remke, M.; Ramaswamy, V.; Peacock, J.; Shih, D.J.; Koelsche, C.; Northcott, P.A.; Hill, N.; Cavalli, F.M.; Kool, M.; Wang, X.; et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013, 126, 917–929. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Kieran, M.W. Targeted treatment for sonic hedgehog-dependent medulloblastoma. Neuro Oncol. 2014, 16, 1037–1047. [Google Scholar] [CrossRef]
- Menyhart, O.; Gyorffy, B. Principles of tumorigenesis and emerging molecular drivers of SHH-activated medulloblastomas. Ann. Clin. Transl. Neurol. 2019, 6, 990–1005. [Google Scholar] [CrossRef]
- Greuter, L.; Guzman, R.; Soleman, J. Typical Pediatric Brain Tumors Occurring in Adults-Differences in Management and Outcome. Biomedicines 2021, 9, 356. [Google Scholar] [CrossRef]
- Kumar, V.; McGuire, T.; Coulter, D.W.; Sharp, J.G.; Mahato, R.I. Challenges and Recent Advances in Medulloblastoma Therapy. Trends Pharmacol. Sci. 2017, 38, 1061–1084. [Google Scholar] [CrossRef]
- Northcott, P.A.; Lee, C.; Zichner, T.; Stutz, A.M.; Erkek, S.; Kawauchi, D.; Shih, D.J.; Hovestadt, V.; Zapatka, M.; Sturm, D.; et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature 2014, 511, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Northcott, P.A.; Kool, M.; Pfister, S.M. The role of chromatin remodeling in medulloblastoma. Brain Pathol. 2013, 23, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ioris, R.M.; Richardson, S.; Van Ess, A.N.; Vendrell, I.; Kessler, B.M.; Buffa, F.M.; Busino, L.; Clifford, S.C.; Bullock, A.N.; et al. Disease-associated KBTBD4 mutations in medulloblastoma elicit neomorphic ubiquitylation activity to promote CoREST degradation. Cell Death Differ. 2022, 29, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Schwalbe, E.C.; Hicks, D.; Aldinger, K.A.; Lindsey, J.C.; Crosier, S.; Richardson, S.; Goddard, J.; Hill, R.M.; Castle, J.; et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022, 40, 111162. [Google Scholar] [CrossRef]
- Sheng, H.; Li, H.; Zeng, H.; Zhang, B.; Lu, Y.; Liu, X.; Xu, Z.; Zhang, J.; Zhang, L. Heterogeneity and tumoral origin of medulloblastoma in the single-cell era. Oncogene 2024, 43, 839–850. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E. Shedding light on adult medulloblastoma: Current management and opportunities for advances. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e82–e87. [Google Scholar] [CrossRef]
- Choi, J.Y. Medulloblastoma: Current Perspectives and Recent Advances. Brain Tumor Res. Treat. 2023, 11, 28–38. [Google Scholar] [CrossRef]
- Archer, T.C.; Mahoney, E.L.; Pomeroy, S.L. Medulloblastoma: Molecular Classification-Based Personal Therapeutics. Neurotherapeutics 2017, 14, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.O.; Borgenvik, A.; Zhao, M.; Giraud, G.; Swartling, F.J. Drivers Underlying Metastasis and Relapse in Medulloblastoma and Targeting Strategies. Cancers 2024, 16, 1752. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Cohen, S.; Gudenas, B.L.; Husain, S.; Carlson, A.; Westelman, S.; Wang, L.; Phillips, J.J.; Northcott, P.A.; Weiss, W.A.; et al. PRDM6 promotes medulloblastoma by repressing chromatin accessibility and altering gene expression. Sci. Rep. 2024, 14, 16074. [Google Scholar] [CrossRef]
- Lee, J.J.Y.; Tao, R.; You, Z.; Haldipur, P.; Erickson, A.W.; Farooq, H.; Hendriske, L.D.; Abeysundara, N.; Richman, C.M.; Wang, E.Y.; et al. ZIC1 is a context-dependent medulloblastoma driver in the rhombic lip. Nat. Genet. 2025, 57, 88–102. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Joshi, P.; Korber, V.; Rademacher, A.; Bortolomeazzi, M.; Mallm, J.P.; Vaillant, J.; da Silva, P.B.G.; Statz, B.; Sepp, M.; et al. Oncogene aberrations drive medulloblastoma progression, not initiation. Nature 2025, 642, 1062–1072. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, A.P.Y.; Northcott, P.A. Medulloblastoma genomics in the modern molecular era. Brain Pathol. 2020, 30, 679–690. [Google Scholar] [CrossRef]
- Dhar, S.S.; Lee, M.G. Cancer-epigenetic function of the histone methyltransferase KMT2D and therapeutic opportunities for the treatment of KMT2D-deficient tumors. Oncotarget 2021, 12, 1296–1308. [Google Scholar] [CrossRef]
- Robinson, G.; Parker, M.; Kranenburg, T.A.; Lu, C.; Chen, X.; Ding, L.; Phoenix, T.N.; Hedlund, E.; Wei, L.; Zhu, X.; et al. Novel mutations target distinct subgroups of medulloblastoma. Nature 2012, 488, 43–48. [Google Scholar] [CrossRef]
- Ciobanu-Caraus, O.; Czech, T.; Peyrl, A.; Haberler, C.; Kasprian, G.; Furtner, J.; Kool, M.; Sill, M.; Frischer, J.M.; Cho, A.; et al. The Site of Origin of Medulloblastoma: Surgical Observations Correlated to Molecular Groups. Cancers 2023, 15, 4877. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.; Robinson, G.W.; Smith, K.S.; Lin, T.; Merchant, T.E.; Chintagumpala, M.; Mahajan, A.; Su, J.; Bouffet, E.; Bartels, U.; et al. Outcomes by Clinical and Molecular Features in Children with Medulloblastoma Treated with Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J. Clin. Oncol. 2021, 39, 822–835. [Google Scholar] [CrossRef]
- Mushtaq, N.; Ul Ain, R.; Hamid, S.A.; Bouffet, E. Evolution of Systemic Therapy in Medulloblastoma Including Irradiation-Sparing Approaches. Diagnostics 2023, 13, 3680. [Google Scholar] [CrossRef]
- AbdelBaki, M.S.; Boue, D.R.; Finlay, J.L.; Kieran, M.W. Desmoplastic nodular medulloblastoma in young children: A management dilemma. Neuro Oncol. 2018, 20, 1026–1033. [Google Scholar] [CrossRef]
- Mazewski, C.; Kang, G.; Kellie, S.; Gossett, J.; Leary, S.; Li, B.; Aridgides, P.; Hayes, L.; Reddy, A.; Shaw, D.; et al. MBCL-34. Efficacy of methotrexate (MTX) according to molecular sub-type in young children with medulloblastoma (MB): A report from children’s oncology group phase III trial ACNS0334. Neuro Oncol. 2020, 22, iii396. [Google Scholar] [CrossRef]
- Robinson, G.W.; Rudneva, V.A.; Buchhalter, I.; Billups, C.A.; Waszak, S.M.; Smith, K.S.; Bowers, D.C.; Bendel, A.; Fisher, P.G.; Partap, S.; et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 768–784. [Google Scholar] [CrossRef]
- Mizumoto, M.; Oshiro, Y.; Yamamoto, T.; Kohzuki, H.; Sakurai, H. Proton Beam Therapy for Pediatric Brain Tumor. Neurol. Med. Chir. 2017, 57, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Remke, M.; Benner, A.; Mendrzyk, F.; Toedt, G.; Felsberg, J.; Wittmann, A.; Devens, F.; Gerber, N.U.; Joos, S.; et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J. Clin. Oncol. 2009, 27, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.L.; Muraszko, K.M.; Holmes, E.J.; Sposto, R.; Packer, R.J.; Gajjar, A.; Dias, M.S.; Allen, J.C. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: A prospective study by the Children’s Oncology Group. J. Neurosurg. 2006, 105 (Suppl. S6), 444–451. [Google Scholar] [CrossRef]
- Khan, R.B.; Patay, Z.; Klimo, P.; Huang, J.; Kumar, R.; Boop, F.A.; Raches, D.; Conklin, H.M.; Sharma, R.; Simmons, A.; et al. Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: A prospective study. Neuro Oncol. 2021, 23, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, C.V.; Dhakoji, A.; Menon, G.; Nair, S. Factors predicting the need for cerebrospinal fluid diversion following posterior fossa tumor surgery in children. Pediatr. Neurosurg. 2012, 48, 93–101. [Google Scholar] [CrossRef]
- Michalski, J.M.; Janss, A.J.; Vezina, L.G.; Smith, K.S.; Billups, C.A.; Burger, P.C.; Embry, L.M.; Cullen, P.L.; Hardy, K.K.; Pomeroy, S.L.; et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2021, 39, 2685–2697. [Google Scholar] [CrossRef]
- Leary, S.E.S.; Packer, R.J.; Li, Y.; Billups, C.A.; Smith, K.S.; Jaju, A.; Heier, L.; Burger, P.; Walsh, K.; Han, Y.; et al. Efficacy of Carboplatin and Isotretinoin in Children with High-risk Medulloblastoma: A Randomized Clinical Trial from the Children’s Oncology Group. JAMA Oncol. 2021, 7, 1313–1321. [Google Scholar] [CrossRef]
- Dhall, G.; O’Neil, S.H.; Ji, L.; Haley, K.; Whitaker, A.M.; Nelson, M.D.; Gilles, F.; Gardner, S.L.; Allen, J.C.; Cornelius, A.S.; et al. Excellent outcome of young children with nodular desmoplastic medulloblastoma treated on “Head Start” III: A multi-institutional, prospective clinical trial. Neuro Oncol. 2020, 22, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Dhall, G.; Stanek, J.; Blue, M.; Patel, P.; Thomas, D.; Pierson, C.; Tamrazi, B.; Mahadeo, K.M.; Fleming, J.; Bell, E.; et al. LTBK-05. Outcomes of infants and young children with newly diagnosed localized (M0) SHH medulloblastoma treated on the NEXT Consortium “Head Start” 4 protocol. Neuro Oncol. 2022, 24, i192. [Google Scholar]
- Prados, M.D. Current Strategies for Management of Medulloblastoma. Diagnostics 2023, 13, 2622. [Google Scholar] [CrossRef]
- Shalaby, T.; von Bueren, A.O.; Hurlimann, M.L.; Fiaschetti, G.; Castelletti, D.; Masayuki, T.; Nagasawa, K.; Arcaro, A.; Jelesarov, I.; Sin-ya, K.; et al. Disabling c-Myc in childhood medulloblastoma and atypical teratoid/rhabdoid tumor cells by the potent G-quadruplex interactive agent S2T1-6OTD. Mol. Cancer Ther. 2010, 9, 167–179. [Google Scholar] [CrossRef]
- Sarvode, S.; Gajjar, A. Review of the impact of molecular analysis on the therapy of medulloblastoma. Pediatr. Hematol. Oncol. J. 2023, 8, 121–128. [Google Scholar] [CrossRef]
- Sursal, T.; Ronecker, J.S.; Dicpinigaitis, A.J.; Mohan, A.L.; Tobias, M.E.; Gandhi, C.D.; Jhanwar-Uniyal, M. Molecular Stratification of Medulloblastoma: Clinical Outcomes and Therapeutic Interventions. Anticancer Res. 2022, 42, 2225–2239. [Google Scholar] [CrossRef]
- Mani, S.; Chatterjee, A.; Dasgupta, A.; Shirsat, N.; Epari, S.; Chinnaswamy, G.; and Gupta, T. WNT-pathway medulloblastoma: What constitutes low-risk and how low can one go? Oncotarget 2023, 14, 105–110. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Jonchere, B.; Williams, J.; Zindy, F.; Liu, J.; Robinson, S.; Farmer, D.M.; Min, J.; Yang, L.; Stripay, J.L.; Wang, Y.; et al. Combination of Ribociclib with BET-Bromodomain and PI3K/mTOR Inhibitors for Medulloblastoma Treatment In Vitro and In Vivo. Mol. Cancer Ther. 2023, 22, 37–51. [Google Scholar] [CrossRef]
- Khatua, S.; Song, A.; Citla Sridhar, D.; Mack, S.C. Childhood Medulloblastoma: Current Therapies, Emerging Molecular Landscape and Newer Therapeutic Insights. Curr. Neuropharmacol. 2018, 16, 1045–1058. [Google Scholar] [CrossRef]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef]
- Pereira, V.; Torrejon, J.; Kariyawasam, D.; Berlanga, P.; Guerrini-Rousseau, L.; Ayrault, O.; Varlet, P.; Tauziede-Espariat, A.; Puget, S.; Bolle, S.; et al. Clinical and molecular analysis of smoothened inhibitors in Sonic Hedgehog medulloblastoma. Neuro Oncol. Adv. 2021, 3, vdab097. [Google Scholar] [CrossRef]
- Qin, C.; Pan, Y.; Li, Y.; Long, W.; Liu, Q. Novel Molecular Hallmarks of Group 3 Medulloblastoma by Single-Cell Transcriptomics. Front. Oncol. 2021, 11, 622430. [Google Scholar] [CrossRef] [PubMed]
- Kijima, N.; Kanemura, Y. Molecular Classification of Medulloblastoma. Neurol. Med. Chir. 2016, 56, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.F.; Robinson, G.W. Role of MYC in Medulloblastoma. Cold Spring Harb. Perspect. Med. 2013, 3, a014308. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, E.C.; Lindsey, J.C.; Danilenko, M.; Hill, R.M.; Crosier, S.; Ryan, S.L.; Williamson, D.; Castle, J.; Hicks, D.; Kool, M.; et al. Molecular and clinical heterogeneity within MYC-family amplified medulloblastoma is associated with survival outcomes: A multicenter cohort study. Neuro Oncol. 2025, 27, 222–236. [Google Scholar] [CrossRef]
- Nazam, N.; Erwin, M.H.; Julson, J.R.; Quinn, C.H.; Beierle, A.M.; Bownes, L.V.; Stewart, J.E.; Kang, K.D.; Butey, S.; Mroczek-Musulman, E.; et al. PP2A activation overcomes leptomeningeal dissemination in group 3 medulloblastoma. J. Biol. Chem. 2024, 300, 107892. [Google Scholar] [CrossRef]
- Gatto, L.; Franceschi, E.; Tosoni, A.; Di Nunno, V.; Bartolini, S.; Brandes, A.A. Molecular Targeted Therapies: Time for a Paradigm Shift in Medulloblastoma Treatment? Cancers 2022, 14, 333. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior Intellectual Outcomes After Proton Radiotherapy Compared with Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2020, 38, 454–461. [Google Scholar] [CrossRef]
- Li, Y.; Lim, C.; Dismuke, T.; Malawsky, D.S.; Oasa, S.; Bruce, Z.C.; Offenhauser, C.; Baumgartner, U.; D’Souza, R.C.J.; Edwards, S.L.; et al. Preventing recurrence in Sonic Hedgehog Subgroup Medulloblastoma using the OLIG2 inhibitor CT-179. Nat. Commun. 2023, 16, 1091. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Liu, K.W.; Wang, J.; Garancher, A.; Tao, R.; Esparza, L.A.; Maier, D.L.; Udaka, Y.T.; Murad, N.; Morrissy, S.; et al. HDAC and PI3K Antagonists Cooperate to Inhibit Growth of MYC-Driven Medulloblastoma. Cancer Cell 2016, 29, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, A.; Jaeger, M.; Brunetto, A.L.; Brunetto, A.T.; Gregianin, L.; de Farias, C.B.; Ramaswamy, V.; Nor, C.; Taylor, M.D.; Roesler, R. Neurotrophin Signaling in Medulloblastoma. Cancers 2020, 12, 2542. [Google Scholar] [CrossRef] [PubMed]
- Frederico, S.C.; Sharma, N.; Darling, C.; Taori, S.; Dubinsky, A.C.; Zhang, X.; Raphael, I.; Kohanbash, G. Myeloid cells as potential targets for immunotherapy in pediatric gliomas. Front. Pediatr. 2024, 12, 1346493. [Google Scholar] [CrossRef]
- Frederico, S.C.; Zhang, X.; Hu, B.; Kohanbash, G. Pre-clinical models for evaluating glioma targeted immunotherapies. Front. Immunol. 2022, 13, 1092399. [Google Scholar] [CrossRef]
- Margol, A.S.; Robison, N.J.; Gnanachandran, J.; Hung, L.T.; Kennedy, R.J.; Vali, M.; Dhall, G.; Finlay, J.L.; Erdreich-Epstein, A.; Krieger, M.D.; et al. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin. Cancer Res. 2015, 21, 1457–1465. [Google Scholar] [CrossRef]
- Pham, C.D.; Flores, C.; Yang, C.; Pinheiro, E.M.; Yearley, J.H.; Sayour, E.J.; Pei, Y.; Moore, C.; McLendon, R.E.; Huang, J.; et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin. Cancer Res. 2016, 22, 582–595. [Google Scholar] [CrossRef]
- Bockmayr, M.; Mohme, M.; Klauschen, F.; Winkler, B.; Budczies, J.; Rutkowski, S.; Schuller, U. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 2018, 7, e1462430. [Google Scholar] [CrossRef]
- Maximov, V.; Chen, Z.; Wei, Y.; Robinson, M.H.; Herting, C.J.; Shanmugam, N.S.; Rudneva, V.A.; Goldsmith, K.C.; MacDonald, T.J.; Northcott, P.A.; et al. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat. Commun. 2019, 10, 2410. [Google Scholar] [CrossRef]
- Riemondy, K.A.; Venkataraman, S.; Willard, N.; Nellan, A.; Sanford, B.; Griesinger, A.M.; Amani, V.; Mitra, S.; Hankinson, T.C.; Handler, M.H.; et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro Oncol. 2022, 24, 273–286. [Google Scholar] [CrossRef]
- Dang, M.T.; Gonzalez, M.V.; Gaonkar, K.S.; Rathi, K.S.; Young, P.; Arif, S.; Zhai, L.; Alam, Z.; Devalaraja, S.; To, T.K.J.; et al. Macrophages in SHH subgroup medulloblastoma display dynamic heterogeneity that varies with treatment modality. Cell Rep. 2021, 34, 108917. [Google Scholar] [CrossRef]
- Ciccone, R.; Quintarelli, C.; Camera, A.; Pezzella, M.; Caruso, S.; Manni, S.; Ottaviani, A.; Guercio, M.; Del Bufalo, F.; Quadraccia, M.C.; et al. GD2-Targeting CAR T-cell Therapy for Patients with GD2+ Medulloblastoma. Clin. Cancer Res. 2024, 30, 2545–2557. [Google Scholar] [CrossRef]
- Schakelaar, M.Y.; Monnikhof, M.; Crnko, S.; Pijnappel, E.W.; Meeldijk, J.; Ten Broeke, T.; Bovenschen, N. Cellular immunotherapy for medulloblastoma. Neuro Oncol. 2023, 25, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Kunkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: An interim analysis. Nat. Med. 2021, 27, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Reitberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- Li, Y.; Gibson, A.; Saeed, H.N.; Tahboub, M.A.; Li, D.; Ostrov, D.A.; Krantz, M.S.; Mallal, S.A.; Alves, E.; Chopra, A.; et al. HLA-B Alleles with Shared Peptide Binding Specificities Define Global Risk of Cotrimoxazole-induced SCAR. J. Allergy Clin. Immunol. Pract. 2025. [Google Scholar] [CrossRef]
- Monnikhof, M.; Schakelaar, M.Y.; Meulenbroeks, C.; Quist, M.; Perzolli, A.; Selten, A.; Koster, C.J.M.; Maassen, D.S.C.; Montoro Canelo, A.; Fredriks, M.; et al. Dual targeting of CD155/TIGIT and PD-L1/PD-1 immune checkpoints potentiates NK cell-mediated cytotoxicity in medulloblastoma. Neurooncol Adv. 2025, 7, vdaf099. [Google Scholar] [CrossRef]
- Peyrl, A.; Chocholous, M.; Sabel, M.; Lassaletta, A.; Sterba, J.; Leblond, P.; Sterba, J.; Leblond, P.; Nysom, K.; Torsvik, I.; et al. Sustained Survival Benefit in Recurrent Medulloblastoma by a Metronomic Antiangiogenic Regimen: A Nonrandomized Controlled Trial. JAMA Oncol. 2023, 9, 1688–1695. [Google Scholar] [CrossRef]
- Kinders, R.; Parchment, R.E.; Ji, J.; Kummar, S.; Murgo, A.J.; Gutierrez, M.; Collins, J.; Rubenstein, L.; Pickeral, O.; Steinberg, S.M.; et al. Phase 0 clinical trials in cancer drug development: From FDA guidance to clinical practice. Mol. Interv. 2007, 7, 325–334. [Google Scholar] [CrossRef]
- El-Osta, H.; Hong, D.; Wheler, J.; Fu, S.; Naing, A.; Falchook, G.; Hicks, M.; Wen, S.; Tsimberidou, A.M.; Kurzrock, R. Outcomes of research biopsies in phase I clinical trials: The MD anderson cancer center experience. Oncologist 2011, 16, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roca, C.A.; Lacroix, L.; Massard, C.; De Baere, T.; Deschamps, F.; Pramod, R.; Bahleda, R.; Deutsch, E.; Bourgier, C.; Angevin, E.; et al. Sequential research-related biopsies in phase I trials: Acceptance, feasibility and safety. Ann. Oncol. 2012, 23, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Escudero, L.; Llort, A.; Arias, A.; Diaz-Navarro, A.; Martinez-Ricarte, F.; Rubio-Perez, C.; Mayor, R.; Caratu, G.; Martinez-Saez, E.; Vazquez-Mendez, E.; et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat. Commun. 2020, 11, 5376. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.Y.; Smith, K.S.; Kumar, R.; Paul, L.; Bihannic, L.; Lin, T.; Maass, K.K.; Pajtler, K.W.; Chintagumpala, M.; Su, J.M.; et al. Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell 2021, 39, 1519–1530.e4. [Google Scholar] [CrossRef]
- Jaafari, M.; Razine, R.; Haroun, A.E.; Tahiri, Z.; Hessissen, L. Childhood medulloblastoma in Morocco (middle-income country): Therapeutic outcomes and survival. Ther. Radiol. Oncol. 2023, 7, 10. [Google Scholar] [CrossRef]
- Bailey, S.; Davidson, A.; Parkes, J.; Tabori, U.; Figaji, A.; Epari, S.; Chinnaswamy, G.; Diaz-Coronado, R.; Casavilca-Zambrano, S.; Amayiri, N.; et al. How can genomic innovations in pediatric brain tumors transform outcomes in low- and middle-income countries? JCO Glob. Oncol. 2022, 9, e2200400. [Google Scholar] [CrossRef]
| Total # Cases 2017–2021 | Age Range | |
|---|---|---|
| Medulloblastoma | 1395 | 0–14 |
| Total # Cases 2018–2021 | Age Range | |
| SHH Subtype | 397 | 5–31 |
| WNT Subtype | 74 | 8–14 |
| Non-SHH/WNT | 295 | 8–12 |
| WHO CNS5 2021 Medulloblastoma Classification Overview | ||
|---|---|---|
| WHO CNS4 (2016) | WHO CNS5 (2021) | |
| Knowledge of Genes/Molecular Profiles Characteristically Altered |
| WNT-activated: CTNNB1, APC SHH-activated: TP53, PTCH1, SUFU, SMO, MYCN, GLI2 (methylome)
Non-WNT/non-SHH: MYC, MYCN, PRDM6, KDM6A (methylome)
|
| Morphology |
| Combined into 1 section (Medulloblastoma, histologically defined) that describes them as morphologic patterns of an inclusive tumor type. Most Common Associations:
|
| Remaining the same |
| |
| New |
| |
| Subgroup | 2012 Taylor et al. Classification of MB [12] | 2017 Cavalli et al. Classification of MB [11] |
|---|---|---|
| WNT | Single subgroup characterized by CTNNB1 mutations and monosomy 6. Excellent prognosis. | Split into WNTα (pediatric, monosomy 6) and WNTβ (older patients, no chr6 loss). Both have excellent outcomes. |
| SHH | Single SHH subgroup, known age bimodality (infants & adults). TP53 mutation linked to poor outcome. | Refined into 4 subtypes: • SHHα (children, TP53 mutations, poor prognosis) • SHHβ (infants, poor outcome + frequent copy number alterations) • SHHγ (infants, desmoplastic/nodular, good prognosis, MBEN-enriched, genomic quietness) • SHHδ (adults, TERT mutations) |
| Group 3 | Defined as aggressive, MYC-amplified, high metastasis. Poor prognosis. | Refined into 3 subtypes: • Group 3α (infants, 8q-loss + lack major driver amplifications) • Group 3β (GFI1/GFI1B-driven) • Group 3γ (MYC-driven, worst prognosis) |
| Group 4 | Most common subgroup; less well understood. Isochromosome 17q frequent. Intermediate prognosis. | Refined into 3 subtypes: • Group 4α (MYCN, CDK6 amplification) • Group 4β (SNCAIP tandem duplication) • Group 4γ (8p loss) |
| Summary of New Therapeutic Directions | |
|---|---|
| Targeted Therapies | CT179: A novel small molecule inhibitor of OLIG2 (a helix-loop-helix (HLH) transcription factor), a key factor in maintaining tumor cells in certain medulloblastoma subtypes. Pairing of CT179-mediated OLIG2 inhibition with CDK4/6 inhibition has had a synergistic effect and significant suppression of tumor growth (63). |
| Immunotherapy | Powerful role in combating pediatric low-grade and high-grade gliomas; use is now being explored in Medulloblastoma treatment. |
| GD2 CAR-T cell therapy: Recent success in treating diffuse midline glioma (DMG) with GD2 CAR-T cell therapy. Preclinical findings show promise for using this approach in combating GD2+ medulloblastoma (66–68). | |
| Natural Killer (NK) cell: A broader immunotherapy approach is being explored. | |
| Challenges for immunotherapy: The blood-brain barrier (BBB) and localized immunosuppression. | |
| Metronomic Dosing of Chemotherapy | It has been shown that low-dose (metronomic) oral etoposide and cyclophosphamide supplemented with intravenous bevacizumab has led to an overall five-year progression-free survival of 49.7% (71). |
| Addition of carboplatin (a radiosensitizer): Shown to improve event-free survival by 19% at five years in children with high-risk Group 3 disease. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.T.; Uloho-Okundaye, M.; Frederico, S.C.; Guru, S.; Kim, M.J.; Chang, S.D. Advancing Medulloblastoma Therapy in Pediatrics: Integrative Molecular Classification and Emerging Treatments. Brain Sci. 2025, 15, 896. https://doi.org/10.3390/brainsci15080896
Kim DT, Uloho-Okundaye M, Frederico SC, Guru S, Kim MJ, Chang SD. Advancing Medulloblastoma Therapy in Pediatrics: Integrative Molecular Classification and Emerging Treatments. Brain Sciences. 2025; 15(8):896. https://doi.org/10.3390/brainsci15080896
Chicago/Turabian StyleKim, David T., Michaela Uloho-Okundaye, Stephen C. Frederico, Santosh Guru, Min J. Kim, and Steven D. Chang. 2025. "Advancing Medulloblastoma Therapy in Pediatrics: Integrative Molecular Classification and Emerging Treatments" Brain Sciences 15, no. 8: 896. https://doi.org/10.3390/brainsci15080896
APA StyleKim, D. T., Uloho-Okundaye, M., Frederico, S. C., Guru, S., Kim, M. J., & Chang, S. D. (2025). Advancing Medulloblastoma Therapy in Pediatrics: Integrative Molecular Classification and Emerging Treatments. Brain Sciences, 15(8), 896. https://doi.org/10.3390/brainsci15080896





