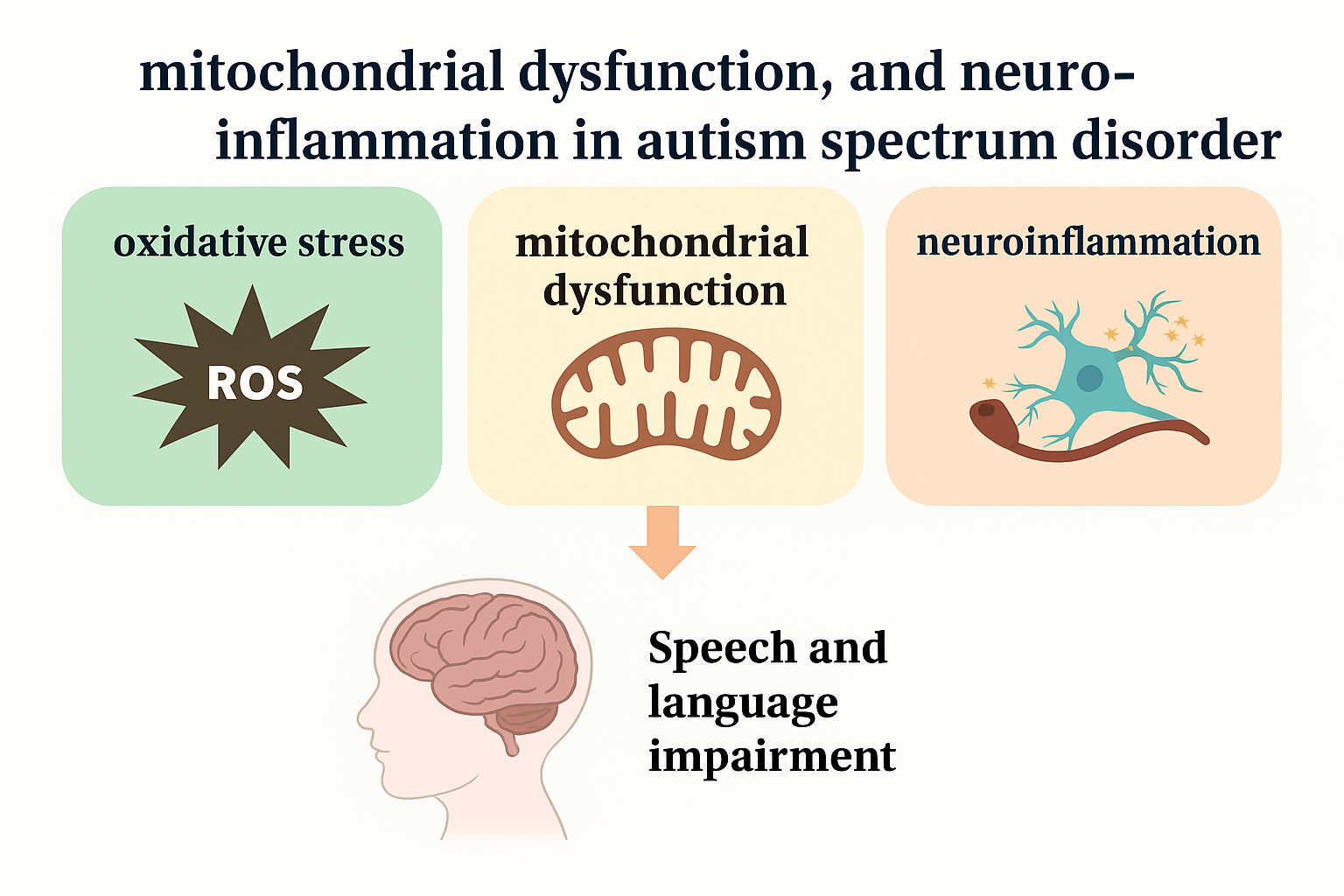
The Interplay of Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation in Autism Spectrum Disorder: Behavioral Implications and Therapeutic Strategies
Abstract
1. Introduction
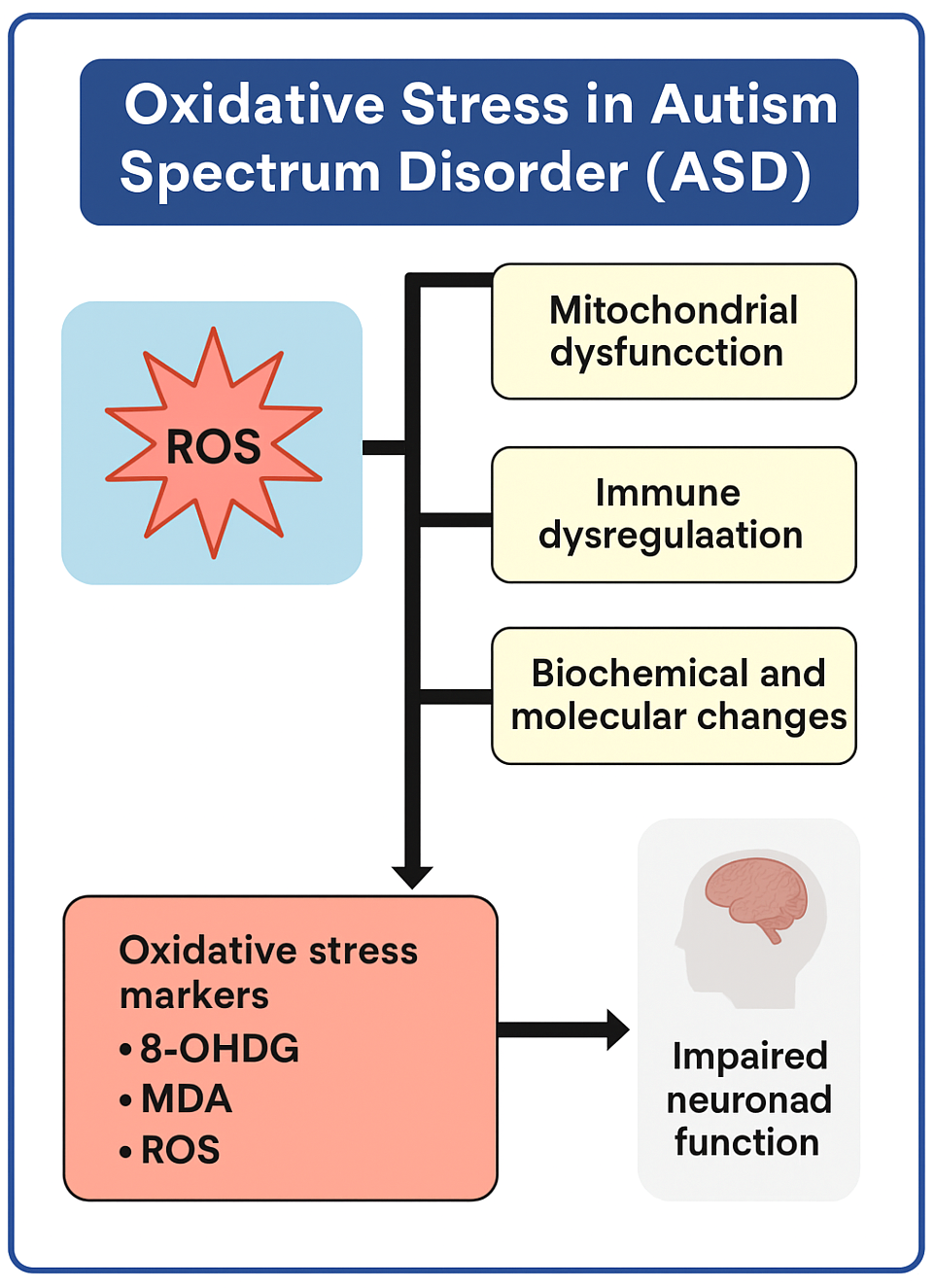
2. Oxidative Stress in Autism Spectrum Disorder
2.1. Malondialdehyde (MDA)
2.2. 8-Hydroxy-2′-deoxyguanosine (8-OHdG)
2.3. Protein Carbonyls
2.4. Glutathione (GSH/GSSH)
3. Impact of Oxidative Stress on Speech and Language Development
4. Mitochondrial Dysfunction in ASD-Related Neurological Disorders
5. Mitochondrial Dysfunction and Speech and Language Development
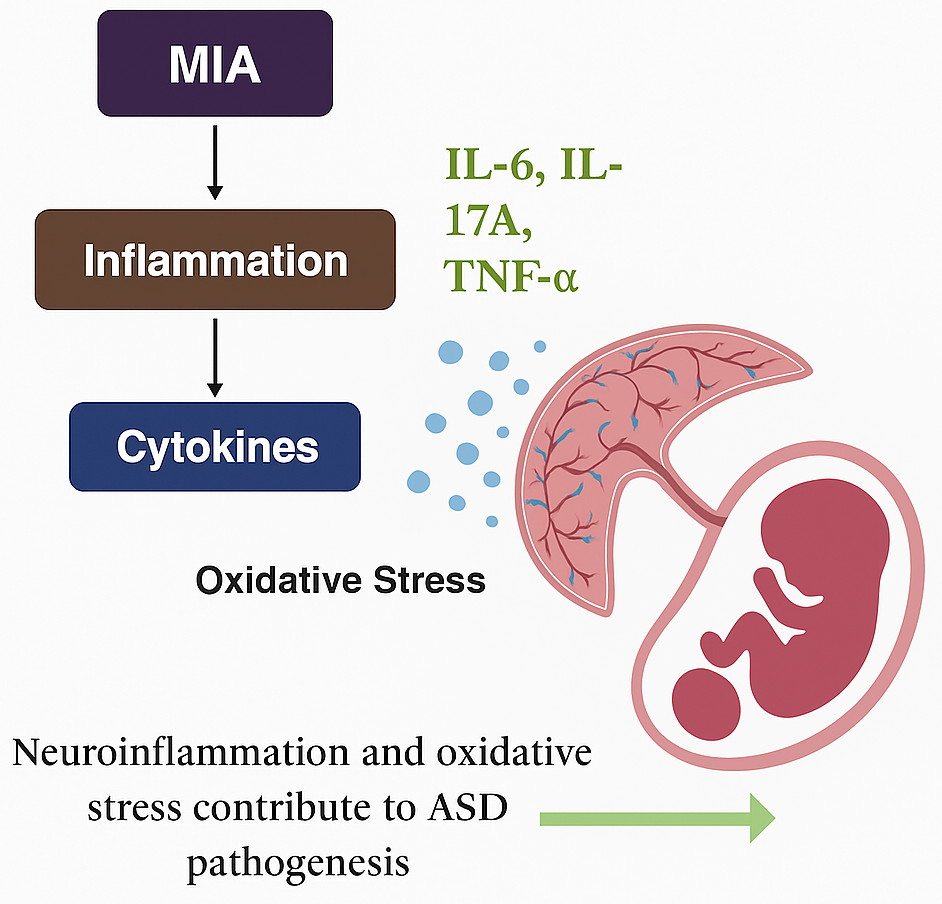
6. Neuroinflammation in Autism Spectrum Disorder
6.1. Maternal Immune Activation-Mediated Neuroinflammation
6.2. Microglial Activation
7. Antioxidant, Mitochondrial, and Anti-Inflammatory Therapy
8. Environmental Factors in Autism Spectrum Disorder
9. Gut–Brain Axis in Autism Spectrum Disorder
10. Genetics and Epigenetics in Autism Spectrum Disorder
11. Recent Improvements in the Evaluation and Management of Autism Spectrum Disorders: Diagnosis and Treatment
| Gene Function | Genetic Variant/Mutation Type | Behavioral Changes | Neurological Effects | Molecular and Cellular Alterations | Possible Treatments |
|---|---|---|---|---|---|
| Dup 15q11-q13 [88] | Chromosome 15 portion duplication | Reduced social interaction, fewer vocalizations, developmental delays | Rigid behavior patterns | Impaired serotonin signaling, enhanced spine density | Behavioral and speech therapy, no specific gene manipulation therapy |
| TBR11 [89] | Missense mutation | Reduced social interaction, altered food preference learning, intellectual disability | Rigid behavior, learning difficulties | Abnormal axonal links in amygdala, reduced NMDAR activity | Lithium chloride, D-cycloserine rescues synaptic function in Tbr1 mutant mice |
| FMR1 [90] | Trinucleotide repeat expansion | Reduced social interaction, fewer vocalizations | Repetitive movements, hypersensitivity to sound, learning issues | Disrupted neural connectivity, increased mGluR activity, and replaced synaptic plasticity | Pirenperone reduces hyperactivity in Fmr1 KO mice |
| SHANK2 [91] | Frameshift mutation | Reduced social behaviors, hyperactivity | Repetitive movements, cognitive difficulties | Impaired excitatory, synaptic dysfunction | Downstream of SHANK2 such as NMDA receptor and ERK pathway can be targeted |
| SHANK3 [92] | Frameshift mutation | Reduced social behaviors | Excessive grooming, anxiety | Insufficient striatal activation and decreased AMPAR function | IGF1 enhances long-term potentiation and motor function in SHANK-deficient mouse model |
| SCN1A [93] | Missense mutation | Reduced social behaviors | Seizures, learning deficits, grooming repetition | Reduced activity of inhibitory interneurons | Clonazepam improves neurobehavioral activities by targeting the Scn1 gene in PFC |
| CNTNAP2 [94] | Deletion | Reduced social behaviors, intellectual slowness, fewer vocalizations | Hyperactivity, repetitive grooming, seizures | Reduced interneuron population, disrupted neuronal migration | Risperidone and Oxytocin alleviate repetitive behavior in Cntnap2 −/− mice |
| TSC1, TSC2 [95] | Loss-of-function mutation | Reduced social interaction, increased vocalizations | Enhanced repetitive behaviors, coordination difficulties, cognitive impairment | Enlarged brain size, overactive mTOR signaling, impaired autophagy | Rapamycin targets mTOR (overactivated by Tsc1 and tsc2) and hence decreases ASD neuropathology |
| PTEN [96] | Frameshift mutation | Reduced social interaction | Learning impairment, seizures, anxiety | Abnormal neuronal growth, PI3K pathway overactivation | Rapamycin improves social and stereotypic behavior in Pten KO mice |
| NLGN3 [97] | Point mutation | Reduced social interaction, fewer vocalizations | Increased motor activity | Increased mTOR/Akt activation, impaired GABAergic transmission in striatal neurons, synaptic dysfunction | There are no specific drugs to target Nlgn3, but rapamycin can be tested |
| NRXN1A [98] | Exonic deletion | Reduced social behaviors, aggression | Motor learning issues, sensory processing deficits, spatial learning deficits | Lowered glutamate transmission, decreased synaptic density | No specific drugs, but extensive research is ongoing to target Nrxn1 |
| MECP2 [99] | Loss-of-function mutation | Reduced social interaction | Repetitive movements, motor deficits, seizures | Synaptic dysfunction, increased microglia activation, BDNF activation | IGF1, Clenbuterol, Fingolimod can enhance neuronal plasticity by targeting Mecp2 |
| CX3CR1 [100] | Deletion | Reduced social interaction | Impairment in learning and memory, anxiety | Impaired synaptic pruning, overactive microglia, neuroinflammation | Microglial modulators, E6130 as anti-inflammatory, and AZD8797 to inhibit CX3CR1 |
| CHD8 [101] | Loss-of-function mutation | Social deficit, repetitive behavior | Repetitive grooming, increased brain size, learning impairment | Disrupted neuronal differentiation, abnormal cortical development | Fluoxetine partially restores neurogenesis in CHD8-ablated mice |
| TCF4 [102] | Point mutation | Restlessness | Abnormal neuronal migration and excitability | Impaired neuronal plasticity, altered brain connectivity | Nicradipine improves learning, memory, and restlessness in TCF4 +/− mice |
| EIF4E KO [103] | Missense mutation | Reduced sociability | Self-grooming, contextual fear memory | Dysregulated protein synthesis, abnormal synaptic function | Mnk inhibitors dephosphorylate EIF4E to downregulate it |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- St Amant, H.G.; Schrager, S.M.; Peña-Ricardo, C.; Williams, M.E.; Vanderbilt, D.L. Language barriers impact access to services for children with autism spectrum disorders. J. Autism Dev. Disord. 2018, 48, 333–340. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.-A.; Waly, M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology 2008, 29, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative stress in autism spectrum disorder. Frye 2020, 57, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar-Pałka, A. The Role of Oxidative Stress in Autism Spectrum Disorder Pathophysiology, Diagnosis and Treatment. Biomedicines 2025, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.S.; Luca, A.-C.; Ciobica, A.; Mosca, L. Potential novel therapies for neurodevelopmental diseases targeting oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 6640206. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of oxidative stress and antioxidants in autism. Pers. Food Interv. Ther. Autism Spectr. Disord. Manag. 2020, 24, 193–206. [Google Scholar]
- Membrino, V.; Di Paolo, A.; Alia, S.; Papiri, G.; Vignini, A. The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review. Oxygen 2023, 3, 34–44. [Google Scholar] [CrossRef]
- Del Casale, A.; Ferracuti, S.; Alcibiade, A.; Simone, S.; Modesti, M.N.; Pompili, M. Neuroanatomical correlates of autism spectrum disorders: A meta-analysis of structural magnetic resonance imaging (MRI) studies. Psychiatry Res. Neuroimaging 2022, 325, 111516. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial dysfunction in autism spectrum disorder: Unique abnormalities and targeted treatments. In Seminars in Pediatric Neurology; WB Saunders: Philadelphia, PA, USA, 2020; Volume 35, p. 100829. [Google Scholar]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative stress in autism spectrum disorder—Current progress of mechanisms and biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; E Frye, R.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, 134. [Google Scholar] [CrossRef]
- Yui, K.; Sato, A.; Imataka, G. Mitochondrial dysfunction and its relationship with mTOR signaling and oxidative damage in autism spectrum disorders. Mini Rev. Med. Chem. 2015, 15, 373–389. [Google Scholar] [CrossRef] [PubMed]
- E Frye, R.; DeLaTorre, R.; Taylor, H.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl. Psychiatry 2013, 3, 273. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, I.-L.; Dirven, H.; Couderq, S.; David, A.; D’cruz, S.C.; Fernández, M.F.; Mustieles, V.; Rodríguez-Carrillo, A.; Hofer, T. Bisphenols and oxidative stress biomarkers—Associations found in human studies, evaluation of methods used, and strengths and weaknesses of the biomarkers. Int. J. Environ. Res. Public Health 2020, 17, 3609. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Chauhan, V.; Gu, F.; Chauhan, A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic. Biol. Med. 2014, 76, 25–33. [Google Scholar] [CrossRef]
- Bjørklund, G.; Tinkov, A.A.; Hosnedlová, B.; Kizek, R.; Ajsuvakova, O.P.; Chirumbolo, S.; Skalnaya, M.G.; Peana, M.; Dadar, M.; El-Ansary, A.; et al. The role of glutathione redox imbalance in autism spectrum disorder: A review. Free Radic. Biol. Med. 2020, 160, 149–162. [Google Scholar] [CrossRef]
- Bjørklund, G.; Waly, M.I.; Al-Farsi, Y.; Saad, K.; Dadar, M.; Rahman, M.M.; Elhoufey, A.; Chirumbolo, S.; Jóźwik-Pruska, J.; Kałużna-Czaplińska, J. The role of vitamins in autism spectrum disorder: What do we know? J. Mol. Neurosci. 2019, 67, 373–387. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Habib, R.; Noureen, N.; Nadeem, N. Decoding Common Features of Neurodegenerative Disorders: From Differentially Expressed Genes to Pathways. Curr. Genom. 2018, 19, 300–312. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, 647. [Google Scholar] [CrossRef]
- Castora, F.J. Mitochondrial function and abnormalities implicated in the pathogenesis of ASD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 83–108. [Google Scholar] [CrossRef]
- Costa, L.G.; Chang, Y.C.; Cole, T.B. Developmental neurotoxicity of traffic-related air pollution: Focus on autism. Curr. Environ. Health Rep. 2017, 4, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hodgson, N.W.; Trivedi, M.S.; Abdolmaleky, H.M.; Fournier, M.; Cuenod, M.; Do, K.Q.; Deth, R.C.; Bauer, J.A. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLoS ONE 2016, 11, e0146797. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Durand, T.; Galano, J.M.; Oger, C.; Leoncini, S.; Ciccoli, L.; Carone, M.; Ulivelli, M.; Manna, C.; et al. Relevance of 4-F4t-neuroprostane and 10-F4t-neuroprostane to neurological diseases. Free Radic. Biol. Med. 2018, 115, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Hollis, F.; Kanellopoulos, A.K.; Bagni, C. Kanellopoulos, and Claudia Bagni. Mitochondrial dysfunction in Autism Spectrum Disorder: Clinical features and perspectives. Curr. Opin. Neurobiol. 2017, 45, 178–187. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Du, Y.; Shi, S.; Cheng, Y. Antioxidant interventions in autism spectrum disorders: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 113, 110476. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: Alterations in the activities and protein expression of glutathione-related enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef]
- González-Fraguela, M.E.; Hung, M.-L.D.; Vera, H.; Maragoto, C.; Noris, E.; Blanco, L.; Galvizu, R.; Robinson, M. Oxidative stress markers in children with autism spectrum disorders. Br. J. Med. Med. Res. 2013, 3, 307–317. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Sasaki, H.; Shiroki, R. The role of lipid peroxidation in individuals with autism spectrum disorders. Metab. Brain Dis. 2020, 35, 1101–1108. [Google Scholar] [CrossRef]
- Zaky, E.A.; Elhameed, S.A.A.; Ismail, S.M.; Eldamer, N.M.; Abdelaziz, A.W. Analysis of urinary 8-hydroxy-2-deoxyguanosine as a biomarker of oxidative DNA damage in pediatric children with autism spectrum disorder. Res. Autism Spectr. Disord. 2023, 102, 102129. [Google Scholar] [CrossRef]
- Ming, X.; Stein, T.P.; Brimacombe, M.; Johnson, W.G.; Lambert, G.H.; Wagner, G.C. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 379–384. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Y.; Pan, J.; Yang, A.; Niu, L.; Min, J.; Meng, X.; Liao, L.; Zhang, K.; Shen, L. Redox proteomic identification of carbonylated proteins in autism plasma: Insight into oxidative stress and its related biomarkers in autism. Clin. Proteom. 2017, 14, 2. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Harutyunyan, H.; Harutyunyan, A. A certain role of SOD/CAT imbalance in pathogenesis of autism spectrum disorders. Free Radic. Biol. Med. 2018, 123, 85–95. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Xu, M.; McGinnis, W.; Koibuchi, N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum 2011, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Long, C.; Yang, L. Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. BioMed Res. Int. 2015, 2015, 810548. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef] [PubMed]
- Modafferi, S.; Lupo, G.; Tomasello, M.; Rampulla, F.; Ontario, M.; Scuto, M.; Salinaro, A.T.; Arcidiacono, A.; Anfuso, C.D.; Legmouz, M.; et al. Antioxidants, hormetic nutrition, and autism. Curr. Neuropharmacol. 2024, 22, 1156–1168. [Google Scholar] [CrossRef]
- Penna, E.; Pizzella, A.; Cimmino, F.; Trinchese, G.; Cavaliere, G.; Catapano, A.; Allocca, I.; Chun, J.T.; Campanozzi, A.; Messina, G.; et al. Neurodevelopmental disorders: Effect of high-fat diet on synaptic plasticity and mitochondrial functions. Brain Sci. 2020, 10, 805. [Google Scholar] [CrossRef]
- McKenna, K.; Prasad, S.; Cooper, J.; King, A.M.; Shahzeidi, S.; Mittal, J.; Zalta, M.; Mittal, R.; Eshraghi, A.A. Incidence of Otolaryngological Manifestations in Individuals with Autism Spectrum Disorder: A Special Focus on Auditory Disorders. Audiol. Res. 2024, 14, 35–61. [Google Scholar] [CrossRef]
- Khaliulin, I.; Hamoudi, W.; Amal, H. The multifaceted role of mitochondria in autism spectrum disorder. Mol. Psychiatry 2024, 30, 629–650. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Elwell, C.; Johnson, M.H. Mitochondrial dysfunction in autism spectrum disorders. Autism-Open Access 2016, 6, 1000190. [Google Scholar] [CrossRef]
- Oyarzábal, A.; Musokhranova, U.; Barros, L.F.; García-Cazorla, A. Energy metabolism in childhood neurodevelopmental disorders. EBioMedicine 2021, 69, 103474. [Google Scholar] [CrossRef]
- Varga, N.Á.; Pentelényi, K.; Balicza, P.; Gézsi, A.; Reményi, V.; Hársfalvi, V.; Bencsik, R.; Illés, A.; Prekop, C.; Molnár, M.J. Mitochondrial dysfunction and autism: Comprehensive genetic analyses of children with autism and mtDNA deletion. Behav. Brain Funct. 2018, 14, 4. [Google Scholar] [CrossRef]
- Kępka, A.; Ochocińska, A.; Chojnowska, S.; Borzym-Kluczyk, M.; Skorupa, E.; Knaś, M.; Waszkiewicz, N. Potential role of L-carnitine in autism spectrum disorder. J. Clin. Med. 2021, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Ren, G.; Steiner, R.D.; Merkens, L.; Roullet, J.B.; Korade, Z.; DiMuzio, P.J.; Tulenko, T.N. Elevated autophagy and mitochondrial dysfunction in the Smith–Lemli–Opitz syndrome. Mol. Genet. Metab. Rep. 2014, 1, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Alishvandi, A.; Hanaei, S. Helsmoortel-Van Der Aa Syndrome (HVDAS). In Genetic Syndromes: A Comprehensive Reference Guide; Springer Nature: Cham, Switzerland, 2025; pp. 1–6. [Google Scholar]
- Kasprzyk-Pawelec, A.; Tan, M.; Rahhal, R.; McIntosh, A.; Fernandez, H.R.; Mosaoa, R.M.; Jiang, L.; Pearson, G.W.; Glasgow, E.; Vockley, J.; et al. Inactivation of the SLC25A1 gene during embryogenesis induces a unique senescence program controlled by p53. Cell Death Differ. 2024, 32, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Feuermann, Y.; Kaphzan, H. Dynamic shift in localization of UBE3A across developmental stages in an Angelman syndrome mouse model. Neurobiol. Dis. 2025, 210, 106912. [Google Scholar] [CrossRef]
- Caron, C.; McCullagh, E.A.; Bertolin, G. Sex-specific loss of mitochondrial membrane integrity in the auditory brainstem of a mouse model of Fragile X Syndrome. Open Biol. 2025, 15, 240384. [Google Scholar] [CrossRef]
- Mietto, M.; Montanari, S.; Falzarano, M.S.; Manzati, E.; Rimessi, P.; Fabris, M.; Selvatici, R.; Gualandi, F.; Neri, M.; Fortunato, F.; et al. MECP2 mRNA Profile in Brain Tissues from a Rett Syndrome Patient and Three Human Controls: Mutated Allele Preferential Transcription and In Situ RNA Mapping. Biomolecules 2025, 15, 687. [Google Scholar] [CrossRef]
- Uwibambe, E.; Yalcouyé, A.; Aboagye, E.T.; Xhakaza, L.; Popel, K.; Dukuze, N.; Bharadwaj, T.; de Kock, C.; Schrauwen, I.; Leal, S.M.; et al. Exome sequencing revealed a novel homozygous variant in TRMT61 A in a multiplex family with atypical Cornelia de Lange Syndrome from Rwanda. BMC Med. Genom. 2025, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Giona, F.; Beretta, S.; Zippo, A.; Stefanoni, A.; Tomasoni, Z.; Vicidomini, C.; Ponzoni, L.; Sala, M.; Jones, C.K.; Conn, P.J.; et al. Shank3 modulates Rpl3 expression and protein synthesis via mGlu5: Implications for Phelan McDermid syndrome. Mol. Psychiatry 2025, 30, 3599–3614. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef]
- Rexrode, L.E.; Hartley, J.; Showmaker, K.C.; Challagundla, L.; Vandewege, M.W.; Martin, B.E.; Blair, E.; Bollavarapu, R.; Antonyraj, R.B.; Hilton, K.; et al. Molecular profiling of the hippocampus of children with autism spectrum disorder. Mol. Psychiatry 2024, 29, 1968–1979. [Google Scholar] [CrossRef]
- Hughes, H.K.; RJMoreno Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J. Psychiatr. Res. 2019, 115, 90–102. [Google Scholar] [CrossRef]
- Gilbert, J.; Man, H.Y. Fundamental elements in autism: From neurogenesis and neurite growth to synaptic plasticity. Front. Cell. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Bordt, E.A.; Polster, B.M. NADPH oxidase-and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Block, C.L.; Bolton, J.L.; Hanamsagar, R.; Tran, P.K. Beyond infection-Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018, 299, 241–251. [Google Scholar] [CrossRef]
- Di, J.; Li, J.; O’hAra, B.; Alberts, I.; Xiong, L.; Li, J.; Li, X. The role of GABAergic neural circuits in the pathogenesis of autism spectrum disorder. Int. J. Dev. Neurosci. 2020, 80, 73–85. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural antioxidants: A novel therapeutic approach to autism spectrum disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Bernardina, B.D.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, N.; Wang, X.; Zhang, J.; Li, K.; Lei, T. Epothilone B inactivation of Sirtuin1 promotes mitochondrial reactive oxygen species to induce dysfunction and ferroptosis of Schwann cells. Eur. J. Pharm. Sci. 2023, 181, 106350. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, H.Y.; Lee, J.; Seo, J. Mitochondrion-targeting peptides and peptidomimetics: Recent progress and design principles. Biochemistry 2019, 59, 270–284. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, Y.; Duan, Z.; Yi, Y.; Wang, H. Mitochondria-targeted self-assembly of peptide-based nanomaterials. Front. Bioeng. Biotechnol. 2021, 9, 782234. [Google Scholar] [CrossRef] [PubMed]
- rteaga-Henríquez, G.; Gisbert, L.; Ramos-Quiroga, J.A. Immunoregulatory and/or anti-inflammatory agents for the management of core and associated symptoms in individuals with autism spectrum disorder: A narrative review of randomized, placebo-controlled trials. CNS Drugs 2023, 37, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kisku, A.; Kungumaraj, H.; Nagaraj, V.; Pal, A.; Kumar, S.; Sulakhiya, K. Autism spectrum disorders: A recent update on targeting inflammatory pathways with natural anti-inflammatory agents. Biomedicines 2023, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H. Autism spectrum disorder and a possible role of anti-inflammatory treatments: Experience in the pediatric allergy/immunology clinic. Front. Psychiatry 2024, 15, 1333717. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Needleman, H.L. Prenatal and early postnatal exposure to lead: Developmental effects, correlates, and implications. Int. J. Ment. Health 1985, 14, 78–111. [Google Scholar] [CrossRef]
- Yu, X.; Mostafijur Rahman, M.; Carter, S.A.; Lin, J.C.; Zhuang, Z.; Chow, T.; Lurmann, F.W.; Kleeman, M.J.; Martinez, M.P.; van Donkelaar, A.; et al. Prenatal air pollution, maternal immune activation, and autism spectrum disorder. Environ. Int. 2023, 179, 108148. [Google Scholar] [CrossRef]
- Sato, A.; Kotajima-Murakami, H.; Tanaka, M.; Katoh, Y.; Ikeda, K. Influence of prenatal drug exposure, maternal inflammation, and parental aging on the development of autism spectrum disorder. Front. Psychiatry 2022, 13, 821455. [Google Scholar] [CrossRef]
- Kern, J.K.; A Geier, D.; Sykes, L.K.; Geier, M.R. Evidence of neurodegeneration in autism spectrum disorder. Transl. Neurodegener. 2013, 2, 17. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, F. Microbiota-gut-brain axis in autism spectrum disorder. J. Genet. Genom. 2021, 48, 755–762. [Google Scholar] [CrossRef]
- Młynarska, E.; Barszcz, E.; Budny, E.; Gajewska, A.; Kopeć, K.; Wasiak, J.; Rysz, J.; Franczyk, B. The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients 2025, 17, 1135. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R.; Alhawamdeh, R. Role of gastrointestinal health in managing children with autism spectrum disorder. World J. Clin. Pediatr. 2023, 12, 171. [Google Scholar] [CrossRef]
- Gonçalves, C.L.; Doifode, T.; Rezende, V.L.; Costa, M.A.; Rhoads, J.M.; Soutullo, C.A. The many faces of microbiota-gut-brain axis in autism spectrum disorder. Life Sci. 2024, 337, 122357. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Liu, G.; Kay, S.-I.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and autism spectrum disorder: Is there a correlation? Front. Cell. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef]
- Oztenekecioglu, B.; Mavis, M.; Osum, M.; Kalkan, R. Genetic and epigenetic alterations in autism spectrum disorder. Glob. Med. Genet. 2021, 8, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Magielski, J.H.; Ruggiero, S.M.; Xian, J.; Parthasarathy, S.; Galer, P.D.; Ganesan, S.; Back, A.; McKee, J.L.; McSalley, I.; Gonzalez, A.K.; et al. The clinical and genetic spectrum of paediatric speech and language disorders. Brain 2025, 148, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhu, F.; Chen, K.; Zhang, Y. Genetic analysis of a child with severe intellectual disability caused by a novel variant in the FERM domain of the FRMPD4 protein. J. Genet. 2024, 103, 14. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.J.; Schreiner, J.D.; Bloom, J.E.; James, J.; Baker, D.S.; Robinson, T.M.; Lieberman, R.; Loew, L.M.; Chamberlain, S.J.; Levine, E.S. Hyperexcitable phenotypes in induced pluripotent stem cell–derived neurons from patients with 15q11-q13 duplication syndrome, a genetic form of autism. Biol. Psychiatry 2021, 90, 756–765. [Google Scholar] [CrossRef]
- Hsu, T.-T.; Wang, C.-Y.; Hsueh, P.-P. Tbr1 autism mouse model displays altered structural and functional amygdalar connectivity and abnormal whole-brain synchronization. bioRxiv 2023, 14, 548970. [Google Scholar]
- Kim, Y.; Jeon, S.J.; Gonzales, E.L.; Shin, D.; Remonde, C.G.; Ahn, T.; Shin, C.Y. Pirenperone relieves the symptoms of fragile X syndrome in Fmr1 knockout mice. Sci. Rep. 2022, 12, 20966. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.K.; Pérez Arévalo, A.; Ioannidis, V.; Stirmlinger, N.; Demestre, M.; Delorme, R.; Bourgeron, T.; Boeckers, T.M. SHANK2 mutations result in dysregulation of the ERK1/2 pathway in human induced pluripotent stem cells-derived neurons and Shank2 (−/−) mice. Front. Mol. Neurosci. 2021, 14, 773571. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, L.; Yang, Y.; Zhou, M.; Xu, S.; Zhang, W.; Zhang, C. Insulin-like growth factor 1 has the potential to be used as a diagnostic tool and treatment target for autism spectrum disorders. Cureus 2024, 16, e65393. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Zhong, Z.; Chen, H.; Ding, W.; Hoi, M.P.M. Clonazepam attenuates neurobehavioral abnormalities in offspring exposed to maternal immune activation by enhancing GABAergic neurotransmission. Biochem. Pharmacol. 2021, 192, 114711. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, F.; Lu, R.; Xing, X.; Xu, L.; Wu, K.; Gong, Z.; Zhang, Q.; Zhang, Y.; Xing, M.; et al. CNTNAP2 intracellular domain (CICD) generated by γ-secretase cleavage improves autism-related behaviors. Signal Transduct. Target. Ther. 2023, 8, 219. [Google Scholar] [CrossRef]
- Bassetti, D.; Luhmann, H.J.; Kirischuk, S. Effects of mutations in TSC genes on neurodevelopment and synaptic transmission. Int. J. Mol. Sci. 2021, 22, 7273. [Google Scholar] [CrossRef]
- Narvaiz, D.A.; Nolan, S.O.; Smith, G.D.; Holley, A.J.; Reynolds, C.D.; Blandin, K.J.; Nguyen, P.H.; Tran, D.L.K.; Lugo, J.N. Rapamycin improves social and stereotypic behavior abnormalities induced by pre-mitotic neuronal subset specific Pten deletion. Genes Brain Behav. 2023, 22, 12854. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Shi, X.; Zhang, X.-X.; Yang, F.-F.; Li, K.-R.; Fang, Q.; Cao, C.; Chen, X.-H.; Peng, Y. Neuron-secreted NLGN3 ameliorates ischemic brain injury via activating Gαi1/3-Akt signaling. Cell Death Dis. 2023, 14, 700. [Google Scholar] [CrossRef]
- Tromp, A.; Mowry, B.; Giacomotto, J. Neurexins in autism and schizophrenia—A review of patient mutations, mouse models and potential future directions. Mol. Psychiatry 2021, 26, 747–760. [Google Scholar] [CrossRef]
- Fuchs, C.; ‘t Hoen, P.A.C.; Müller, A.R.; Ehrhart, F.; Van Karnebeek, C.D.M. Drug repurposing in Rett and Rett-like syndromes: A promising yet underrated opportunity? Front. Med. 2024, 11, 1425038. [Google Scholar] [CrossRef]
- Huang, J.M.; Zhao, N.; Hao, X.N.; Li, S.Y.; Wei, D.; Pu, N.; Peng, G.H.; Tao, Y. CX3CL1/CX3CR1 signaling mediated neuroglia activation is implicated in the retinal degeneration: A potential therapeutic target to prevent photoreceptor death. Investig. Ophthalmol. Vis. Sci. 2024, 65, 29. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, C.; Chen, X.; Berry, K.; Wang, J.; Zhang, F.; Liao, Y.; Han, R.; Ogurek, S.; Xu, L.; et al. Conserved and distinct functions of the autism-related chromatin remodeler CHD8 in embryonic and adult forebrain neurogenesis. J. Neurosci. 2022, 42, 8373–8392. [Google Scholar] [CrossRef] [PubMed]
- Somorai, M.A.; Ekins, S.E.; Rupprecht, C.; Lettl, C.; Mall, V. Repurposing nicardipine leads to improved development in a young patient with Pitt-Hopkins syndrome. Front. Pharmacol. 2025, 16, 1592011. [Google Scholar] [CrossRef]
- Xu, W.; Kannan, S.; Verma, C.S.; Nacro, K. Update on the development of MNK inhibitors as therapeutic agents. J. Med. Chem. 2021, 65, 983–1007. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Keil-Stietz, K.; Lein, P.J. Gene× environment interactions in autism spectrum disorders. Curr. Top. Dev. Biol. 2023, 152, 221–284. [Google Scholar]
- Li, X.; Zhang, K.; He, X.; Zhou, J.; Jin, C.; Shen, L.; Gao, Y.; Tian, M.; Zhang, H. Structural, functional, and molecular imaging of autism spectrum disorder. Neurosci. Bull. 2021, 37, 1051–1071. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Senan, E.M.; Rassem, T.H.; Ali, M.A.H.; Shatnawi, H.S.A.; Alwazer, S.M.; Alshahrani, M. Eye tracking-based diagnosis and early detection of autism spectrum disorder using machine learning and deep learning techniques. Electronics 2022, 11, 530. [Google Scholar] [CrossRef]
- Du, G.; Guo, Y.; Xu, W. The effectiveness of applied behavior analysis program training on enhancing autistic children’s emotional-social skills. BMC Psychol. 2024, 12, 568. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Sahraeizadeh, A.; Berk, M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 2014, 45, 185–192. [Google Scholar] [CrossRef]
- Smith, J.R.; DiSalvo, M.; Green, A.; Ceranoglu, T.A.; Anteraper, S.A.; Croarkin, P.; Joshi, G. Treatment response of transcranial magnetic stimulation in intellectually capable youth and young adults with autism spectrum disorder: A systematic review and meta-analysis. Neuropsychol. Rev. 2023, 33, 834–855. [Google Scholar] [CrossRef]
- Hartman, R.E.; Patel, D. Dietary approaches to the management of autism spectrum disorders. Pers. Food Interv. Ther. Autism Spectr. Disord. Manag. 2020, 24, 547–571. [Google Scholar]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef]
- Wang, P.; Mokhtari, R.; Pedrosa, E.; Kirschenbaum, M.; Bayrak, C.; Zheng, D.; Lachman, H.M. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol. Autism 2017, 8, 11. [Google Scholar] [CrossRef]
| Syndrome | Mitochondrial Process Affected | Energy Metabolism Impact | Sample Source | Gene/Protein Involvement | Mitochondrial Impact | Neurological and Developmental Effects |
|---|---|---|---|---|---|---|
| Smith–Lemli–Opitz Syndrome (SLOS) [47] | Mitochondrial function and cholesterol metabolism | Disturbance of cholesterol processing, energy dysregulation | Fibroblasts from affected individuals | DHCR7 | Accumulation of mitochondria dysfunctional substances | Developmental delay, weak muscle tone, behavioral differences |
| Helsmoortel–Van der Aa Syndrome (HVDAS) [48] | Cellular respiration efficiency | Deficiency of energy to operate cells | Fibroblasts from affected individuals | ADNP | Suppressed mitochondrial oxygen utilization, decreased production of ATP | Autism-like characteristics, retarded brain development |
| DiGeorge Syndrome (DGS) [49] | Mitochondrial transport and ion balance | Faulty mitochondrial integrity, increased oxidative load | Fibroblasts from patients and mouse models | SLC25A1, SLC25A4 | Disruptions in mitochondrial carrier proteins, disturbances in calcium homeostasis | Deficits in learning, increased risk of psychiatric diseases such as schizophrenia |
| Angelman Syndrome (AS) [50] | Gene regulation and electron transport | Low levels of ATP production, mitochondrial shortages | Fibroblasts and hippocampal cells from UBE3A mutant mice | UBE3A | ETC complex III dysfunction, irregular expression of mitochondrial genes | Serious lag of mental development, epilepsy, movement disorders |
| Fragile X Syndrome (FXS) [51] | Mitochondrial structure and dynamics | A loss in cellular energy and oxidative stress | Brain tissue from FMR1 knockout mice | FMR1, MFN1, MFN2, OPA1 | Loss of fusion protein levels, abridged ATP production, and lesser action in ETC complexes I and II | Impaired cognitive abilities, disrupted synapses in neurons, neurodegeneration |
| Rett Syndrome (RS) [52] | Mitochondrial structure and oxidative balance | More oxidative stress, poor energy consumption | Brain tissue from MECP2 knockout mice | MECP2 | Mitochondria having aberrant morphology, long length of mitochondria | Motor impairment, intellectual disability, the likelihood of a seizure |
| Cornelia de Lange Syndrome (CdLS) [53] | Mitochondrial protein synthesis | Deficient mitochondrial protein synthesis | Skin fibroblasts from ASD patient | TRMT61A, MRPS22 | Ribosomal mitochondrial defects and compromised complexes of ETC I, III, and IV | Height deficiencies, intellectual disabilities, unusual facial appearance |
| Phelan–McDermid Syndrome (PMS) [54] | Electron transport chain performance | Reduced supply of ATP, harm by ROS | Oral samples from PMS patients | SHANK3 | Nonfunctioning complexes I and IV, and imbalance in the energy schedule of mitochondria | Learning disorders, deficit in neuronal signaling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, A.; Rahaman, S.B. The Interplay of Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation in Autism Spectrum Disorder: Behavioral Implications and Therapeutic Strategies. Brain Sci. 2025, 15, 853. https://doi.org/10.3390/brainsci15080853
Akhtar A, Rahaman SB. The Interplay of Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation in Autism Spectrum Disorder: Behavioral Implications and Therapeutic Strategies. Brain Sciences. 2025; 15(8):853. https://doi.org/10.3390/brainsci15080853
Chicago/Turabian StyleAkhtar, Ansab, and SK Batin Rahaman. 2025. "The Interplay of Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation in Autism Spectrum Disorder: Behavioral Implications and Therapeutic Strategies" Brain Sciences 15, no. 8: 853. https://doi.org/10.3390/brainsci15080853
APA StyleAkhtar, A., & Rahaman, S. B. (2025). The Interplay of Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation in Autism Spectrum Disorder: Behavioral Implications and Therapeutic Strategies. Brain Sciences, 15(8), 853. https://doi.org/10.3390/brainsci15080853







