AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington’s Disease
Abstract
1. Huntington’s Disease: From the First Clinical Report to the Discovery of Its Etiology
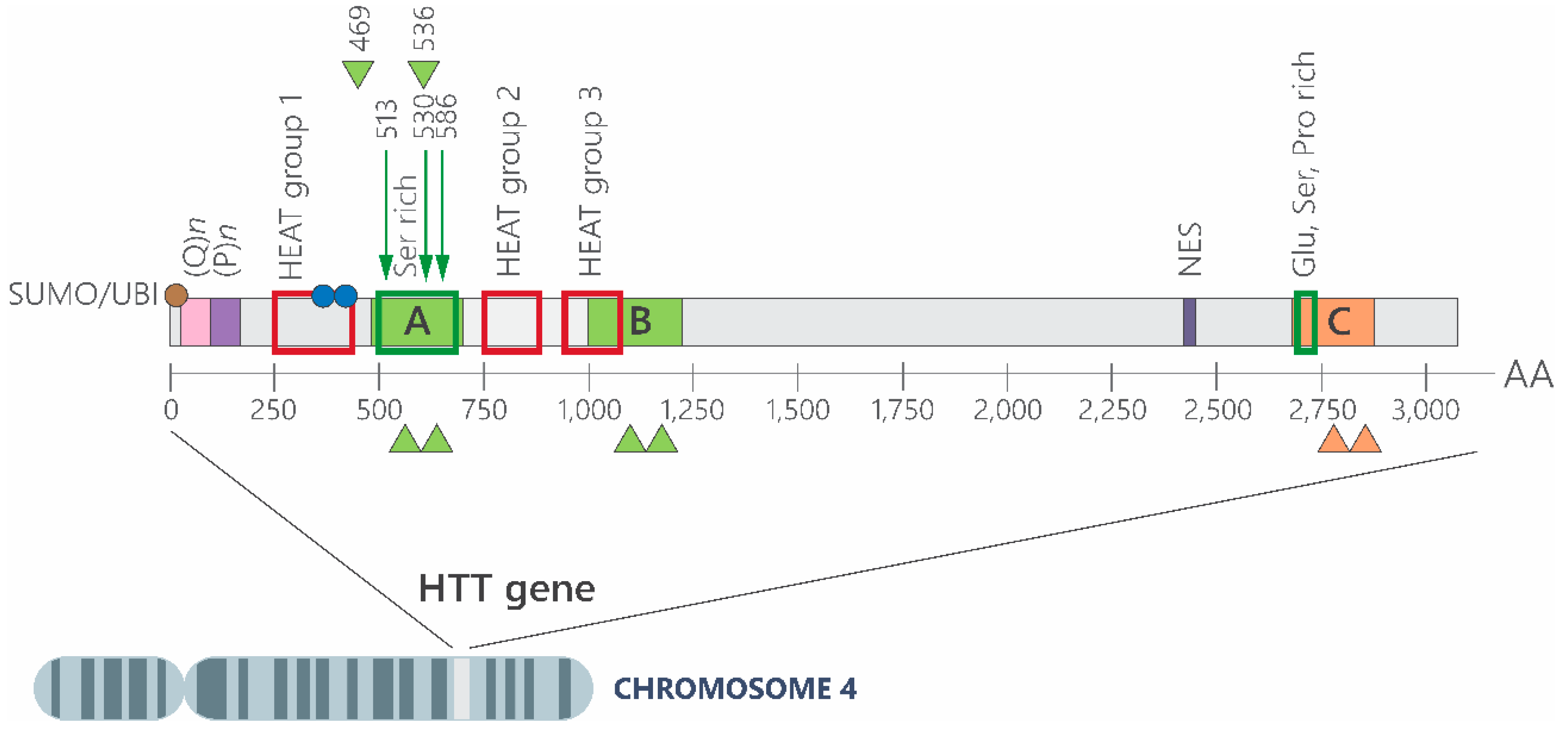
2. How Is the CAG Expansion Involved in HD Physiopathology?
2.1. mHTT Contributes to Mitochondrial Dysfunction and Increased Oxidative Stress
2.2. Mutant Huntingtin (HTT) Protein Leads to Dysregulation of Metal Homeostasis
2.3. mHTT and Its Role in Excitotoxicity
2.4. Mutated HTT Promotes Cholesterol Metabolism Deregulation
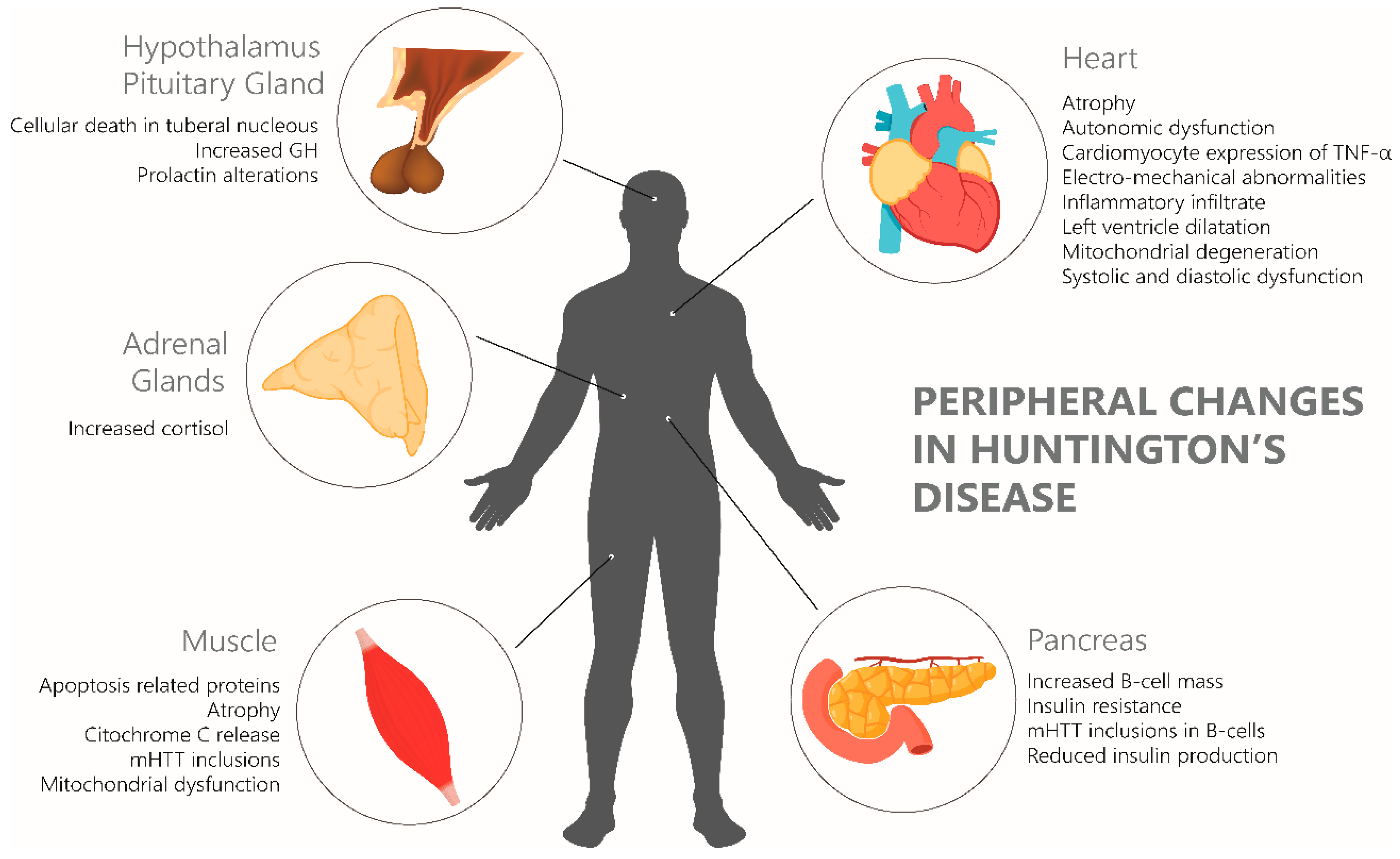
2.5. Systemic Effects of Mutated Huntingtin
3. Therapeutic Opportunities Targeting HD Pathophysiology
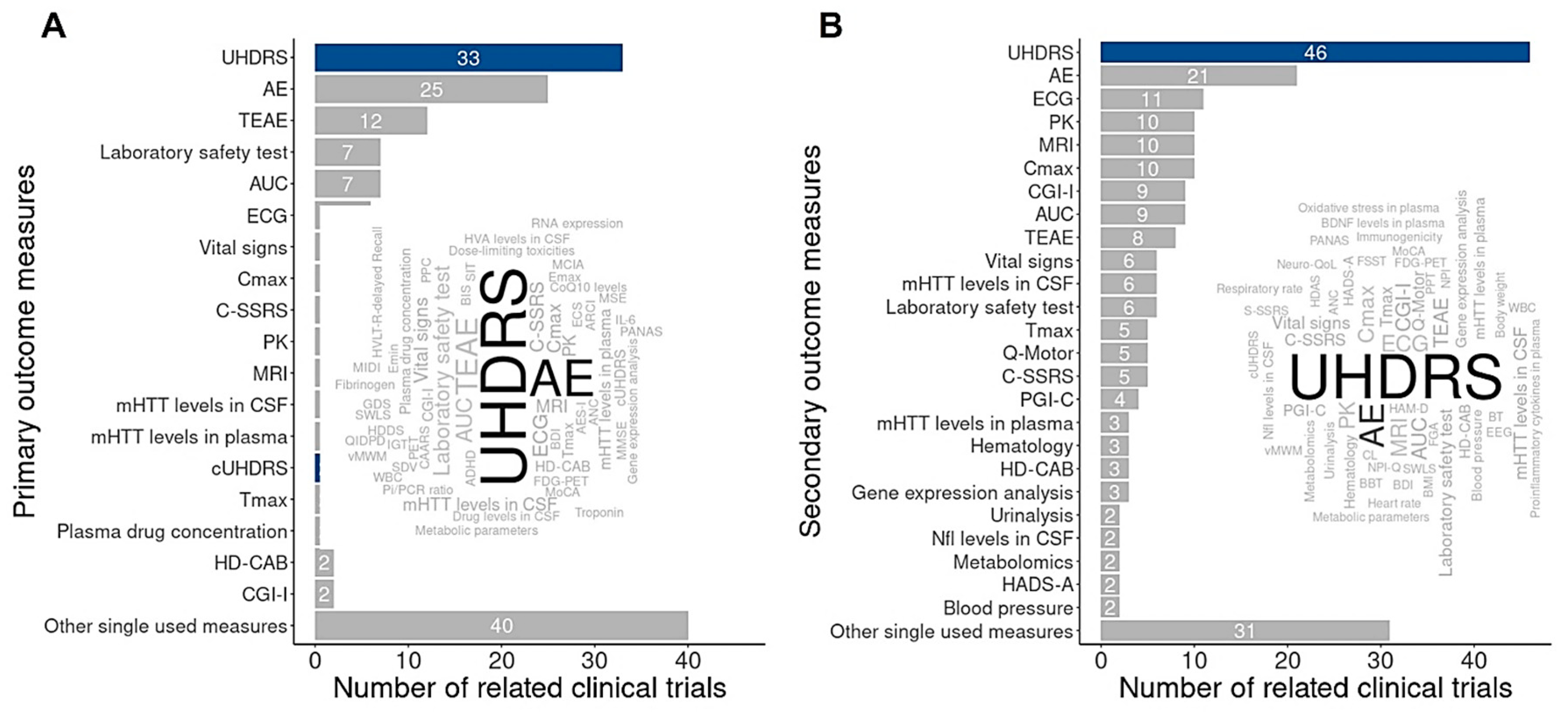
4. Transcriptomic Studies of HD
5. Approved Drugs for HD Treatment
6. Translational Bioinformatics and Pharma Intelligence Bring New Horizons for HD Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABH | Brazil Huntington Association |
| BDASeq® | Biomarker Discovery Algorithm |
| CADD | Computer-aided drug design |
| CAG | Cytosine–adenine–guanine |
| DEA | Differential expression analysis |
| DEG | Differentially expressed genes |
| HD | Huntington’s disease |
| RMC | Recursive Method Combination |
| TFC | Total Function Capacity |
| TMS | Total Motor Score |
| UHDRS | Unified Huntington’s Disease Rate Scale |
References
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Jurcau, A.; Jurcau, C. Mitochondria in Huntington’s Disease: Implications in Pathogenesis and Mitochondrial-Targeted Therapeutic Strategies. Neural Regen. Res. 2023, 18, 1472–1477. [Google Scholar] [CrossRef]
- Kay, C.; Hayden, M.R.; Leavitt, B.R. Epidemiology of Huntington Disease. Handb. Clin. Neurol. 2017, 144, 31–46. [Google Scholar]
- Alencar, M.A.; Figueiredo, E.; Porciúncula, C.; Monlleó, I. I5 Huntington’s Disease and Prevalence in Brazil. J. Neurol. Neurosurg. Psychiatry 2016, 87, A60–A61. [Google Scholar] [CrossRef]
- Gatto, E.M.; Rojas, N.G.; Persi, G.; Etcheverry, J.L.; Cesarini, M.E.; Perandones, C. Huntington Disease: Advances in the Understanding of Its Mechanisms. Clin. Park. Relat. Disord. 2020, 3, 100056. [Google Scholar] [CrossRef]
- Rodríguez-Santana, I.; Mestre, T.; Squitieri, F.; Willock, R.; Arnesen, A.; Clarke, A.; D’Alessio, B.; Fisher, A.; Fuller, R.; Hamilton, J.L.; et al. Economic Burden of Huntington Disease in Europe and the USA: Results from the Huntington’s Disease Burden of Illness Study. Eur. J. Neurol. 2023, 30, 1109–1117. [Google Scholar] [CrossRef]
- Kerkis, I.; Araldi, R.; Wenceslau, C.; Mendes, T. Advances in Cellular and Cell-Free Therapy Medicinal Products for Huntigton’s Disease Treatment. In From Physiopatology to Treatment of Huntigton’s Disease; InTech: London, UK, 2022; pp. 1–27. [Google Scholar]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Harper, P.S. The Epidemiology of Huntington’s Disease. Hum. Genet. 1992, 89, 365–376. [Google Scholar] [CrossRef]
- Bhattacharyya, K. The Story of George Huntington and His Disease. Ann. Indian Acad. Neurol. 2016, 19, 25–28. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Arning, L.; Nguyen, H.P. Huntington Disease Update: New Insights into the Role of Repeat Instability in Disease Pathogenesis. Med. Genet. 2022, 33, 293–300. [Google Scholar] [CrossRef]
- de Sabando, A.R.; Ciosi, M.; Galbete, A.; Cumming, S.A.; Álvarez, V.; Martinez-Descals, A.; Mila, M.; Trujillo-Tiebas, M.J.; López-Sendón, J.L.; Fenollar-Cortés, M.; et al. Somatic CAG Repeat Instability in Intermediate Alleles of the HTT Gene and Its Potential Association with a Clinical Phenotype. Eur. J. Hum. Genet. 2024, 32, 770–778. [Google Scholar] [CrossRef]
- Semaka, A.; Kay, C.; Doty, C.; Collins, J.A.; Bijlsma, E.K.; Richards, F.; Goldberg, Y.P.; Hayden, M.R. CAG Size-Specific Risk Estimates for Intermediate Allele Repeat Instability in Huntington Disease. J. Med. Genet. 2013, 50, 696–703. [Google Scholar] [CrossRef]
- Martí-Martínez, S.; Valor, L.M. A Glimpse of Molecular Biomarkers in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 5411. [Google Scholar] [CrossRef]
- Kenney, C.; Powell, S.; Jankovic, J. Autopsy-proven Huntington’s Disease with 29 Trinucleotide Repeats. Mov. Disord. 2007, 22, 127–130. [Google Scholar] [CrossRef]
- Killoran, A.; Biglan, K.M.; Jankovic, J.; Eberly, S.; Kayson, E.; Oakes, D.; Young, A.B.; Shoulson, I. Characterization of the Huntington Intermediate CAG Repeat Expansion Phenotype in PHAROS. Neurology 2013, 80, 2022–2027. [Google Scholar] [CrossRef]
- Cubo, E.; Ramos-Arroyo, M.A.; Martinez-Horta, S.; Martínez-Descalls, A.; Calvo, S.; Gil-Polo, C.; Bachoud-Lévi, A.-C.; Bentivoglio, A.R.; Biunno, I.; Bonelli, R.M.; et al. Clinical Manifestations of Intermediate Allele Carriers in Huntington Disease. Neurology 2016, 87, 571–578. [Google Scholar] [CrossRef]
- Savitt, D.; Jankovic, J. Clinical Phenotype in Carriers of Intermediate Alleles in the Huntingtin Gene. J. Neurol. Sci. 2019, 402, 57–61. [Google Scholar] [CrossRef]
- Acuña, A.R.; Martín, E.S.S.; Fernández, C.G.; Menéndez, S.F.; Estrada, M.B.; Díaz, M.A.; González, M.M.; Martínez, V.Á. A Series of Cases with Huntington-like Phenotype and Intermediate Repeats in HTT. J. Neurol. Sci. 2021, 425, 117452. [Google Scholar] [CrossRef]
- Alcalá-Vida, R.; Seguin, J.; Lotz, C.; Molitor, A.M.; Irastorza-Azcarate, I.; Awada, A.; Karasu, N.; Bombardier, A.; Cosquer, B.; Skarmeta, J.L.G.; et al. Age-Related and Disease Locus-Specific Mechanisms Contribute to Early Remodelling of Chromatin Structure in Huntington’s Disease Mice. Nat. Commun. 2021, 12, 364. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, B.; Cheng, J.; Seefelder, M.; Engler, T.; Pfeifer, G.; Oeckl, P.; Otto, M.; Moser, F.; Maurer, M.; et al. The Cryo-Electron Microscopy Structure of Huntingtin. Nature 2018, 555, 117–120. [Google Scholar] [CrossRef]
- Imarisio, S.; Carmichael, J.; Korolchuk, V.; Chen, C.-W.; Saiki, S.; Rose, C.; Krishna, G.; Davies, J.E.; Ttofi, E.; Underwood, B.R.; et al. Huntington’s Disease: From Pathology and Genetics to Potential Therapies. Biochem. J. 2008, 412, 191–209. [Google Scholar] [CrossRef]
- Duyao, M.P.; Auerbach, A.B.; Ryan, A.; Persichetti, F.; Barnes, G.T.; McNeil, S.M.; Ge, P.; Vonsattel, J.-P.; Gusella, J.F.; Joyner, A.L.; et al. Inactivation of the Mouse Huntington’s Disease Gene Homolog Hdh. Science 1995, 269, 407–410. [Google Scholar] [CrossRef]
- Nasir, J.; Floresco, S.B.; O’Kusky, J.R.; Diewert, V.M.; Richman, J.M.; Zeisler, J.; Borowski, A.; Marth, J.D.; Phillips, A.G.; Hayden, M.R. Targeted Disruption of the Huntington’s Disease Gene Results in Embryonic Lethality and Behavioral and Morphological Changes in Heterozygotes. Cell 1995, 81, 811–823. [Google Scholar] [CrossRef]
- Zeitlin, S.; Liu, J.-P.; Chapman, D.L.; Papaioannou, V.E.; Efstratiadis, A. Increased Apoptosis and Early Embryonic Lethality in Mice Nullizygous for the Huntington’s Disease Gene Homologue. Nat. Genet. 1995, 11, 155–163. [Google Scholar] [CrossRef]
- O’Kusky, J.R.; Nasir, J.; Cicchetti, F.; Parent, A.; Hayden, M.R. Neuronal Degeneration in the Basal Ganglia and Loss of Pallido-Subthalamic Synapses in Mice with Targeted Disruption of the Huntington’s Disease Gene. Brain Res. 1999, 818, 468–479. [Google Scholar] [CrossRef]
- Barron, J.C.; Hurley, E.P.; Parsons, M.P. Huntingtin and the Synapse. Front. Cell. Neurosci 2021, 15, 689332. [Google Scholar] [CrossRef]
- Tartari, M.; Gissi, C.; Lo Sardo, V.; Zuccato, C.; Picardi, E.; Pesole, G.; Cattaneo, E. Phylogenetic Comparison of Huntingtin Homologues Reveals the Appearance of a Primitive PolyQ in Sea Urchin. Mol. Biol. Evol. 2008, 25, 330–338. [Google Scholar] [CrossRef]
- Atwal, R.S.; Xia, J.; Pinchev, D.; Taylor, J.; Epand, R.M.; Truant, R. Huntingtin Has a Membrane Association Signal That Can Modulate Huntingtin Aggregation, Nuclear Entry and Toxicity. Hum. Mol. Genet. 2007, 16, 2600–2615. [Google Scholar] [CrossRef]
- Rockabrand, E.; Slepko, N.; Pantalone, A.; Nukala, V.N.; Kazantsev, A.; Marsh, J.L.; Sullivan, P.G.; Steffan, J.S.; Sensi, S.L.; Thompson, L.M. The First 17 Amino Acids of Huntingtin Modulate Its Sub-Cellular Localization, Aggregation and Effects on Calcium Homeostasis. Hum. Mol. Genet. 2007, 16, 61–77. [Google Scholar] [CrossRef]
- Harjes, P.; Wanker, E.E. The Hunt for Huntingtin Function: Interaction Partners Tell Many Different Stories. Trends Biochem. Sci. 2003, 28, 425–433. [Google Scholar] [CrossRef]
- Maiuri, T.; Mocle, A.J.; Hung, C.L.; Xia, J.; van Roon-Mom, W.M.C.; Truant, R. Huntingtin Is a Scaffolding Protein in the ATM Oxidative DNA Damage Response Complex. Hum. Mol. Genet. 2016, 26, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.-N.; Xu, Z.; Patel, B.; Chen, Z.; Chen, D.; Tito, A.; David, G.; Sun, Y.; Stimming, E.F.; Bellen, H.J.; et al. Huntingtin Functions as a Scaffold for Selective Macroautophagy. Nat. Cell Biol. 2015, 17, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.J.; Deme, J.C.; Hevler, J.F.; Tamara, S.; Lemak, A.; Cantle, J.P.; Szewczyk, M.M.; Begeja, N.; Goss, S.; Zuo, X.; et al. Huntingtin Structure Is Orchestrated by HAP40 and Shows a Polyglutamine Expansion-Specific Interaction with Exon 1. Commun. Biol. 2021, 4, 1374. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, M. Huntingtin Regulates RE1-Silencing Transcription Factor/Neuron-Restrictive Silencer Factor (REST/NRSF) Nuclear Trafficking Indirectly through a Complex with REST/NRSF-Interacting LIM Domain Protein (RILP) and Dynactin P150Glued. J. Biol. Chem. 2008, 283, 34880–34886. [Google Scholar] [CrossRef]
- Ravache, M.; Weber, C.; Mérienne, K.; Trottier, Y. Transcriptional Activation of REST by Sp1 in Huntington’s Disease Models. PLoS ONE 2010, 5, e14311. [Google Scholar] [CrossRef]
- Schulte, J.; Littleton, J.T. The Biological Function of the Huntingtin Protein and Its Relevance to Huntington’s Disease Pathology. Curr. Trends Neurol. 2011, 5, 65–78. [Google Scholar]
- Lorenzetti, V.; Cousijn, J. Cannabis Use Disorders and Brain Morphology. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 773–785. [Google Scholar]
- Wenceslau, C.V.; de Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; da Silva, J.M.; Pagani, E.; Haddad, M.S.; Kerkis, I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells 2022, 11, 1664. [Google Scholar] [CrossRef]
- Davies, S.W.; Turmaine, M.; Cozens, B.A.; DiFiglia, M.; Sharp, A.H.; Ross, C.A.; Scherzinger, E.; Wanker, E.E.; Mangiarini, L.; Bates, G.P. Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 1997, 90, 537–548. [Google Scholar] [CrossRef]
- DiFiglia, M.; Sapp, E.; Chase, K.O.; Davies, S.W.; Bates, G.P.; Vonsattel, J.P.; Aronin, N. Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science 1997, 277, 1990–1993. [Google Scholar] [CrossRef]
- Becher, M.W.; Kotzuk, J.A.; Sharp, A.H.; Davies, S.W.; Bates, G.P.; Price, D.L.; Ross, C.A. Intranuclear Neuronal Inclusions in Huntington’s Disease and Dentatorubral and Pallidoluysian Atrophy: Correlation between the Density of Inclusions andIT15CAG Triplet Repeat Length. Neurobiol. Dis. 1998, 4, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ordway, J.M.; Tallaksen-Greene, S.; Gutekunst, C.-A.; Bernstein, E.M.; Cearley, J.A.; Wiener, H.W.; Dure, L.S.; Lindsey, R.; Hersch, S.M.; Jope, R.S.; et al. Ectopically Expressed CAG Repeats Cause Intranuclear Inclusions and a Progressive Late Onset Neurological Phenotype in the Mouse. Cell 1997, 91, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.B.; Cicchetti, F.; Hauser, R.A.; Deacon, T.W.; Li, X.-J.; Hersch, S.M.; Nauert, G.M.; Sanberg, P.R.; Kordower, J.H.; Saporta, S.; et al. Transplanted Fetal Striatum in Huntington’s Disease: Phenotypic Development and Lack of Pathology. Proc. Natl. Acad. Sci. USA 2000, 97, 13877–13882. [Google Scholar] [CrossRef] [PubMed]
- Arrasate, M.; Finkbeiner, S. Protein Aggregates in Huntington’s Disease. Exp. Neurol. 2012, 238, 1–11. [Google Scholar] [CrossRef]
- Ratovitski, T.; Gucek, M.; Jiang, H.; Chighladze, E.; Waldron, E.; D’Ambola, J.; Hou, Z.; Liang, Y.; Poirier, M.A.; Hirschhorn, R.R.; et al. Mutant Huntingtin N-Terminal Fragments of Specific Size Mediate Aggregation and Toxicity in Neuronal Cells. J. Biol. Chem. 2009, 284, 10855–10867. [Google Scholar] [CrossRef]
- Marcellin, D.; Abramowski, D.; Young, D.; Richter, J.; Weiss, A.; Marcel, A.; Maassen, J.; Kauffmann, M.; Bibel, M.; Shimshek, D.R.; et al. Fragments of HdhQ150 Mutant Huntingtin Form a Soluble Oligomer Pool That Declines with Aggregate Deposition upon Aging. PLoS ONE 2012, 7, e44457. [Google Scholar] [CrossRef]
- Lunkes, A.; Lindenberg, K.S.; Ben-Haïem, L.; Weber, C.; Devys, D.; Landwehrmeyer, G.B.; Mandel, J.-L.; Trottier, Y. Proteases Acting on Mutant Huntingtin Generate Cleaved Products That Differentially Build Up Cytoplasmic and Nuclear Inclusions. Mol. Cell 2002, 10, 259–269. [Google Scholar] [CrossRef]
- Tian, J.; Yan, Y.-P.; Zhou, R.; Lou, H.-F.; Rong, Y.; Zhang, B.-R. Soluble N-Terminal Fragment of Mutant Huntingtin Protein Impairs Mitochondrial Axonal Transport in Cultured Hippocampal Neurons. Neurosci. Bull. 2014, 30, 74–80. [Google Scholar] [CrossRef]
- Bhat, K.P.; Yan, S.; Wang, C.-E.; Li, S.; Li, X.-J. Differential Ubiquitination and Degradation of Huntingtin Fragments Modulated by Ubiquitin-Protein Ligase E3A. Proc. Natl. Acad. Sci. USA 2014, 111, 5706–5711. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s Disease: From Molecular Pathogenesis to Clinical Treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Johnson, G.V.W. Role of Mitochondrial Dysfunction in the Pathogenesis of Huntington’s Disease. Brain Res. Bull. 2009, 80, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rosas, H.D.; Chen, Y.I.; Doros, G.; Salat, D.H.; Chen, N.; Kwong, K.K.; Bush, A.; Fox, J.; Hersch, S.M. Alterations in Brain Transition Metals in Huntington Disease. Arch. Neurol. 2012, 69, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Yan, Y.; Kang, H.; Totten, M.; Silverman, J.; Bowman, A.B.; Erikson, K.; Claassen, D.O. Alterations in Metal Homeostasis Occur Prior to Canonical Markers in Huntington Disease. Sci. Rep. 2022, 12, 10373. [Google Scholar] [CrossRef] [PubMed]
- Hands, S.L.; Mason, R.; Sajjad, M.U.; Giorgini, F.; Wyttenbach, A. Metallothioneins and Copper Metabolism Are Candidate Therapeutic Targets in Huntington’s Disease. Biochem. Soc. Trans. 2010, 38, 552–558. [Google Scholar] [CrossRef]
- González-Guevara, E.; Cárdenas, G.; Pérez-Severiano, F.; Martínez-Lazcano, J.C. Dysregulated Brain Cholesterol Metabolism Is Linked to Neuroinflammation in Huntington’s Disease. Mov. Disord. 2020, 35, 1113–1127. [Google Scholar] [CrossRef]
- Kacher, R.; Mounier, C.; Caboche, J.; Betuing, S. Altered Cholesterol Homeostasis in Huntington’s Disease. Front. Aging Neurosci. 2022, 14, 797220. [Google Scholar] [CrossRef]
- Phillips, G.R.; Hancock, S.E.; Brown, S.H.J.; Jenner, A.M.; Kreilaus, F.; Newell, K.A.; Mitchell, T.W. Cholesteryl Ester Levels Are Elevated in the Caudate and Putamen of Huntington’s Disease Patients. Sci. Rep. 2020, 10, 20314. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A Mitochondria-Associated Oxidative Stress Perspective on Huntington’s Disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef]
- Sánchez, A.M.E.; Mejía-Toiber, J.; Massieu, L. Excitotoxic Neuronal Death and the Pathogenesis of Huntington’s Disease. Arch. Med. Res. 2008, 39, 265–276. [Google Scholar] [CrossRef]
- Glaser, T.; Silva, J.B.; Juvenal, G.; Maiolini, P.N.; Turrini, N.; Petiz, L.L.; Marques, L.B.; Ribeiro, D.E.; Ye, Q.; Tang, Y.; et al. Various Facets of Excitotoxicity. Explor. Neuroprot. Ther. 2022, 2, 36–64. [Google Scholar] [CrossRef]
- Yablonska, S.; Ganesan, V.; Ferrando, L.M.; Kim, J.; Pyzel, A.; Baranova, O.V.; Khattar, N.K.; Larkin, T.M.; Baranov, S.V.; Chen, N.; et al. Mutant Huntingtin Disrupts Mitochondrial Proteostasis by Interacting with TIM23. Proc. Natl. Acad. Sci. USA 2019, 116, 16593–16602. [Google Scholar] [CrossRef]
- Soares, T.R.; Reis, S.D.; Pinho, B.R.; Duchen, M.R.; Oliveira, J.M.A. Targeting the Proteostasis Network in Huntington’s Disease. Ageing Res. Rev. 2019, 49, 92–103. [Google Scholar] [CrossRef]
- Young, D.; Mayer, F.; Vidotto, N.; Schweizer, T.; Berth, R.; Abramowski, D.; Shimshek, D.R.; van der Putten, P.H.; Schmid, P. Mutant Huntingtin Gene-Dose Impacts on Aggregate Deposition, DARPP32 Expression and Neuroinflammation in HdhQ150 Mice. PLoS ONE 2013, 8, e75108. [Google Scholar] [CrossRef]
- Crotti, A.; Benner, C.; Kerman, B.E.; Gosselin, D.; Lagier-Tourenne, C.; Zuccato, C.; Cattaneo, E.; Gage, F.H.; Cleveland, D.W.; Glass, C.K. Mutant Huntingtin Promotes Autonomous Microglia Activation via Myeloid Lineage-Determining Factors. Nat. Neurosci. 2014, 17, 513–521. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It Is All about Energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A Prebiotic Basis for ATP as the Universal Energy Currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Araldi, R.; Módolo, D.; De-Sá-Júnior, P.; Consonni, S.; Carvalho, R.; Roperto, F.; Beçak, W.; Stocco, R. Genetics and Metabolic Deregulation Following Cancer Initiation: A World to Explore. Biomed. Pharmacother. 2016, 82, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J. Huntingt. Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Ratovitski, T.; Chighladze, E.; Arbez, N.; Boronina, T.; Herbrich, S.; Cole, R.N.; Ross, C.A. Huntingtin Protein Interactions Altered by Polyglutamine Expansion as Determined by Quantitative Proteomic Analysis. Cell Cycle 2012, 11, 2006–2021. [Google Scholar] [CrossRef]
- Damiano, M.; Diguet, E.; Malgorn, C.; D’Aurelio, M.; Galvan, L.; Petit, F.; Benhaim, L.; Guillermier, M.; Houitte, D.; Dufour, N.; et al. A Role of Mitochondrial Complex II Defects in Genetic Models of Huntington’s Disease Expressing N-Terminal Fragments of Mutant Huntingtin. Hum. Mol. Genet. 2013, 22, 3869–3882. [Google Scholar] [CrossRef] [PubMed]
- De Marco, F. Oxidative Stress and HPV Carcinogenesis. Viruses 2013, 5, 708–731. [Google Scholar] [CrossRef] [PubMed]
- Damiano, M.; Galvan, L.; Déglon, N.; Brouillet, E. Mitochondria in Huntington’s Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2010, 1802, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Petrilli, A.; Knott, A.B. Mutant Huntingtin and Mitochondrial Dysfunction. Trends Neurosci. 2008, 31, 609–616. [Google Scholar] [CrossRef]
- Oliveira, J.M.A.; Jekabsons, M.B.; Chen, S.; Lin, A.; Rego, A.C.; Gonçalves, J.; Ellerby, L.M.; Nicholls, D.G. Mitochondrial Dysfunction in Huntington’s Disease: The Bioenergetics of Isolated and in Situ Mitochondria from Transgenic Mice. J. Neurochem. 2007, 101, 241–249. [Google Scholar] [CrossRef]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 59–83. [Google Scholar]
- Paul, B.D.; Sbodio, J.I.; Xu, R.; Vandiver, M.S.; Cha, J.Y.; Snowman, A.M.; Snyder, S.H. Cystathionine γ-Lyase Deficiency Mediates Neurodegeneration in Huntington’s Disease. Nature 2014, 509, 96–100. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Neurodegeneration in Huntington’s Disease Involves Loss of Cystathionine γ-Lyase. Cell Cycle 2014, 13, 2491–2493. [Google Scholar] [CrossRef]
- Sugars, K.L.; Rubinsztein, D.C. Transcriptional Abnormalities in Huntington Disease. Trends Genet. 2003, 19, 233–238. [Google Scholar] [CrossRef]
- Cha, J.-H.J. Transcriptional Signatures in Huntington’s Disease. Prog. Neurobiol. 2007, 83, 228–248. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Hennessey, T.; Johri, A.; Tiwari, S.K.; Mishra, D.; Agarwal, S.; Kim, Y.S.; Beal, M.F. Transducer of Regulated CREB-Binding Proteins (TORCs) Transcription and Function Is Impaired in Huntington’s Disease. Hum. Mol. Genet. 2012, 21, 3474–3488. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Basso, M.; Niatsetskaya, Z.V.; Sleiman, S.F.; Smirnova, N.A.; Langley, B.C.; Mahishi, L.; Cooper, A.J.L.; Antonyak, M.A.; Cerione, R.A.; et al. Inhibition of Transglutaminase 2 Mitigates Transcriptional Dysregulation in Models of Huntington Disease. EMBO Mol. Med. 2010, 2, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Cohen, D.E.; Cui, L.; Supinski, A.; Savas, J.N.; Mazzulli, J.R.; Yates, J.R.; Bordone, L.; Guarente, L.; Krainc, D. Sirt1 Mediates Neuroprotection from Mutant Huntingtin by Activation of the TORC1 and CREB Transcriptional Pathway. Nat. Med. 2012, 18, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jeong, H.; Borovecki, F.; Parkhurst, C.N.; Tanese, N.; Krainc, D. Transcriptional Repression of PGC-1α by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell 2006, 127, 59–69. [Google Scholar] [CrossRef]
- Tobore, T.O. Towards a Comprehensive Understanding of the Contributions of Mitochondrial Dysfunction and Oxidative Stress in the Pathogenesis and Pathophysiology of Huntington’s Disease. J. Neurosci. Res. 2019, 97, 1455–1468. [Google Scholar] [CrossRef]
- Perluigi, M.; Poon, H.F.; Maragos, W.; Pierce, W.M.; Klein, J.B.; Calabrese, V.; Cini, C.; De Marco, C.; Butterfield, D.A. Proteomic Analysis of Protein Expression and Oxidative Modification in R6/2 Transgenic Mice. Mol. Cell. Proteom. 2005, 4, 1849–1861. [Google Scholar] [CrossRef]
- Feigin, A.; Kieburtz, K.; Como, P.; Hickey, C.; Abwender, D.; Zimmerman, C.; Steinberg, K.; Shoulson, I. Assessment of Coenzyme Q10 Tolerability in Huntington’s Disease. Mov. Disord. 1996, 11, 321–323. [Google Scholar] [CrossRef]
- The Huntington Study Group. A Randomized, Placebo-Controlled Trial of Coenzyme Q10 and Remacemide in Huntington’s Disease. Neurology 2001, 57, 397–404. [Google Scholar] [CrossRef]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington Disease Is Safe, Tolerable, Bioavailable in Brain and Reduces Serum 8OH2′dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef]
- Huntington Study Group TREND-HD Investigators. Randomized Controlled Trial of Ethyl-Eicosapentaenoic Acid in Huntington Disease. Arch. Neurol. 2008, 65, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Ranen, N.G.; Peyser, C.E.; Coyle, J.T.; Bylsma, F.W.; Sherr, M.; Day, L.; Folstein, M.F.; Brandt, J.; Ross, C.A.; Folstein, S.E. A Controlled Trial of Idebenone in Huntington’s Disease. Mov. Disord. 1996, 11, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Luthi-Carter, R. Decreased Expression of Striatal Signaling Genes in a Mouse Model of Huntington’s Disease. Hum. Mol. Genet. 2000, 9, 1259–1271. [Google Scholar] [CrossRef]
- Achour, M.; Le Gras, S.; Keime, C.; Parmentier, F.; Lejeune, F.-X.; Boutillier, A.-L.; Neri, C.; Davidson, I.; Merienne, K. Neuronal Identity Genes Regulated by Super-Enhancers Are Preferentially down-Regulated in the Striatum of Huntington’s Disease Mice. Hum. Mol. Genet. 2015, 24, 3481–3496. [Google Scholar] [CrossRef]
- Iannicola, C.; Moreno, S.; Oliverio, S.; Nardacci, R.; Ciofi-Luzzatto, A.; Piacentini, M. Early Alterations in Gene Expression and Cell Morphology in a Mouse Model of Huntington’s Disease. J. Neurochem. 2000, 75, 830–839. [Google Scholar] [CrossRef]
- Kumar, A.; Vaish, M.; Ratan, R.R. Transcriptional Dysregulation in Huntington’s Disease: A Failure of Adaptive Transcriptional Homeostasis. Drug Discov. Today 2014, 19, 956–962. [Google Scholar] [CrossRef]
- Bartzokis, G.; Lu, P.H.; Geschwind, D.H.; Tingus, K.; Huang, D.; Mendez, M.F.; Edwards, N.; Mintz, J. Apolipoprotein E Affects Both Myelin Breakdown and Cognition: Implications for Age-Related Trajectories of Decline into Dementia. Biol. Psychiatry 2007, 62, 1380–1387. [Google Scholar] [CrossRef]
- Fox, J.H.; Kama, J.A.; Lieberman, G.; Chopra, R.; Dorsey, K.; Chopra, V.; Volitakis, I.; Cherny, R.A.; Bush, A.I.; Hersch, S. Mechanisms of Copper Ion Mediated Huntington’s Disease Progression. PLoS ONE 2007, 2, e334. [Google Scholar] [CrossRef]
- Brazier, M.; Wedd, A.; Collins, S. Antioxidant and Metal Chelation-Based Therapies in the Treatment of Prion Disease. Antioxidants 2014, 3, 288–308. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P.; Schapira, A.H.V.; Marsden, C.D. Alterations in Levels of Iron, Ferritin, and Other Trace Metals in Neurodegenerative Diseases Affecting the Basal Ganglia. Ann. Neurol. 1992, 32, S94–S100. [Google Scholar] [CrossRef]
- Bartzokis, G.; Lu, P.H.; Tishler, T.A.; Fong, S.M.; Oluwadara, B.; Finn, J.P.; Huang, D.; Bordelon, Y.; Mintz, J.; Perlman, S. Myelin Breakdown and Iron Changes in Huntington’s Disease: Pathogenesis and Treatment Implications. Neurochem. Res. 2007, 32, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Leavitt, B.R. Iron Dysregulation in Huntington’s Disease. J. Neurochem. 2014, 130, 328–350. [Google Scholar] [CrossRef]
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron Accumulates in Huntington’s Disease Neurons: Protection by Deferoxamine. PLoS ONE 2013, 8, e77023. [Google Scholar] [CrossRef]
- García-Bueno, B.; Caso, J.R. Cannabis, Cannabinoid Receptors, and Stress-Induced Excitotoxicity. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 731–737. [Google Scholar]
- Fox, J.H.; Connor, T.; Stiles, M.; Kama, J.; Lu, Z.; Dorsey, K.; Liebermann, G.; Sapp, E.; Cherny, R.A.; Banks, M.; et al. Cysteine Oxidation within N-Terminal Mutant Huntingtin Promotes Oligomerization and Delays Clearance of Soluble Protein. J. Biol. Chem. 2011, 286, 18320–18330. [Google Scholar] [CrossRef]
- Xiao, G.; Fan, Q.; Wang, X.; Zhou, B. Huntington Disease Arises from a Combinatory Toxicity of Polyglutamine and Copper Binding. Proc. Natl. Acad. Sci. USA 2013, 110, 14995–15000. [Google Scholar] [CrossRef]
- Mitomi, Y.; Nomura, T.; Kurosawa, M.; Nukina, N.; Furukawa, Y. Post-Aggregation Oxidation of Mutant Huntingtin Controls the Interactions between Aggregates. J. Biol. Chem. 2012, 287, 34764–34775. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Bush, A.I. Biological Metals and Metal-Targeting Compounds in Major Neurodegenerative Diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Alim, I.; Khim, S.J.; Bourassa, M.W.; Sleiman, S.F.; John, R.; Thinnes, C.C.; Yeh, T.-L.; Demetriades, M.; Neitemeier, S.; et al. Therapeutic Targeting of Oxygen-Sensing Prolyl Hydroxylases Abrogates ATF4-Dependent Neuronal Death and Improves Outcomes after Brain Hemorrhage in Several Rodent Models. Sci. Transl. Med. 2016, 8, 328ra29. [Google Scholar] [CrossRef] [PubMed]
- Raymond, L.A. Excitotoxicity in Huntington Disease. Clin. Neurosci. Res. 2003, 3, 121–128. [Google Scholar] [CrossRef]
- Block, R.C.; Dorsey, E.R.; Beck, C.A.; Brenna, J.T.; Shoulson, I. Altered Cholesterol and Fatty Acid Metabolism in Huntington Disease. J. Clin. Lipidol. 2010, 4, 17–23. [Google Scholar] [CrossRef]
- Valenza, M.; Cattaneo, E. Cholesterol Dysfunction in Neurodegenerative Diseases: Is Huntington’s Disease in the List? Prog. Neurobiol. 2006, 80, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zeron, M.M.; Chen, N.; Moshaver, A.; Ting-Chun Lee, A.; Wellington, C.L.; Hayden, M.R.; Raymond, L.A. Mutant Huntingtin Enhances Excitotoxic Cell Death. Mol. Cell. Neurosci. 2001, 17, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Pouladi, M.A.; Talantova, M.; Yao, D.; Xia, P.; Ehrnhoefer, D.E.; Zaidi, R.; Clemente, A.; Kaul, M.; Graham, R.K.; et al. Balance between Synaptic versus Extrasynaptic NMDA Receptor Activity Influences Inclusions and Neurotoxicity of Mutant Huntingtin. Nat. Med. 2009, 15, 1407–1413. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1α Regulates the Mitochondrial Antioxidant Defense System in Vascular Endothelial Cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Sankaram, M.B.; Thompson, T.E. Modulation of Phospholipid Acyl Chain Order by Cholesterol. A Solid-State Deuterium Nuclear Magnetic Resonance Study. Biochemistry 1990, 29, 10676–10684. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Smith, B.A.; McConnell, H.M. Lateral Diffusion in Binary Mixtures of Cholesterol and Phosphatidylcholines. Proc. Natl. Acad. Sci. USA 1979, 76, 15–18. [Google Scholar] [CrossRef]
- Gimpl, G.; Burger, K.; Fahrenholz, F. Cholesterol as Modulator of Receptor Function. Biochemistry 1997, 36, 10959–10974. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular Anatomy of a Trafficking Organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- van der Kant, R.; Fish, A.; Janssen, L.; Janssen, H.; Krom, S.; Ho, N.; Brummelkamp, T.; Carette, J.; Rocha, N.; Neefjes, J. Late Endosomal Transport and Tethering Are Coupled Processes Controlled by RILP and the Cholesterol Sensor ORP1L. J. Cell Sci. 2013, 126, 3462–3474. [Google Scholar] [CrossRef]
- Koseoglu, S.; Love, S.A.; Haynes, C.L. Cholesterol Effects on Vesicle Pools in Chromaffin Cells Revealed by Carbon-Fiber Microelectrode Amperometry. Anal. Bioanal. Chem. 2011, 400, 2963–2971. [Google Scholar] [CrossRef]
- Korinek, M.; Gonzalez-Gonzalez, I.M.; Smejkalova, T.; Hajdukovic, D.; Skrenkova, K.; Krusek, J.; Horak, M.; Vyklicky, L. Cholesterol Modulates Presynaptic and Postsynaptic Properties of Excitatory Synaptic Transmission. Sci. Rep. 2020, 10, 12651. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Lin, C.-C.; Sheng, M. Lipid Rafts in the Maintenance of Synapses, Dendritic Spines, and Surface AMPA Receptor Stability. J. Neurosci. 2003, 23, 3262–3271. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Sheng, M.; Hanson, J.E. Targeting Synapse Function and Loss for Treatment of Neurodegenerative Diseases. Nat. Rev. Drug Discov. 2024, 23, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Plinta, K.; Plewka, A.; Pawlicki, K.; Zmarzły, N.; Wójcik-Pędziwiatr, M.; Rudziński, M.; Krzak-Kubica, A.; Doręgowska-Stachera, M.; Rudzińska-Bar, M. The Utility of BDNF Detection in Assessing Severity of Huntington’s Disease. J. Clin. Med. 2021, 10, 5181. [Google Scholar] [CrossRef]
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of Huntingtin-Mediated BDNF Gene Transcription in Huntington’s Disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef]
- Giralt, A.; Carretón, O.; Lao-Peregrin, C.; Martín, E.D.; Alberch, J. Conditional BDNF Release under Pathological Conditions Improves Huntington’s Disease Pathology by Delaying Neuronal Dysfunction. Mol. Neurodegener. 2011, 6, 71. [Google Scholar] [CrossRef]
- Xie, Y.; Hayden, M.R.; Xu, B. BDNF Overexpression in the Forebrain Rescues Huntington’s Disease Phenotypes in YAC128 Mice. J. Neurosci. 2010, 30, 14708–14718. [Google Scholar] [CrossRef]
- Markianos, M.; Panas, M.; Kalfakis, N.; Vassilopoulos, D. Low Plasma Total Cholesterol in Patients with Huntington’s Disease and First-Degree Relatives. Mol. Genet. Metab. 2008, 93, 341–346. [Google Scholar] [CrossRef]
- Lütjohann, D.; Breuer, O.; Ahlborg, G.; Nennesmo, I.; Sidén, A.; Diczfalusy, U.; Björkhem, I. Cholesterol Homeostasis in Human Brain: Evidence for an Age-Dependent Flux of 24S-Hydroxycholesterol from the Brain into the Circulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9799–9804. [Google Scholar] [CrossRef]
- Valenza, M.; Rigamonti, D.; Goffredo, D.; Zuccato, C.; Fenu, S.; Jamot, L.; Strand, A.; Tarditi, A.; Woodman, B.; Racchi, M.; et al. Dysfunction of the Cholesterol Biosynthetic Pathway in Huntington’s Disease. J. Neurosci. 2005, 25, 9932–9939. [Google Scholar] [CrossRef]
- Torrejon, C.; Jung, U.J.; Deckelbaum, R.J. N-3 Fatty Acids and Cardiovascular Disease: Actions and Molecular Mechanisms. Prostagland. Leukot. Essent. Fat. Acids 2007, 77, 319–326. [Google Scholar] [CrossRef]
- Yanai, A.; Huang, K.; Kang, R.; Singaraja, R.R.; Arstikaitis, P.; Gan, L.; Orban, P.C.; Mullard, A.; Cowan, C.M.; Raymond, L.A.; et al. Palmitoylation of Huntingtin by HIP14is Essential for Its Trafficking and Function. Nat. Neurosci. 2006, 9, 824–831. [Google Scholar] [CrossRef]
- Singaraja, R.R. HIP14, a Novel Ankyrin Domain-Containing Protein, Links Huntingtin to Intracellular Trafficking and Endocytosis. Hum. Mol. Genet. 2002, 11, 2815–2828. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, M.; Adachi, H.; Sobue, G. Getting a Handle on Huntington’s Disease: The Case for Cholesterol. Nat. Med. 2009, 15, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kiyosue, K.; Hazama, S.; Ogura, A.; Kashihara, M.; Hara, T.; Koshimizu, H.; Kojima, M. Brain-Derived Neurotrophic Factor Regulates Cholesterol Metabolism for Synapse Development. J. Neurosci. 2007, 27, 6417–6427. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, S.; Lahaye, R.A.; Vitet, H.; Scaramuzzino, C.; Virlogeux, A.; Capellano, L.; Genoux, A.; Gershoni-Emek, N.; Geva, M.; Hayden, M.R.; et al. Pridopidine Rescues BDNF/TrkB Trafficking Dynamics and Synapse Homeostasis in a Huntington Disease Brain-on-a-Chip Model. Neurobiol. Dis. 2022, 173, 105857. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, I.; Noh, J.-E.; Lee, H.; Hong, K.S.; Lee, N.; Pei, Z.; Song, J. Intracerebral Transplantation of BDNF-Overexpressing Human Neural Stem Cells (HB1.F3.BDNF) Promotes Migration, Differentiation and Functional Recovery in a Rodent Model of Huntington’s Disease. Exp. Neurobiol. 2020, 29, 130–137. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Conforti, P.; MacDonald, M.E.; Tartari, M.; Cattaneo, E. Systematic Assessment of BDNF and Its Receptor Levels in Human Cortices Affected by Huntington’s Disease. Brain Pathol. 2007, 18, 225–238. [Google Scholar] [CrossRef]
- Yu, C.; Li, C.H.; Chen, S.; Yoo, H.; Qin, X.; Park, H. Decreased BDNF Release in Cortical Neurons of a Knock-in Mouse Model of Huntington’s Disease. Sci. Rep. 2018, 8, 16976. [Google Scholar] [CrossRef]
- Maloney, M.T.; Wang, W.; Bhowmick, S.; Millan, I.; Kapur, M.; Herrera, N.; Frost, E.; Zhang, E.Y.; Song, S.; Wang, M.; et al. Failure to Thrive: Impaired BDNF Transport along the Cortical–Striatal Axis in Mouse Q140 Neurons of Huntington’s Disease. Biology 2023, 12, 157. [Google Scholar] [CrossRef]
- Lejkowska, R.; Kawa, M.; Pius-Sadowska, E.; Rogińska, D.; Łuczkowska, K.; Machaliński, B.; Machalińska, A. Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. Int. J. Mol. Sci. 2019, 20, 777. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Schwieger, J.; Hamm, A.; Lenarz, T.; Hoffmann, A. BDNF-overexpressing Human Mesenchymal Stem Cells Mediate Increased Neuronal Protection in Vitro. J. Neurosci. Res. 2019, 97, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Pan, B.-S.; Tsai, S.-F.; Chiang, Y.-T.; Huang, B.-M.; Mo, F.-E.; Kuo, Y.-M. BDNF Reverses Aging-Related Microglial Activation. J. Neuroinflamm. 2020, 17, 210. [Google Scholar] [CrossRef]
- Deng, P.; Anderson, J.D.; Yu, A.S.; Annett, G.; Fink, K.D.; Nolta, J.A. Engineered BDNF Producing Cells as a Potential Treatment for Neurologic Disease. Expert Opin. Biol. Ther. 2016, 16, 1025–1033. [Google Scholar] [CrossRef]
- Valadão, P.A.C.; Santos, K.B.S.; Vieira, T.H.F.E.; Cordeiro, T.M.E.; Teixeira, A.L.; Guatimosim, C.; de Miranda, A.S. Inflammation in Huntington’s Disease: A Few New Twists on an Old Tale. J. Neuroimmunol. 2020, 348, 577380. [Google Scholar] [CrossRef]
- van der Burg, J.M.; Björkqvist, M.; Brundin, P. Beyond the Brain: Widespread Pathology in Huntington’s Disease. Lancet Neurol. 2009, 8, 765–774. [Google Scholar] [CrossRef]
- Zuccato, C.; Valenza, M.; Cattaneo, E. Molecular Mechanisms and Potential Therapeutical Targets in Huntington’s Disease. Physiol. Rev. 2010, 90, 905–981. [Google Scholar] [CrossRef]
- Gómez-Jaramillo, L.; Cano-Cano, F.; del Carmen González-Montelongo, M.; Campos-Caro, A.; Aguilar-Diosdado, M.; Arroba, A.I. A New Perspective on Huntington’s Disease: How a Neurological Disorder Influences the Peripheral Tissues. Int. J. Mol. Sci. 2022, 23, 6089. [Google Scholar] [CrossRef]
- Aziz, N.A.; Pijl, H.; Frolich, M.; Snel, M.; Streefland, T.C.M.; Roelfsema, F.; Roos, R.A.C. Systemic Energy Homeostasis in Huntington’s Disease Patients. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1233–1237. [Google Scholar] [CrossRef]
- Burtscher, J.; Strasser, B.; Pepe, G.; Burtscher, M.; Kopp, M.; Di Pardo, A.; Maglione, V.; Khamoui, A.V. Brain–Periphery Interactions in Huntington’s Disease: Mediators and Lifestyle Interventions. Int. J. Mol. Sci. 2024, 25, 4696. [Google Scholar] [CrossRef]
- Paraskevopoulou, F.; Herman, M.A.; Rosenmund, C. Glutamatergic Innervation onto Striatal Neurons Potentiates GABAergic Synaptic Output. J. Neurosci. 2019, 39, 4448–4460. [Google Scholar] [CrossRef]
- Tunstall, M.J.; Oorschot, D.E.; Kean, A.; Wickens, J.R. Inhibitory Interactions Between Spiny Projection Neurons in the Rat Striatum. J. Neurophysiol. 2002, 88, 1263–1269. [Google Scholar] [CrossRef]
- Lassus, B.; Naudé, J.; Faure, P.; Guedin, D.; Von Boxberg, Y.; la Cour, C.M.; Millan, M.J.; Peyrin, J.-M. Glutamatergic and Dopaminergic Modulation of Cortico-Striatal Circuits Probed by Dynamic Calcium Imaging of Networks Reconstructed in Microfluidic Chips. Sci. Rep. 2018, 8, 17461. [Google Scholar] [CrossRef]
- Kubota, Y.; Inagaki, S.; Shimada, S.; Kito, S.; Eckenstein, F.; Tohyama, M. Neostriatal Cholinergic Neurons Receive Direct Synaptic Inputs from Dopaminergic Axons. Brain Res. 1987, 413, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.R.; Charrin, B.C.; Borrell-Pagès, M.; Dompierre, J.P.; Rangone, H.; Cordelières, F.P.; De Mey, J.; MacDonald, M.E.; Leßmann, V.; Humbert, S.; et al. Huntingtin Controls Neurotrophic Support and Survival of Neurons by Enhancing BDNF Vesicular Transport along Microtubules. Cell 2004, 118, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Maki, T.; Shindo, A.; Liang, A.C.; Maeda, M.; Egawa, N.; Itoh, K.; Lo, E.K.; Lok, J.; Ihara, M.; et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J. Neurosci. 2015, 35, 14002–14008. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, P.S.; Dallas, S.; Wilson, B.; Block, M.L.; Wang, C.-C.; Kinyamu, H.; Lu, N.; Gao, X.; Leng, Y.; et al. Histone Deacetylase Inhibitors Up-Regulate Astrocyte GDNF and BDNF Gene Transcription and Protect Dopaminergic Neurons. Int. J. Neuropsychopharmacol. 2008, 11, 1123–1134. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021, 13, 654931. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF Overexpression in Mouse Hippocampal Astrocytes Promotes Local Neurogenesis and Elicits Anxiolytic-like Activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef]
- Wang, L.; Lin, F.; Wang, J.; Wu, J.; Han, R.; Zhu, L.; Zhang, G.; DiFiglia, M.; Qin, Z. Truncated N-Terminal Huntingtin Fragment with Expanded-Polyglutamine (Htt552-100Q) Suppresses Brain-Derived Neurotrophic Factor Transcription in Astrocytes. Acta Biochim. Biophys. Sin. 2012, 44, 249–258. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Fang, Z.-H.; Yu, Z.-X.; Wang, C.-E.; Li, S.-H.; Li, X.-J. Expression of Mutant Huntingtin in Glial Cells Contributes to Neuronal Excitotoxicity. J. Cell Biol. 2005, 171, 1001–1012. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Striatal Plasticity and Basal Ganglia Circuit Function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef]
- Park, H.; Poo, M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF Signaling and Survival of Striatal Neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Caettaneo, E. Role of Brain-Derived Neurotrophic Factor in Huntington’s Disease. Prog. Neurobiol. 2007, 81, 294–330. [Google Scholar] [CrossRef]
- Hermel, E.; Gafni, J.; Propp, S.S.; Leavitt, B.R.; Wellington, C.L.; Young, J.E.; Hackam, A.S.; Logvinova, A.V.; Peel, A.L.; Chen, S.F.; et al. Specific Caspase Interactions and Amplification Are Involved in Selective Neuronal Vulnerability in Huntington’s Disease. Cell Death Differ. 2004, 11, 424–438. [Google Scholar] [CrossRef]
- Shin, C.; Kim, R.; Yoo, D.; Oh, E.; Moon, J.; Kim, M.; Lee, J.-Y.; Kim, J.-M.; Koh, S.-B.; Kim, M.; et al. A Practical Guide for Clinical Approach to Patients with Huntington’s Disease in Korea. J. Mov. Disord. 2024, 17, 138–149. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, T.; Li, X.-J.; Li, S. Mutant Huntingtin Impairs BDNF Release from Astrocytes by Disrupting Conversion of Rab3a-GTP into Rab3a-GDP. J. Neurosci. 2016, 36, 8790–8801. [Google Scholar] [CrossRef]
- Sauer, H.; Fischer, W.; Nikkhah, G.; Wiegand, S.J.; Brundin, P.; Lindsay, R.M.; Björklund, A. Brain-Derived Neurotrophic Factor Enhances Function Rather than Survival of Intrastriatal Dopamine Cell-Rich Grafts. Brain Res. 1993, 626, 37–44. [Google Scholar] [CrossRef]
- van der Zwaan, K.F.; Feleus, S.; Dekkers, O.M.; Roos, R.A.C.; de Bot, S.T. Total Functioning Capacity Scale in Huntington’s Disease: Natural Course over Time. J. Neurol. 2025, 272, 140. [Google Scholar] [CrossRef]
- Shoulson, I. Huntington Disease: Functional Capacities in Patients Treated with Neuroleptic and Antidepressant Drugs. Neurology 1981, 31, 1333–1335. [Google Scholar] [CrossRef]
- Fujisawa, M.; Takeshita, Y.; Fujikawa, S.; Matsuo, K.; Okamoto, M.; Tamada, M.; Shimizu, F.; Sano, Y.; Koga, M.; Kanda, T. Exploring Lipophilic Compounds That Induce BDNF Secretion in Astrocytes beyond the BBB Using a New Multi-Cultured Human in Vitro BBB Model. J. Neuroimmunol. 2022, 362, 577783. [Google Scholar] [CrossRef]
- Saylor, A.J.; McGinty, J.F. An Intrastriatal Brain-Derived Neurotrophic Factor Infusion Restores Striatal Gene Expression in Bdnf Heterozygous Mice. Brain Struct. Funct. 2010, 215, 97–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakao, N.; Brundin, P.; Funa, K.; Lindvall, O.; Odin, P. Trophic and Protective Actions of Brain-Derived Neurotrophic Factor on Striatal DARPP-32-Containing Neurons in Vitro. Dev. Brain Res. 1995, 90, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Palizvan, M.R.; Sohya, K.; Kohara, K.; Maruyama, A.; Yasuda, H.; Kimura, F.; Tsumoto, T. Brain-Derived Neurotrophic Factor Increases Inhibitory Synapses, Revealed in Solitary Neurons Cultured from Rat Visual Cortex. Neuroscience 2004, 126, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Rauskolb, S.; Zagrebelsky, M.; Dreznjak, A.; Deogracias, R.; Matsumoto, T.; Wiese, S.; Erne, B.; Sendtner, M.; Schaeren-Wiemers, N.; Korte, M.; et al. Global Deprivation of Brain-Derived Neurotrophic Factor in the CNS Reveals an Area-Specific Requirement for Dendritic Growth. J. Neurosci. 2010, 30, 1739–1749. [Google Scholar] [CrossRef]
- Login, I.S.; Cronin, M.J.; MacLeod, R.M. Tetrabenazine Has Properties of a Dopamine Receptor Antagonist. Ann. Neurol. 1982, 12, 257–262. [Google Scholar] [CrossRef]
- Shahani, L. Tetrabenazine and Suicidal Ideation. J. Neuropsychiatry Clin. Neurosci. 2013, 25, E30. [Google Scholar] [CrossRef]
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: Reliability and Consistency. Mov. Disord. 1996, 11, 136–142. [Google Scholar] [CrossRef]
- Mestre, T.A.; Forjaz, M.J.; Mahlknecht, P.; Cardoso, F.; Ferreira, J.J.; Reilmann, R.; Sampaio, C.; Goetz, C.G.; Cubo, E.; Martinez-Martin, P.; et al. Rating Scales for Motor Symptoms and Signs in Huntington’s Disease: Critique and Recommendations. Mov. Disord. Clin. Pract. 2018, 5, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.T.; Krier, I.; Arjomand, J.; Borowsky, B.; Tabrizi, S.J.; Leavitt, B.R.; Arran, N.; Axelson, E.; Bardinet, E.; Bechtel, N.; et al. Longitudinal Expression Changes Are Weak Correlates of Disease Progression in Huntington’s Disease. Brain Commun. 2020, 2, fcaa172. [Google Scholar] [CrossRef] [PubMed]
- Labadorf, A.; Choi, S.H.; Myers, R.H. Evidence for a Pan-Neurodegenerative Disease Response in Huntington’s and Parkinson’s Disease Expression Profiles. Front. Mol. Neurosci. 2018, 10, 430. [Google Scholar] [CrossRef]
- Agus, F.; Crespo, D.; Myers, R.H.; Labadorf, A. The Caudate Nucleus Undergoes Dramatic and Unique Transcriptional Changes in Human Prodromal Huntington’s Disease Brain. BMC Med. Genom. 2019, 12, 137. [Google Scholar] [CrossRef]
- Labadorf, A.; Hoss, A.G.; Lagomarsino, V.; Latourelle, J.C.; Hadzi, T.C.; Bregu, J.; MacDonald, M.E.; Gusella, J.F.; Chen, J.F.; Akbarian, S.; et al. RNA Sequence Analysis of Human Huntington Disease Brain Reveals an Extensive Increase in Inflammatory and Developmental Gene Expression. PLoS ONE 2015, 10, e0143563, Erratum in PLoS ONE 2016, 11, e0160295. [Google Scholar] [CrossRef]
- Dias-Pinto, J.R.; Faustinoni-Neto, B.; Sanches-Fernandes, J.M.; Kerkis, I.; Araldi, R.P. How Does the Age of Control Individuals Hinder the Identification of Target Genes for Huntington’s Disease? Front. Genet. 2024, 15, 1377237. [Google Scholar] [CrossRef]
- Dias-Pinto, J.R.; Faustinoni-Neto, B.; Munhoz, L.; Kerkis, I.; Araldi, R.P. From Brain to Blood: Uncovering Potential Therapeutical Targets and Biomarkers for Huntington’s Disease Using an Integrative RNA-Seq Analytical Platform (BDASeq®). Cells 2025, 14, 976. [Google Scholar] [CrossRef]
- Lin, L.; Park, J.W.; Ramachandran, S.; Zhang, Y.; Tseng, Y.T.; Shen, S.; Waldvogel, H.J.; Curtis, M.A.; Richard, R.L.; Troncoso, J.C.; et al. Transcriptome Sequencing Reveals Aberrant Alternative Splicing in Huntington’s Disease. Hum. Mol. Genet. 2016, 25, 3454–3466. [Google Scholar] [CrossRef]
- Selemon, L.D.; Rajkowska, G.; Goldman-Rakic, P.S. Evidence for Progression in Frontal Cortical Pathology in Late-stage Huntington’s Disease. J. Comp. Neurol. 2004, 468, 190–204. [Google Scholar] [CrossRef]
- Delmaire, C.; Dumas, E.M.; Sharman, M.A.; van den Bogaard, S.J.A.; Valabregue, R.; Jauffret, C.; Justo, D.; Reilmann, R.; Stout, J.C.; Craufurd, D.; et al. The Structural Correlates of Functional Deficits in Early Huntington’s Disease. Hum. Brain Mapp. 2013, 34, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Furr Stimming, E.; Claassen, D.O.; Kayson, E.; Goldstein, J.; Mehanna, R.; Zhang, H.; Liang, G.S.; Haubenberger, D.; Adams, J.; Beck, C.; et al. Safety and Efficacy of Valbenazine for the Treatment of Chorea Associated with Huntington’s Disease (KINECT-HD): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2023, 22, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Heim, B.; Seppi, K. Valbenazine as Treatment for Huntington’s Disease Chorea. Lancet Neurol. 2023, 22, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on Drug Review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.H.; Sparks, B.F.; Field, K.M.; Yallapragada, V.; Shpritz, B.D.; Rosenblatt, A.; Brandt, J.; Gourley, L.M.; Liang, K.; Zhou, H.; et al. Onset and Rate of Striatal Atrophy in Preclinical Huntington Disease. Neurology 2004, 63, 66–72. [Google Scholar] [CrossRef]
- Aylward, E.H.; Nopoulos, P.C.; Ross, C.A.; Langbehn, D.R.; Pierson, R.K.; Mills, J.A.; Johnson, H.J.; Magnotta, V.A.; Juhl, A.R.; Paulsen, J.S. Longitudinal Change in Regional Brain Volumes in Prodromal Huntington Disease. J. Neurol. Neurosurg. Psychiatry 2011, 82, 405–410. [Google Scholar] [CrossRef]
- Sneha, N.P.; Dharshini, S.A.P.; Taguchi, Y.-h.; Gromiha, M.M. Investigating Neuron Degeneration in Huntington’s Disease Using RNA-Seq Based Transcriptome Study. Genes 2023, 14, 1801. [Google Scholar] [CrossRef]
- Seefelder, M.; Kochanek, S. A Meta-Analysis of Transcriptomic Profiles of Huntington’s Disease Patients. PLoS ONE 2021, 16, e0253037. [Google Scholar] [CrossRef]
- Research and Markets. Global Huntington’s Disease Treatment Market Forecast Report by Drug Type (Approved Drugs, Off-Label Drugs) End User (Hospital Pharmacy, Drug Store & Retail Pharmacy, Online Pharmacy) Countries and Company Analysis, 2024–2032; Research and Markets: Dublin, Ireland, 2024; Available online: https://www.researchandmarkets.com/report/huntington-disease-drug?srsltid=AfmBOop1kZhsccNpDXkzXcUx9MvGnn1EBOuWOCFGtC96HHJEb7RSILHR (accessed on 1 August 2025).
- Claassen, D.O.; Ayyagari, R.; Garcia-Horton, V.; Zhang, S.; Alexander, J.; Leo, S. Real-World Adherence to Tetrabenazine or Deutetrabenazine Among Patients with Huntington’s Disease: A Retrospective Database Analysis. Neurol. Ther. 2022, 11, 435–448. [Google Scholar] [CrossRef]
- Frank, S.; Testa, C.; Edmondson, M.C.; Goldstein, J.; Kayson, E.; Leavitt, B.R.; Oakes, D.; O’Neill, C.; Vaughan, C.; Whaley, J.; et al. The Safety of Deutetrabenazine for Chorea in Huntington Disease: An Open-Label Extension Study. CNS Drugs 2022, 36, 1207–1216. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Costa, J.; Ferreira, J.J.; Wild, E.J. Tetrabenazine Versus Deutetrabenazine for Huntington’s Disease: Twins or Distant Cousins? Mov. Disord. Clin. Pract. 2017, 4, 582–585. [Google Scholar] [CrossRef]
- Claassen, D.O.; Carroll, B.; De Boer, L.M.; Wu, E.; Ayyagari, R.; Gandhi, S.; Stamler, D. Indirect Tolerability Comparison of Deutetrabenazine and Tetrabenazine for Huntington Disease. J. Clin. Mov. Disord. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.P.; Cheng, M.H.; Bahar, I.; Coleman, J.A. Structural Mechanisms for VMAT2 Inhibition by Tetrabenazine. eLife 2024, 12, RP91973. [Google Scholar] [CrossRef]
- Alothman, D.; Marshall, C.R.; Tyrrell, E.; Lewis, S.; Card, T.; Fogarty, A. Risk of Mortality from Suicide in Patients with Huntington’s Disease Is Increased Compared to the General Population in England. J. Neurol. 2022, 269, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.J.; Molnar, V.; Fedor, M.; Csehi, R.; Acsai, K.; Borsos, B.; Grosz, Z. Improving Mood and Cognitive Symptoms in Huntington’s Disease with Cariprazine Treatment. Front. Psychiatry 2022, 12, 825532. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, E. Treatment of Irritability in Huntington’s Disease. Curr. Treat. Options Neurol. 2010, 12, 424–433. [Google Scholar] [CrossRef]
- Andriessen, R.L.; Oosterloo, M.; Hollands, A.; Linden, D.E.J.; de Greef, B.T.A.; Leentjens, A.F.G. Psychotropic Medication Use in Huntington’s Disease: A Retrospective Cohort Study. Parkinsonism Relat. Disord. 2022, 105, 69–74. [Google Scholar] [CrossRef]
- Kerkis, I.; Wenceslau, C.V.; Araldi, R.P. Stem Cells from Dental Pulp of Deciduous Teeth: Twenty Years of Experience. In Recent Update on Mesenchymal Stem Cells; IntechOpen: London, UK, 2024. [Google Scholar]
- Araldi, R.P.; Dias-Pinto, J.; Kerkis, I. Pharma Intelligence Applied to the Research and Development of Cell and Cell-Free Therapy: A New Era of the Medicine. In Recent Update on Mesenchymal Stem Cells; IntechOpen: London, UK, 2024. [Google Scholar]
- Araldi, R.P.; Viana, M.; Colozza-Gama, G.A.; Dias-Pinto, J.R.; Ankol, L.; Valverde, C.W.; Perlson, E.; Kerkis, I. Unique Transcriptional Signatures Observed in Stem Cells from the Dental Pulp of Deciduous Teeth Produced on a Large Scale. Pharmacologia 2023, 14, 72–95. [Google Scholar] [CrossRef]
- Araldi, R.P.; D’Amelio, F.; Vigerelli, H.; de Melo, T.C.; Kerkis, I. Stem Cell-Derived Exosomes as Therapeutic Approach for Neurodegenerative Ddsorders: From Biology to Biotechnology. Cells 2020, 9, 2663. [Google Scholar] [CrossRef]
- Kerkis, I.; Wenceslau, C.V.; Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; Silva, J.M.; Pagani, E.; Haddad, M.S. Preclincal Assessment of NestaCell® in Huntigton’s Disease 3-NP Rat Model Demonstrates Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Single versus Multiple Injections. Cytotherapy 2022, 24, S5. [Google Scholar] [CrossRef]
- Araldi, R.P.; Ramos, A.T.; Alievi, A.L.; Policíquio, B.; Teixei, M.R.; Mendes, T.B.; Gongaza, V.; Costa, V.R.; Troncone, L.R.; Kerkis, I. NestaCell® Promotes Motor, Cognitive and Neuropsychiatric Functions Amelioration and Dopaminergic Neurons Restoration in a Preclinical Model of Parkinson’s Disease. Cytotherapy 2022, 24, S3. [Google Scholar] [CrossRef]
- da Silva, J.M.; Araldi, R.P.; Colozza-Gama, G.A.; Pagani, E.; Sid, A.; Valverde, C.W.; Kerkis, I. Human Immature Dental Pulp Stem Cells Did Not Graft into a Preexisting Human Lung Adenocarcinoma. Case Rep. Oncol. 2022, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Mihai, D.P.; Nitulescu, G.M. Computer-Aided Drug Design and Drug Discovery. Pharmaceuticals 2025, 18, 436. [Google Scholar] [CrossRef]
- Faustinoni-Neto, B.; Dias-Pinto, J.R.; Araldi, R.P. Pharma Data Analytics (Pharma Intelligence): Opportunities and Challenges in the Multi-Omics Era of Drug Discovery and Development. Pharmacologia 2023, 14, 29–39. [Google Scholar] [CrossRef]
- Dias-Pinto, J.R.; Faustinoni-Neto, B.; Araldi, R.P. BDASeq: A Novel and Poweful Tool Able to Accurately Discover Theraeputic Targets for the Treatment of Rare Diseases. In Proceedings of the MENA Rare Disease Congress, Abu Dhabi, United Arab Emirates, 16–19 May 2024; p. 5. [Google Scholar]
- Araldi, R.P.; Faustinoni-Neto, B.; Dias-Pinto, J.R. Novel Pharmaceutical Tragets and Prognostic Biomarkers for Huntington’s Disease: New Perspectives for the Drug Development. In Proceedings of the MENA Rare Disease Congress, Abu Dhabi, United Arab Emirates, 16–19 May 2024; pp. 16–19. [Google Scholar]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA Sequencing: The Teenage Years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A Survey of Best Practices for RNA-Seq Data Analysis. Genome Biol. 2016, 17, 1–19. [Google Scholar] [CrossRef]
- Lamarre, S.; Frasse, P.; Zouine, M.; Labourdette, D.; Sainderichin, E.; Hu, G.; Le Berre-Anton, V.; Bouzayen, M.; Maza, E. Optimization of an RNA-Seq Differential Gene Expression Analysis Depending on Biological Replicate Number and Library Size. Front. Plant Sci. 2018, 9, 108. [Google Scholar] [CrossRef]
- Maza, E. In Papyro Comparison of TMM (EdgeR), RLE (DESeq2), and MRN Normalization Methods for a Simple Two-Conditions-Without-Replicates RNA-Seq Experimental Design. Front. Genet. 2016, 7, 164. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.-C.; Konaté, M.M.; Chen, L.; Das, B.; Karlovich, C.; Williams, P.M.; Evrard, Y.A.; Doroshow, J.H.; McShane, L.M. TPM, FPKM, or Normalized Counts? A Comparative Study of Quantification Measures for the Analysis of RNA-Seq Data from the NCI Patient-Derived Models Repository. J. Transl. Med. 2021, 19, 269. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Dias-Pinto, J.R.; Araldi, R.P.; Faustinoni-Neto, B. Biomarker Discovery Algorithm (BDASeq): An Integrative Analytics-Based Tool Designed to Identify Potential Diagnostic and Therapeutic Targets for Diseases. In Proceedings of the MENA Rare Disease Congress, Abu Dhabi, United Arab Emirates, 16–19 May 2024; p. 1. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Tarazona, S.; García, F.; Ferrer, A.; Dopazo, J.; Conesa, A. NOIseq: A RNA-Seq Differential Expression Method Robust for Sequencing Depth Biases. EMBnet J. 2012, 17, 18. [Google Scholar] [CrossRef]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An Empirical Bayes Hierarchical Model for Inference in RNA-Seq Experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Agniel, D.; Thiébaut, R.; Hejblum, B.P. Dearseq: A Variance Component Score Test for RNA-Seq Differential Analysis That Effectively Controls the False Discovery Rate. NAR Genom. Bioinform. 2020, 2, lqaa093. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Firth, D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Unti, E.; Mazzucchi, S.; Kiferle, L.; Bonuccelli, U.; Ceravolo, R. Q09 Valproic Acid for the Treatment of Aggressiveness in Huntington’s Disease: 1-Year Follow-Up. J. Neurol. Neurosurg. Psychiatry 2012, 83, A57. [Google Scholar] [CrossRef]
- Saft, C.; Lauter, T.; Kraus, P.H.; Przuntek, H.; Andrich, J.E. Dose-Dependent Improvement of Myoclonic Hyperkinesia Due to Valproic Acid in Eight Huntington’s Disease Patients: A Case Series. BMC Neurol. 2006, 6, 11. [Google Scholar] [CrossRef]
- Pearce, I.; Heathfield, K.W.G.; Pearce, J.M.S. Valproate Sodium in Huntington Chorea. Arch. Neurol. 1977, 34, 308–309. [Google Scholar] [CrossRef]
- Scholefield, M.; Patassini, S.; Xu, J.; Cooper, G.J.S. Widespread Selenium Deficiency in the Brain of Cases with Huntington’s Disease Presents a New Potential Therapeutic Target. EBioMedicine 2023, 97, 104824. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Marks, E.; Chen, J.; Moline, J.; Barrows, L.; Raisbeck, M.; Volitakis, I.; Cherny, R.A.; Chopra, V.; Bush, A.I.; et al. Altered Selenium Status in Huntington’s Disease: Neuroprotection by Selenite in the N171-82Q Mouse Model. Neurobiol. Dis. 2014, 71, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kalonia, H.; Kumar, A. Cyclosporine A Attenuates 3-Nitropropionic Acid–Induced Huntington-Like Symptoms in Rats: Possible Nitric Oxide Mechanism. Int. J. Toxicol. 2010, 29, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Jin, Y.N.; von Bernhardi, R.; Johnson, G.V. Mitochondrial Permeability Transition Pore Induces Mitochondria Injury in Huntington Disease. Mol. Neurodegener. 2013, 8, 45. [Google Scholar] [CrossRef]
- Cong, W.; Bai, R.; Li, Y.-F.; Wang, L.; Chen, C. Selenium Nanoparticles as an Efficient Nanomedicine for the Therapy of Huntington’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 34725–34735. [Google Scholar] [CrossRef]
- Koller, W.C.; Barr, A.; Biary, N. Estrogen Treatment of Dyskinetic Disorders. Neurology 1982, 32, 547. [Google Scholar] [CrossRef]
- Nuzzo, M.; Marino, M. Estrogen/Huntingtin: A Novel Pathway Involved in Neuroprotection. Neural Regen. Res. 2016, 11, 402–403. [Google Scholar] [CrossRef]
- Nilsen, J. Estradiol and Neurodegenerative Oxidative Stress. Front. Neuroendocr. 2008, 29, 463–475. [Google Scholar] [CrossRef]
- Bassani, T.B.; Bartolomeo, C.S.; Oliveira, R.B.; Ureshino, R.P. Progestogen-Mediated Neuroprotection in Central Nervous System Disorders. Neuroendocrinology 2023, 113, 14–35. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Deshmukh, R.; Sharma, P. P2–044: Neuroprotective Role of Progesterone against 3-nitropropionic-acid–Induced Huntington’s Disease-like Symptoms in Rats. Alzheimer’s Dement. 2013, 9, P360. [Google Scholar] [CrossRef]
- Theis, V.; Theiss, C. Progesterone: A Universal Stimulus for Neuronal Cells? Neural Regen. Res. 2015, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, R.; Ma, W.; Yan, J.; Li, Y.; Chen, G.; Pan, J. Pain in Huntington’s Disease and Its Potential Mechanisms. Front. Aging Neurosci. 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Roos, R.A.C.; Steenvoorden, J.M.C.; Mulder, G.J.; Van Kempen, G.M.J. Acetaminophen Sulfation in Patients with Parkinson’s Disease or Huntington’s Disease Is Not Impaired. Neurology 1993, 43, 1373. [Google Scholar] [CrossRef] [PubMed]
- Siebzehnrübl, F.A.; Raber, K.A.; Urbach, Y.K.; Schulze-Krebs, A.; Canneva, F.; Moceri, S.; Habermeyer, J.; Achoui, D.; Gupta, B.; Steindler, D.A.; et al. Early Postnatal Behavioral, Cellular, and Molecular Changes in Models of Huntington Disease Are Reversible by HDAC Inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, E8765–E8774. [Google Scholar] [CrossRef]
- Athira, K.V.; Sadanandan, P.; Chakravarty, S. Repurposing Vorinostat for the Treatment of Disorders Affecting Brain. Neuromol. Med. 2021, 23, 449–465. [Google Scholar] [CrossRef]
- Shukla, S.; Shariat-Madar, Z.; Walker, L.A.; Tekwani, B.L. Mechanism for Neurotropic Action of Vorinostat, a Pan Histone Deacetylase Inhibitor. Mol. Cell. Neurosci. 2016, 77, 11–20. [Google Scholar] [CrossRef]
- Gray, S.G. Targeting Huntington’s Disease through Histone Deacetylases. Clin. Epigenet. 2011, 2, 257–277. [Google Scholar] [CrossRef]
- Kang, J.; Park, M.; Lee, E.; Jung, J.; Kim, T. The Role of Vitamin D in Alzheimer’s Disease: A Transcriptional Regulator of Amyloidopathy and Gliopathy. Biomedicines 2022, 10, 1824. [Google Scholar] [CrossRef]
- Jeong, S.P.; Sharma, N.; An, S.S.A. Role of Calcitriol and Vitamin D Receptor (VDR) Gene Polymorphisms in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 4806. [Google Scholar] [CrossRef]
- Fryer, M.J. Vitamin E Status and Neurodegenerative Disease. Nutr. Neurosci. 1998, 1, 327–351. [Google Scholar] [CrossRef]
- Kašparová, S.; Sumbalová, Z.; Bystrický, P.; Kucharská, J.; Liptaj, T.; Mlynárik, V.; Gvozdjáková, A. Effect of Coenzyme Q10 and Vitamin E on Brain Energy Metabolism in the Animal Model of Huntington’s Disease. Neurochem. Int. 2006, 48, 93–99. [Google Scholar] [CrossRef]
- Dickey, A.S.; Pineda, V.V.; Tsunemi, T.; Liu, P.P.; Miranda, H.C.; Gilmore-Hall, S.K.; Lomas, N.; Sampat, K.R.; Buttgereit, A.; Torres, M.-J.M.; et al. PPAR-δ Is Repressed in Huntington’s Disease, Is Required for Normal Neuronal Function and Can Be Targeted Therapeutically. Nat. Med. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Cyske, Z.; Węgrzyn, G. Genistein Induces Degradation of Mutant Huntingtin in Fibroblasts from Huntington’s Disease Patients. Metab. Brain Dis. 2019, 34, 715–720. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.; Kumar, A.; Singh, T.G. Genistein: A Promising Ally in Combating Neurodegenerative Disorders. Eur. J. Pharmacol. 2025, 991, 177273. [Google Scholar] [CrossRef] [PubMed]
- Menze, E.T.; Esmat, A.; Tadros, M.G.; Abdel-Naim, A.B.; Khalifa, A.E. Genistein Improves 3-NPA-Induced Memory Impairment in Ovariectomized Rats: Impact of Its Antioxidant, Anti-Inflammatory and Acetylcholinesterase Modulatory Properties. PLoS ONE 2015, 10, e0117223. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A Focus on Several Neurodegenerative Diseases. J. Food Biochem. 2022, 46, e14155. [Google Scholar] [CrossRef] [PubMed]
- Vuu, Y.M.; Kadar Shahib, A.; Rastegar, M. The Potential Therapeutic Application of Simvastatin for Brain Complications and Mechanisms of Action. Pharmaceuticals 2023, 16, 914. [Google Scholar] [CrossRef]
- da Cruz, J.N.; Magro, D.D.D.; de Lima, D.D.; da Cruz, J.G.P. Simvastatin Treatment Reduces the Cholesterol Content of Membrane/Lipid Rafts, Implicating the N-Methyl-D-Aspartate Receptor in Anxiety: A Literature Review. Braz. J. Pharm. Sci. 2017, 53, e16102. [Google Scholar] [CrossRef]
- Maheshwari, M.; Bhutani, S.; Das, A.; Mukherjee, R.; Sharma, A.; Kino, Y.; Nukina, N.; Jana, N.R. Dexamethasone Induces Heat Shock Response and Slows down Disease Progression in Mouse and Fly Models of Huntington’s Disease. Hum. Mol. Genet. 2014, 23, 2737–2751. [Google Scholar] [CrossRef]
- Haddad, M.S.; Wenceslau, C.V.; Pompeia, C.; Kerkis, I. Cell-Based Technologies for Huntington’s Disease. Dement. Neuropsychol. 2016, 10, 287–295. [Google Scholar] [CrossRef]
- Macedo, J.; Pagani, E.; Wenceslau, C.; Ferrara, L.; Kerkis, I. A Pahse I Clinical Trial on Intravenous Administration of Immature Human Dental Pulp Stem Cells (NestaCell) to Huntington’s Disease Patients. Cytotherapy 2021, 23, 1. [Google Scholar] [CrossRef]



| Direct Analyses 1 | Indirect Analyses 2 | ||
|---|---|---|---|
| Outcomes/Adverse Effects | TBZ-Placebo | DEU-Placebo | TBZ-DEU |
| UHDRS (chorea) | −3.20 3 | −2.50 4 | −1.00 |
| UHDRS-TMS | −3.30 | −4.00 4 | 0.70 |
| Depression scale | 0.76 5 | −0.18 4 | 0.94 |
| Insomnia scale | 1.80 5 | −0.30 4 | 2.10 |
| Severe adverse effects | 5.44 | 1.00 | 5.44 |
| Drowsiness | 13.32 3 | 2.69 | 4.95 |
| Diarrhea | 0.72 | 9.87 | 0.07 |
| Insomnia | 21.84 3 | 1.54 | 14.18 |
| Fatigue | 1.86 | 1.54 | 1.21 |
| Fall | 1.30 | 0.48 | 2.71 |
| Depression | 11.15 | 0.65 | 17.15 |
| Drug | Mechanism of Action | HD-Relevant Effects | Evidence Level | References |
|---|---|---|---|---|
| Valproic acid | GABAergic activity; HDAC inhibition | Reduces aggression, improves motor symptoms (e.g., myoclonic hyperkinesia); potential mood stabilizer | Preclinical 1 & clinical 2 | [237,238,239] |
| Cyclosporine A | Calcineurin inhibition; mitochondrial protection | Attenuates mitochondrial dysfunction and oxidative stress; improves behavior in HD models | Preclinical | [240,241] |
| Selenium | Antioxidant; glutathione peroxidase cofactor | Addresses selenium deficiency in HD brains; reduces oxidative damage | Preclinical and Post-mortem Human 3 | [242,243,244] |
| Estradiol | Estrogen receptor activation; mitochondrial regulation | Reduces oxidative stress and inflammation; modulates energy metabolism | Preclinical | [245,246,247] |
| Progesterone | Neurosteroid; anti-inflammatory | Reduces oxidative stress; neuroprotective in HD models | Preclinical | [247,248,249,250] |
| Acetaminophen | COX inhibition; analgesic | Pain relief for muscle aches in early-stage HD | Clinical Use (Non-HD-specific) | [251,252] |
| Panobinostat | HDAC inhibition | Modulates gene expression; potential to reduce neurodegeneration | Preclinical | [253] |
| Vorinostat (SAHA) | HDAC inhibition | Improves neuronal survival; reduces oxidative stress | Preclinical | [254,255,256] |
| Entinostat | HDAC1/3 selective inhibitor | Putative neuroprotective, but not yet studied in HD models | Theoretical 4 | — |
| Calcitriol | Vitamin D receptor activation | Reduces oxidative stress, inflammation; modulates calcium homeostasis and mitochondria | Preclinical | [257,258] |
| Vitamin E | Antioxidant | Protects neurons from oxidative stress and lipid peroxidation | Preclinical and Clinical (limited) | [259,260] |
| Troglitazone | PPAR-γ agonist | Reduces oxidative stress; improves mitochondrial and energy metabolism | Preclinical | [261] |
| Genistein | Autophagy induction; estrogenic activity | Clears mHTT aggregates; improves motor, cognitive and behavioral outcomes | Preclinical | [262,263,264,265] |
| Simvastatin | Cholesterol modulation; anti-inflammatory | Crosses BBB; reduces oxidative stress; potential neuroprotection | Preclinical and Observational | [58,266,267] |
| Dexamethasone | Glucocorticoid receptor agonist; heat shock response | Reduces mHTT aggregation; improves behavioral phenotype | Preclinical | [268] |
| Feature | Synthetic Drugs | hDPSC-Based Treatments |
|---|---|---|
| Targets | Narrow (few genes or pathways) | Broad (multi-pathway modulation) |
| Neuroprotection | Limited | Strong (via trophic support) |
| Neuroregeneration | Absent | Potential for neuronal replacement |
| Immunomodulatory action | Indirect | Direct immunomodulatory effects |
| Disease modification | Unlikely | Promising in preclinical models and Phase I/II clinical trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araldi, R.P.; Dias Pinto, J.R.; Kerkis, I. AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington’s Disease. Brain Sci. 2025, 15, 865. https://doi.org/10.3390/brainsci15080865
Araldi RP, Dias Pinto JR, Kerkis I. AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington’s Disease. Brain Sciences. 2025; 15(8):865. https://doi.org/10.3390/brainsci15080865
Chicago/Turabian StyleAraldi, Rodrigo Pinheiro, João Rafael Dias Pinto, and Irina Kerkis. 2025. "AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington’s Disease" Brain Sciences 15, no. 8: 865. https://doi.org/10.3390/brainsci15080865
APA StyleAraldi, R. P., Dias Pinto, J. R., & Kerkis, I. (2025). AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington’s Disease. Brain Sciences, 15(8), 865. https://doi.org/10.3390/brainsci15080865





