Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications
Abstract
1. Introduction
2. Materials and Methods
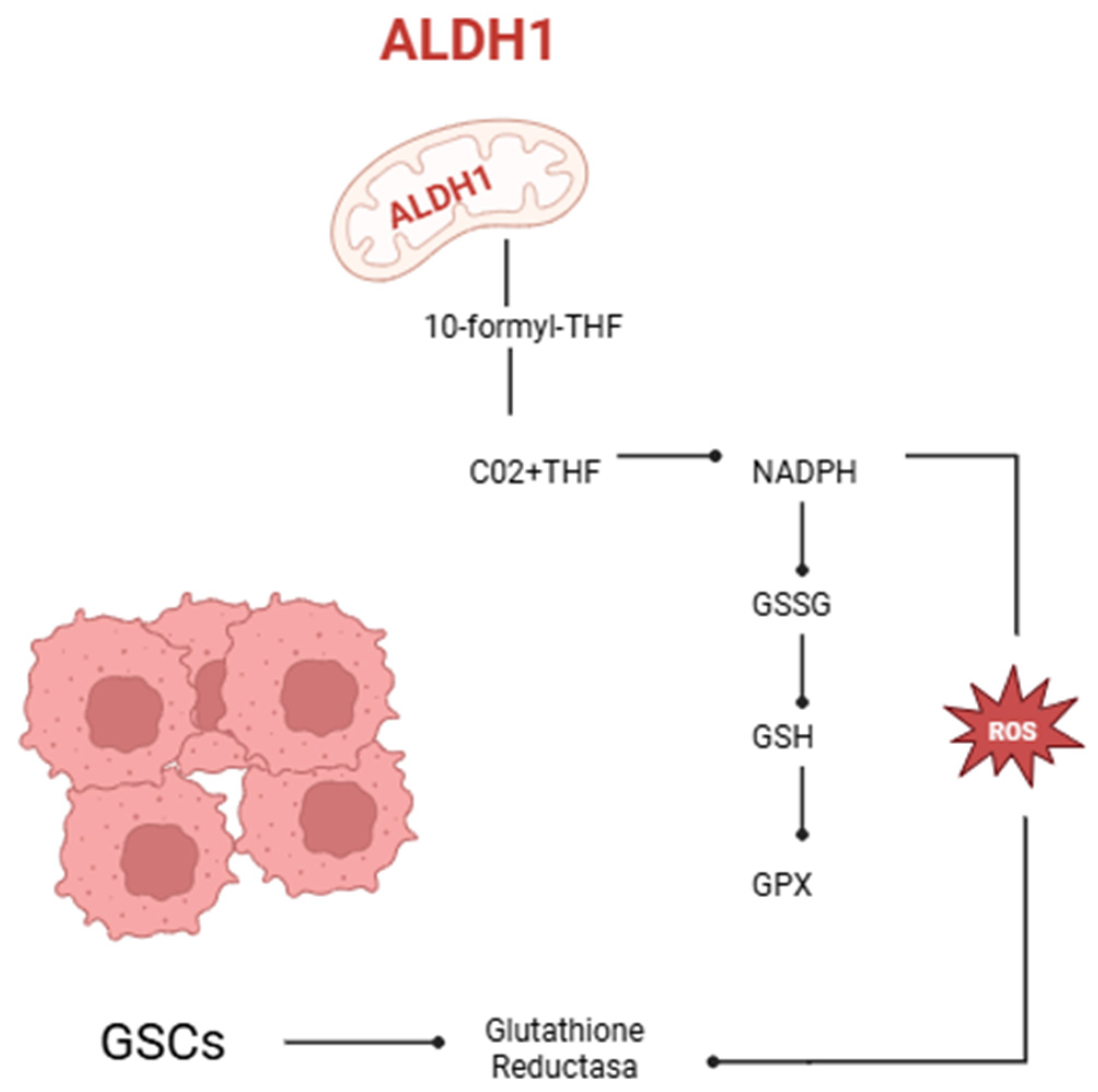
3. The Function of Human Aldehyde Dehydrogenase in Redox Equilibrium in Oncology
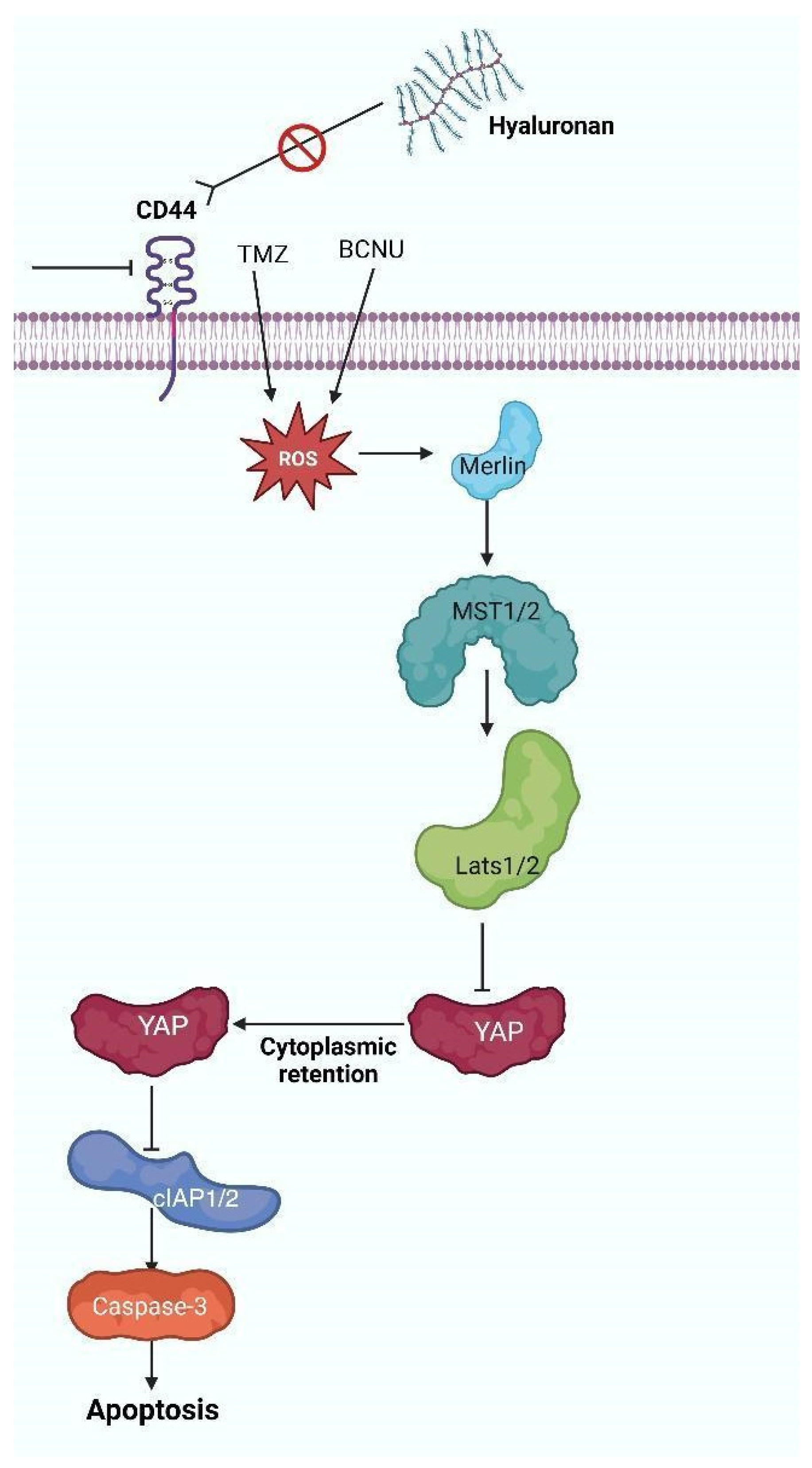
4. The HA-CD44 Axis Regulates the Oxidative Stress Response in Glioblastoma
5. Genetics and Oxidative Pathways in Glioblastoma Multiforme
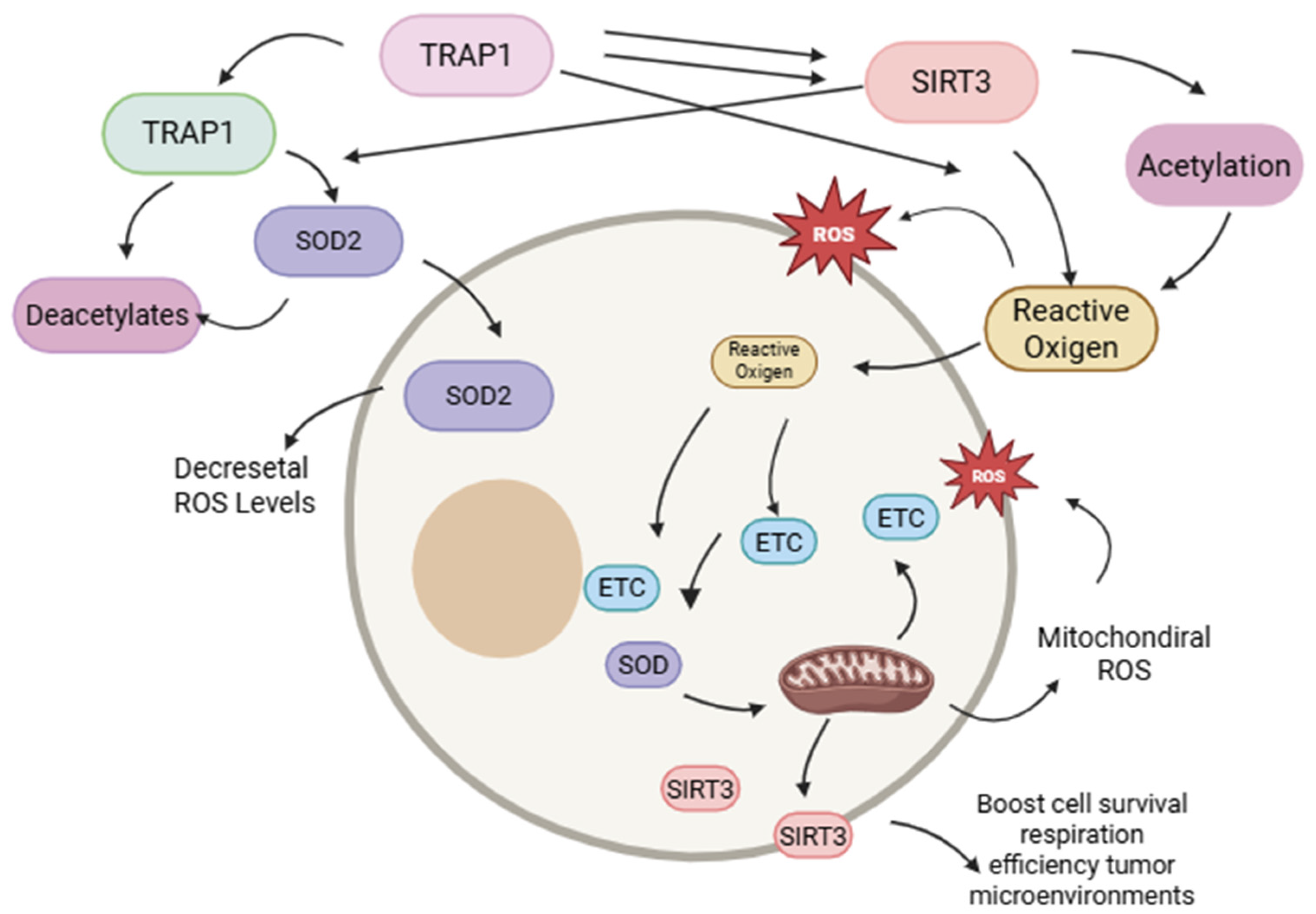
6. SIRT3: Mitochondrial Regulator of Redox Equilibrium and Cellular Oxidative Stress
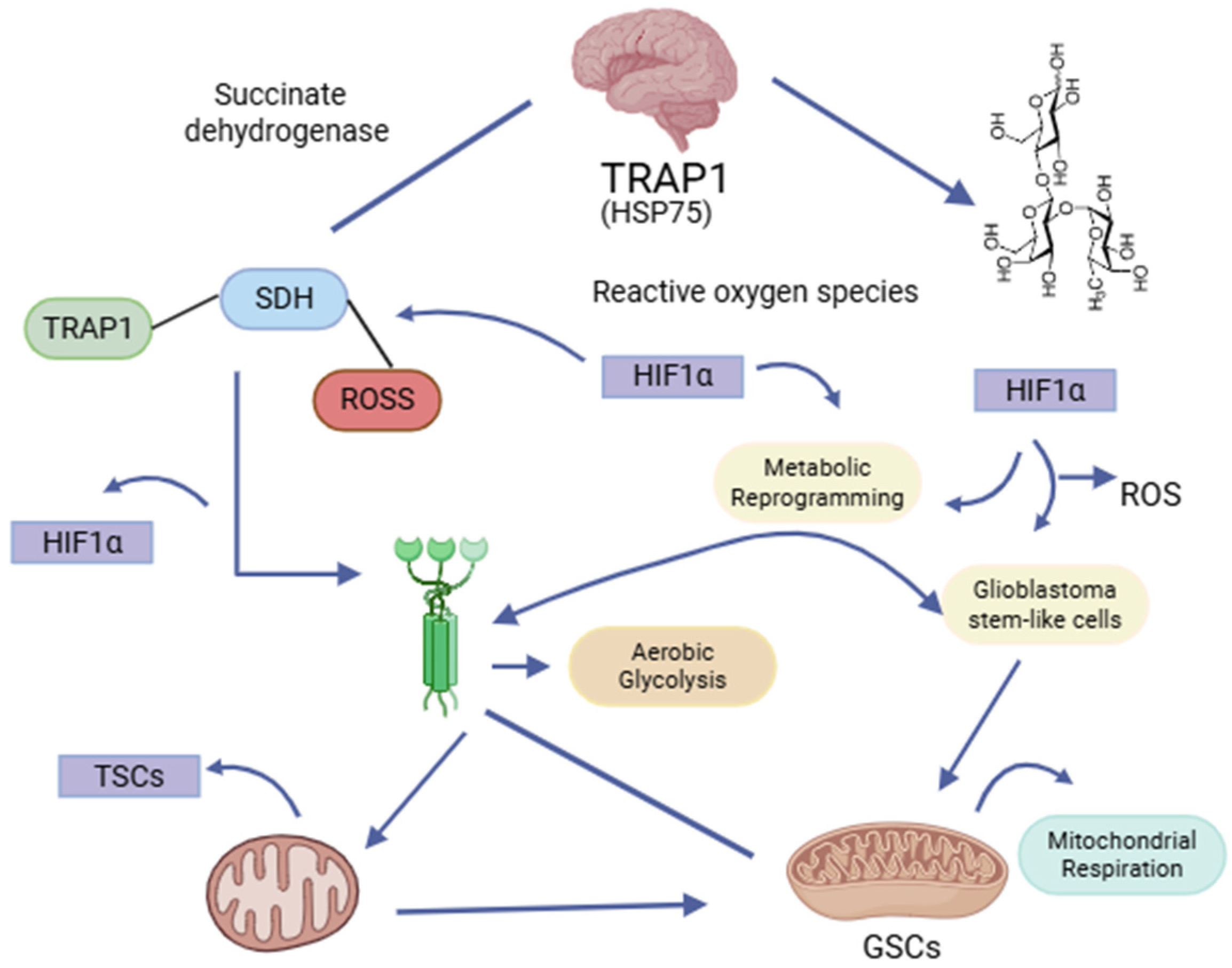
7. Functional Interdependence of TRAP1 and SIRT3 in the Metabolic Adaptation of Glial Stem Cells in Glioblastoma
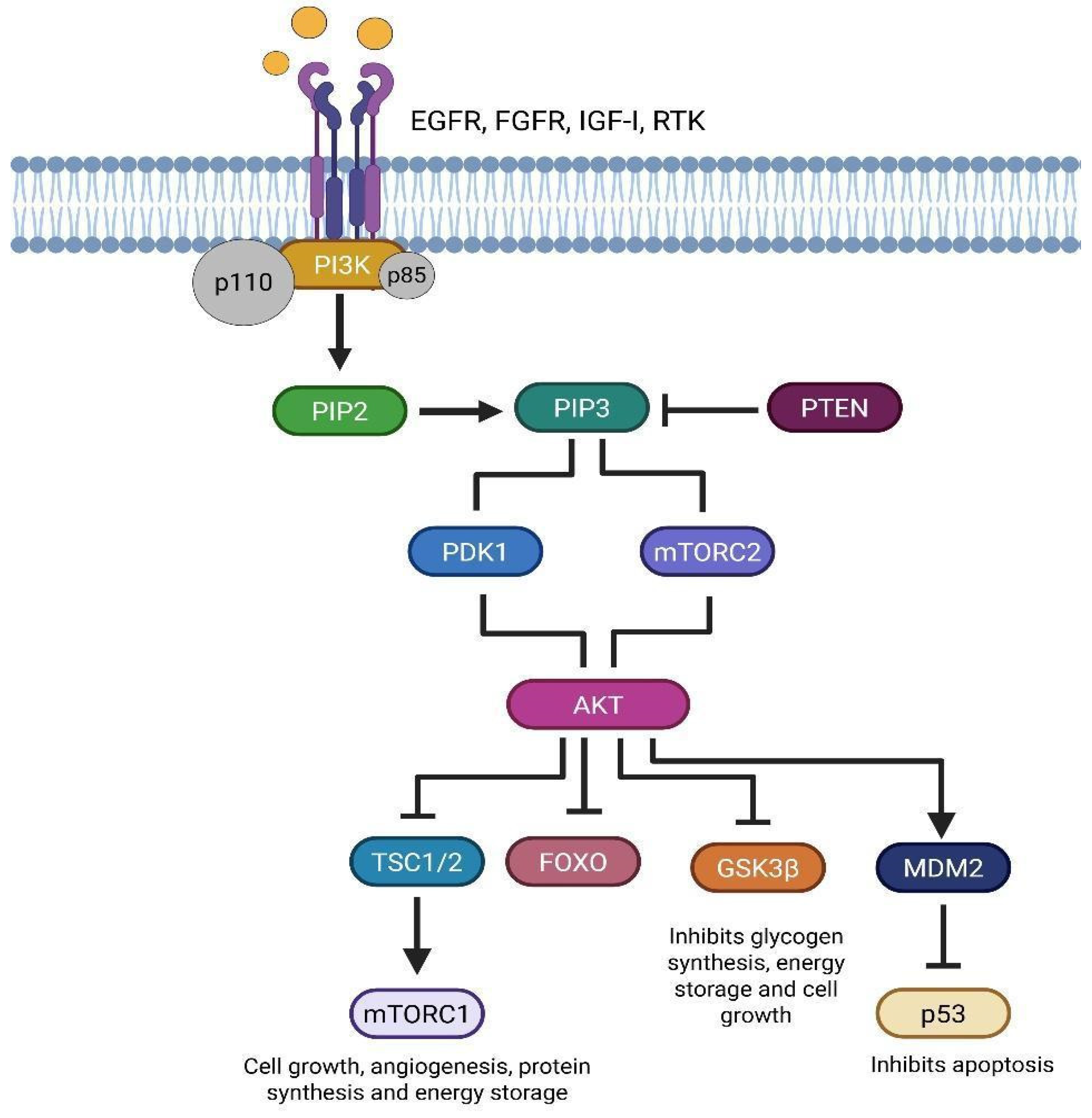
8. Modifications in the PI3K/AKT/mTOR Pathway in Glioblastoma Molecular and Prognostic Significance
9. Targeting the PI3K/AKT/mTOR Pathway in Glioblastoma Stem Cells for Therapeutic Intervention
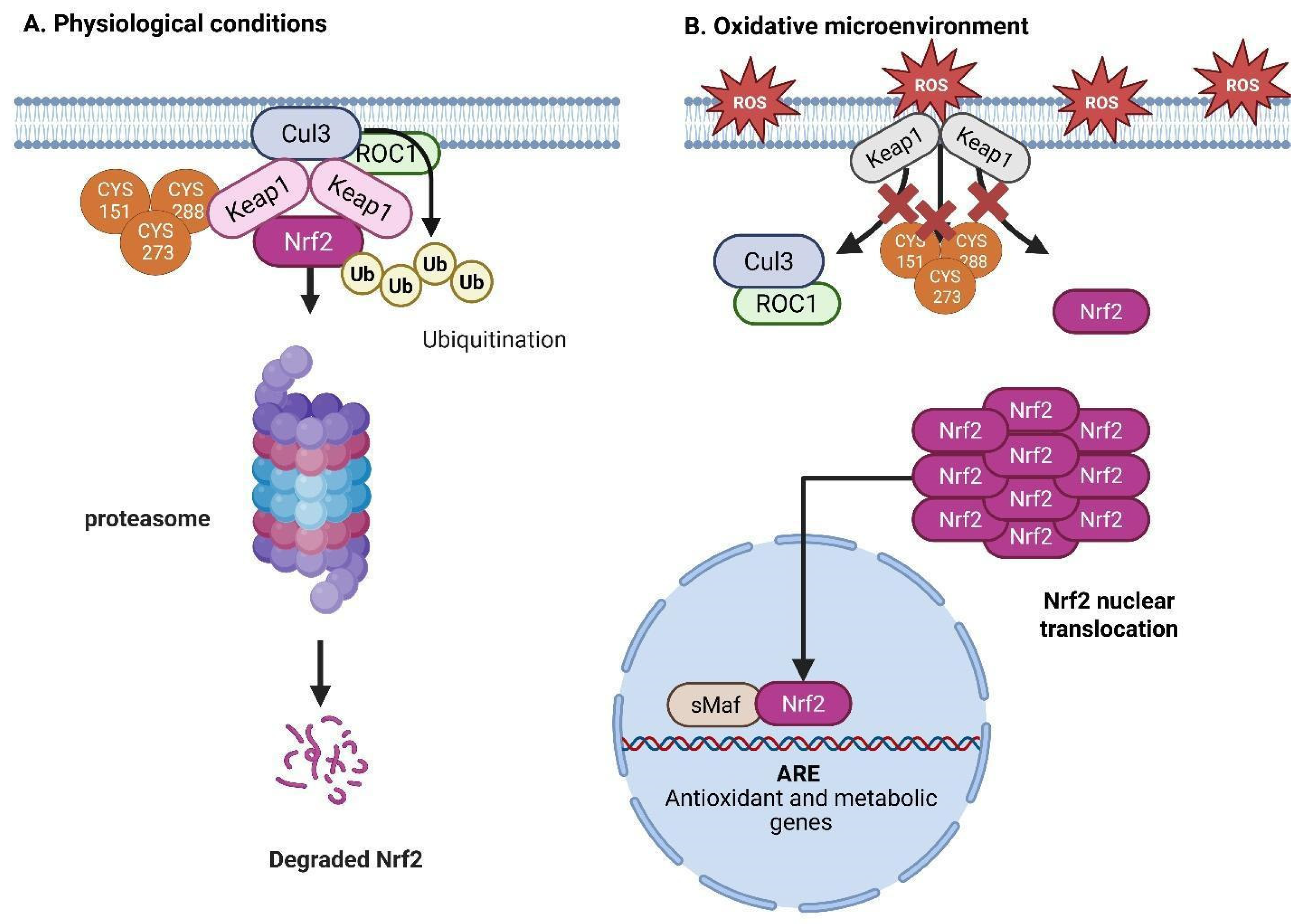
10. The Nrf2/Keap1 Pathway and Its Significance in Oxidative Stress and Tumor Proliferation
11. Impact of Oxidative Stress on the Wnt/β-Catenin Signaling Pathway in Neoplastic Cells
12. Oxidative Stress Induced by Hydrogen Peroxide
13. Therapeutic Approaches for Tumor Immune Microenvironment
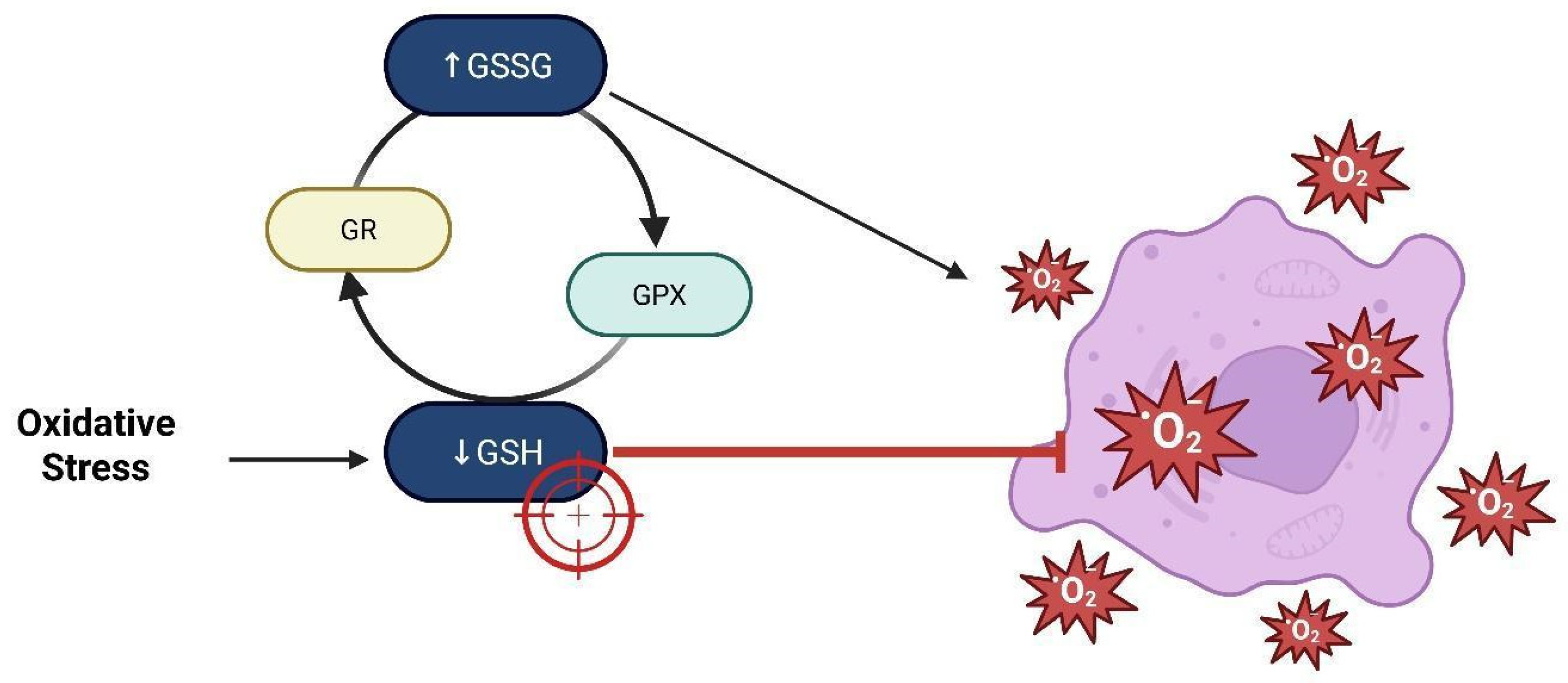
14. Depletion of Glutathione
15. Inhibition of the EGFR/AKT Pathway
16. Future Directions
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxy-2-Nonenal (4-hidroxi-2-nonenal) |
| ACSS3 | Acyl-CoA Synthetase Short-Chain Family Member 3 |
| ACSL3 | Acyl-CoA Synthetase Long-Chain Family Member 3 |
| AKR1C1 | Aldo-Keto Reductase Family 1 Member C1 |
| AKT | Protein Kinase B |
| ALDH | Aldehyde Dehydrogenase (Aldehído Deshidrogenasa) |
| ALDH1L2 | Aldehyde Dehydrogenase 1 Family Member L2 |
| APC | Adenomatous Polyposis Coli |
| ARE | Antioxidant Response Element (Elemento de Respuesta Antioxidante) |
| CAMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| CAT | Catalase (Catalasa) |
| CD44 | Cluster of Differentiation 44 |
| CD44v | CD44 Variant Isoform |
| CD44-ICD | CD44 Intracellular Domain (Dominio intracelular de CD44) |
| CK-1 | Casein Kinase 1 |
| CNS | Central Nervous System (Sistema Nervioso Central) |
| CO2 | Carbon Dioxide (Dióxido de Carbono) |
| CypD | Cyclophilin D |
| DVL | Dishevelled Protein |
| EGFR | Epidermal Growth Factor Receptor (Receptor del Factor de Crecimiento Epidérmico) |
| EGFRvIII | Epidermal Growth Factor Receptor Variant III |
| ELOVL2 | Elongation of Very Long Chain Fatty Acids Protein 2 |
| ERM | Ezrin./Radixin/Moesin Proteins |
| ETC | Electron Transport Chain (Cadena de Transporte de Electrones) |
| FOXO | Forkhead Box O Transcription Factors |
| FZD | Frizzled Receptor |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GBM | Glioblastoma |
| GCL | γ-Glutamylcysteinyl Ligase |
| GPX | Glutathione Peroxidase (Glutatión Peroxidasa) |
| GR | Glutathione Reductase |
| GSC | Glial Stem-like Cell (Célula Glial con características de Célula Madre) |
| GSH | Reduced Glutathione (Glutatión reducido) |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| GSSG | Oxidized Glutathione (Glutatión oxidado) |
| HIF1α | Hypoxia-Inducible Factor 1 Alpha |
| HO-1 | Heme Oxygenase 1 |
| IDH | Isocitrate Dehydrogenase |
| IP3 | Inositol 1,4,5-Trisphosphate |
| JNK | c-Jun N-terminal Kinase |
| Keap1 | Kelch-like ECH-associated Protein 1 |
| LATS1/2 | Large Tumor Suppressor Kinases 1 and 2 |
| LRP5/6 | Low-Density Lipoprotein Receptor-Related Protein 5/6 |
| MAPK | Mitogen-Activated Protein Kinase |
| mPTP | Mitochondrial Permeability Transition Pore |
| mTOR | Mechanistic Target of Rapamycin (Objetivo mecanístico de la rapamicina) |
| MST1/2 | Mammalian Sterile 20-like Kinases 1 and 2 |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF2 | Neurofibromin 2 (Merlin) |
| NF-κB | Nuclear Factor Kappa B |
| NFAT | Nuclear Factor of Activated T Cells |
| NFE2L2 | Nuclear Factor, Erythroid 2-Like 2 (gene encoding Nrf2) |
| NQO1 | Quinone Oxidoreductase 1 |
| NR4A2 | Nuclear Receptor Subfamily 4 Group A Member 2 |
| NRX | Nucleoredoxin |
| Nrf2 | Nuclear Factor Erythroid 2-related Factor 2 |
| Nox1 | NADPH Oxidase 1 |
| OS | Oxidative Stress (Estrés Oxidativo) |
| OXPHOS | Oxidative Phosphorylation |
| PIP2 | Phosphatidylinositol 4,5-Bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-Trisphosphate |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| PMT | Phenotypic Mesenchymal Transition (Transición Fenotípica Mesenquimal) |
| PTEN | Phosphatase and Tensin Homolog |
| RAS | Rat Sarcoma Virus Oncogene (Oncogén Ras) |
| RAC3 | Receptor-Associated Coactivator 3 / |
| SRC-3 | Steroid Receptor Coactivator-3 |
| RHEB | Ras Homolog Enriched in Brain |
| RIOK1 | Right Open Reading Frame Kinase 1 |
| RIOK2 | Right Open Reading Frame Kinase 2 |
| ROS | Reactive Oxygen Species (Especies Reactivas de Oxígeno) |
| RTK | Receptor Tyrosine Kinase |
| RXRα | Retinoid X Receptor Alpha |
| SDH | Succinate Dehydrogenase |
| sMaf | Small Maf Proteins |
| SIRT3 | Sirtuin 3 |
| SOD | Superoxide Dismutase |
| SQLE | Squalene Monooxygenase |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TCF/LEF | T-Cell Factor/Lymphoid Enhancer-Binding Factor |
| TAK1 | Transforming Growth Factor Beta-Activated Kinase 1 |
| THF | Tetrahydrofolate (Tetrahidrofolato) |
| TMZ | Temozolomide |
| TME | Tumor Microenvironment |
| TSC2 | Tuberous Sclerosis Complex 2 |
| TRAP1 | Tumor Necrosis Factor Receptor-Associated Protein 1 |
| Trx | Thioredoxin |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization (Organización Mundial de la Salud) |
| Wnt/β | Wnt/β-catenin signaling pathway |
| YAP | Yes-Associated Protein |
References
- Finch, A.; Solomou, G.; Wykes, V.; Pohl, U.; Bardella, C.; Watts, C. Advances in research of adult gliomas. Int. J. Mol. Sci. 2021, 22, 924. [Google Scholar] [CrossRef]
- Baker, S.J.; Zong, H.; Monje, M. Glial Malignancies. Cold Spring Harb. Perspect. Biol. 2024, 17, a041373. [Google Scholar] [CrossRef] [PubMed]
- Aiman, W.; Gasalberti, D.P.; Rayi, A. Low-Grade Gliomas. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, M.A.; Sotelo, J. Brain tumors in Mexico: Characteristics and prognosis of glioblastoma. Surg. Neurol. 2000, 53, 157–162. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.; Lawrie, T.A.; Rogozińska, E.; Kernohan, A.; Robinson, T.; Jefferies, S. Treatment options for progression or recurrence of glioblastoma: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, CD013579. [Google Scholar] [CrossRef] [PubMed]
- Adegboyega, G.; Kanmounye, U.S.; Petrinic, T.; Ozair, A.; Bandyopadhyay, S.; Kuri, A.; Zolo, Y.; Marks, K.; Ramjee, S.; Baticulon, R.E.; et al. Global Landscape of Glioblastoma Multiforme Management in the Stupp Protocol Era: Systematic Review Protocol. Int. J. Surg. Protoc. 2021, 25, 108–113. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Hennequart, M.; Pilley, S.E.; Labuschagne, C.F.; Coomes, J.; Mervant, L.; Driscoll, P.C.; Legrave, N.M.; Lee, Y.; Kreuzaler, P.; Macintyre, B.; et al. ALDH1L2 regulation of formate, formyl-methionine, and ROS controls cancer cell migration and metastasis. Cell Rep. 2023, 42, 112562. [Google Scholar] [CrossRef]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Gillespie, G.Y.; Friedman, G.K.; Hjelmeland, A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics 2021, 11, 665–683. [Google Scholar] [CrossRef]
- Salazar-Ramiro, A.; Ramírez-Ortega, D.; de la Cruz, V.P.; Hérnandez-Pedro, N.Y.; González-Esquivel, D.F.; Sotelo, J.; Pineda, B. Role of Redox Status in Development of Glioblastoma. Front. Immunol. 2016, 7, 156. [Google Scholar] [CrossRef]
- Li, C.; Teng, P.; Sun, S.; Cui, K.; Yao, S.; Fei, B.; Ling, F.; Huang, Z. Acetylation of aldehyde dehydrogenase ALDH1L2 regulates cellular redox balance and the chemosensitivity of colorectal cancer to 5-fluorouracil. J. Biol. Chem. 2023, 299, 105090. [Google Scholar] [CrossRef]
- Quéré, M.; Alberto, J.-M.; Broly, F.; Hergalant, S.; Christov, C.; Gauchotte, G.; Guéant, J.-L.; Namour, F.; Battaglia-Hsu, S.-F. ALDH1L2 Knockout in U251 Glioblastoma Cells Reduces Tumor Sphere Formation by Increasing Oxidative Stress and Suppressing Methionine Dependency. Nutrients 2022, 14, 1887. [Google Scholar] [CrossRef]
- Krupenko, S.A.; Krupenko, N.I. ALDH1L1 and ALDH1L2 folate regulatory enzymes in cancer. In Alcohol and Cancer; Springer: Berlin/Heidelberg, Germany, 2018; pp. 127–143. [Google Scholar] [CrossRef]
- Xia, J.; Li, S.; Liu, S.; Zhang, L. Aldehyde dehydrogenase in solid tumors and other diseases: Potential biomarkers and therapeutic targets. Medcomm 2023, 4, e195. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Matsumoto, S.; Suehiro, S.; Yamashita, D.; Ozaki, S.; Watanabe, H.; et al. Significance of Glioma Stem-Like Cells in the Tumor Periphery That Express High Levels of CD44 in Tumor Invasion, Early Progression, and Poor Prognosis in Glioblastoma. Stem Cells Int. 2018, 2018, 5387041. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Li, Q.; Wang, M.; Hu, J.; Dai, J.; Niu, L.; Yuan, G.; Pan, Y. Elevated CD44 expression predicts poor prognosis in patients with low-grade glioma. Oncol. Lett. 2019, 18, 3698–3704. [Google Scholar] [CrossRef]
- Kolliopoulos, C.; Ali, M.M.; Castillejo-Lopez, C.; Heldin, C.-H.; Heldin, P. CD44 depletion in glioblastoma cells suppresses growth and stemness and induces senescence. Cancers 2022, 14, 3747. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X. Targeting the Hippo pathway to improve response to chemotherapy. In Targeting Cell Survival Pathways to Enhance Response to Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–185. [Google Scholar] [PubMed] [PubMed Central]
- Chow, C.Y.; Zhang, Y.; Dowling, J.J.; Jin, N.; Adamska, M.; Shiga, K.; Szigeti, K.; Shy, M.E.; Li, J.; Zhang, X.; et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 2007, 448, 68–72. [Google Scholar] [CrossRef]
- Mao, Y.; Tournier, A.L.; Hoppe, A.; Kester, L.; Thompson, B.J.; Tapon, N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013, 32, 2790–2803. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 2019, 8, e44235. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Shamseldin, H.E.; Fogle, H.M.; Rushing, B.R.; AlMalki, R.H.; Jaafar, A.; Hashem, M.; Abdulwahab, F.; Abdel Rahman, A.M.; Krupenko, N.I.; et al. Further delineation of the phenotypic and metabolomic profile of ALDH1L2-related neurodevelopmental disorder. Clin. Genet. 2024, 105, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Björklund, M.; Caldez, M.J.; Zheng, J.; Kaldis, P. Therapeutic targeting of the mitochondrial one-carbon pathway: Perspectives, pitfalls, and potential. Oncogene 2021, 40, 2339–2354. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-Q.; Lin, J.-F.; Tian, T.; Xie, D.; Xu, R.-H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef]
- Ma, I.; Allan, A.L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011, 7, 292–306. [Google Scholar] [CrossRef]
- Chen, J.L.; Villa, K.L.; Cha, J.W.; So, P.T.C.; Kubota, Y.; Nedivi, E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 2012, 74, 361–373. [Google Scholar] [CrossRef]
- Pibuel, M.A.; Poodts, D.; Díaz, M.; SE, H.; Lompardía, S.L. The scrambled story between hyaluronan and glioblastoma. J. Biol. Chem. 2021, 296, 100549. [Google Scholar] [CrossRef] [PubMed Central]
- Xu, Y.; Stamenkovic, I.; Yu, Q. CD44 Attenuates Activation of the Hippo Signaling Pathway and Is a Prime Therapeutic Target for Glioblastoma. Cancer Res. 2010, 70, 2455–2464. [Google Scholar] [CrossRef] [PubMed Central]
- Inoue, A.; Ohnishi, T.; Nishikawa, M.; Ohtsuka, Y.; Kusakabe, K.; Yano, H.; Tanaka, J.; Kunieda, T. A Narrative Review on CD44’s Role in Glioblastoma Invasion, Proliferation, and Tumor Recurrence. Cancers 2023, 15, 4898. [Google Scholar] [CrossRef] [PubMed Central]
- Shoaib, F.; Furrukh, M.; Mushtaq, F.; Sayeed, S.; Nasir, H.; Ghafoor, H. Extra Neural Metastasis Of Glioblastoma Multiforme: A Literature Review. J. Pak. Med. Assoc. 2023, 73, 1869–1873. [Google Scholar] [CrossRef] [PubMed]
- Klank, R.L.; Grunke, S.A.D.; Bangasser, B.L.; Forster, C.L.; Price, M.A.; Odde, T.J.; SantaCruz, K.S.; Rosenfeld, S.S.; Canoll, P.; Turley, E.A.; et al. Biphasic Dependence of Glioma Survival and Cell Migration on CD44 Expression Level. Cell Rep. 2017, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Duca, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models. Cancers 2022, 14, 2128. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Eyler, C.E.; Rich, J.N. Survival of the Fittest: Cancer Stem Cells in Therapeutic Resistance and Angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef]
- Derouiche, A.; Geiger, K.D. Perspectives for Ezrin and Radixin in Astrocytes: Kinases, Functions and Pathology. Int. J. Mol. Sci. 2019, 20, 3776. [Google Scholar] [CrossRef]
- Vivanco, I.; Chen, Z.; Tanos, B.; Oldrini, B.; Hatanpaa, K.J.; Gray, N.S. Differential sensitivity of glioma- versus lung cancer–specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2014, 2, 458–471. [Google Scholar] [CrossRef]
- Wu, G.; Song, X.; Liu, J.; Li, S.; Gao, W.; Qiu, M.; Yang, C.; Ma, Y.; Chen, Y. Expression of CD44 and the survival in glioma: A meta-analysis. Biosci. Rep. 2020, 40, BSR20200520. [Google Scholar] [CrossRef]
- Pontes, B.; Mendes, F.A. Mechanical Properties of Glioblastoma: Perspectives for YAP/TAZ Signaling Pathway and Beyond. Diseases 2023, 11, 86. [Google Scholar] [CrossRef]
- Hou, C.; Lin, H.; Wang, P.; Yang, Y.; Cen, S.; Zhou, D. Comment on “Expression of CD44 and the survival in glioma: A meta-analysis”. Biosci. Rep. 2020, 40, BSR20202812. [Google Scholar] [CrossRef]
- Loftus, A.E.P.; Romano, M.S.; Phuong, A.N.; McKinnel, B.J.; Muir, M.T.; Furqan, M.; Dawson, J.C.; Avalle, L.; Douglas, A.T.; Mort, R.L.; et al. An ILK/STAT3 pathway controls glioblastoma stem cell plasticity. Dev. Cell 2024, 59, 3197–3212.e7. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.K.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef]
- Chen, S.-M.; Li, Y.-Y.; Tu, C.-H.; Salazar, N.; Tseng, Y.-Y.; Huang, S.-F.; Hsieh, L.-L.; Lui, T.-N.; Lebedeva, I.V. Blockade of Inhibitors of Apoptosis Proteins in Combination with Conventional Chemotherapy Leads to Synergistic Antitumor Activity in Medulloblastoma and Cancer Stem-Like Cells. PLoS ONE 2016, 11, e0161299. [Google Scholar] [CrossRef]
- Bourguignon, T.; Lo, N.; Dietrich, C.; Šobotník, J.; Sidek, S.; Roisin, Y.; Brune, A.; Evans, T.A. Rampant Host Switching Shaped the Termite Gut Microbiome. Curr. Biol. 2018, 28, 649–654.e2. [Google Scholar] [CrossRef]
- Yusupov, M.; Privat-Maldonado, A.; Cordeiro, R.M.; Verswyvel, H.; Shaw, P.; Razzokov, J.; Smits, E.; Bogaerts, A. Oxidative damage to hyaluronan–CD44 interactions as an underlying mechanism of action of oxidative stress-inducing cancer therapy. Redox Biol. 2021, 43, 101968. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Du, M.; Zhang, J.; Wicha, M.S.; Luo, M. Redox regulation of cancer stem cells: Biology and therapeutic implications. MedComm Oncology 2024, 3, e70005. [Google Scholar] [CrossRef]
- Ramos Rego, I.; Santos Cruz, B.; Ambrósio, A.F.; Alves, C.H. TRAP1 in oxidative stress and neurodegeneration. Antioxidants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Kang, S.; Kang, B.H. Structure, function, and inhibitors of the mitochondrial chaperone TRAP1. J. Med. Chem. 2022, 65, 16155–16172. [Google Scholar] [CrossRef]
- Park, H.-K.; Hong, J.-H.; Oh, Y.T.; Kim, S.S.; Yin, J.; Lee, A.-J.; Chae, Y.C.; Kim, J.H.; Park, S.-H.; Park, C.-K.; et al. Interplay between TRAP1 and Sirtuin-3 Modulates Mitochondrial Respiration and Oxidative Stress to Maintain Stemness of Glioma Stem Cells. Cancer Res. 2019, 79, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, E.; Lagadec, C.; Vergnes, L.; Matsutani, T.; Masui, K.; Poulou, M.; Popescu, R.; Della Donna, L.; Evers, P.; Dekmezian, C.; et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16062–16067. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of cancer stem cell metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Roca, P.; Sastre-Serra, J. Importancia de la sirtuina 3 en el estrés oxidativo y el cáncer. Med. Balear. 2017, 32, 47–52. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. SIRT3 Sirtuin 3 [Homo Sapiens (Human)]. Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/23410/ (accessed on 7 April 2025).
- Zhang, H.; Dai, S.; Yang, Y.; Wei, J.; Li, X.; Luo, P.; Jiang, X. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules 2023, 13, 735. [Google Scholar] [CrossRef]
- Nowacka, A.; Śniegocka, M.; Śniegocki, M.; Ziółkowska, E.A. Sirtuins in Central Nervous System Tumors—Molecular Mechanisms and Therapeutic Targeting. Cells 2025, 14, 1113. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Li, J. A review on SIRT3 and its natural small molecule activators as a potential Preventive and therapeutic target. Eur. J. Pharmacol. 2023, 963, 176155. [Google Scholar] [CrossRef]
- Agosti, E.; Antonietti, S.; Ius, T.; Fontanella, M.M.; Zeppieri, M.; Panciani, P.P. Glioma Stem Cells as Promoter of Glioma Progression: A Systematic Review of Molecular Pathways and Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 7979. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- PIK3R1 Phosphoinositide-3-Kinase Regulatory Subunit 1 [Homo Sapiens (human)]-Gene-. N.C.B.I. Available online: https://www.ncbi.nlm.nih.gov/gene/5295 (accessed on 28 May 2025).
- De Rosa, V.; Galgani, M.; Porcellini, A.; Colamatteo, A.; Santopaolo, M.; Zuchegna, C.; Romano, A.; De Simone, S.; Procaccini, C.; La Rocca, C.; et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015, 16, 1174–1184. [Google Scholar] [CrossRef]
- AKT1 AKTserine/threonine Kinase 1 [Homo Sapiens (human)]-Gene-, N.C.B.I. Nih.gov. 2025. Available online: https://www.ncbi.nlm.nih.gov/gene/207 (accessed on 28 May 2025).
- AKT2 AKTserine/threonine Kinase 2 [Homo Sapiens (human)]-Gene-, N.C.B.I. Available online: https://www.ncbi.nlm.nih.gov/gene/208 (accessed on 28 May 2025).
- AKT3 AKTserine/threonine Kinase 3 [Homo Sapiens (human)]-Gene-, N.C.B.I. Available online: https://www.ncbi.nlm.nih.gov/gene/10000 (accessed on 28 May 2025).
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- PTENphosphatase Tensin Homolog [Homo Sapiens (human)]-Gene-, N.C.B.I. Available online: https://www.ncbi.nlm.nih.gov/gene/5728 (accessed on 12 August 2025).
- Li, Y.; Liu, X.; Dong, Y.; Zhou, Y. Angiogenesis causes and vasculogenic mimicry formation in the context of cancer stem cells. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189323. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Zhao, D.; Chen, J.; Alcantara, S. PI3K pathway inhibition disrupts GSC maintenance and enhances radiosensitivity. Neuro Oncology 2015, 17, 810–820. [Google Scholar]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Luongo, F.; Colonna, F.; Calapà, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef]
- Chinchu Praisthy, L.J.; Kushwah, R.; Dubey, S.; Labhade, S.; Karwa, P.; Jain, S. Daidzein targets PI3K-Akt signaling and oxidative stress in glioblastoma: An Integrated pharmacological and in vitro study. Brain Res. 2025, 1863, 149840. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Mureb, M.C.; Zeller, S.L.; Rosberger, H.T.; Spirollari, E.; Das, M.; Hanft, S.J.; Gandhi, C.D.; Jhanwar-Uniyal, M. Cancer Stem Cells in Glioblastoma: The Role of the mTOR Pathway. Anticancer Res. 2025, 45, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- El-Khayat, S.M.; Arafat, W.O. Therapeutic strategies of recurrent glioblastoma and its molecular pathways ‘Lock up the beast’. Ecancermedicalscience 2021, 15, 1176. [Google Scholar] [CrossRef]
- N.C.B.I. Bethesda (MD): National Library of Medicine (US). NFE2L2 NFE2 Like bZIPtranscription Factor 2 [Homo Sapiens (human)]-Gene-. 2025. Available online: https://www.ncbi.nlm.nih.gov/gene/4780 (accessed on 30 May 2025).
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- KEAP1 Kelch Like ECH Associated Protein 1 [Homo Sapiens (human)]-Gene-NCBI [Internet]. Bethesda (MD): National Library of Medicine (US). 2025. Available online: https://www.ncbi.nlm.nih.gov/gene/9817 (accessed on 30 May 2025).
- Jia, Y.; Wang, H.D.; Wang, Q.; Ding, H.; Wu, H.M.; Pan, H. GSH depletion and consequent AKT inhibition contribute to the Nrf2 knockdown-induced decrease in proliferation in glioblastoma U251 cells. Oncol. Rep. 2017, 37, 2252–2260. [Google Scholar] [CrossRef]
- Godoy, P.R.D.V.; Pour Khavari, A.; Rizzo, M.; Sakamoto-Hojo, E.T.; Haghdoost, S. Targeting NRF2, Regulator of Antioxidant System, to Sensitize Glioblastoma Neurosphere Cells to Radiation-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 2534643. [Google Scholar] [CrossRef]
- Hammad, M.; Salma, R.; Balosso, J.; Rezvani, M.; Haghdoost, S. Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-Rays, Protons and Carbon Ions. Antioxidants 2024, 13, 1035. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, J.; Liu, X.M.; Zhao, R.; Zhe, H. Nrf2-Mediated Metabolic Reprogramming in Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9304091. [Google Scholar] [CrossRef]
- Panieri, E.; Pinho, S.A.; Afonso, G.J.M.; Oliveira, P.J.; Cunha-Oliveira, T.; Saso, L. NRF2 and Mitochondrial Function in Cancer and Cancer Stem Cells. Cells 2022, 11, 2401. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 00745. [Google Scholar] [CrossRef]
- Tompa, M.; Kajtar, B.; Galik, B.; Gyenesei, A.; Kalman, B. DNA methylation and protein expression of Wnt pathway markers in progressive glioblastoma. Pathol. Res. Pract. 2021, 222, 153429. [Google Scholar] [CrossRef]
- Zuccarini, M.; Giuliani, P.; Ziberi, S.; Carluccio, M.; Iorio, P.D.; Caciagli, F.; Ciccarelli, R. The role of Wnt signal in glioblastoma development and progression: A possible new pharmacological target for the therapy of this tumor. Genes 2018, 9, 105. [Google Scholar] [CrossRef]
- Haseeb, M.; Pirzada, R.H.; Ain, Q.U.; Choi, S. Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells 2019, 8, 1380. [Google Scholar] [CrossRef]
- Guan, R.; Zhang, X.; Guo, M. Glioblastoma stem cells and Wnt signaling pathway: Molecular mechanisms and therapeutic targets. Chin. Neurosurg. J. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.-K.; Ahn, S.H.; Lee, J.; Nam, D.-H. WNT signaling in glioblastoma and therapeutic opportunities. Mod. Pathol. 2016, 96, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Guo, J.; Chen, H.; Yao, C.-J.; Zhuang, D.-X.; Wang, Y.; Tang, W.-J.; Ren, G.; Yao, Y.; Wu, J.-S.; et al. Gene mutation profiling of primary glioblastoma through multiple tumor biopsy guided by 1H-magnetic resonance spectroscopy. Int. J. Clin. Exp. Pathol. 2015, 8, 5327–5335. [Google Scholar] [PubMed]
- Kahlert, U.D.; Maciaczyk, D.; Doostkam, S.; Orr, B.A.; Simons, B.; Bogiel, T.; Reithmeier, T.; Prinz, M.; Schubert, J.; Niedermann, G.; et al. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012, 325, 42–53. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Zhang, L.; Yang, L.; Zhou, M.; Li, X.; Li, Y.; Li, G.; Zeng, Z.; Xiong, W.; et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer 2019, 10, 3789–3797. [Google Scholar] [CrossRef]
- Kim, J.E.; Patel, M.; Ruzevick, J.; Jackson, C.M.; Lim, M. STAT3 Activation in Glioblastoma: Biochemical and Therapeutic Implications. Cancers 2014, 6, 376–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoogeboom, D.; Essers, M.A.G.; Polderman, P.E.; Voets, E.; Smits, L.M.M.; Burgering, B.M.T. Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J. Biol. Chem. 2008, 283, 9224–9230. [Google Scholar] [CrossRef]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef]
- Kajla, S.; Mondol, A.S.; Nagasawa, A.; Zhang, Y.; Kato, M.; Matsuno, K.; Kamata, T. A crucial role for Nox1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J. 2012, 26, 2049–2059. [Google Scholar] [CrossRef]
- Funato, Y.; Miki, H. Redox regulation of Wnt signalling via nucleoredoxin. Free. Radic. Res. 2010, 44, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Kim, S.; Hsieh, J.T.; Baek, S.T. Wnt/β-catenin signaling pathway induces autophagy-mediated temozolomide-resistance in human glioblastoma. Cell Death Dis. 2020, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lu, B.; Zamponi, R.; Yang, Z.; Wetzel, K.; Loureiro, J.; Mohammadi, S.; Beibel, M.; Bergling, S.; Reece-Hoyes, J.; et al. mTORC1 signaling suppresses Wnt/β-catenin signaling through DVL-dependent regulation of Wnt receptor FZD level. Proc. Natl. Acad. Sci. USA 2018, 115, E10362–E10369. [Google Scholar] [CrossRef] [PubMed]
- Rasper, M.; Schäfer, A.; Piontek, G.; Teufel, J.; Brockhoff, G.; Ringel, F.; Heindl, S.; Zimmer, C.; Schlegel, J. Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro Oncology 2010, 12, 1024–1033. [Google Scholar] [CrossRef]
- Katz, J.L.; Geng, Y.; Billingham, L.K.; Sadagopan, N.S.; DeLay, S.L.; Subbiah, J.; Chia, T.-Y.; McManus, G.; Wei, C.; Wang, H.; et al. A covalent creatine kinase inhibitor ablates glioblastoma migration and sensitizes tumors to oxidative stress. Sci. Rep. 2024, 14, 21959. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xiang, W.; Zhu, Q.; Ren, N. NRF1 Alleviated Oxidative Stress of Glioblastoma Cells by Regulating NOR1. Folia Biol. 2023, 69, 13–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Wang, C.; Wu, Y.; Chen, D.; Lee, T.H. Potential implications of hydrogen peroxide in the pathogenesis and therapeutic strategies of gliomas. Arch. Pharm. Res. 2020, 43, 187–203. [Google Scholar] [CrossRef]
- Nakayama, N.; Yamaguchi, S.; Sasaki, Y.; Chikuma, T. Hydrogen Peroxide-Induced Oxidative Stress Activates Proteasomal Trypsin-Like Activity in Human U373 Glioma Cells. J. Mol. Neurosci. 2015, 58, 297–305. [Google Scholar] [CrossRef]
- Mikeladze, M.A.; Dutysheva, E.A.; Kartsev, V.G.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Disruption of the complex between GAPDH and Hsp70 sensitizes C6 glioblastoma cells to hypoxic stress. Int. J. Mol. Sci. 2021, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Nikotina, A.D.; Mikhaylova, E.R.; Nudler, E.; Polonik, S.G.; Guzhova, I.V.; Margulis, B.A. Hsp70 chaperone rescues C6 rat glioblastoma cells from oxidative stress by sequestration of aggregating GAPDH. Biochem. Biophys. Res. Commun. 2016, 470, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.A.; Kleyn, M.V.; Schiller, N.; King, A.G.; Flores, G.; Engelsman, S.B.; Bowles, C.; Smith, S.L.; Robinson, A.E.; Rothstein, J. Hydrogen peroxide triggers an increase in cell surface expression of system xc−in cultured human glioma cells. Neurochem. Int. 2020, 134, 104648. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.K.; Palma, L.; Yim, I.; Bullen, N.; Barnett, D.; Walsh, A.; Bhinder, B.; Benedetti, E.; Krumsiek, J.; Gurvitch, J.; et al. Cysteine induces mitochondrial reductive stress in glioblastoma through hydrogen peroxide production. Proc. Natl. Acad. Sci. USA 2024, 121, e2317343121. [Google Scholar] [CrossRef]
- Burster, T.; Gärtner, F.; Bulach, C.; Zhanapiya, A.; Gihring, A.; Knippschild, U. Regulation of MHC I molecules in glioblastoma cells and the sensitizing of NK cells. Pharmaceuticals 2021, 14, 236. [Google Scholar] [CrossRef]
- Lei, F.-J.; Chiang, J.-Y.; Chang, H.-J.; Chen, D.-C.; Wang, H.-L.; Yang, H.-A.; Wei, K.-Y.; Huang, Y.; Wang, C.-C.; Wei, S.-T.; et al. Cellular and exosomal GPx1 are essential for controlling hydrogen peroxide balance and alleviating oxidative stress in hypoxic glioblastoma. Redox Biol. 2023, 65, 102831. [Google Scholar] [CrossRef]
- Ye, Z.; Ai, X.; Yang, K.; Yang, Z.; Fei, F.; Liao, X.; Qiu, Z.; Gimple, R.C.; Yuan, H.; Huang, H.; et al. Targeting Microglial Metabolic Rewiring Synergizes with Immune-Checkpoint Blockade Therapy for Glioblastoma. Cancer Discov. 2023, 13, 974–1001. [Google Scholar] [CrossRef]
- Upadhyay, S.; Lee, M.; Zhang, L.; Oany, A.R.; Mikheeva, S.A.; Mikheev, A.M.; Rostomily, R.C.; Safe, S. Dual nuclear receptor 4A1 (NR4A1/NR4A2) ligands inhibit glioblastoma growth and target TWIST1. Mol. Pharmacol. 2024, 107, 100009. [Google Scholar] [CrossRef]
- Yesudhas, D.; Dharshini, S.A.P.; Taguchi, Y.-H.; Gromiha, M.M. Tumor heterogeneity and molecular characteristics of glioblastoma revealed by single-cell RNA-seq data analysis. Genes 2022, 13, 428. [Google Scholar] [CrossRef]
- Naulaerts, S.; Datsi, A.; Borras, D.M.; Martinez, A.A.; Messiaen, J.; Vanmeerbeek, I.; Sprooten, J.; Laureano, R.S.; Govaerts, J.; Panovska, D.; et al. Multiomics and spatial mapping characterizes human CD8+ T cell states in cancer. Sci. Transl. Med. 2023, 15, eadd1016. [Google Scholar] [CrossRef]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 2020, 32, 229–242.e8. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. 2022, 31, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, J.; Chen, Y.; Zheng, J.; Li, X.; Fu, T.; Xie, S.; Liu, X.; Tan, W. Transferrin Receptor-Targeted Aptamer–Drug Conjugate Overcomes Blood–Brain Barrier for Potent Glioblastoma Therapy. Bioconjugate Chem. 2025, 36, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Obara-Michlewska, M. The tryptophan metabolism, kynurenine pathway and oxidative stress—Implications for glioma pathobiology. Neurochem Int. 2022, 158, 105363. [Google Scholar] [CrossRef]
- König, S.; Strassheimer, F.; Brandner, N.I.; Schröder, J.-H.; Urban, H.; Harwart, L.F.; Hehlgans, S.; Steinbach, J.P.; Ronellenfitsch, M.W.; Luger, A.-L. Superoxide dismutase 1 mediates adaptation to the tumor microenvironment of glioma cells via mammalian target of rapamycin complex 1. Cell Death Discov. 2024, 10, 379. [Google Scholar] [CrossRef]
- Ma, T.; Li, W.; Ye, J.; Huang, C.; Li, Y.; Qiu, H.; Yin, S. GSH/pH dual response drug delivery system for photothermal enhanced gene-immunotherapy. Nanoscale 2023, 15, 16947–16958. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Wang, Z.; Li, C.; Zhang, Z.; Lu, S.; Wang, X.; Liang, Q.; Zhu, X.; Pan, C.; Wang, Q.; et al. SIRT1 activated by AROS sensitizes glioma cells to ferroptosis via induction of NAD+ depletion-dependent activation of ATF3. Redox Biol. 2024, 69, 103030. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Liping, Y.; Jian, H.; Zhenchao, T.; Yan, Z.; Jing, Y.; Yangyang, Z.; Jing, G.; Liting, Q. GSH-responsive poly-resveratrol based nanoparticles for effective drug delivery and reversing multidrug resistance. Drug Deliv. 2022, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Arasaratnam, M.; Chan, D.L.H.; Khasraw, M.; Howell, V.M.; Wheeler, H. Anti-epidermal growth factor receptor therapy for glioblastoma in adults. Cochrane Database Syst. Rev. 2020, 5, CD013238. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, Z.; Liang, W.; Hu, W.; Zhou, S.; Yang, Z.; Tao, Y.; Hou, X.; Xing, Z.; Mao, J.; et al. SOCS proteins and their roles in the development of glioblastoma (Review). Oncol. Lett. 2021, 23, 5. [Google Scholar] [CrossRef]
- Yakubov, R.; Kaloti, R.; Persaud, P.; McCracken, A.; Zadeh, G.; Bunda, S. It’s all downstream from here: RTK/Raf/MEK/ERK pathway resistance mechanisms in glioblastoma. J. Neuro Oncol. 2025, 172, 327–345. [Google Scholar] [CrossRef]
- Hashemi, M.; Etemad, S.; Rezaei, S.; Ziaolhagh, S.; Rajabi, R.; Rahmanian, P.; Abdi, S.; Koohpar, Z.K.; Rafiei, R.; Raei, B.; et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomed. Pharmacother. 2023, 158, 114204. [Google Scholar] [CrossRef]
- Bikfalvi, A.; da Costa, C.A.; Avril, T.; Barnier, J.-V.; Bauchet, L.; Brisson, L.; Cartron, P.F.; Castel, H.; Chevet, E.; Chneiweiss, H.; et al. Challenges in glioblastoma research: Focus on the tumor microenvironment. Trends Cancer 2022, 9, 9–27. [Google Scholar] [CrossRef]
- Xiao, F.; Zhu, H.; Xiong, Y.; Guo, Y.; Zhang, Z.; Zeng, J.; Xiao, Y.; Liao, B.; Shang, X.; Zhao, S.; et al. Positive feedback loop of c-myc/XTP6/NDH2/NF-κB to promote malignant progression in glioblastoma. J. Exp. Clin. Cancer Res. 2024, 43, 187. [Google Scholar] [CrossRef]
- Cai, J.; Ye, Z.; Hu, Y.; Ye, L.; Gao, L.; Wang, Y.; Sun, Q.; Tong, S.; Zhang, S.; Wu, L.; et al. Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. Cell Death Dis. 2023, 14, 211. [Google Scholar] [CrossRef]
- Read, R.D.; Tapp, Z.M.; Rajappa, P.; Hambardzumyan, D. Glioblastoma microenvironment-from biology to therapy. Genes Dev. 2024, 38, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chernysheva, A.A.; Abramova, O.V.; Naumenko, V.A.; Chekhonin, V.P. The need for paradigm shift: Prognostic significance and implications of standard therapy-related systemic immunosuppression in glioblastoma for immunotherapy and oncolytic virotherapy. Front. Immunol. 2024, 15, 1326757. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef]
- Mirhadi, E.; Mashreghi, M.; Faal Maleki, M.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Rohit Kumar, P.; Aran, K.R. Targeting EGFR and PI3K/mTOR pathways in glioblastoma: Innovative therapeutic approaches. Med. Oncol. 2025, 42, 97. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Kothari, S.; Rahman, R.; Lee, E.Q.; Dunn, G.P.; Galanis, E.; Chang, S.M.; Nabors, L.B.; Ahluwalia, M.S.; Stupp, R.; et al. Glioblastoma Clinical Trials: Current Landscape and Opportunities for Improvement. Clin. Cancer Res. 2021, 28, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Huang, J.; Inkman, M.; Zhang, J.; Thotala, S.; Tikhonova, E.; Miheecheva, N.; Frenkel, F.; Ataullakhanov, R.; Wang, X.; et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci. Transl. Med. 2023, 15, 14. [Google Scholar] [CrossRef]


| Main Function | Interaction with AKT | Effects in Glioblastoma | Consequences of Inhibition | |
|---|---|---|---|---|
| RIOK1 | Participates in the final maturation of the 40S ribosomal subunit in the cytoplasm. | Its expression is induced by AKT; correlates with AKT activation. | Overexpressed in high-grade gliomas; associated with increased migration and invasiveness. | Decreased levels of AKT1 and c-Myc; reduced tumor cell migration and invasiveness. |
| RIOK2 | Facilitates nuclear export of the pre-40S subunit and its maturation in the cytoplasm (via ATPase activity after phosphorylation). | Positive feedback loop: RIOK2 activates AKT and vice versa. | Overexpressed in glioblastoma cells; contributes to tumor progression. | Indirect reduction of AKT activation and proliferative signals. |
| c-Myc | Regulates pericellular adhesion and genes associated with invasiveness and metastasis. | Activated by AKT. | Associated with tumor progression and aggressiveness in glioblastoma. | Decreased expression following RIOK1 inhibition. |
| Therapeutic Strategy | Main Mechanism of Action | Specific Focus/Objective | Implication in Glioblastoma |
|---|---|---|---|
| Degradation of oxidized proteins | Removal of damaged proteins like 4-HNE-GAPDH | Proteasome, cathepsin G | Crucial for removing proteins damaged by oxidative stress |
| Targeting NR4A2 in the tumor immune microenvironment | Modulation of microglial plasticity; reduction of tumor proliferation | NR4A2, SQLE, c-Myc | Enhances antigen-presenting capability of microglia; reduces tumor proliferation |
| Glutathione (GSH) depletion | Interference with the GSH antioxidant system | Use of nanoparticles with disulfide bonds | Increases chemotherapy sensitivity in GSCs |
| Inhibition of the EGFR/AKT pathway | Disruption of energy metabolism | EGFR, EGFRvIII, MK-2206, MK-803, TCA cycle, ATP synthesis | Decreases tumor growth; enhances temozolomide efficacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Román, N.F.; Taddei, E.; Castro-Velázquez, E.; Villafuentes-Vidal, L.; Velez-Herrera, A.; Rubio-Osornio, M.; Rubio, C. Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications. Brain Sci. 2025, 15, 884. https://doi.org/10.3390/brainsci15080884
Esteban-Román NF, Taddei E, Castro-Velázquez E, Villafuentes-Vidal L, Velez-Herrera A, Rubio-Osornio M, Rubio C. Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications. Brain Sciences. 2025; 15(8):884. https://doi.org/10.3390/brainsci15080884
Chicago/Turabian StyleEsteban-Román, Nadia Fernanda, Elisa Taddei, Edson Castro-Velázquez, Lorna Villafuentes-Vidal, Alejandra Velez-Herrera, Moisés Rubio-Osornio, and Carmen Rubio. 2025. "Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications" Brain Sciences 15, no. 8: 884. https://doi.org/10.3390/brainsci15080884
APA StyleEsteban-Román, N. F., Taddei, E., Castro-Velázquez, E., Villafuentes-Vidal, L., Velez-Herrera, A., Rubio-Osornio, M., & Rubio, C. (2025). Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications. Brain Sciences, 15(8), 884. https://doi.org/10.3390/brainsci15080884






