TARPγ2-Derived Peptide Enhances Early-Phase Long-Term Potentiation and Impairs Memory Retention in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Animals
2.3. LTP Paradigm
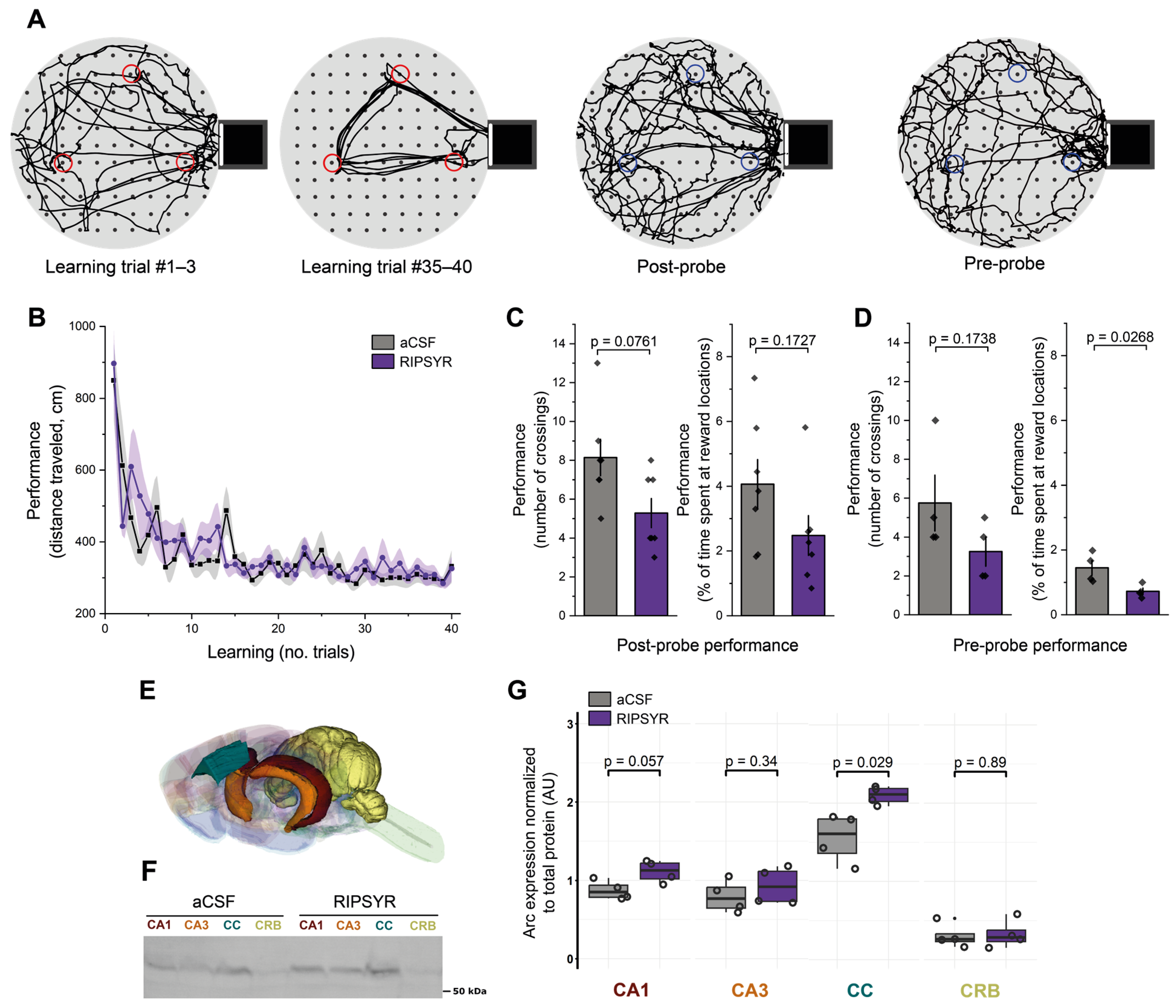
2.4. Cheeseboard Maze Task
2.5. Pose Estimation and Behavioral Analysis
2.6. Arc Level Measured by Western Blot
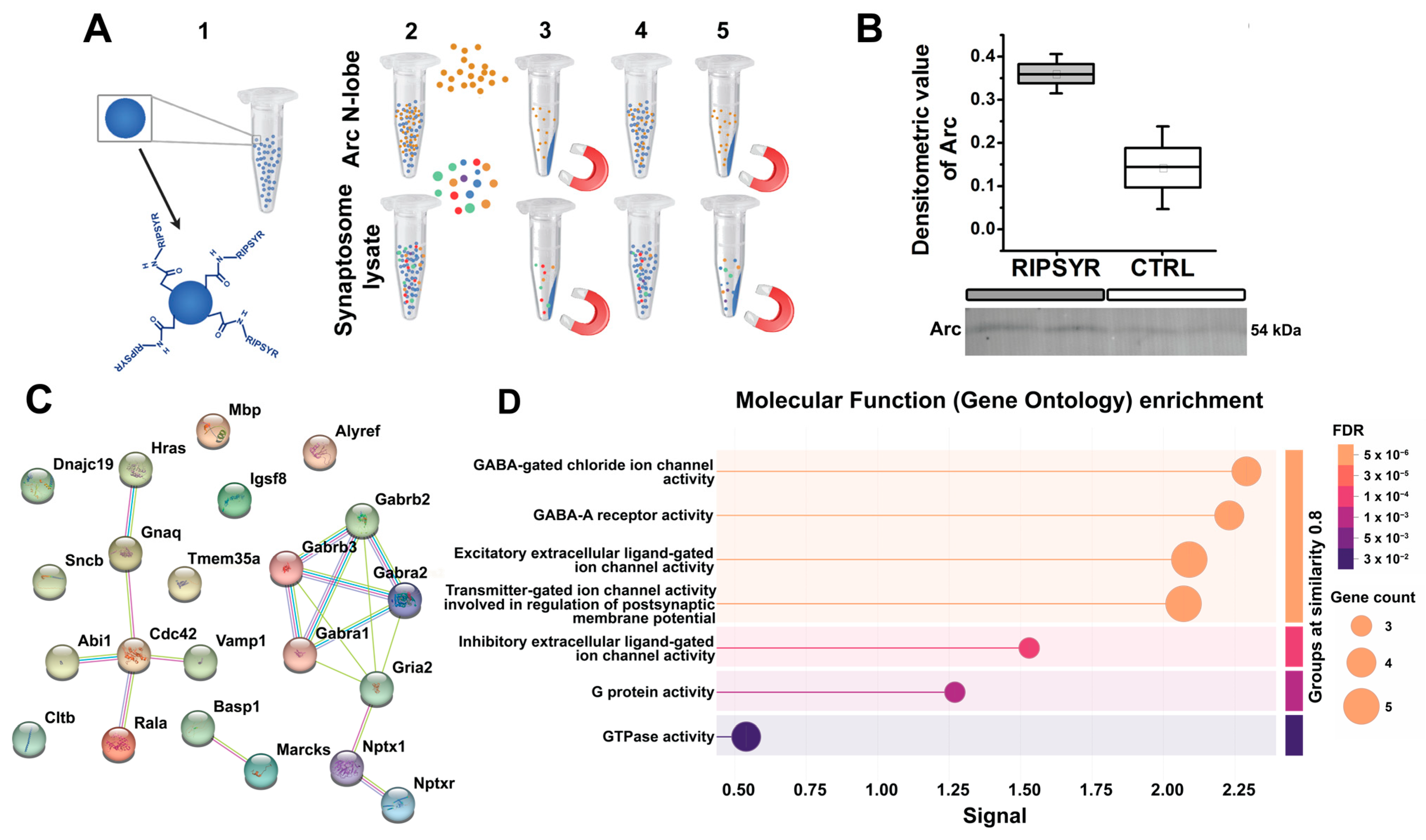
2.7. Peptide Coupling to Magnetic Beads
2.8. Recombinant Arc Protein Expression and Purification
2.9. Synaptosome Preparation
2.10. Protein Capturing by RIPSYR Peptide
2.11. Liquid Chromatography–Mass Spectrometry (LC-MS)
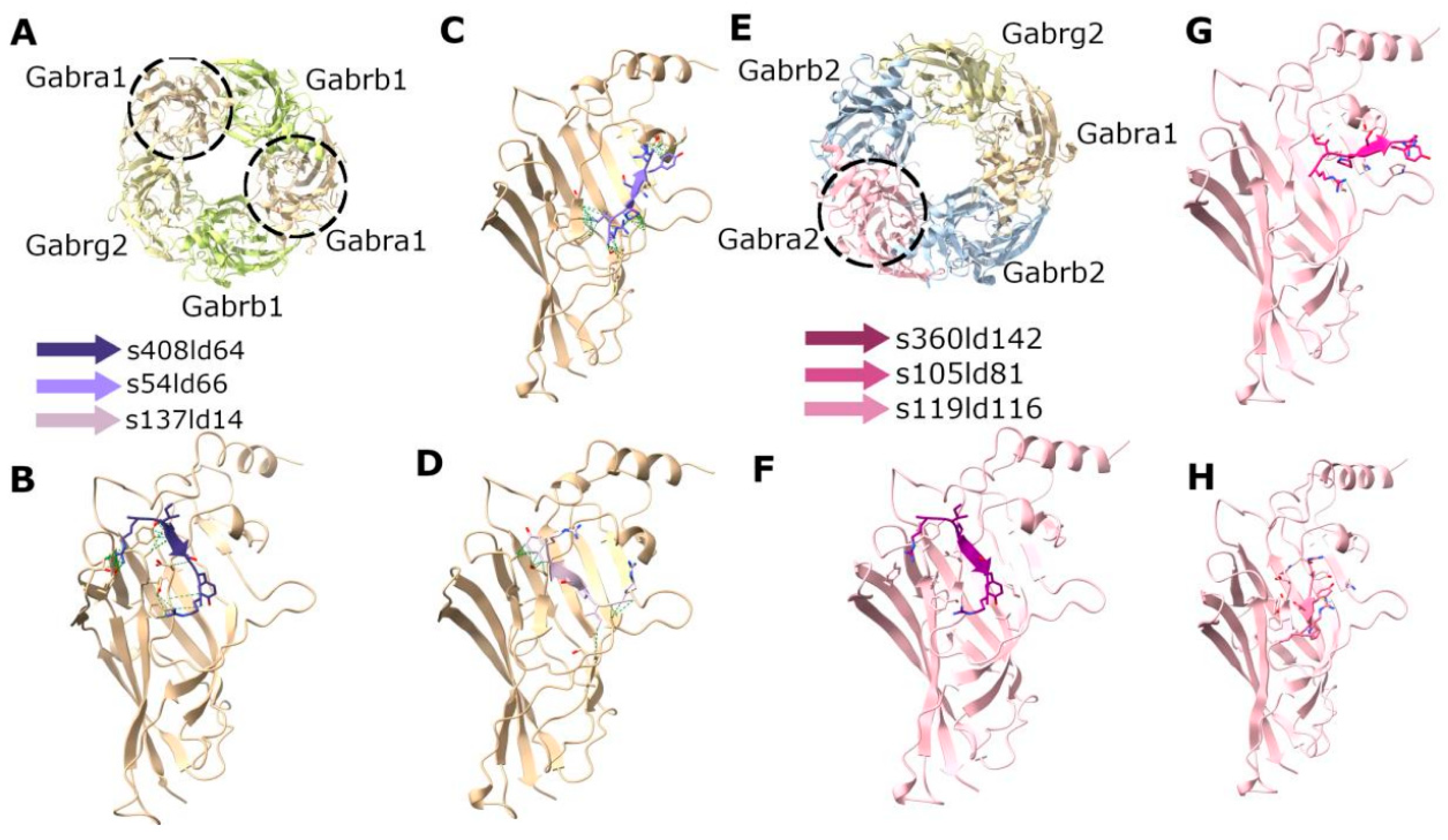
2.12. In Silico Peptide Docking
2.13. Molecular Dynamics and Enthalpy Estimation
3. Results
3.1. RIPSYR Effect on High Frequency Stimulation-Induced LTP
3.2. Arc Levels Are Elevated in RIPSYR Treatment
3.3. RIPSYR Treatment Affects Memory Retention on Cheeseboard
3.4. Arc Levels Are Increased upon RIPSYR Treatment in the Cingulate Cortex of Rats in Cheeseboard Maze Tasks
3.5. Putative Binding Partners of RIPSYR
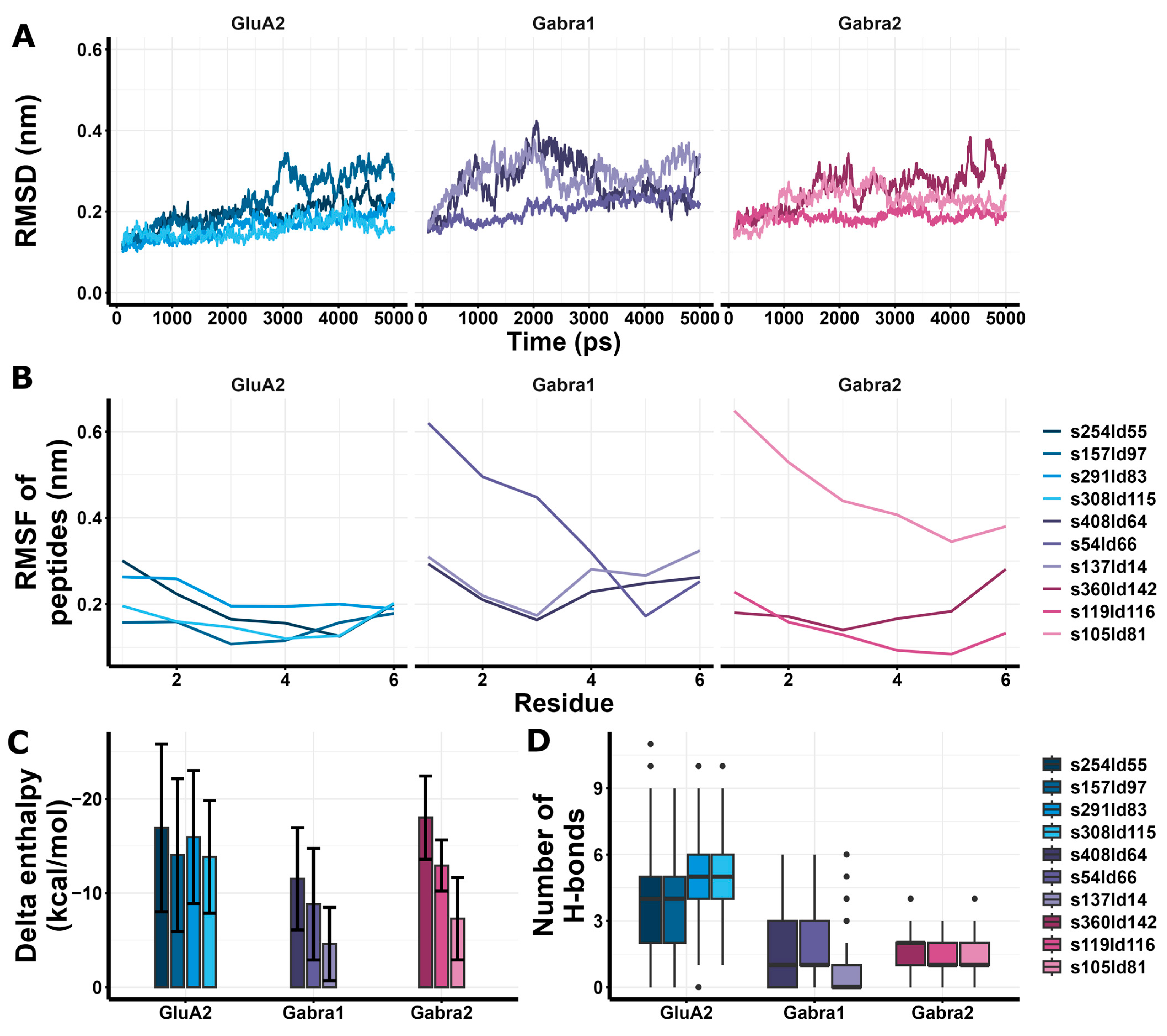
3.6. In Silico Peptide Docking and Estimation of Interaction Energies Suggest Possible GluA2 LBD Dimer Binding of RIPSYR Peptide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| Arc | Activity-regulated cytoskeleton associated protein |
| TARP | Transmembrane AMPAR regulatory protein |
| LC-MS | Liquid chromatography–mass spectrometry |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| PSD95 | Postsynaptic density protein 95 |
| NMDAR | N-methyl-D-aspartate receptor |
| GluA2 | AMPA-selective glutamate receptor 2 |
| Gabra1 | GABA receptor subunit alpha 1 |
| HFS | High-frequency stimulation |
| PTP | Post-tetanic potentiation |
| eLTP | Early phase of LTP |
| fEPSP | Field excitatory postsynaptic potential |
| aCSF | Artificial cerebrospinal fluid |
| CC | Cingulate cortex |
| CRB | Cerebellum |
| LBD | Ligand binding domain |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
References
- Small, S.A.; Schobel, S.A.; Buxton, R.B.; Witter, M.P.; Barnes, C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011, 12, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Eichenbaum, H. The Episodic Memory System: Neurocircuitry and Disorders. Neuropsychopharmacology 2010, 35, 86–104. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Dringenberg, H.C. The history of LONG-TERM potentiation as a memory mechanism: Controversies, confirmation, and some lessons to remember. Hippocampus 2020, 30, 987–1012. [Google Scholar] [CrossRef]
- Nair, D.; Hosy, E.; Petersen, J.D.; Constals, A.; Giannone, G.; Choquet, D.; Sibarita, J.-B. Super-Resolution Imaging Reveals That AMPA Receptors Inside Synapses Are Dynamically Organized in Nanodomains Regulated by PSD95. J. Neurosci. 2013, 33, 13204–13224. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.-Z.; Ramsey, A.M.; Tang, A.-H. Re-examination of the determinants of synaptic strength from the perspective of superresolution imaging. Curr. Opin. Neurobiol. 2022, 74, 102540. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Kamboj, S.; Ehlers, M.D.; Rosen, K.R.; Fischbach, G.D.; Huganir, R.L. Activity-Dependent Modulation of Synaptic AMPA Receptor Accumulation. Neuron 1998, 21, 1067–1078. [Google Scholar] [CrossRef]
- Roth, R.H.; Zhang, Y.; Huganir, R.L. Dynamic imaging of AMPA receptor trafficking in vitro and in vivo. Curr. Opin. Neurobiol. 2017, 45, 51–58. [Google Scholar] [CrossRef]
- Ashby, M.C.; Maier, S.R.; Nishimune, A.; Henley, J.M. Lateral Diffusion Drives Constitutive Exchange of AMPA Receptors at Dendritic Spines and Is Regulated by Spine Morphology. J. Neurosci. 2006, 26, 7046–7055. [Google Scholar] [CrossRef]
- Heine, M.; Groc, L.; Frischknecht, R.; BéïqUe, J.-C.; Lounis, B.; Rumbaugh, G.; Huganir, R.L.; Cognet, L.; Choquet, D. Surface Mobility of Postsynaptic AMPARs Tunes Synaptic Transmission. Science 2008, 320, 201–205. [Google Scholar] [CrossRef]
- Chen, H.; Roth, R.H.; Lopez-Ortega, E.; Tan, H.L.; Huganir, R.L. AMPA Receptors Exist in Tunable Mobile and Immobile Synaptic Fractions In Vivo. Eneuro 2021, 8, ENEURO.0015–21.2021. [Google Scholar] [CrossRef]
- Bissen, D.; Foss, F.; Acker-Palmer, A. AMPA receptors and their minions: Auxiliary proteins in AMPA receptor trafficking. Cell Mol. Life Sci. 2019, 76, 2133–2169. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.S.; Zeng, M.; Chen, X.; Sandoval, G.; Diaz-Alonso, J.; Zhang, M.; Nicoll, R.A. Long-term potentiation reconstituted with an artificial TARP/PSD-95 complex. Cell Rep. 2022, 41, 111483. [Google Scholar] [CrossRef]
- Fukata, Y.; Tzingounis, A.V.; Trinidad, J.C.; Fukata, M.; Burlingame, A.L.; Nicoll, R.A.; Bredt, D.S. Molecular constituents of neuronal AMPA receptors. J. Cell Biol. 2005, 169, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Ziff, E.B. TARPs and the AMPA Receptor Trafficking Paradox. Neuron 2007, 53, 627–633. [Google Scholar] [CrossRef]
- Schnell, E.; Sizemore, M.; Karimzadegan, S.; Chen, L.; Bredt, D.S.; Nicoll, R.A. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA 2002, 99, 13902–13907. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Adesnik, H.; Sekiguchi, M.; Zhang, W.; Wada, K.; Howe, J.R.; Nicoll, R.A.; Bredt, D.S. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 2005, 435, 1052–1058. [Google Scholar] [CrossRef]
- Bats, C.; Groc, L.; Choquet, D. The Interaction between Stargazin and PSD-95 Regulates AMPA Receptor Surface Trafficking. Neuron 2007, 53, 719–734. [Google Scholar] [CrossRef]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Sweeney, N.T.; Kawasaki, Y.; Wenthold, R.J.; Bredt, D.S.; Nicoll, R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Ward, M.D.; Yang, S.; Chuang, Y.-A.; Xiao, M.; Li, R.; Leahy, D.J.; Worley, P.F. Structural Basis of Arc Binding to Synaptic Proteins: Implications for Cognitive Disease. Neuron 2015, 86, 490–500. [Google Scholar] [CrossRef]
- Chen, X.; Jia, B.; Araki, Y.; Liu, B.; Ye, F.; Huganir, R.; Zhang, M. Arc weakens synapses by dispersing AMPA receptors from postsynaptic density via modulating PSD phase separation. Cell Res. 2022, 32, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Okuno, H.; Minatohara, K.; Bito, H. Inverse synaptic tagging: An inactive synapse-specific mechanism to capture activity-induced Arc/arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin. Cell Dev. Biol. 2018, 77, 43–50. [Google Scholar] [CrossRef]
- Fila, M.; Diaz, L.; Szczepanska, J.; Pawlowska, E.; Blasiak, J. mRNA Trafficking in the Nervous System: A Key Mechanism of the Involvement of Activity-Regulated Cytoskeleton-Associated Protein (Arc) in Synaptic Plasticity. Neural Plast. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.D.; Rumbaugh, G.; Wu, J.; Chowdhury, S.; Plath, N.; Kuhl, D.; Huganir, R.L.; Worley, P.F. Arc/Arg3.1 Mediates Homeostatic Synaptic Scaling of AMPA Receptors. Neuron 2006, 52, 475–484. [Google Scholar] [CrossRef]
- Pastuzyn, E.D.; Day, C.E.; Kearns, R.B.; Kyrke-Smith, M.; Taibi, A.V.; McCormick, J.; Yoder, N.; Belnap, D.M.; Erlendsson, S.; Morado, D.R.; et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 2018, 173, 275. [Google Scholar] [CrossRef]
- Nielsen, L.D.; Pedersen, C.P.; Erlendsson, S.; Teilum, K. The Capsid Domain of Arc Changes Its Oligomerization Propensity through Direct Interaction with the NMDA Receptor. Structure 2019, 27, 1071–1081.e5. [Google Scholar] [CrossRef]
- Hallin, E.I.; Bramham, C.R.; Kursula, P. Structural properties and peptide ligand binding of the capsid homology domains of human Arc. Biochem. Biophys. Rep. 2021, 26, 100975. [Google Scholar] [CrossRef]
- Xiang, J.; Keep, R.F. Proton-Coupled Oligopeptide Transport (Slc15) in the Brain: Past and Future Research. Pharm. Res. 2023, 40, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Farkas, V.; Ferentzi, K.; Horváti, K.; Perczel, A. Cost-Effective Flow Peptide Synthesis: Metamorphosis of HPLC. Org. Process Res. Dev. 2021, 25, 182–191. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Kesner, R.P.; Farnsworth, G.; Kametani, H. Role of parietal cortex and hippocampus in representing spatial information. Cereb. Cortex 1991, 1, 367–373. [Google Scholar] [CrossRef]
- Dupret, D.; O’Neill, J.; Pleydell-Bouverie, B.; Csicsvari, J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 2010, 13, 995–1002. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Nath, T.; Mathis, A.; Chen, A.C.; Patel, A.; Bethge, M.; Mathis, M.W. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 2019, 14, 2152–2176. [Google Scholar] [CrossRef]
- Insafutdinov, E.; Pishchulin, L.; Andres, B.; Andriluka, M.; Schiele, B. DeeperCut: A Deeper, Stronger, and Faster Multi-Person Pose Estimation Model. arXiv 2016, arXiv:1605.03170. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar] [CrossRef]
- Goodwin, N.L.; Choong, J.J.; Hwang, S.; Pitts, K.; Bloom, L.; Islam, A.; Zhang, Y.Y.; Szelenyi, E.R.; Tong, X.; Newman, E.L.; et al. Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience. Nat. Neurosci. 2024, 27, 1411–1424. [Google Scholar] [CrossRef]
- Wartman, B.C.; Gabel, J.; Holahan, M.R. Inactivation of the Anterior Cingulate Reveals Enhanced Reliance on Cortical Networks for Remote Spatial Memory Retrieval after Sequential Memory Processing. PLoS ONE 2014, 9, e108711. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 5 February 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 17 January 2025).
- Constantin, A.; Patil, I. ggsignif: R Package for Displaying Significance Brackets for ‘ggplot2’. PsyArxiv 2021. [Google Scholar] [CrossRef]
- Phillips, G.R.; Huang, J.K.; Wang, Y.; Tanaka, H.; Shapiro, L.; Zhang, W.; Shan, W.-S.; Arndt, K.; Frank, M.; Gordon, R.E.; et al. The Presynaptic Particle Web. Neuron 2001, 32, 63–77. [Google Scholar] [CrossRef]
- Hahn, C.-G.; Banerjee, A.; MacDonald, M.L.; Cho, D.-S.; Kamins, J.; Nie, Z.; Borgmann-Winter, K.E.; Grosser, T.; Pizarro, A.; Ciccimaro, E.; et al. The Post-Synaptic Density of Human Postmortem Brain Tissues: An Experimental Study Paradigm for Neuropsychiatric Illnesses. PLoS ONE 2009, 4, e5251. [Google Scholar] [CrossRef]
- Jin, R.; Clark, S.; Weeks, A.M.; Dudman, J.T.; Gouaux, E.; Partin, K.M. Mechanism of Positive Allosteric Modulators Acting on AMPA Receptors. J. Neurosci. 2005, 25, 9027–9036. [Google Scholar] [CrossRef]
- Phulera, S.; Zhu, H.; Yu, J.; Claxton, D.P.; Yoder, N.; Yoshioka, C.; Gouaux, E. Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA. eLife 2018, 7, e39383. [Google Scholar] [CrossRef]
- Zhou, J.; Noviello, C.M.; Teng, J.; Moore, H.; Lega, B.; Hibbs, R.E. Resolving native GABAA receptor structures from the human brain. Nature 2025, 638, 562–568. [Google Scholar] [CrossRef]
- Jiménez-García, B.; Roel-Touris, J.; Romero-Durana, M.; Vidal, M.; Jiménez-González, D.; Fernández-Recio, J. LightDock: A new multi-scale approach to protein–protein docking. Bioinformatics 2018, 34, 49–55. [Google Scholar] [CrossRef]
- Roel-Touris, J.; Bonvin, A.M.J.J.; Jiménez-García, B. LightDock goes information-driven. Bioinformatics 2020, 36, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Gohlke, H.; Case, D.A. Converging free energy estimates: MM-PB(GB)SA studies on the protein–protein complex Ras–Raf. J. Comput. Chem. 2004, 25, 238–250. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Penn, A.C.; Zhang, C.L.; Georges, F.; Royer, L.; Breillat, C.; Hosy, E.; Petersen, J.D.; Humeau, Y.; Choquet, D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 2017, 549, 384–388. [Google Scholar] [CrossRef]
- Kleven, H.; Bjerke, I.E.; Clascá, F.; Groenewegen, H.J.; Bjaalie, J.G.; Leergaard, T.B. Waxholm Space atlas of the rat brain: A 3D atlas supporting data analysis and integration. Nat. Methods 2023, 20, 1822–1829. [Google Scholar] [CrossRef]
- Schuman, E.M.; Dynes, J.L.; Steward, O. Synaptic Regulation of Translation of Dendritic mRNAs. J. Neurosci. 2006, 26, 7143–7146. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Fox, R.; Proulx, C.D.; Lin, J.Y.; Tsien, R.Y.; Malinow, R. Engineering a memory with LTD and LTP. Nature 2014, 511, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, I.; Malinow, R. Postsynaptic Density 95 controls AMPA Receptor Incorporation during Long-Term Potentiation and Experience-Driven Synaptic Plasticity. J. Neurosci. 2004, 24, 916–927. [Google Scholar] [CrossRef]
- Farkas, Ö.; Lajkó, E.; Kalabay, M.; Enyedi, K.N. How to Scramble? A ‘Wise Way’ to Achieve a Useful Control Peptide Sequence. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Link, W.; Konietzko, U.; Kauselmann, G.; Krug, M.; Schwanke, B.; Frey, U.; Kuhl, D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA 1995, 92, 5734–5738. [Google Scholar] [CrossRef]
- Avallone, M.; Pardo, J.; Mergiya, T.F.; Rájová, J.; Räsänen, A.; Davidsson, M.; Åkerblom, M.; Quintino, L.; Kumar, D.; Bramham, C.R.; et al. Visualizing Arc protein dynamics and localization in the mammalian brain using AAV-mediated in situ gene labeling. Front. Mol. Neurosci. 2023, 16, 1140785. [Google Scholar] [CrossRef]
- El Oussini, H.; Zhang, C.-L.; François, U.; Castelli, C.; Lampin-Saint-Amaux, A.; Lepleux, M.; Molle, P.; Velez, L.; Dejean, C.; Lanore, F.; et al. CA3 hippocampal synaptic plasticity supports ripple physiology during memory consolidation. Nat. Commun. 2023, 14, 8312. [Google Scholar] [CrossRef]
- Buzsáki, G. Two-stage model of memory trace formation: A role for ‘noisy’ brain states. Neuroscience 1989, 31, 551–570. [Google Scholar] [CrossRef]
- Bollmann, L.; Baracskay, P.; Stella, F.; Csicsvari, J. Sleep stages antagonistically modulate reactivation drift. Neuron 2025, 113, 1446–1459.e6. [Google Scholar] [CrossRef]
- Korshunova, I.; Caroni, P.; Kolkova, K.; Berezin, V.; Bock, E.; Walmod, P.S. Characterization of BASP1-mediated neurite outgrowth. J. Neurosci. Res. 2008, 86, 2201–2213. [Google Scholar] [CrossRef]
- Tanabe, A.; Shiraishi, M.; Negishi, M.; Saito, N.; Tanabe, M.; Sasaki, Y. MARCKS dephosphorylation is involved in bradykinin-induced neurite outgrowth in neuroblastoma SH-SY5Y cells. J. Cell Physiol. 2012, 227, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Cornejo, B.J.; Kuhn, T.B.; Bamburg, J.R. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol. 2000, 43, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, H.; Wang, H.; Yasuda, R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 2011, 472, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.; Madeira, N.; Fonseca, R. Cdc42 activation is necessary for heterosynaptic cooperation and competition. Mol. Cell Neurosci. 2024, 129, 103921. [Google Scholar] [CrossRef]

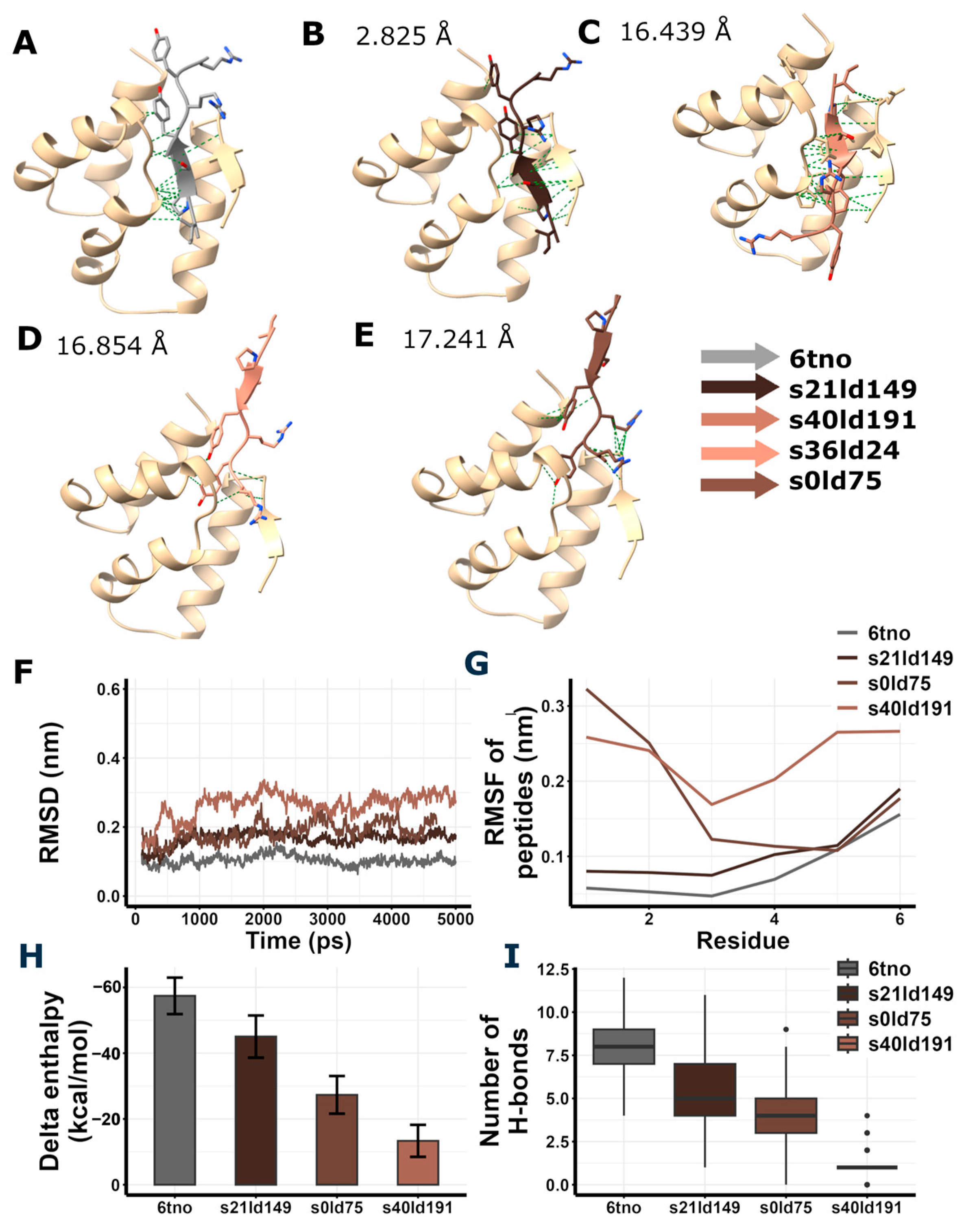

| Model ID | fastdfire Score | RMSD (Å) |
|---|---|---|
| s21ld149 | 13.02 | 2.825 |
| s40ld191 | 13.25 | 16.439 |
| s36ld24 | 13.23 | 16.854 |
| s0ld75 | 13.40 | 17.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mátyás, D.; Tukacs, V.; Tóth, V.; Baracskay, P.; Pap, S.K.; Stráner, P.; Hiền, T.M.; Hunyadi-Gulyás, É.; Darula, Z.; Perczel, A.; et al. TARPγ2-Derived Peptide Enhances Early-Phase Long-Term Potentiation and Impairs Memory Retention in Male Rats. Brain Sci. 2025, 15, 881. https://doi.org/10.3390/brainsci15080881
Mátyás D, Tukacs V, Tóth V, Baracskay P, Pap SK, Stráner P, Hiền TM, Hunyadi-Gulyás É, Darula Z, Perczel A, et al. TARPγ2-Derived Peptide Enhances Early-Phase Long-Term Potentiation and Impairs Memory Retention in Male Rats. Brain Sciences. 2025; 15(8):881. https://doi.org/10.3390/brainsci15080881
Chicago/Turabian StyleMátyás, Dominik, Vanda Tukacs, Vilmos Tóth, Péter Baracskay, Stefánia Krisztina Pap, Pál Stráner, Trần Minh Hiền, Éva Hunyadi-Gulyás, Zsuzsanna Darula, András Perczel, and et al. 2025. "TARPγ2-Derived Peptide Enhances Early-Phase Long-Term Potentiation and Impairs Memory Retention in Male Rats" Brain Sciences 15, no. 8: 881. https://doi.org/10.3390/brainsci15080881
APA StyleMátyás, D., Tukacs, V., Tóth, V., Baracskay, P., Pap, S. K., Stráner, P., Hiền, T. M., Hunyadi-Gulyás, É., Darula, Z., Perczel, A., Kékesi, K. A., & Juhász, G. (2025). TARPγ2-Derived Peptide Enhances Early-Phase Long-Term Potentiation and Impairs Memory Retention in Male Rats. Brain Sciences, 15(8), 881. https://doi.org/10.3390/brainsci15080881






