1. Introduction and Background
Optic pathway gliomas (OPGs) are rare, low-grade astrocytic tumors that occur in the optic nerve, chiasm, or both, most commonly affecting children under 10 years old. Most cases are associated with neurofibromatosis type 1 (NF–1), an autosomal-dominant genetic disorder caused by mutations in the NF-1 gene, leading to abnormal regulation of cell growth. NF–1 is characterized by skin manifestations (e.g., café-au-lait spots, neurofibromas), learning difficulties, and predisposition to tumors such as OPGs [
1]. While many OPGs are indolent and can remain stable without immediate intervention, others may cause significant morbidity, particularly through visual impairment, proptosis, or even neurological decline [
2]. Managing OPG poses a unique challenge, requiring a delicate balance between tumor control and preservation of vision and quality of life.
Treating OPG is influenced by factors such as tumor size, growth rate, patient age, tumor location in the optic pathway, visual status, and the presence of an NF–1 diagnosis. Traditional management strategies include observation for asymptomatic or stable tumors, chemotherapy for symptomatic cases, and surgery or radiation for more aggressive lesions. Chemotherapy protocols like carbo-vincristine vinblastine single agent; and thioguanine, procarbazine, lomustine (CCNU), and vincristine (TPCV) protocols have proven effective in stabilizing or reducing tumor size, particularly for pediatric patients [
3,
4]. Surgical resection is reserved for certain indications but carries risks of vision loss and neurological complications [
5]. Radiation therapy (RT) is avoided for young children, particularly those younger than 5 years old, because of its long-term developmental effects. RT is also avoided in NF-1 patients due to the risk of secondary malignancies in this patient population [
6].
Despite these options, treating OPG remains challenging because of issues such as treatment resistance, side effects, and long-term complications. Advances in molecular genetics and targeted therapies offer hope for more effective, personalized approaches [
7]. Ongoing clinical trials are exploring innovative treatments, including new drug combinations, targeted agents, and immunotherapies, aiming to improve tumor control, minimize side effects, and enhance long-term outcomes. This review highlights the current treatment landscape and emerging therapeutic options, focusing on clinical trials that represent the future of OPG management.
A version of this manuscript was previously posted as a preprint on Research Square on 27 June 2025 [
8].
2. Review
2.1. Current Treatment Approaches for OPG
The current treatment options for OPG include observation, chemotherapy, RT, and surgery. Treatment decisions should be made by a multidisciplinary team, including pediatric oncologists, neuro-oncologists, neurosurgeons, ophthalmologists, and radiation oncologists, to ensure comprehensive care and minimize long-term adverse effects. Screening for NF–1 is essential for patients diagnosed with OPG.
Figure 1 shows a suggested algorithm for treating OPGs.
2.1.1. Observation
Observation is reserved for patients who are asymptomatic or have mild symptoms, especially for patients with NF–1. Observation may be recommended to avoid treatment-related morbidity. This approach is often chosen when the tumor is not causing immediate threats to vision or other neurological functions.
Scheduling observation visits is advised every 3 to 6 months. These visits usually involve clinical assessments and imaging tests, such as magnetic resonance imaging (MRI), to monitor for any changes in the tumor that could require treatment. The specific frequency of visits may differ based on the patient’s individual circumstances and the judgment of the treating physician. Additionally, regular ophthalmological assessments are an integral part of observation for early detection of visual changes.
The natural course of OPG among children is highly unpredictable. While some tumors remain stable for years or even regress spontaneously, other tumors grow progressively. Active treatment is typically reserved for patients experiencing progressive vision loss or those with existing visual impairment who are at high risk of further deterioration, regardless of substantial tumor progression on MRI. Tumor growth alone is not a criterion for initiating therapy. Nicolin et al. reported that approximately 48% of patients do not require immediate treatment. However, these patients still need regular neuroradiological and ophthalmological monitoring, with intervals determined by factors such as tumor location, symptoms, and the presence or absence of NF–1 association [
9,
10].
2.1.2. Chemotherapy
Chemotherapy is typically the first-line treatment for symptomatic OPG, particularly for young children. Although many OPGs appear benign histologically, chemotherapy surprisingly yields high response rates. Consequently, chemotherapy is frequently chosen as the initial treatment over radiation, particularly for children under 5 years old, as radiotherapy can significantly harm cognitive development. By using chemotherapy, the need for radiation can often be delayed, which may lessen neurocognitive side effects without compromising the survival rates [
11].
The most-used first-line chemotherapy regimen available for treating OPG is the combination of vincristine and carboplatin, which is the preferred first-line therapy, achieving a 3-year progression-free survival (PFS) rate of 77% and a 5-year PFS rate of 69% [
12]. Vinblastine, as a single agent, is widely used as a first-line chemotherapy protocol in Canada, based on a phase II study from The Hospital for Sick Children in Toronto, which supports weekly vinblastine as an effective first-line or salvage monotherapy for pediatric low-grade gliomas; this approach has been broadly adopted across Canadian centers [
13].
A multicenter study led by Institut Gustave Roussy in France, involving 85 children with progressive OPGs (median age, 33 months), demonstrated the effectiveness of using chemotherapy as an initial treatment to delay the need for RT. The children were treated with alternating chemotherapy regimens every 3 weeks, such as procarbazine and carboplatin, etoposide and cisplatin, or vincristine and cyclophosphamide. This strategy successfully deferred the need for RT among 75% of cases at 3 years old and 61% at 5 years old. For the 25 children who eventually required RT, the median interval from the start of treatment to the commencement of RT was 35 months. Postponing RT did not adversely affect outcomes, as the 5-year overall survival rate was 89%. This result of postponed radiation was similar to that achieved with initial radiation, and visual results were comparable [
14].
Between 40% and 60% of patients eventually progress after chemotherapy and require further salvage therapies [
15]. These may include RT, bevacizumab with or without irinotecan, BRAF and/or MEK inhibitors, immunotherapy, and participation in clinical trials.
The benefits of chemotherapy come with significant risks, as its side effects can be serious, including severe bone marrow suppression.
Table 1 shows the most commonly used chemotherapy regimen in treating OPG, including the dosage and potential side effects.
2.1.3. Radiation Therapy
Several studies have investigated the role of radiation therapy in the management of low-grade gliomas, including optic pathway gliomas (OPGs). The typical radiation dose ranges from 45 Gy to 54 Gy, delivered in fractions of 1.8 to 2 Gy. Current recommendations suggest reserving radiation therapy for children older than 8–10 years or for those with tumors resistant to chemotherapy, due to the potential for long-term complications such as cognitive impairment, secondary malignancies, and vascular injury. Additionally, radiation therapy is generally avoided in patients with neurofibromatosis type 1 (NF–1) because of the increased risk of secondary malignancies [
11,
16].
Radiation therapy can halt the progression of visual decline, though the full impact of RT may take years to manifest. In a retrospective analysis involving 42 patients with OPG, with a median age of 6.6 years (1.25–19 years), 29 patients received radiation because of disease progression. Tumor reduction was noted for 18% of patients at 24 months and 46% at 60 months, with a median response time of 62 months. Additionally, vision stabilization or improvement was achieved in 81% of cases. The rate of freedom from disease progression at 10 years was 89%, and the rate of overall survival at 10 years was 100% [
17].
2.1.4. Surgery
Surgery is not commonly used as a primary treatment but may be considered for specific cases, such as patients with single-nerve involvement causing progressive proptosis and blindness, patients with exophytic tumors involving the optic chiasm that cause mass effect or hydrocephalus. Furthermore, surgical debulking may be performed to relieve symptoms or improve vision, but complete resection is rarely possible without significant neurological injury [
18].
2.2. Promising Treatment Options
2.2.1. Mitogen-Activated Protein Kinase (MAPK) Pathway Inhibitors
Targeting the MAPK pathway has emerged as a promising strategy for treating OPG; however, the optimal timing for its implication remains controversial; some pediatric oncologists advocate for using it as a first-line therapy, while others recommend it after failure of chemotherapy or RT. Therefore, participation in clinical trials is highly encouraged [
19].
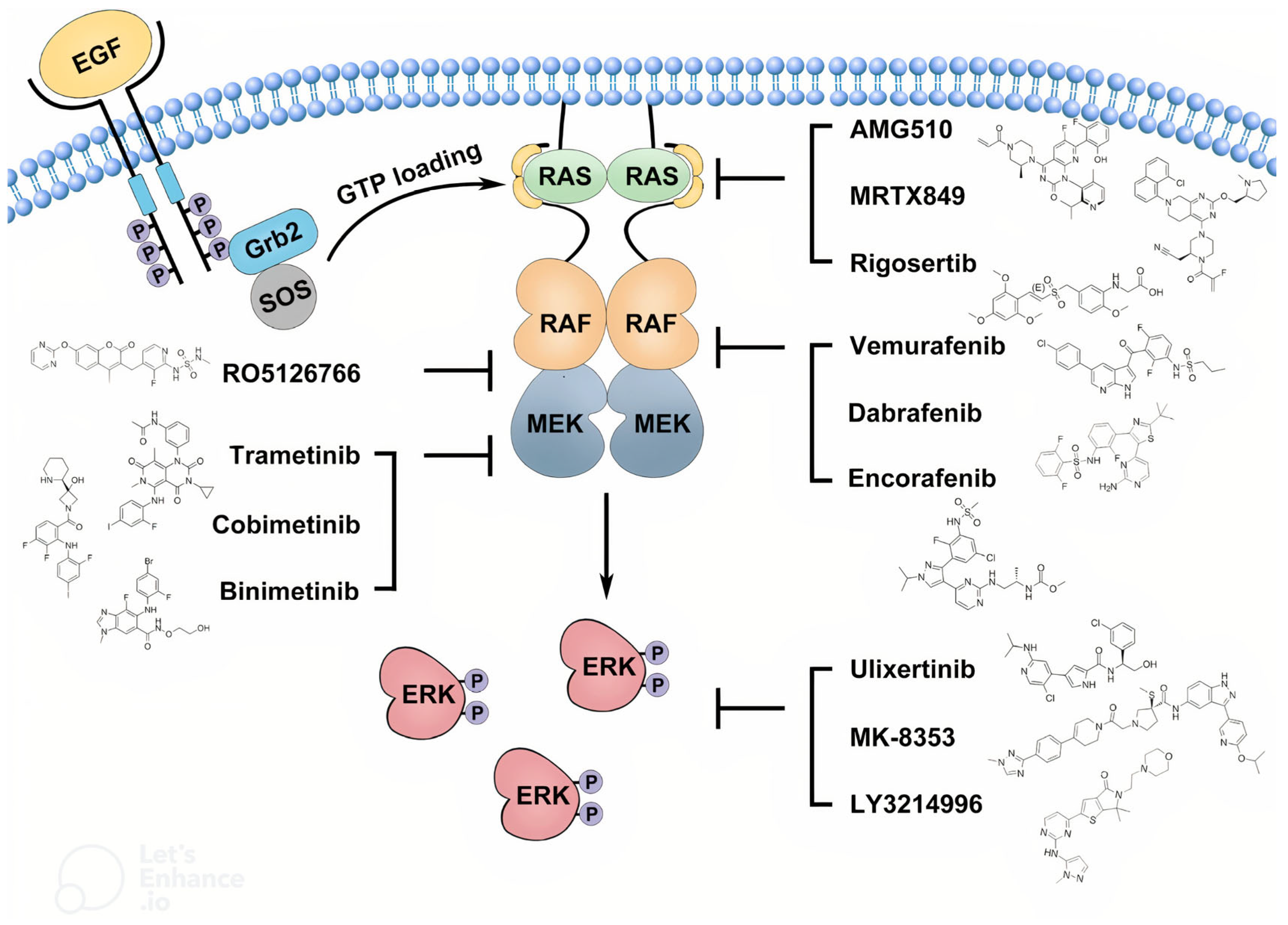
The mitogen-activated protein kinase (MAPK) signaling pathway is a key intracellular cascade that regulates cell proliferation, differentiation, and survival. In its canonical form, activation begins when extracellular growth factors bind to receptor tyrosine kinases (RTKs), leading to recruitment of adaptor proteins and activation of Ras GTPases. Activated Ras triggers phosphorylation and activation of RAF kinases, which in turn phosphorylate MEK1/MEK2, leading to activation of extracellular signal-regulated kinases (ERK1/ERK2). These kinases translocate to the nucleus to regulate gene expression that promotes cell cycle progression and survival. In pediatric low-grade gliomas, including OPGs, aberrations such as BRAF–KIAA1549 fusion and BRAF V600E mutation constitutively activate the MAPK pathway, driving uncontrolled tumor cell proliferation and contributing to treatment resistance. Persistent MAPK signaling can also alter the tumor microenvironment, enhancing angiogenesis and immune evasion, which may further limit the efficacy of conventional therapies. These molecular insights have provided the rationale for the development of targeted inhibitors of BRAF and MEK in OPG management [
20].
Additionally, the overwhelming majority of OPGs are pilocytic astrocytomas which frequently exhibit BRAF alterations. Furthermore, the Pediatric Brain Tumor Consortium (PBTC) data looking at MEKi and OPGs illustrated that even without biopsy, some known pilocytic astrocytomas without a BRAF fusion also had a response. In OPG, the two most common BRAF mutations identified in biopsied cases are the KIAA1549:BRAF fusion and the BRAF V600E point mutation [
21]. These mutations activate the MAPK pathway, a key regulator of cell growth and proliferation.
Figure 2 provides a simplified illustration of the MAPK pathway and its inhibitors [
22].
Interestingly, several MAPK pathway inhibitors have demonstrated activity against OPGs, including selumetinib (MEK1/MEK2 inhibitors), dabrafenib (type I rapidly accelerated fibrosarcoma [RAF] inhibitor) plus trametinib (MEK inhibitor), and tovorafenib (a type II RAF inhibitor) [
23,
24,
25,
26,
27]. Typically, RAF inhibitors are combined with MEK inhibitors to avoid paradoxical MAPK pathway activation.
Table 2 demonstrates a clear overview of the mechanisms, dosages, and side effects for each drug, highlighting their clinical considerations and potential adverse effects.
2.2.2. Evidence Supporting MAPK Pathway Inhibitors
Selumetinib: Selumetinib, a MEK1/MEK2 inhibitor approved for NF–1-associated plexiform neurofibromas, has demonstrated efficacy in both NF–1-associated and sporadic OPG. In a phase II trial by the Pediatric Brain Tumor Consortium, a total of 25 eligible and evaluable patients were included in the study, with a median of 4 prior treatment regimens (ranging from 1 to 11). Among them, 24% (6 patients) achieved a partial response, 56% (14 patients) maintained stable disease, and 20% (5 patients) experienced disease progression during therapy. The median number of treatment cycles administered was 26 (range: 2–26), and over half (14 of 25) completed the full course. The two-year progression-free survival (PFS) rate was 78% (±8.5%). Of the 19 patients assessable for visual acuity, 21% (4 patients) showed improvement, 68% (13 patients) remained stable, and 11% (2 patients) experienced deterioration. Visual field enhancement was observed in 26% (5 patients), while 74% (14 patients) had no change. The most frequently reported adverse effects were mild to moderate (grade 1/2) and included elevated creatine phosphokinase (CPK), anemia, gastrointestinal symptoms (diarrhea, nausea/vomiting), fatigue, headaches, liver enzyme elevations (AST, ALT), hypoalbuminemia, and dermatologic reactions such as rash [
23,
24].
Tovorafenib: Tovorafenib is a type II RAF inhibitor, which was approved by the FDA in 2024 for children 6 months and older with relapsed/refractory low-grade gliomas harboring BRAF V600E mutations or fusions. In the phase II FIREFLY-1 trial (
n = 137), tovorafenib achieved an objective response rate of 67% (Response Assessment in Neuro-Oncology criteria) and a median duration of response of 16.6 months. Common adverse events included hair-color changes, elevated creatine phosphokinase, and anemia, with toxicities of grade 3 or higher among 42% of patients [
25].
According to the FDA prescribing information, tovorafenib is approved for the treatment of pediatric patients 6 months of age and older with relapsed or refractory low-grade glioma harboring a BRAF fusion or rearrangement, not associated with neurofibromatosis type 1 (NF–1). In vitro studies demonstrated that tovorafenib increased ERK phosphorylation at clinically relevant concentrations in cells with neurofibromatosis type 1 loss-of-function (NF–1-LOF), indicating activation rather than inhibition of the MAP kinase pathway. In an NF–1 genetically engineered mouse model of plexiform neurofibroma lacking BRAF alterations, tovorafenib showed no antitumor activity; moreover, although not statistically significant, tumor volume increased in 2 out of 12 mice (approximately 17%) [
26].
Dabrafenib Plus Trametinib: This combination is FDA-approved for BRAF V600E-mutant low-grade gliomas among children 1 year and older. A randomized phase II trial demonstrated its superiority over carboplatin/vincristine, with significant activity in tumors involving the optic pathway or hypothalamus [
27].
MAPK pathway inhibitors are emerging as a promising treatment option for optic pathway glioma (OPG), offering the advantage of oral administration over traditional chemotherapy. However, most available studies are phase II trials with small patient cohorts. Therefore, these agents can be considered as potential salvage or second-line therapies, with careful consideration of possible serious adverse effects such as cardiomyopathy and severe skin reactions.
2.3. Other Treatment Options
2.3.1. Bevacizumab
Bevacizumab is an anti-VEGF (vascular endothelial growth factor) monoclonal antibody that has a significant role in managing brain tumors such as recurrent high-grade gliomas. Several reports have documented qualitative improvements in vision among children with OPG following bevacizumab-based treatment [
28].
Multiple retrospective studies consistently show that bevacizumab, either as a stand-alone treatment or in combination with therapies like irinotecan, can lead to significant visual improvement and radiological responses among 50% to 70% of children with recurrent OPGs, regardless of NF–1 status. Even though bevacizumab shows effectiveness and manageable toxicity, such as systemic hypertension and proteinuria, it has decreased efficacy with prolonged treatment beyond 12 months. Furthermore, over 40% of children experienced recurrence within a few months after discontinuation of bevacizumab, indicating that its effects are not long-lasting [
29].
Looking into possible mechanisms of resistance or recurrence, bevacizumab’s anti-angiogenic effect in OPG appears to provide only temporary disease control in many patients, with relapses often occurring within months of discontinuation. At the macro level, this may reflect the drug’s inability to eradicate tumor cells, instead reducing edema and tumor bulk without altering the underlying disease biology. At the micro level, adaptive resistance can occur via activation of alternative pro-angiogenic pathways (e.g., fibroblast growth factor, platelet-derived growth factor), and changes in the tumor microenvironment that restore blood supply despite VEGF blockade [
30].
Based on the available evidence, MAPK pathway inhibitors are preferred as second-line systemic therapy for OPG, as their efficacy and safety have been evaluated in at least phase II clinical trials, whereas the evidence supporting bevacizumab in this setting is limited to retrospective studies.
2.3.2. Lenalidomide
Researchers are investigating immune modulators like lenalidomide among pediatric patients whose conditions have worsened despite standard treatments. In a phase II study involving 74 children with pilocytic astrocytoma or OPG, both high-dose and low-dose lenalidomide were tested, resulting in four partial responses in each group. The low-dose treatment was found to be better tolerated by the children [
31]. Therefore, the clinical benefit of lenalidomide was not sufficient to support its use as a preferred option for second-line systemic therapy.
2.3.3. Pegylated Interferon α-2b
Pegylated interferon α-2b works by binding to and activating the human type 1 interferon receptors, causing them to dimerize, and this activates the JAK/STAT pathway. Peginterferon α-2b may also activate the nuclear factor κB pathway.
A phase II clinical trial of pegylated interferon α-2B for patients with unresectable juvenile pilocytic astrocytomas and OPGs enrolled nine subjects with a median age of 11 years. The trial demonstrated prolonged stable disease among two patients (75+ and 66+ months) and event-free survival rates of 76.2% at 12 and 24 months, with median event-free survival and overall survival not reached. Side effects were mostly mild (grade 1–2), with no severe drug-related events reported. While the treatment showed safety and potential for delaying disease progression in selected cases, its overall efficacy as a therapeutic option remains uncertain because of the lack of significant tumor responses and small number of enrolled patients [
32].
2.3.4. Immunotherapy
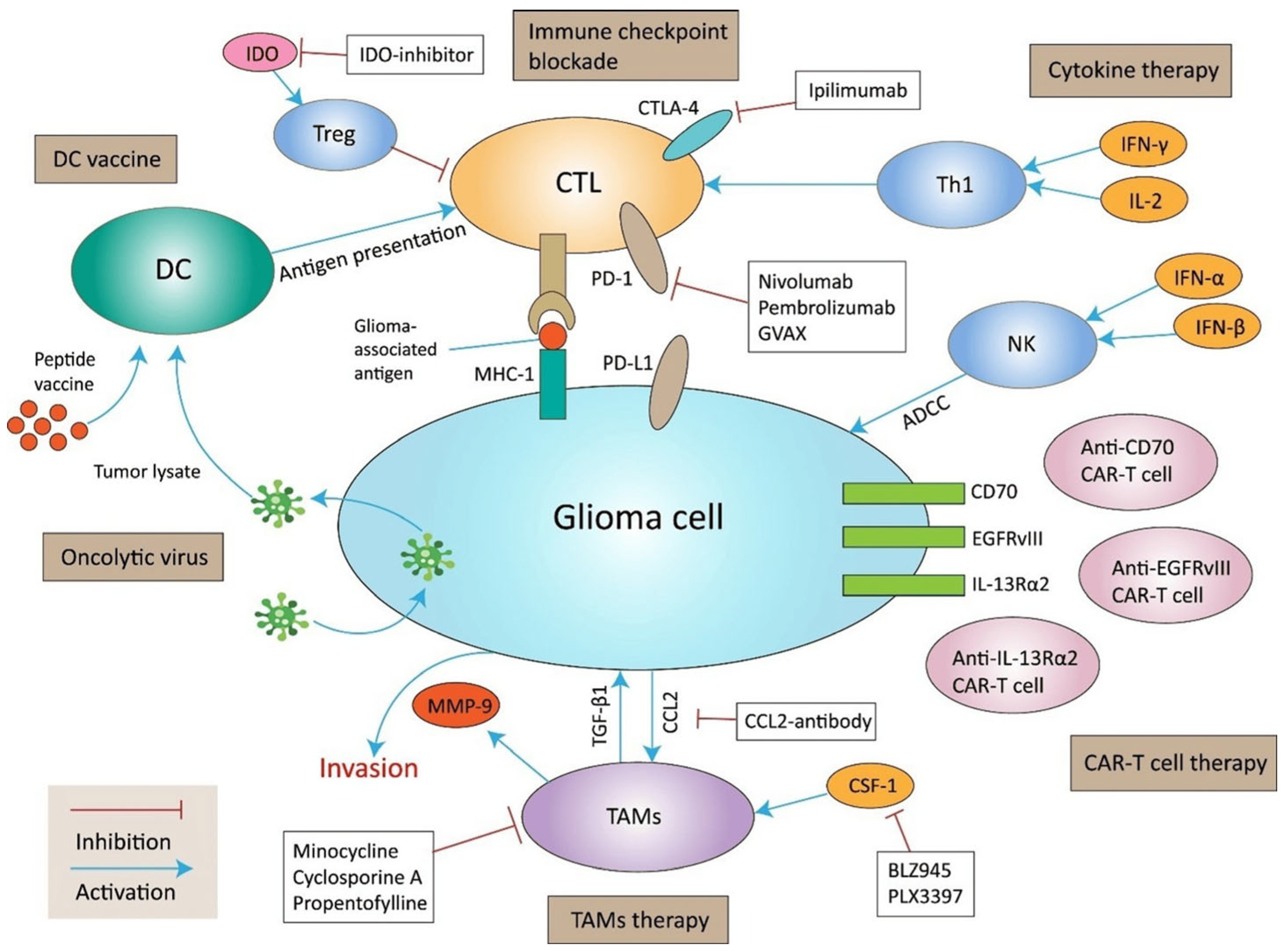
To date, no compelling evidence supports immunotherapy as a treatment for OPG. However, there are ongoing clinical trials testing the PD1/PD–L1 inhibitors and CAR T in gliomas [
33]. Current immunotherapy approaches for glioma are summarized in
Figure 3.
The role of tumor-associated microglia and macrophages (TAMs) in glioma has been increasingly recognized and discussed in literature. TAMs are abundant in the microenvironment of pediatric gliomas and contribute to tumor progression through the secretion of pro-inflammatory cytokines, growth factors, and matrix-remodeling enzymes. These cells can promote glioma cell proliferation, enhance angiogenesis, and suppress anti-tumor immune responses, thereby supporting tumor maintenance and potentially contributing to treatment resistance [
34]. Therefore, modulating tumor-associated macrophages (TAMs) has the potential to enhance anti-tumor immune responses and warrants further investigation as a therapeutic strategy.
2.4. Ongoing Clinical Trials
These are examples of actively recruiting clinical trials that will assist physicians, patients, and their families in making treatment decisions. These clinical trials are divided into three categories: systemic therapy trials, vision-improvement trials, and CAR T-cell therapy trials, as follows:
2.4.1. Systemic Therapy Trials
Selumetinib: Three phase III clinical trials are investigating selumetinib for low-grade glioma (LGG) at multiple locations in the United States. NCT04166409 compares the efficacy of selumetinib to the standard chemotherapy combination of carboplatin and vincristine (CV) for patients with newly diagnosed LGG that lacks the BRAFV600E mutation and is not linked to NF–1. The trial aims to determine whether selumetinib can provide comparable or improved outcomes, including tumor control and quality of life, compared with CV [
35].
NCT04576117 examines the addition of vinblastine to selumetinib in treating recurrent or progressive LGG. This trial seeks to determine whether the combination therapy is more effective than selumetinib alone [
36]. Similarly, NCT03871257 evaluates selumetinib vs. CV for treating LGG for patients with NF–1, focusing on tumor control and its potential to improve vision for those patients with OPG [
37].
Tovorafenib: Tovorafenib is being compared with chemotherapy in an ongoing two-arm, randomized, open-label, multicenter, global, phase III trial to evaluate the efficacy, safety, and tolerability of tovorafenib monotherapy vs. standard-of-care chemotherapy among patients with pediatric LGG harboring an activating RAF alteration requiring first-line systemic therapy (NCT05566795) [
38].
Avutometinib: NCT06104488 is a multicenter phase I dose-escalation study of avutometinib, a RAF/MEK clamp, in pediatric patients with refractory or recurrent solid tumors harboring activating MAPK pathway alterations [
39].
2.4.2. Vision-Improvement Trials
The NCT05278715 trial evaluates a modified chemotherapy regimen combining increased-dose carboplatin (220 mg/m
2), vincristine, and recombinant human endostatin (rh-ES). The trial includes pediatric and adult patients and focuses on visual acuity improvement and response rates over 3 years [
40]. Meanwhile, the NCT05733572 trial assesses the safety and efficacy of CHF6467, a recombinant mutated nerve growth factor, for improving visual function among OPG patients. This randomized, double-blind, placebo-controlled study uses CHF6467 eye drops and measures visual-field and visual-evoked potentials over 6 months. The trial targets children (ages 6–17 years) and young adults (ages 18–39 years) [
41].
2.4.3. CAR T-Cell Therapy Trials
Three innovative clinical trials are investigating CAR T-cell therapy in gliomas. The first trial (NCT06355908) is a phase I study assessing the safety and feasibility of IL13Ra2-targeted CAR T cells in patients with recurrent or refractory glioma [
42]. This trial is focusing on glioma-specific CAR T-cell therapies. Similarly, the second trial (NCT04099797) explores C7R-GD2 CAR T cells for treating GD2- expressing brain tumors, including diffuse intrinsic pontine glioma, high-grade glioma, and medulloblastoma [
43]. This phase I study combines i.v. and intracranial infusions to determine the largest safe dose while enhancing T-cell survival with cytokine support.
The third trial (NCT06640582) is a phase I/II study that investigates a combination of tumor-infiltrating lymphocyte therapy with pembrolizumab in advanced brain cancers, including glioblastoma and meningioma [
44].
3. Conclusions
Managing OPGs is challenging because of their complex location, association with NF-1, and diverse clinical progression. Current treatments, including chemotherapy, targeted therapy, and RT, provide reasonable control in many cases but are limited by issues such as treatment resistance, long-term toxicities, and variability in outcomes. Emerging strategies, including bevacizumab for symptom control and MEK inhibitors for NF–1-associated gliomas, show promise, while immunotherapy and CAR T-cell therapy hold potential to transform the therapeutic landscape. However, the safety and efficacy of these treatments, particularly among pediatric populations, require further investigation.
Future research should focus on developing durable therapies to reduce relapse risk, minimizing toxicities through targeted approaches, and identifying predictive biomarkers for personalized treatment. Integrative care models that address the multifaceted needs of OPG patients and the expansion of clinical trials exploring next-generation therapeutics, such as immune-based therapies and gene-editing techniques, are vital. Collaborations among researchers, clinicians, and patient-advocacy groups will be essential in translating preclinical advancements into effective clinical practices, improving outcomes and quality of life for OPG patients.
Author Contributions
Conceptualization, O.E., S.E., and S.M.; methodology, O.E. and S.E. validation, O.E., S.E., A.R., and S.M.; formal analysis, O.E. and S.M.; investigation, O.E.; resources, O.E. and S.E.; data curation, O.E. and S.E.; writing—original draft preparation, O.E.; writing—review and editing, S.E., A.R., V.D., and S.M.; visualization, O.E.; supervision, S.M.; project administration, O.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review article did not generate any new data. All data discussed in this manuscript are derived from previously published studies, which are cited accordingly in the references section.
Acknowledgments
Editorial assistance was provided by Moffitt Cancer Center’s Office of Scientific Publishing by Gerard Hebert; no compensation was given beyond his regular salary.
Conflicts of Interest
In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services information: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Yousefi, O.; Azami, P.; Sabahi, M.; Dabecco, R.; Adada, B.; Borghei-Razavi, H. Management of optic pathway glioma: A systematic review and meta-analysis. Cancers 2022, 14, 4781. [Google Scholar] [CrossRef]
- Packer, R.J. Optic pathway gliomas: Long-term outcomes and challenges. Neuro-Oncology 2024, 26, 1325–1326. [Google Scholar] [CrossRef] [PubMed]
- Fried, I.; Tabori, U.; Tihan, T.; Reginald, A.; Bouffet, E. Optic pathway gliomas: A review. CNS Oncol. 2013, 2, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Chintagumpala, M.; Eckel, S.P.; Krailo, M.; Morris, M.; Adesina, A.; Packer, R.; Lau, C.; Gajjar, A. A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: A Children’s Oncology Group study. Neuro-Oncology 2015, 17, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, C.; Zong, S.; Piao, J.; Zhao, Y.; Chen, X. A review on surgical treatment options in gliomas. Front. Oncol. 2023, 13, 1088484. [Google Scholar] [CrossRef]
- Kim, N.; Lim, D.H. Recent updates on radiation therapy for pediatric optic pathway glioma. Brain Tumor. Res. Treat. 2022, 10, 94–100. [Google Scholar] [CrossRef]
- Farazdaghi, M.K.; Katowitz, W.R.; Avery, R.A. Current treatment of optic nerve gliomas. Curr. Opin. Ophthalmol. 2019, 30, 356–363. [Google Scholar] [CrossRef]
- Elzaafarany, O.; Elhomosany, S.; Rincones, A.; Dlugi, V.; Mokhtari, S. Optic Pathway Glioma: Current Treatment Approaches and Ongoing Clinical Trials—A Review Article. PREPRINT (Version 1). Available online: https://www.researchsquare.com/article/rs-6882947/v1 (accessed on 16 June 2025).
- Nicolin, G.; Parkin, P.; Mabbott, D.; Hargrave, D.; Bartels, U.; Tabori, U.; Rutka, J.; Buncic, J.R.; Bouffet, E. Natural history and outcome of optic pathway gliomas in children. Pediatr. Blood. Cancer 2009, 53, 1231–1237. [Google Scholar] [CrossRef]
- Bello, S.D.; Martinuzzi, D.; Tereshko, Y.; Veritti, D.; Sarao, V.; Gigli, G.L.; Lanzetta, P.; Valente, M. The present and future of optic pathway glioma therapy. Cells 2023, 12, 2380. [Google Scholar] [CrossRef]
- Optic Pathway Glioma. 2025. Available online: https://www.uptodate.com/contents/optic-pathway-glioma (accessed on 7 July 2025).
- Packer, R.J.; Ater, J.; Allen, J.; Phillips, P.; Geyer, R.; Nicholson, H.S.; Jakacki, R.; Kurczynski, E.; Needle, M.; Finlay, J.; et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 1997, 86, 747–754. [Google Scholar] [CrossRef]
- Bouffet, E.; Jakacki, R.; Goldman, S.; Hargrave, D.; Hawkins, C.; Shroff, M.; Hukin, J.; Bartels, U.; Foreman, N.; Kellie, S.; et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J. Clin. Oncol. 2012, 30, 1358–1363. [Google Scholar] [CrossRef]
- Laithier, V.; Grill, J.; Le Deley, M.-C.; Ruchoux, M.-M.; Couanet, D.; Doz, F.; Pichon, F.; Rubie, H.; Frappaz, D.; Vannier, J.-P.; et al. French Society of Pediatric Oncology. Progression-free survival in children with optic pathway tumors: Dependence on age and the quality of the response to chemotherapy—Results of the first French prospective study for the French Society of Pediatric Oncology. J. Clin. Oncol. 2003, 21, 4572–4578. [Google Scholar] [CrossRef]
- Janss, A.J.; Grundy, R.; Cnaan, A.; Packer, R.J.; Zackai, E.H.; Sutton, L.N.; Molloy, P.T.; Phillips, P.C.; Lange, B.; Savino, P.J.; et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer 1995, 7575, 1051–1059. [Google Scholar] [CrossRef]
- Saran, F.H.; Baumert, B.G.; Khoo, V.S.; Adams, E.J.; Garré, M.L.; Warrington, A.P.; Brada, M. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 43–51. [Google Scholar] [CrossRef]
- Tao, M.L.; Barnes, P.D.; Billett, A.L.; Leong, T.; Shrieve, D.C.; Scott, R.; Tarbell, N.J. Childhood optic chiasm gliomas: Radiographic response following radiotherapy and long-term clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 1997, 3900359, 9336136. [Google Scholar] [CrossRef]
- Konovalov, A.; Gorelyshev, S.; Serova, N. Surgery of giant gliomas of chiasma and IIIrd ventricle. Acta Neurochir. 1994, 130, 71–79. [Google Scholar] [CrossRef]
- Cooney, T.; Yeo, K.K.; Kline, C.; Prados, M.; Haas-Kogan, D.; Chi, S.; Mueller, S. Neuro-Oncology Practice Clinical Debate: Targeted therapy vs conventional chemotherapy in pediatric low-grade glioma. Neuro-Oncol. Pract. 2020, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Crotty, E.E.; Sato, A.A.; Abdelbaki, M.S. Integrating MAPK pathway inhibition into standard-of-care therapy for pediatric low-grade glioma. Front. Oncol. 2025, 15, 1520316. [Google Scholar] [CrossRef] [PubMed]
- Nobre, L.; Zapotocky, M.; Ramaswamy, V.; Ryall, S.; Bennett, J.; Alderete, D.; Guill, J.B.; Baroni, L.; Bartels, U.; Bavle, A.; et al. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis. Oncol. 2020, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Poussaint, T.Y.; Wu, S.; Ligon, A.H.; Lindeman, N.; Banerjee, A.; Packer, R.J.; Kilburn, L.B.; Goldman, S.; et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019, 20, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Onar-Thomas, A.; Poussaint, T.Y.; Wu, S.; Ligon, A.H.; Lindeman, N.; Campagne, O.; Banerjee, A.; Gururangan, S.; Kilburn, L.B.; et al. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: A pediatric brain tumor consortium study. Neuro-Oncology 2021, 23, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, L.B.; Khuong-Quang, D.-A.; Hansford, J.R.; Landi, D.; van der Lugt, J.; Leary, S.E.S.; Driever, P.H.; Bailey, S.; Perreault, S.; McCowage, G.; et al. The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: The phase 2 FIREFLY-1 trial. Nat. Med. 2024, 30, 207–217. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. DAY101 (Tovorafenib) Prescribing Information; FDA: Silver Spring, MD, USA, 2024; pp. 1–33. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217700s001,218033s001lbl.pdf (accessed on 8 August 2025).
- Bouffet, E.; Hansford, J.R.; Garrè, M.L.; Hara, J.; Plant-Fox, A.; Aerts, I.; Locatelli, F.; van der Lugt, J.; Papusha, L.; Sahm, F.; et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N. Engl. J. Med. 2023, 389, 1108–1120. [Google Scholar] [CrossRef]
- Yamasaki, F.; Takano, M.; Yonezawa, U.; Taguchi, A.; Kolakshyapati, M.; Okumichi, H.; Kiuchi, Y.; Kurisu, K. Bevacizumab for optic pathway glioma with worsening visual field in absence of imaging progression: 2 case reports and literature review. Childs Nerv. Syst. 2020, 36, 635–639. [Google Scholar] [CrossRef]
- Bennebroek, C.A.M.; van Zwol, J.; Porro, G.L.; Oostenbrink, R.; Dittrich, A.T.M.; Groot, A.L.W.; Pott, J.W.; Janssen, E.J.M.; Bauer, N.J.; van Genderen, M.M.; et al. Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study. Cancers 2022, 14, 6087. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Reardon, D.A.; de Groot, J.F.; Wick, W.; Weller, M. Antiangiogenic therapy for glioblastoma: Current status and future prospects. Clin. Cancer Res. 2014, 20, 5612–5619. [Google Scholar] [CrossRef]
- Warren, K.E.; Vezina, G.; Krailo, M.; Springer, L.; Buxton, A.; Peer, C.J.; Figg, W.D.; William-Hughes, C.; Kessel, S.; Fouladi, M.; et al. Phase II randomized trial of lenalidomide in children with pilocytic astrocytomas and optic pathway gliomas: A report from the Children’s Oncology Group. J. Clin. Oncol. 2023, 41, 18. [Google Scholar] [CrossRef]
- Aguilera, D.; Mazewski, C.; Janss, A.; MacDonald, T.; Goldman, A.; Leong, T.; Castellino, R.C. LGG-64. A Phase II Study of Pegylated Interferon in Children with Recurrent or Refractory and Radiographically or Clinically Progressive Juvenile Pilocytic Astrocytomas and Optic Pathway Gliomas (NCT02343224). Neuro-Oncology 2022, 24, i103. [Google Scholar] [CrossRef]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef]
- Pires-Afonso, Y.; Niclou, S.P.; Michelucci, A. Revealing and harnessing tumour-associated microglia/macrophage heterogeneity in glioblastoma. Int. J. Mol. Sci. 2020, 21, 689. [Google Scholar] [CrossRef]
- A Study of the Drugs Selumetinib vs. Carboplatin and Vincristine in Patients with Low-Grade Glioma. 2025. Available online: https://clinicaltrials.gov/study/NCT04166409 (accessed on 7 July 2025).
- A Study to Compare Treatment with the Drug Selumetinib Alone Versus Selumetinib and Vinblastine in Patients with Recurrent or Progressive Low-Grade Glioma. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT04576117 (accessed on 7 July 2025).
- A Study of the Drugs Selumetinib Versus Carboplatin/Vincristine in Patients with Neurofibromatosis and Low-Grade Glioma. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT03871257 (accessed on 7 July 2025).
- DAY101 vs. Standard of Care Chemotherapy in Pediatric Patients with Low-Grade Glioma Requiring First-Line Systemic Therapy (LOGGIC/FIREFLY-2). 2025. Available online: https://www.clinicaltrials.gov/study/NCT05566795 (accessed on 7 July 2025).
- A Study of Avutometinib for People with Solid Tumor Cancers. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT06104488 (accessed on 7 July 2025).
- Modified CV Regimen in Optic Pathway Glioma. 2025. Available online: https://clinicaltrials.gov/study/NCT05278715 (accessed on 7 July 2025).
- Safety and Efficacy of the PAINLESS Nerve Growth Factor CHF6467 in Optic Pathway Glioma. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT05733572 (accessed on 7 July 2025).
- IL13Rα2 CAR-T for Patients with r/r Glioma. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT06355908 (accessed on 7 July 2025).
- C7R-GD2. CAR T Cells for GD2-Expressing Brain Tumors. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT04099797 (accessed on 7 July 2025).
- TIL Therapy + Pembrolizumab for Advanced Brain Cancer. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT06640582 (accessed on 7 July 2025).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).