The Crucial Interplay Between the Lungs, Brain, and Heart to Understand Epilepsy-Linked SUDEP: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
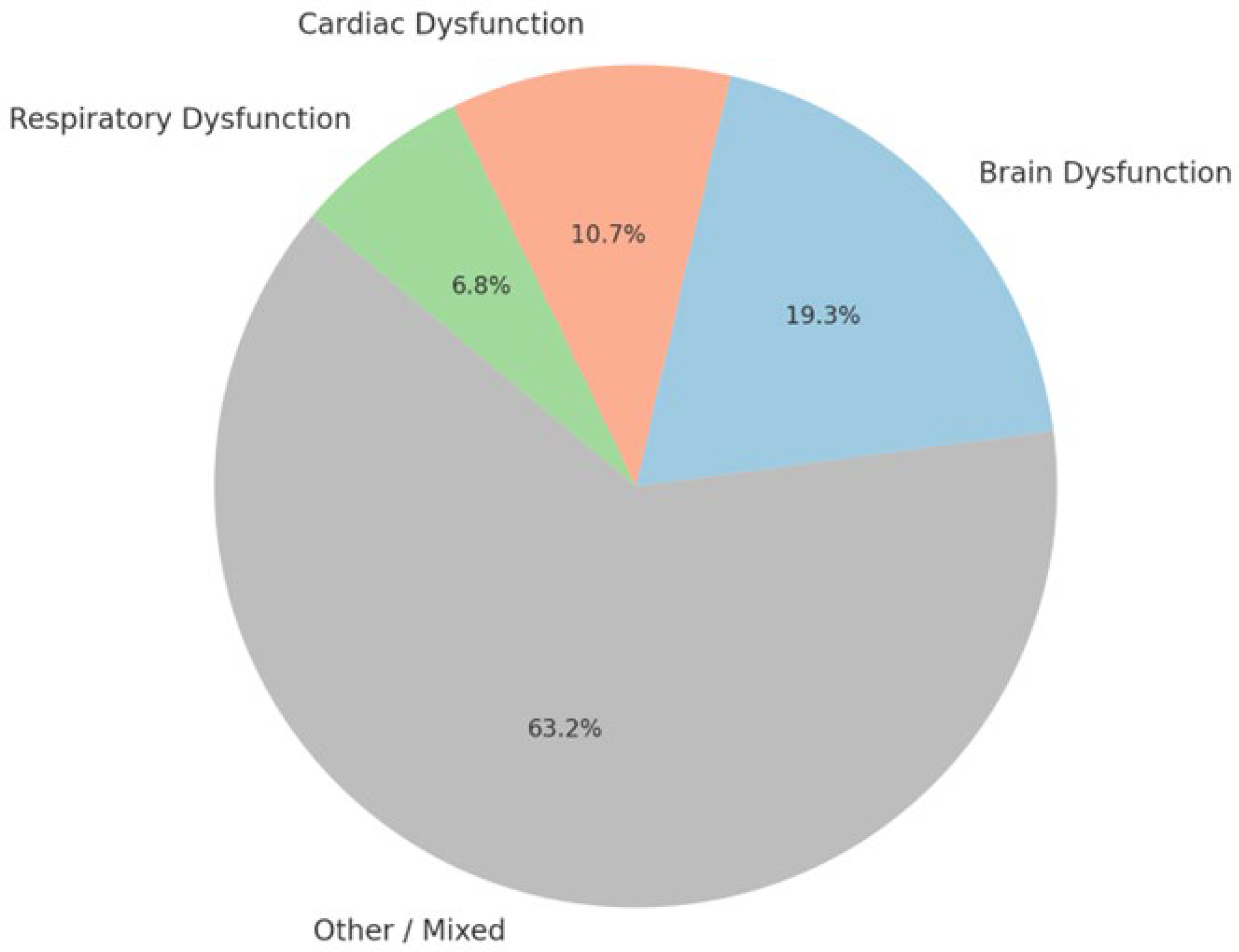
3. Results
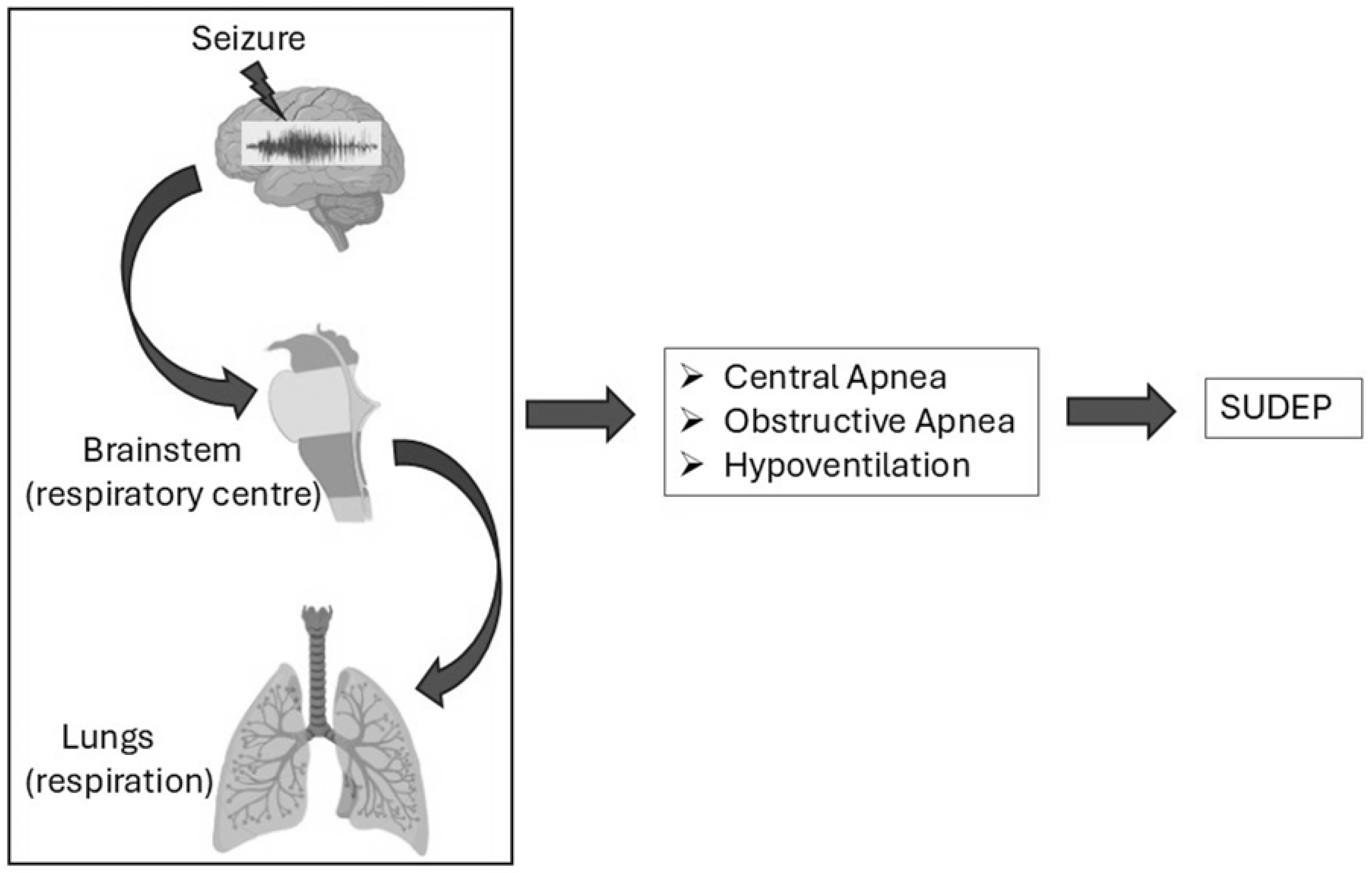
3.1. Respiratory Dysfunction in Epilepsy-Related Sudden Deaths
3.1.1. Central Apnea
3.1.2. Obstructive Apnea
3.1.3. Hyperventilation
3.1.4. Hypoventilation
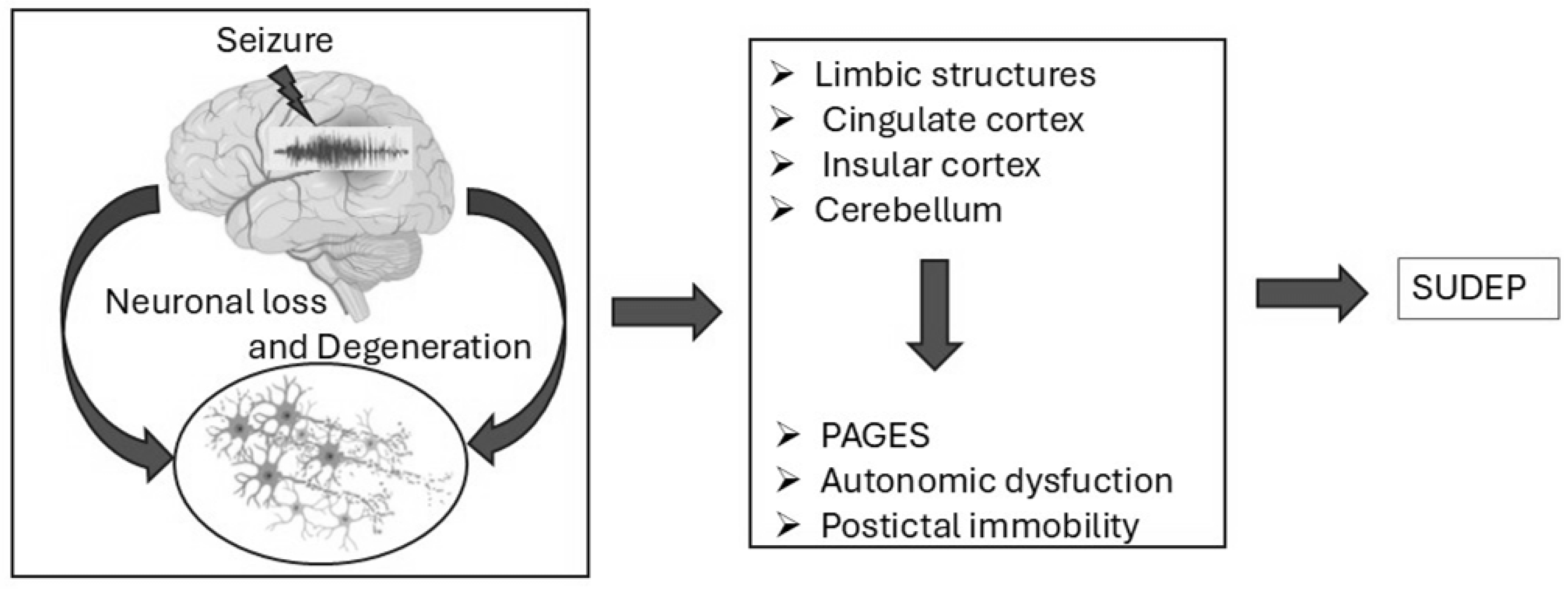
3.2. Brain Dysfunction in Epilepsy-Related Sudden Deaths
3.2.1. Neurotransmitter Imbalances
3.2.2. Neurodegeneration and Brain Damage
3.2.3. Genetic Mutations and Aberrant Neurogenesis
3.2.4. Role of Adenosine and Purinergic Signaling
3.2.5. Mossy Fiber Sprouting, Dynorphin, and CDKL5 Deficiency
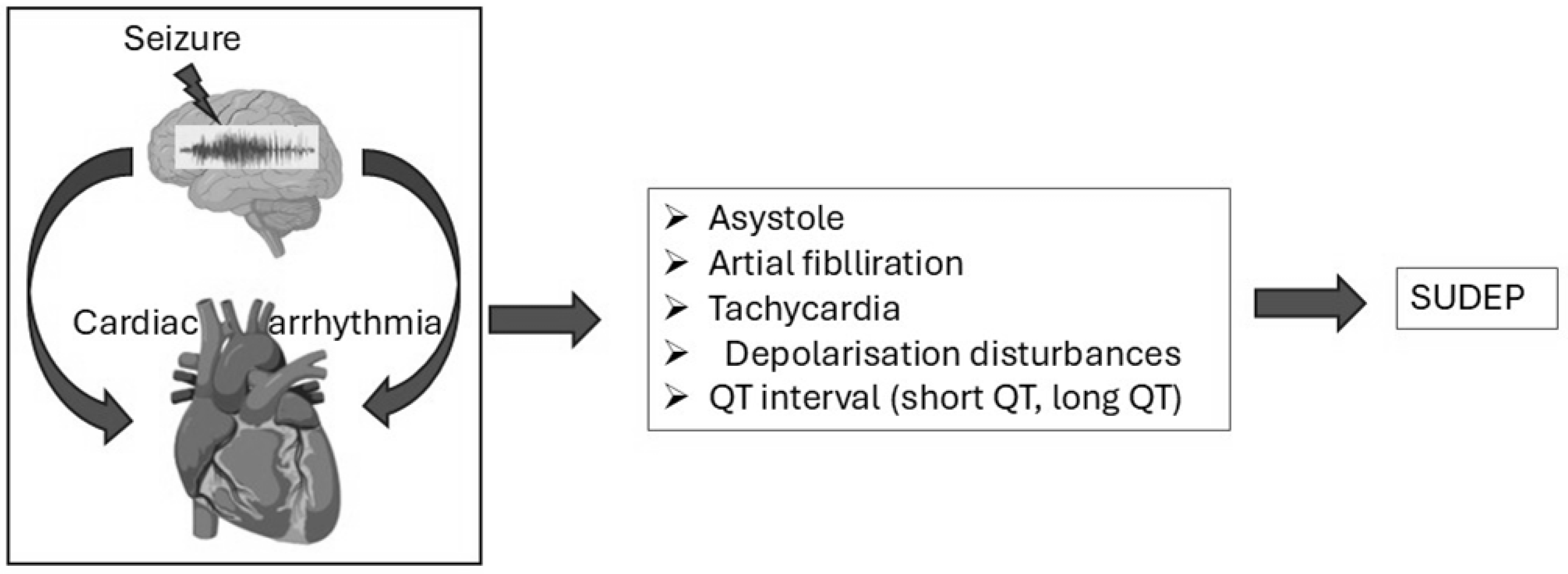
3.3. Cardiac Dysfunction in Epilepsy-Related Sudden Deaths
3.3.1. Autonomic Nervous System Dysregulation and SUDEP Risk
3.3.2. Genetic Contributions to Autonomic Dysregulation
3.3.3. Monitoring and Biomarkers of ANS Dysregulation
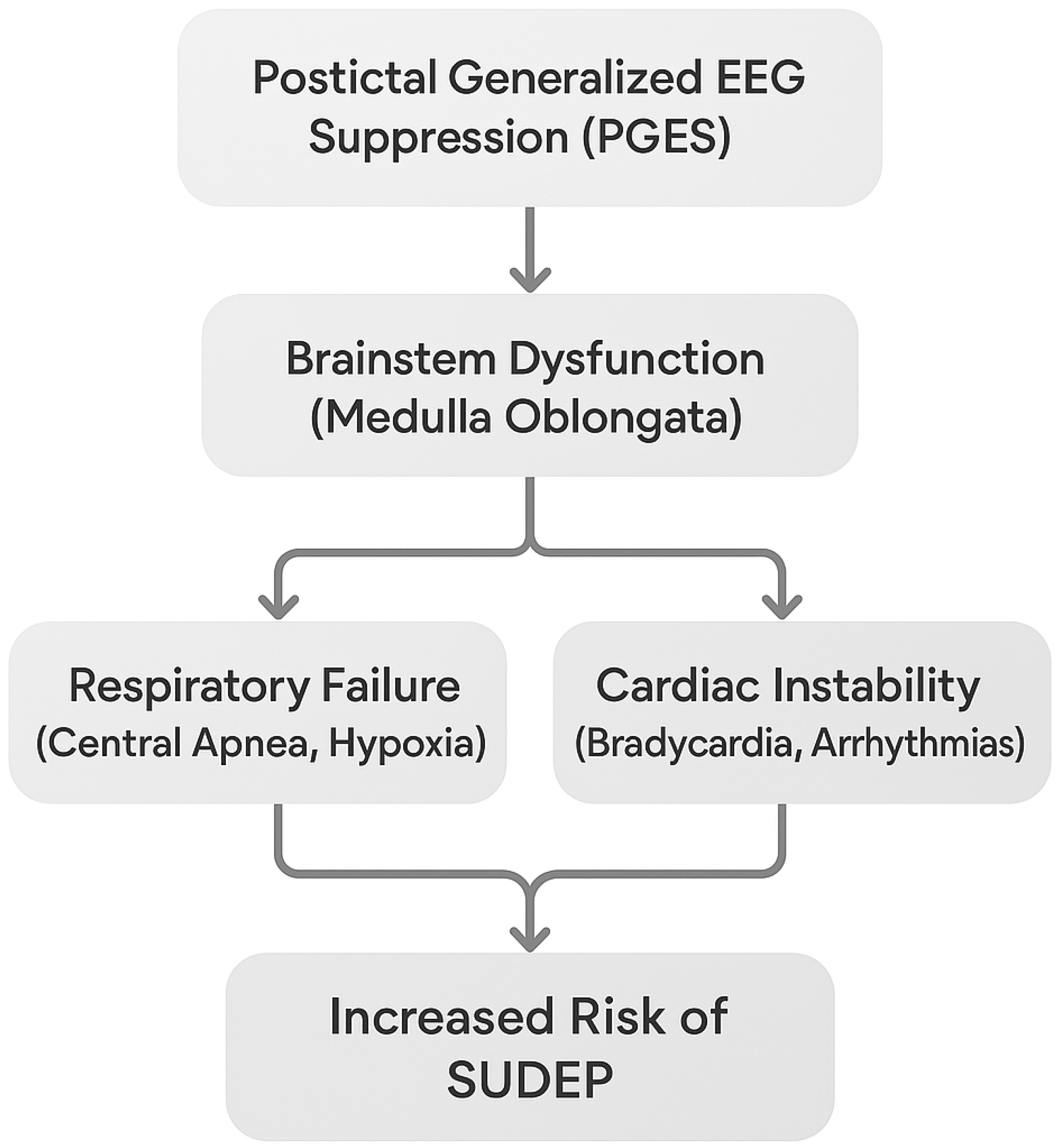
3.3.4. Postictal Cardiac Dysfunction and the Role of Continuous Monitoring
Postictal Phase and Cardiac Health
Importance of Postictal Monitoring
Emerging Role of Wearable Monitoring Devices
3.3.5. Mechanisms Linking Respiratory and Cardiac Dysfunction in SUDEP
Overlap Between Respiratory and Cardiac Dysfunction
Neurotransmitter Release and Autonomic Imbalance
Importance of Multimodal Monitoring
4. Discussion
5. Conclusions
6. Limitations and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SUDEP | Sudden Unexpected Death in Epilepsy |
| EEG | Electroencephalography |
| GTCS | Generalized Tonic–Clonic Seizures |
| ANS | Autonomic Nervous System |
| PGES | Postictal Generalized EEG Suppression |
| RLN | Recurrent Laryngeal Nerve |
| HVR | Hypoxic Ventilatory Response |
| TLE | Temporal Lobe Epilepsy |
| DEE | Developmental and Epileptic Encephalopathy |
| GABA | Gamma-Aminobutyric Acid |
| VPA | Valproic Acid |
| VGB | Vigabatrin |
| TGB | Tiagabine |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| SIDS | Sudden Infant Death Syndrome |
| 5-HT | Serotonin (5-Hydroxytryptamine) |
| Sxc | System x-c (Cystine-Glutamate Antiporter) |
| SAS | Sulfasalazine |
| A1R | Adenosine A1 Receptor |
| A2AR | Adenosine A2A Receptor |
| MTLE | Mesial Temporal Lobe Epilepsy |
| SE | Status Epilepticus |
| FBCTS | Focal to Bilateral Tonic–Clonic Seizures |
| CDKL5 | Cyclin-Dependent Kinase-Like 5 |
| KD | Ketogenic Diet |
| HRV | Heart Rate Variability |
References
- Devinsky, O.; Bundock, E.; Hesdorffer, D.; Donner, E.; Moseley, B.; Cihan, E.; Hussain, F.; Friedman, D. Resolving Ambiguities in SUDEP Classification. Epilepsia 2018, 59, 1220–1233. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shinnar, S. Long-Term Mortality in Childhood-Onset Epilepsy. N. Engl. J. Med. 2010, 363, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Sveinsson, O.; Andersson, T.; Carlsson, S.; Tomson, T. The Incidence of SUDEP: A Nationwide Population-Based Cohort Study. Neurology 2017, 89, 170–177. [Google Scholar] [CrossRef]
- Nashef, L.; Walker, F.; Allen, P.; Sander, J.W.; Shorvon, S.D.; Fish, D.R. Apnoea and Bradycardia During Epileptic Seizures: Relation to Sudden Death in Epilepsy. J. Neurol. Neurosurg. Psychiatry 1996, 60, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.C.; Tomson, T.; Benn, E.; Sander, J.W.; Nilsson, L.; Langan, Y.; Walczak, T.S.; Beghi, E.; Brodie, M.J.; Hauser, A.; et al. Combined Analysis of Risk Factors for SUDEP: Combined SUDEP Analysis. Epilepsia 2011, 52, 1150–1159. [Google Scholar] [CrossRef]
- Appleton, R.E. Mortality in Paediatric Epilepsy. Arch. Dis. Child. 2003, 88, 1091–1094. [Google Scholar] [CrossRef]
- Sveinsson, O.; Andersson, T.; Mattsson, P.; Carlsson, S.; Tomson, T. Clinical Risk Factors in SUDEP: A Nationwide Population-Based Case-Control Study. Neurology 2020, 94, e419–e429. [Google Scholar] [CrossRef]
- Harden, C.; Tomson, T.; Gloss, D.; Buchhalter, J.; Cross, J.H.; Donner, E.; French, J.A.; Gil-Nagel, A.; Hesdorffer, D.C.; Smithson, W.H.; et al. Practice Guideline Summary: Sudden Unexpected Death in Epilepsy Incidence Rates and Risk Factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2017, 88, 1674–1680. [Google Scholar] [CrossRef]
- Saxena, A.; Jones, L.; Shankar, R.; McLean, B.; Newman, C.G.; Hamandi, K. Sudden Unexpected Death in Epilepsy in Children: A Focused Review of Incidence and Risk Factors. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1064–1070. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Salloum, M.N.; Alahdab, F.; Gottwald, J.A.; Tester, D.J.; Anwer, L.A.; So, E.L.; Murad, M.H.; St Louis, E.K.; Ackerman, M.J.; et al. Systematic Review of the Genetics of Sudden Unexpected Death in Epilepsy: Potential Overlap With Sudden Cardiac Death and Arrhythmia-Related Genes. J. Am. Heart Assoc. 2020, 9, e012264. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Nashef, L.; Lhatoo, S.D.; Bateman, L.M.; Bird, J.; Bleasel, A.; Boon, P.; Crespel, A.; Dworetzky, B.A.; Høgenhaven, H. Incidence and Mechanisms of Cardiorespiratory Arrests in Epilepsy Monitoring Units (MORTEMUS): A Retrospective Study. Lancet Neurol. 2013, 12, 966–977. [Google Scholar] [CrossRef]
- Michalak, Z.; Obari, D.; Ellis, M.; Thom, M.; Sisodiya, S.M. Neuropathology of SUDEP: Role of Inflammation, Blood-Brain Barrier Impairment, and Hypoxia. Neurology 2017, 88, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Amendola, E.; Zhan, Y.; Mattucci, C.; Castroflorio, E.; Calcagno, E.; Fuchs, C.; Lonetti, G.; Silingardi, D.; Vyssotski, A.L.; Farley, D.; et al. Mapping Pathological Phenotypes in a Mouse Model of CDKL5 Disorder. PLoS ONE 2014, 9, e91613. [Google Scholar] [CrossRef]
- Okuda, K.; Kobayashi, S.; Fukaya, M.; Watanabe, A.; Murakami, T.; Hagiwara, M.; Sato, T.; Ueno, H.; Ogonuki, N.; Komano-Inoue, S. CDKL5 Controls Postsynaptic Localization of GluN2B-Containing NMDA Receptors in the Hippocampus and Regulates Seizure Susceptibility. Neurobiol. Dis. 2017, 106, 158–170. [Google Scholar] [CrossRef]
- Yennawar, M.; White, R.S.; Jensen, F.E. AMPA Receptor Dysregulation and Therapeutic Interventions in a Mouse Model of CDKL5 Deficiency Disorder. J. Neurosci. 2019, 39, 4814–4828. [Google Scholar] [CrossRef]
- Tang, S.; Terzic, B.; Wang, I.-T.J.; Sarmiento, N.; Sizov, K.; Cui, Y.; Takano, H.; Marsh, E.D.; Zhou, Z.; Coulter, D.A. Altered NMDAR Signaling Underlies Autistic-like Features in Mouse Models of CDKL5 Deficiency Disorder. Nat. Commun. 2019, 10, 2655. [Google Scholar] [CrossRef]
- Koubeissi, M.Z.; Abou-Khalil, B. Autonomic and Cardiac Risk Factors for SUDEP. Clin. Neurophysiol. 2020, 131, 1351–1360. [Google Scholar]
- Glasscock, E. Cardiac Dysfunction in Epilepsy: From Cellular Mechanisms to Clinical Risk. Epilepsia 2021, 62, 234–248. [Google Scholar]
- Bagnall, R.D.; Crompton, D.E.; Petrovski, S.; Lam, L.; Cutmore, C.; Garry, S.I.; Sadleir, L.G.; Dibbens, L.M.; Cairns, A.; Kivity, S.; et al. Exome-Based Analysis of Cardiac Arrhythmia, Respiratory Control, and Epilepsy Genes in Sudden Unexpected Death in Epilepsy. Ann. Neurol. 2016, 79, 522–534. [Google Scholar] [CrossRef]
- Massey, C.A.; Sowers, L.P.; Dlouhy, B.J.; Richerson, G.B. Mechanisms of Sudden Unexpected Death in Epilepsy: The Pathway to Prevention. Nat. Rev. Neurol. 2014, 10, 271–282. [Google Scholar] [CrossRef]
- Bozorgi, A.; Jafari, A.; Massey, C.A. Ictal and Postictal Arrhythmias in Epilepsy and SUDEP Risk. Epilepsy Res. 2021, 172, 106577. [Google Scholar]
- Surges, R.; Sander, J.W.; Thijs, R.D.; Tan, H.L.; Sander, J.W. Sudden Unexpected Death in Epilepsy: Risk Factors and Potential Pathomechanisms. Nat. Rev. Neurol. 2009, 5, 492–504. [Google Scholar] [CrossRef]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden Unexpected Death in Epilepsy: Epidemiology, Mechanisms, and Prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Lacuey, N.; Zonjy, B.; Hampson, J.P.; Rani, M.R.S.; Zaremba, A.; Sainju, R.K.; Gehlbach, B.K.; Schuele, S.; Friedman, D.; Devinsky, O.; et al. The Incidence and Significance of Periictal Apnea in Epileptic Seizures. Epilepsia 2018, 59, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.M.; Li, C.-S.; Seyal, M. Ictal Hypoxemia in Localization-Related Epilepsy: Analysis of Incidence, Severity and Risk Factors. Brain 2008, 131, 3239–3245. [Google Scholar] [CrossRef]
- Mulkey, D.K.; Milla, B.M. Perspectives on the Basis of Seizure-Induced Respiratory Dysfunction. Front. Neural Circuits 2022, 16, 1033756. [Google Scholar] [CrossRef]
- Smith, J.C.; Ellenberger, H.H.; Ballanyi, K.; Richter, D.W.; Feldman, J.L. Pre-Bötzinger Complex: A Brainstem Region That May Generate Respiratory Rhythm in Mammals. Science 1991, 254, 726–729. [Google Scholar] [CrossRef]
- Nobis, W.P.; González Otárula, K.A.; Templer, J.W.; Gerard, E.E.; VanHaerents, S.; Lane, G.; Zhou, G.; Rosenow, J.M.; Zelano, C.; Schuele, S. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. J. Neurosurg. 2019, 132, 1313–1323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villiere, S.M.; Nakase, K.; Kollmar, R.; Silverman, J.; Sundaram, K.; Stewart, M. Seizure-Associated Central Apnea in a Rat Model: Evidence for Resetting the Respiratory Rhythm and Activation of the Diving Reflex. Neurobiol. Dis. 2017, 101, 8–15. [Google Scholar] [CrossRef]
- Richter, D.W.; Smith, J.C. Respiratory Rhythm Generation In Vivo. Physiology 2014, 29, 58–71. [Google Scholar] [CrossRef]
- Affleck, V.S.; Coote, J.H.; Pyner, S. The Projection and Synaptic Organisation of NTS Afferent Connections with Presympathetic Neurons, GABA and nNOS Neurons in the Paraventricular Nucleus of the Hypothalamus. Neuroscience 2012, 219, 48–61. [Google Scholar] [CrossRef]
- Browning, K.N.; Travagli, R.A. Plasticity of Vagal Brainstem Circuits in the Control of Gastric Function: Receptor Trafficking in the DVC. Neurogastroenterol. Motil. 2010, 22, 1154–1163. [Google Scholar] [CrossRef]
- Derera, I.D.; Delisle, B.P.; Smith, B.N. Functional Neuroplasticity in the Nucleus Tractus Solitarius and Increased Risk of Sudden Death in Mice with Acquired Temporal Lobe Epilepsy. eNeuro 2017, 4, ENEURO.0319-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.W.; Hermes, S.M.; Whittier, K.L.; Aicher, S.A.; Andresen, M.C. A-Type Potassium Channels Differentially Tune Afferent Pathways from Rat Solitary Tract Nucleus to Caudal Ventrolateral Medulla or Paraventricular Hypothalamus. J. Physiol. 2007, 582, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Strube, C.; Saliba, L.; Moubarak, E.; Penalba, V.; Martin-Eauclaire, M.-F.; Tell, F.; Clerc, N. Kv4 Channels Underlie A-Currents with Highly Variable Inactivation Time Courses but Homogeneous Other Gating Properties in the Nucleus Tractus Solitarii. Pflugers Arch. 2015, 467, 789–803. [Google Scholar] [CrossRef]
- Villa, C.; Combi, R. Potassium Channels and Human Epileptic Phenotypes: An Updated Overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef]
- Lee, H.; Lin, M.A.; Kornblum, H.I.; Papazian, D.M.; Nelson, S.F. Exome Sequencing Identifies de Novo Gain of Function Missense Mutation in KCND2 in Identical Twins with Autism and Seizures That Slows Potassium Channel Inactivation. Hum. Mol. Genet. 2014, 23, 3481–3489. [Google Scholar] [CrossRef]
- Su, T.; Cong, W.D.; Long, Y.S.; Luo, A.H.; Sun, W.W.; Deng, W.Y.; Liao, W.P. Altered Expression of Voltage-Gated Potassium Channel 4.2 and Voltage-Gated Potassium Channel 4-Interacting Protein, and Changes in Intracellular Calcium Levels Following Lithium-Pilocarpine-Induced Status Epilepticus. Neuroscience 2008, 157, 566–576. [Google Scholar] [CrossRef]
- Teran, F.A.; Bravo, E.; Richerson, G.B. Sudden unexpected death in epilepsy: Respiratory mechanisms. Handb. Clin. Neurol. 2022, 189, 153–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derera, I.D.; Blotter, C.A.; Bernard, L.M. Altered A-Type Potassium Channel Function in the Nucleus Tractus Solitarii in Acquired Temporal Lobe Epilepsy. J. Neurophysiol. 2019, 121, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Aiba, I.; Noebels, J.L. Spreading Depolarization in the Brainstem Mediates Sudden Cardiorespiratory Arrest in Mouse SUDEP Models. Sci. Transl. Med. 2015, 7, 282ra46. [Google Scholar] [CrossRef]
- Vanhoof-Villalba, S.L.; Gautier, N.M.; Mishra, V.; Glasscock, E. Pharmacogenetics of KCNQ Channel Activation in 2 Potassium Channelopathy Mouse Models of Epilepsy. Epilepsia 2018, 59, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Römermann, K.; Bankstahl, J.P.; Löscher, W.; Bankstahl, M. Pilocarpine-Induced Convulsive Activity Is Limited by Multidrug Transporters at the Rodent Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 2015, 353, 351–359. [Google Scholar] [CrossRef]
- Patodia, S.; Tachrount, M.; Somani, A.; Scheffer, I.; Yousry, T.; Golay, X.; Sisodiya, S.M.; Thom, M. MRI and Pathology Correlations in the Medulla in Sudden Unexpected Death in Epilepsy (SUDEP): A Postmortem Study. Neuropathol. Appl. Neurobiol. 2021, 47, 157–170. [Google Scholar] [CrossRef]
- Mueller, S.G.; Nei, M.; Bateman, L.M.; Knowlton, R.C.; Laxer, K.D.; Friedman, D.; Devinsky, O.; Goldman, A.M. Evidence for Brainstem Network Disruption in Temporal Lobe Epilepsy and Sudden Unexplained Death in Epilepsy. NeuroImage Clin. 2014, 5, 208–216. [Google Scholar] [CrossRef]
- Patodia, S.; Somani, A.; O’Hare, M.; Venkateswaran, R.; Liu, J.; Michalak, Z.; Ellis, M.; Scheffer, I.E.; Diehl, B.; Sisodiya, S.M. The Ventrolateral Medulla and Medullary Raphe in Sudden Unexpected Death in Epilepsy. Brain 2018, 141, 1719–1733. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, Y.; Wong-Riley, M.T.T.; Ju, G.; Liu, Y. Synaptic Relationship between Somatostatin- and Neurokinin-1 Receptor-Immunoreactive Neurons in the Pre-Bötzinger Complex of Rats. J. Neurochem. 2012, 122, 923–933. [Google Scholar] [CrossRef]
- Murugesan, A.; Rani, M.R.S.; Hampson, J.; Zonjy, B.; Lacuey, N.; Faingold, C.L.; Friedman, D.; Devinsky, O.; Sainju, R.K.; Schuele, S.; et al. Serum Serotonin Levels in Patients with Epileptic Seizures. Epilepsia 2018, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, A.N.; Joyal, K.G.; Chou, J.W.; Li, R.; Vencer, K.M.; Buchanan, G.F. Post-Ictal Generalized EEG Suppression Is Reduced by Enhancing Dorsal Raphe Serotonergic Neurotransmission. Neuroscience 2021, 453, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-J.; Liang, W.-H.; Lam, C.-S.; Huang, X.-F.; Yang, S.-J.; Wong-Riley, M.T.T.; Fung, M.-L.; Liu, Y.-Y. Catecholaminergic Neurons in Synaptic Connections with Pre-Bötzinger Complex Neurons in the Rostral Ventrolateral Medulla in Normoxic and Daily Acute Intermittent Hypoxic Rats. Exp. Neurol. 2017, 287, 165–175. [Google Scholar] [CrossRef]
- Tada, M.; Kakita, A.; Toyoshima, Y.; Onodera, O.; Ozawa, T.; Morita, T.; Nishizawa, M.; Takahashi, H. Depletion of Medullary Serotonergic Neurons in Patients with Multiple System Atrophy Who Succumbed to Sudden Death. Brain 2009, 132, 1810–1819. [Google Scholar] [CrossRef]
- Spirovski, D.; Li, Q.; Pilowsky, P.M. Brainstem Galanin-Synthesizing Neurons Are Differentially Activated by Chemoreceptor Stimuli and Represent a Subpopulation of Respiratory Neurons. J. Comp. Neurol. 2012, 520, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.M.; Silva, T.M.; Fair, S.R.; Liu, J.; Tupal, S.; Kaya, B.; Cowgill, A.; Mahajan, S.; Silva, P.E.; Wang, Y.; et al. The Role of PHOX2B-Derived Astrocytes in Chemosensory Control of Breathing and Sleep Homeostasis. J. Physiol. 2019, 597, 2225–2251. [Google Scholar] [CrossRef]
- Falquetto, B.; Oliveira, L.M.; Takakura, A.C.; Mulkey, D.K.; Moreira, T.S. Inhibition of the Hypercapnic Ventilatory Response by Adenosine in the Retrotrapezoid Nucleus in Awake Rats. Neuropharmacology 2018, 138, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Patodia, S.; Paradiso, B.; Ellis, M.; Somani, A.; Sisodiya, S.M.; Devinsky, O.; Thom, M. Characterisation of Medullary Astrocytic Populations in Respiratory Nuclei and Alterations in Sudden Unexpected Death in Epilepsy. Epilepsy Res. 2019, 157, 106213. [Google Scholar] [CrossRef] [PubMed]
- SheikhBahaei, S.; Morris, B.; Collina, J.; Anjum, S.; Znati, S.; Gamarra, J.; Zhang, R.; Gourine, A.V.; Smith, J.C. Morphometric Analysis of Astrocytes in Brainstem Respiratory Regions. J. Comp. Neurol. 2018, 526, 2032–2047. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Gehlbach, B.K.; Kreple, C.J.; Kawasaki, H.; Oya, H.; Buzza, C.; Granner, M.A.; Welsh, M.J.; Howard, M.A.; Wemmie, J.A. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J. Neurosci. 2015, 35, 10281–10289. [Google Scholar] [CrossRef]
- Lacuey, N.; Zonjy, B.; Londono, L.; Lhatoo, S.D. Amygdala and Hippocampus Are Symptomatogenic Zones for Central Apneic Seizures. Neurology 2017, 88, 701–705. [Google Scholar] [CrossRef]
- Nobis, W.P.; Schuele, S.; Templer, J.W.; Zhou, G.; Lane, G.; Rosenow, J.M.; Zelano, C. Amygdala-Stimulation-Induced Apnea Is Attention and Nasal-Breathing Dependent. Ann. Neurol. 2018, 83, 460–471. [Google Scholar] [CrossRef]
- Gu, J.; Park, S.; Lin, J.S.; Sugimura, Y.K.; Kato, F.; Del Negro, C.A. Central Amygdala-to-Pre-Bötzinger Complex Neurotransmission Is Direct and Inhibitory. Eur. J. Neurosci. 2024, 60, 6799–6811. [Google Scholar] [CrossRef]
- Applegate, C.D.; Kapp, B.S.; Underwood, M.D.; McNall, C.L. Autonomic and Somatomotor Effects of Amygdala Central N. Stimulation in Awake Rabbits. Physiol. Behav. 1983, 31, 353–360. [Google Scholar] [CrossRef]
- Seyal, M.; Bateman, L.M. Ictal Apnea Linked to Contralateral Spread of Temporal Lobe Seizures: Intracranial EEG Recordings in Refractory Temporal Lobe Epilepsy. Epilepsia 2009, 50, 2557–2562. [Google Scholar] [CrossRef]
- Feldman, J.L.; Mitchell, G.S.; Nattie, E.E. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu. Rev. Neurosci. 2003, 26, 239–266. [Google Scholar] [CrossRef]
- Sowers, L.P.; Massey, C.A.; Gehlbach, B.K.; Granner, M.A.; Richerson, G.B. Sudden Unexpected Death in Epilepsy: Fatal Post-Ictal Respiratory and Arousal Mechanisms. Respir. Physiol. Neurobiol. 2013, 189, 315–323. [Google Scholar] [CrossRef]
- Kriegel, M.F.; Roberts, D.W.; Jobst, B.C. Orbitofrontal and Insular Epilepsy. J. Clin. Neurophysiol. 2012, 29, 385–391. [Google Scholar] [CrossRef]
- Isnard, J.; Guénot, M.; Ostrowsky, K.; Sindou, M.; Mauguière, F. The Role of the Insular Cortex in Temporal Lobe Epilepsy. Ann. Neurol. 2000, 48, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P. Avoid Falling into the Depths of the Insular Trap. Epileptic Disord. 2006, 8 (Suppl. S2), S33–S36. [Google Scholar] [CrossRef]
- Sun, T.; Wang, F.; Cui, J. Insular Epilepsy; People’s Medical Publishing House: Beijing, China, 2013. [Google Scholar]
- Oppenheimer, S. Forebrain Lateralization and the Cardiovascular Correlates of Epilepsy. Brain 2001, 124, 2345–2346. [Google Scholar] [CrossRef][Green Version]
- Smart, S.L.; Lopantsev, V.; Zhang, C.L.; Robbins, C.A.; Wang, H.; Chiu, S.Y.; Schwartzkroin, P.A.; Messing, A.; Tempel, B.L. Deletion of the Kv1.1 Potassium Channel Causes Epilepsy in Mice. Neuron 1998, 20, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bravo, E.; Thirnbeck, C.K.; Smith-Mellecker, L.A.; Kim, S.H.; Gehlbach, B.K.; Laux, L.C.; Zhou, X.; Nordli, D.R.; Richerson, G.B. Severe Peri-Ictal Respiratory Dysfunction Is Common in Dravet Syndrome. J. Clin. Investig. 2018, 128, 1141–1153. [Google Scholar] [CrossRef]
- Xie, A.; Wong, B.; Phillipson, E.A.; Slutsky, A.S.; Bradley, T.D. Interaction of Hyperventilation and Arousal in the Pathogenesis of Idiopathic Central Sleep Apnea. Am. J. Respir. Crit. Care Med. 1994, 150, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Rankin, F.; Rutherford, R.; Bradley, T.D. Effects of Inhaled CO2 and Added Dead Space on Idiopathic Central Sleep Apnea. J. Appl. Physiol. 1997, 82, 918–926. [Google Scholar] [CrossRef]
- Li, D.; Mabrouk, O.S.; Liu, T.; Tian, F.; Xu, G.; Rengifo, S.; Choi, S.J.; Mathur, A.; Crooks, C.P.; Kennedy, R.T.; et al. Asphyxia-Activated Corticocardiac Signaling Accelerates Onset of Cardiac Arrest. Proc. Natl. Acad. Sci. USA 2015, 112, 4995–5000. [Google Scholar] [CrossRef]
- Nakase, K.; Kollmar, R.; Lazar, J.; Arjomandi, H.; Sundaram, K.; Silverman, J.; Orman, R.; Weedon, J.; Stefanov, D.; Savoca, E. Laryngospasm, Central and Obstructive Apnea during Seizures: Defining Pathophysiology for Sudden Death in a Rat Model. Epilepsy Res. 2016, 128, 126–139. [Google Scholar] [CrossRef]
- Peng, W.; Danison, J.L.; Seyal, M. Postictal Generalized EEG Suppression and Respiratory Dysfunction Following Generalized Tonic–Clonic Seizures in Sleep and Wakefulness. Epilepsia 2017, 58, 1409–1414. [Google Scholar] [CrossRef]
- Zhan, Q.; Buchanan, G.F.; Motelow, J.E.; Andrews, J.; Vitkovskiy, P.; Chen, W.C.; Serout, F.; Gummadavelli, A.; Kundishora, A.; Furman, M.; et al. Impaired Serotonergic Brainstem Function during and after Seizures. J. Neurosci. 2016, 36, 2711–2722. [Google Scholar] [CrossRef]
- Irizarry, R.; Sukato, D.; Kollmar, R.; Schild, S.; Silverman, J.; Sundaram, K.; Stephenson, S.; Stewart, M. Seizures Induce Obstructive Apnea in DBA/2J Audiogenic Seizure-Prone Mice: Lifesaving Impact of Tracheal Implants. Epilepsia 2020, 61, 365–375. [Google Scholar] [CrossRef]
- Ravindran, M. Temporal Lobe Seizure Presenting as “Laryngospasm”. Clin. Electroencephalogr. 1981, 12, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Amir, J.; Ashkenazi, S.; Schonfeld, T.; Weitz, R.; Nitzan, M. Laryngospasm as a Single Manifestation of Epilepsy. Arch. Dis. Child. 1983, 58, 151–153. [Google Scholar] [CrossRef][Green Version]
- Stewart, M.; Kollmar, R.; Nakase, K.; Silverman, J.; Sundaram, K.; Orman, R.; Lazar, J. Obstructive Apnea Due to Laryngospasm Links Ictal to Postictal Events in SUDEP Cases and Offers Practical Biomarkers for Review of Past Cases and Prevention of New Ones. Epilepsia 2017, 58, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Fan, Z.; Wu, L.; Silverman, J.; Sundaram, K.; Kollmar, R. Causes and Effects Contributing to Sudden Death in Epilepsy and the Rationale for Prevention and Intervention. Front. Neurol. 2020, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.M. Mechanisms of Sudden Unexplained Death in Epilepsy. Curr. Opin. Neurol. 2015, 28, 166–174. [Google Scholar] [CrossRef]
- Guyenet, P.G. Regulation of Breathing and Autonomic Outflows by Chemoreceptors. Compr. Physiol. 2014, 4, 1511–1562. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.C.; Shin, J.; Chimenti, P.C.; Loewy, A.D. Paraventricular Hypothalamic Nucleus: Axonal Projections to the Brainstem. J. Comp. Neurol. 2010, 518, 1460–1499. [Google Scholar] [CrossRef]
- Stewart, M. An Explanation for Sudden Death in Epilepsy (SUDEP). J. Physiol. Sci. 2018, 68, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Richerson, G.B.; Buchanan, G.F. The Serotonin Axis: Shared Mechanisms in Seizures, Depression, and SUDEP. Epilepsia 2011, 52 (Suppl. S1), 28–38. [Google Scholar] [CrossRef]
- Tupal, S.; Faingold, C.L. Evidence Supporting a Role of Serotonin in Modulation of Sudden Death Induced by Seizures in DBA/2 Mice. Epilepsia 2006, 47, 21–26. [Google Scholar] [CrossRef]
- Uteshev, V.V.; Tupal, S.; Mhaskar, Y.; Faingold, C.L. Abnormal Serotonin Receptor Expression in DBA/2 Mice Associated with Susceptibility to Sudden Death Due to Respiratory Arrest. Epilepsy Res. 2010, 88, 183–188. [Google Scholar] [CrossRef]
- Buchanan, G.F.; Murray, N.M.; Hajek, M.A.; Richerson, G.B. Serotonin Neurones Have Anti-Convulsant Effects and Reduce Seizure-Induced Mortality. J. Physiol. 2014, 592, 4395–4410. [Google Scholar] [CrossRef]
- Foerster, O. Hyperventilationsepilepsie. Dtsch. Z. Nervenheilkd. 1925, 83, 347–356. [Google Scholar] [CrossRef]
- Lennox, W.G.; Gibbs, F.A.; Gibbs, E.L. Effect on the Electro-Encephalogram of Drugs and Conditions Which Influence Seizures. Arch. Neurol. Psychiatry 1936, 36, 1236–1250. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Camfield, P.R.; Gordon, K.E.; Camfield, C.S.; Dooley, J.M.; Hanna, B.D. Will a Critical Level of Hyperventilation-Induced Hypocapnia Always Induce an Absence Seizure? Epilepsia 1996, 37, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Guaranha, M.S.B.; Garzon, E.; Buchpiguel, C.A.; Tazima, S.; Yacubian, E.M.T.; Sakamoto, A.C. Hyperventilation Revisited: Physiological Effects and Efficacy on Focal Seizure Activation in the Era of Video-EEG Monitoring. Epilepsia 2005, 46, 69–75. [Google Scholar] [CrossRef]
- Rockstroh, B. Hyperventilation-Induced EEG Changes in Humans and Their Modulation by an Anticonvulsant Drug. Epilepsy Res. 1990, 7, 146–154. [Google Scholar] [CrossRef]
- Lee, J.; Taira, T.; Pihlaja, P.; Ransom, B.R.; Kaila, K. Effects of CO2 on Excitatory Transmission Apparently Caused by Changes in Intracellular pH in the Rat Hippocampal Slice. Brain Res. 1996, 706, 210–216. [Google Scholar] [CrossRef]
- Weinand, M.E.; Carter, L.P.; Oommen, K.J.; Hutzler, R.; Labiner, D.M.; Talwar, D.; El-Saadany, W.; Ahern, G.L. Response of Human Epileptic Temporal Lobe Cortical Blood Flow to Hyperventilation. Epilepsy Res. 1995, 21, 221–226. [Google Scholar] [CrossRef]
- Labuz-Roszak, B.; Pierzchała, K. Assessment of Autonomic Nervous System in Patients with Epilepsy in the Interictal State. A Pilot Study. Neurol. Neurochir. Pol. 2009, 43, 330–336. [Google Scholar]
- Jansen, K.; Vandeput, S.; Milosevic, M.; Ceulemans, B.; Van Huffel, S.; Brown, L.; Penders, J.; Lagae, L. Autonomic Effects of Refractory Epilepsy on Heart Rate Variability in Children: Influence of Intermittent Vagus Nerve Stimulation. Dev. Med. Child Neurol. 2011, 53, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Assenza, G.; Mecarelli, O.; Tombini, M.; Pulitano, P.; Pellegrino, G.; Benvenga, A.; Assenza, F.; Campana, C.; Di Pino, G.; Di Lazzaro, V. Hyperventilation Induces Sympathetic Overactivation in Mesial Temporal Epilepsy. Epilepsy Res. 2015, 110, 221–227. [Google Scholar] [CrossRef]
- Leonhardt, G.; de Greiff, A.; Marks, S.; Ludwig, T.; Doerfler, A.; Forsting, M.; Konermann, S.; Hufnagel, A. Brain Diffusion during Hyperventilation: Diffusion-Weighted MR-Monitoring in Patients with Temporal Lobe Epilepsy and in Healthy Volunteers. Epilepsy Res. 2002, 51, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, U.; Cook, M.; D’Souza, W. Consistent Topography and Amplitude Symmetry Are More Typical than Morphology of Epileptiform Discharges in Genetic Generalized Epilepsy. Clin. Neurophysiol. 2016, 127, 1138–1146. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Madsen, F.F.; Moltke, F.B.; Uldall, P.; Hogenhaven, H. Intraoperative Hyperventilation vs Remifentanil during Electrocorticography for Epilepsy Surgery—A Case Report: Finding Ictal Onset Zones during Surgery. Acta Neurol. Scand. 2010, 121, 413–417. [Google Scholar] [CrossRef]
- Marrosu, F.; Puligheddu, M.; Giagheddu, M.; Cossu, G.; Piga, M. Correlation between Cerebral Perfusion and Hyperventilation Enhanced Focal Spiking Activity. Epilepsy Res. 2000, 40, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Azar, N.J.; Alabsi, A.; Seymour, J.P.; Bateman, L.M.; Hirsch, L.J.; Spencer, D.; Wieser, H.G.; O’Brien, T.J.; Dalmau, J. Postictal Breathing Pattern Distinguishes Epileptic from Nonepileptic Convulsive Seizures. Epilepsia 2008, 49, 132–137. [Google Scholar] [CrossRef]
- Bagnall, R.D.; Crompton, D.E.; Cutmore, C.; Regan, B.M.; Berkovic, S.F.; Scheffer, I.E.; Semsarian, C. Genetic Analysis of PHOX2B in Sudden Unexpected Death in Epilepsy Cases. Neurology 2014, 83, 1018–1021. [Google Scholar] [CrossRef]
- Ward, C.S.; Arvide, E.M.; Huang, T.-W.; Yoo, J.; Noebels, J.L.; Neul, J.L. MeCP2 Is Critical within HoxB1-Derived Tissues of Mice for Normal Lifespan. J. Neurosci. 2011, 31, 10359–10370. [Google Scholar] [CrossRef]
- El-Khoury, R.; Panayotis, N.; Matagne, V.; Ghata, A.; Villard, L.; Roux, J.-C. GABA and Glutamate Pathways Are Spatially and Developmentally Affected in the Brain of Mecp2-Deficient Mice. PLoS ONE 2014, 9, e92169. [Google Scholar] [CrossRef]
- Viemari, J.-C.; Roux, J.-C.; Tryba, A.K.; Saywell, V.; Burnet, H.; Peña, F.; Zanella, S.; Bévengut, M.; Barthelemy-Requin, M.; Herzing, L.B.K.; et al. Mecp2 Deficiency Disrupts Norepinephrine and Respiratory Systems in Mice. J. Neurosci. 2005, 25, 11521–11530. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, X.; Funk, G.D.; Greer, J.J. Anxiety-Related Mechanisms of Respiratory Dysfunction in a Mouse Model of Rett Syndrome. J. Neurosci. 2012, 32, 17230–17240. [Google Scholar] [CrossRef]
- Smith, J.C.; Abdala, A.P.; Borgmann, A.; Rybak, I.A.; Paton, J.F. Brainstem Respiratory Networks: Building Blocks and Microcircuits. Trends Neurosci. 2013, 36, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.-M.; Ward, C.S.; Neul, J.L. Breathing Challenges in Rett Syndrome: Lessons Learned from Humans and Animal Models. Respir. Physiol. Neurobiol. 2013, 189, 280–287. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.D.; Wang, T.; Mike, E.; Herrera, J.; Beavers, D.L.; Huang, T.-W.; Ward, C.S.; Skinner, S.; Percy, A.K.; Glaze, D.G.; et al. Pathogenesis of Lethal Cardiac Arrhythmias in Mecp2 Mutant Mice: Implication for Therapy in Rett Syndrome. Sci. Transl. Med. 2011, 3, 113ra125. [Google Scholar] [CrossRef]
- Seyal, M.; Bateman, L.M.; Li, C.-S. Respiratory Changes with Seizures in Localization-Related Epilepsy: Analysis of Periictal Hypercapnia and Airflow Patterns. Epilepsia 2010, 51, 1359–1364. [Google Scholar] [CrossRef]
- Seyal, M.; Bateman, L.M.; Li, C. Impact of Periictal Interventions on Respiratory Dysfunction, Postictal EEG Suppression, and Postictal Immobility. Epilepsia 2013, 54, 377–382. [Google Scholar] [CrossRef]
- Moore, B.M.; Jou, C.J.; Tatalovic, M.; Kaufman, E.S.; Kline, D.D.; Kunze, D.L. The Kv1.1 Null Mouse, a Model of Sudden Unexpected Death in Epilepsy (SUDEP). Epilepsia 2014, 55, 1808–1816. [Google Scholar] [CrossRef]
- Campos, R.R.; Tolentino-Silva, F.R.P.; Mello, L.E.A.M. Respiratory Pattern in a Rat Model of Epilepsy. Epilepsia 2003, 44, 712–717. [Google Scholar] [CrossRef]
- Richerson, G.B. Serotonergic Neurons as Carbon Dioxide Sensors That Maintain PH Homeostasis. Nat. Rev. Neurosci. 2004, 5, 449–461. [Google Scholar] [CrossRef]
- Johnston, S.C.; Horn, J.K.; Valente, J.; Simon, R.P. The Role of Hypoventilation in a Sheep Model of Epileptic Sudden Death. Ann. Neurol. 1995, 37, 531–537. [Google Scholar] [CrossRef]
- Johnston, S.C.; Siedenberg, R.; Min, J.K.; Jerome, E.H.; Laxer, K.D. Central Apnea and Acute Cardiac Ischemia in a Sheep Model of Epileptic Sudden Death. Ann. Neurol. 1997, 42, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Faingold, C.L.; Randall, M.; Tupal, S. DBA/1 Mice Exhibit Chronic Susceptibility to Audiogenic Seizures Followed by Sudden Death Associated with Respiratory Arrest. Epilepsy Behav. 2010, 17, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-J.; Faingold, C.L. Abnormalities of Serotonergic Neurotransmission in Animal Models of SUDEP. Epilepsy Behav. 2017, 71, 174–180. [Google Scholar] [CrossRef]
- During, M.J.; Spencer, D.D. Adenosine: A Potential Mediator of Seizure Arrest and Postictal Refractoriness. Ann. Neurol. 1992, 32, 618–624. [Google Scholar] [CrossRef]
- Paydarfar, D.; Eldridge, F.L.; Scott, S.C.; Dowell, R.T.; Wagner, P.G. Respiratory Responses to Focal and Generalized Seizures in Cats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991, 260, R934–R940. [Google Scholar] [CrossRef] [PubMed]
- Paydarfar, D.; Eldridge, F.L.; Wagner, P.G.; Dowell, R.T. Neural Respiratory Responses to Cortically Induced Seizures in Cats. Respir. Physiol. 1992, 89, 225–237. [Google Scholar] [CrossRef]
- Lutas, A.; Yellen, G. The Ketogenic Diet: Metabolic Influences on Brain Excitability and Epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Faingold, C.L.; Randall, M.; Mhaskar, Y.; Uteshev, V.V. Differences in Serotonin Receptor Expression in the Brainstem May Explain the Differential Ability of a Serotonin Agonist to Block Seizure-Induced Sudden Death in DBA/2 vs. DBA/1 Mice. Brain Res. 2011, 1418, 104–110. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Feng, H.-J. Atomoxetine, a Norepinephrine Reuptake Inhibitor, Reduces Seizure-Induced Respiratory Arrest. Epilepsy Behav. 2017, 73, 6–9. [Google Scholar] [CrossRef]
- Kuo, F.-S.; Cleary, C.M.; LoTurco, J.J.; Chen, X.; Mulkey, D.K. Disordered Breathing in a Mouse Model of Dravet Syndrome. eLife 2019, 8, e43387. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.Á.; Knape, K.D.; Leland, M.M.; Feldman, J.; McCoy, K.J.M.; Hubbard, G.B.; Williams, J.T. Mortality in Captive Baboons with Seizures: A New Model for SUDEP? Epilepsia 2009, 50, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Kalume, F.; Westenbroek, R.E.; Cheah, C.S.; Yu, F.H.; Oakley, J.C.; Scheuer, T.; Catterall, W.A. Sudden Unexpected Death in a Mouse Model of Dravet Syndrome. J. Clin. Investig. 2013, 123, 1798–1808. [Google Scholar] [CrossRef]
- Auerbach, D.S.; Jones, J.; Clawson, B.C.; Offord, J.; Lenk, G.M.; Ogiwara, I.; Yamakawa, K.; Meisler, M.H.; Parent, J.M.; Isom, L.L. Altered Cardiac Electrophysiology and SUDEP in a Model of Dravet Syndrome. PLoS ONE 2013, 8, e77843. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.H.; Matthews, S.A.; Simeone, T.A.; Maganti, R.; Simeone, K.A. Accumulation of rest deficiency precedes sudden death of epileptic Kv1.1 knockout mice, a model of sudden unexpected death in epilepsy. Epilepsia 2018, 59, 92–105. [Google Scholar] [CrossRef]
- Ren, Y.; Chang, J.; Li, C.; Jia, C.; Li, P.; Wang, Y.; Chu, X.-P. The effects of ketogenic diet treatment in Kcna1-null mouse, a model of sudden unexpected death in epilepsy. Front. Neurol. 2019, 10, 744. [Google Scholar] [CrossRef]
- Foley, J.; Burnham, V.; Tedoldi, M.; Danial, N.N.; Yellen, G. BAD knockout provides metabolic seizure resistance in a genetic model of epilepsy with sudden unexplained death in epilepsy. Epilepsia 2018, 59, e1–e4. [Google Scholar] [CrossRef]
- Mishra, V.; Karumuri, B.K.; Gautier, N.M.; Liu, R.; Hutson, T.N.; Vanhoof-Villalba, S.L.; Vlachos, I.; Iasemidis, L.; Glasscock, E. Scn2a deletion improves survival and brain–heart dynamics in the Kcna1-null mouse model of sudden unexpected death in epilepsy (SUDEP). Hum. Mol. Genet. 2017, 26, 2091–2103. [Google Scholar] [CrossRef]
- Dhaibar, H.; Gautier, N.M.; Chernyshev, O.Y.; Dominic, P.; Glasscock, E. Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: Implications for sudden unexpected death in epilepsy. Neurobiol. Dis. 2019, 127, 502–511. [Google Scholar] [CrossRef]
- Cook, M.J.; O’Brien, T.J.; Berkovic, S.F.; Murphy, M.; Morokoff, A.; Fabinyi, G.; D’Souza, W.; Yerra, R.; Archer, J.; Litewka, L. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study. Lancet Neurol. 2013, 12, 563–571. [Google Scholar] [CrossRef]
- Ng, M.; Pavlova, M. Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res. Treat. 2013, 2013, 932790. [Google Scholar] [CrossRef]
- Kitchigina, V.F.; Butuzova, M.V. Theta activity of septal neurons during different epileptic phases: The same frequency but different significance? Exp. Neurol. 2009, 216, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Roliz, A.H.; Kothare, S. The interaction between sleep and epilepsy. Curr. Neurol. Neurosci. Rep. 2022, 22, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Derry, C.P.; Duncan, S. Sleep and epilepsy. Epilepsy Behav. 2013, 26, 394–404. [Google Scholar] [CrossRef]
- Žiburkus, J.; Cressman, J.R.; Schiff, S.J. Seizures as imbalanced up states: Excitatory and inhibitory conductances during seizure-like events. J. Neurophysiol. 2013, 109, 1296–1306. [Google Scholar] [CrossRef]
- Finnerty, G.T.; Whittington, M.A.; Jefferys, J.G.R. Altered dentate filtering during the transition to seizure in the rat tetanus toxin model of epilepsy. J. Neurophysiol. 2001, 86, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, R.J.; Thijs, R.D.; Laffan, A.; Langan, Y.; Sander, J.W. Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia 2012, 53, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Hajek, M.A.; Buchanan, G.F. Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J. Neurophysiol. 2016, 115, 2286–2293. [Google Scholar] [CrossRef]
- Nobili, L.; Proserpio, P.; Rubboli, G.; Montano, N.; Didato, G.; Tassinari, C.A. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med. Rev. 2011, 15, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Purnell, B.S.; Hajek, M.A.; Buchanan, G.F. Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J. Neurophysiol. 2017, 118, 2592–2600. [Google Scholar] [CrossRef]
- Dutton, S.B.B.; Sawyer, N.T.; Kalume, F.; Jumbo-Lucioni, P.; Borges, K.; Catterall, W.A.; Escayg, A. Protective effect of the ketogenic diet in Scn1a mutant mice. Epilepsia 2011, 52, 2050–2056. [Google Scholar] [CrossRef]
- Richerson, G.B.; Boison, D.; Faingold, C.L.; Ryvlin, P. From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy. Epilepsia 2016, 57 (Suppl. S1), 35–45. [Google Scholar] [CrossRef]
- Dallérac, G.; Moulard, J.; Benoist, J.-F.; Rouach, S.; Auvin, S.; Guilbot, A.; Lenoir, L.; Rouach, N. Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci. Rep. 2017, 7, 5496. [Google Scholar] [CrossRef]
- Szabó, C.Á.; Knape, K.D.; Leland, M.M.; Cwikla, D.J.; Williams-Blangero, S.; Williams, J.T. Epidemiology and characterization of seizures in a pedigreed baboon colony. Comp. Med. 2012, 62, 535–538. [Google Scholar]
- Kloster, R.; Engelskjøn, T. Sudden unexpected death in epilepsy (SUDEP): A clinical perspective and a search for risk factors. J. Neurol. Neurosurg. Psychiatry 1999, 67, 439–444. [Google Scholar] [CrossRef]
- Leung, H.; Kwan, P.; Elger, C.E. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav. 2006, 9, 19–30. [Google Scholar] [CrossRef]
- Earnest, M.P.; Thomas, G.E.; Eden, R.A.; Hossack, K.F. The sudden unexplained death syndrome in epilepsy: Demographic, clinical, and postmortem features. Epilepsia 1992, 33, 310–316. [Google Scholar] [CrossRef]
- Langan, Y.; Nashef, L.; Sander, J.W. Case-control study of SUDEP. Neurology 2005, 64, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.D.; Smith, B.N. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp. Neurol. 2010, 223, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.M.; Glasscock, E.; Yoo, J.; Chen, T.T.; Klassen, T.L.; Noebels, J.L. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci. Transl. Med. 2009, 1, 2ra6. [Google Scholar] [CrossRef] [PubMed]
- Shibley, H.; Smith, B.N. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002, 49, 109–120. [Google Scholar] [CrossRef]
- Bach, E.C.; Halmos, K.C.; Smith, B.N. Enhanced NMDA receptor-mediated modulation of excitatory neurotransmission in the dorsal vagal complex of streptozotocin-treated, chronically hyperglycemic mice. PLoS ONE 2015, 10, e0121022. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, J.; Mifflin, S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1276–R1286. [Google Scholar] [CrossRef][Green Version]
- Glatzer, N.R.; Derbenev, A.V.; Banfield, B.W.; Smith, B.N. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J. Neurophysiol. 2007, 98, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Card, J.P.; Sved, J.C.; Craig, B.; Raizada, M.; Vazquez, J.; Sved, A.F. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J. Comp. Neurol. 2006, 499, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Sawant-Pokam, P.M.; Suryavanshi, P.; Mendez, J.M.; Dudek, F.E.; Brennan, K.C. Mechanisms of neuronal silencing after cortical spreading depression. Cereb. Cortex 2017, 27, 1311–1325. [Google Scholar] [CrossRef]
- Fong, A.Y.; Stornetta, R.L.; Foley, C.M.; Potts, J.T. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: Subregional distribution in the nucleus tractus solitarius. J. Comp. Neurol. 2005, 493, 274–290. [Google Scholar] [CrossRef]
- Naggar, I.; Stewart, M. A rat model for exploring the contributions of ventricular arrhythmias to sudden death in epilepsy. In Sudden Unexpected Death in Epilepsy: Mechanisms and New Methods for Analyzing Risks; Lathers, C.M., Schraeder, P.L., Leestma, J.E., Wannamaker, B.B., Verrier, R.L., Schachter, S.C., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 241–250. [Google Scholar]
- Budde, R.B.; Arafat, M.A.; Pederson, D.J.; Lovick, T.A.; Jefferys, J.G.R.; Irazoqui, P.P. Acid reflux induced laryngospasm as a potential mechanism of sudden death in epilepsy. Epilepsy Res. 2018, 148, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Walczak, T.; Sillanpää, M.; Sander, J.W.A.S. Sudden unexpected death in epilepsy: A review of incidence and risk factors. Epilepsia 2005, 46 (Suppl. S11), 54–61. [Google Scholar] [CrossRef]
- Helbig, I.; Scheffer, I.E.; Mulley, J.C.; Berkovic, S.F. Navigating the channels and beyond: Unravelling the genetics of the epilepsies. Lancet Neurol. 2008, 7, 231–245. [Google Scholar] [CrossRef]
- Allen, L.A.; Harper, R.M.; Kumar, R.; Guye, M.; Ogren, J.A.; Lhatoo, S.D.; Lemieux, L.; Scott, C.A.; Vos, S.B.; Rani, S.; et al. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front. Neurol. 2017, 8, 544. [Google Scholar] [CrossRef]
- Patodia, S.; Somani, A.; Thom, M. Neuropathology findings in autonomic brain regions in SUDEP and future research directions. Auton. Neurosci. 2021, 235, 102862. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, Z.-H.; Liu, C.; Li, G.-Y.; Qiao, X.-Z.; Gan, Y.-J.; Zhang, C.-C.; Deng, Y.-C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Robinson, R.; Taske, N.; Sander, T.; Heils, A.; Whitehouse, W.; Goutières, F.; Aicardi, J.; Lehesjoki, A.E.; Siren, A.; Friis, M.L.; et al. Linkage analysis between childhood absence epilepsy and genes encoding GABAA and GABAB receptors, voltage-dependent calcium channels, and the ECA1 region on chromosome 8q. Epilepsy Res. 2002, 48, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Macdonald, R.L. The GABA Receptor γ2 Subunit R43Q Mutation Linked to Childhood Absence Epilepsy Causes Retention in the Endoplasmic Reticulum. J. Neurosci. 2004, 24, 8672–8677. [Google Scholar] [CrossRef]
- Han, H.A.; Cortez, M.A.; Snead, O.C., III. GABA Receptors and Absence Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Bruno, E.; Simel, D.L.; Giovannini, G.; Sander, T.; Rocamora, R.; Bateman, L.M.; Seyal, M.; Leach, J.; Novy, J.; Wright, S.; et al. Ictal Hypoxemia: A Systematic Review and Meta-Analysis. Seizure 2018, 63, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-W.; Lu, H.-C.; Liao, W.-P.; Huang, Y.-C.; Chen, H.-I.; Lai, H.-H.; Hsu, Y.-T. Progressive Changes in a Distributed Neural Circuit Underlie Breathing Abnormalities in Mice Lacking MeCP2. J. Neurosci. 2016, 36, 5572–5586. [Google Scholar] [CrossRef]
- Jafarian, M.; Ghahremani, M.H.; Bahari, Z.; Shiri, S.; Esmaeili, H.; Emami, M.; Ghazavi, A.; Khaleghi, A.; Mohammadi, M.; Haddad, F.; et al. The effect of GABAergic neurotransmission on the seizure-related activity of the laterodorsal thalamic nuclei and the somatosensory cortex in a genetic model of absence epilepsy. Brain Res. 2021, 1757, 147304. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.B. Design and Mechanism of GABA Aminotransferase Inactivators: Treatments for Epilepsies and Addictions. Chem. Rev. 2018, 118, 4037–4070. [Google Scholar] [CrossRef]
- Crino, P.B.; Jin, H.; Shumate, M.D.; Robinson, M.B.; Coulter, D.A.; Brooks-Kayal, A.R. Increased Expression of the Neuronal Glutamate Transporter (EAAT3/EAAC1) in Hippocampal and Neocortical Epilepsy. Epilepsia 2002, 43, 211–218. [Google Scholar] [CrossRef]
- Dereli, A.S.; Apaire, A.; El Tahry, R. Sudden Unexpected Death in Epilepsy: Central Respiratory Chemoreception. Int. J. Mol. Sci. 2025, 26, 1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- During, M.J. In vivo neurochemistry of the conscious human brain: Intrahippocampal microdialysis in epilepsy. In Techniques in the Behavioral and Neural Sciences; Elsevier: Amsterdam, The Netherlands, 1991; Volume 7, pp. 425–442. [Google Scholar]
- During, M.J.; Spencer, D.D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 1993, 341, 1607–1610. [Google Scholar] [CrossRef]
- Cavus, I.; Kasoff, W.S.; Cassaday, M.P.; Jacob, R.; Gueorguieva, R.; Sherwin, R.S.; Krystal, J.H.; Spencer, D.D.; Abi-Saab, W.M. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 2005, 57, 226–235. [Google Scholar] [CrossRef]
- Pan, J.W.; Cavus, I.; Kim, J.; Hetherington, H.P.; Spencer, D.D. Hippocampal extracellular GABA correlates with metabolism in human epilepsy. Metab. Brain Dis. 2008, 23, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Çavuş, I.; Romanyshyn, J.C.; Kennard, J.T.; Farooque, P.; Williamson, A.; Eid, T.; Spencer, S.S.; Duckrow, R.; Dziura, J.; Spencer, D.D. Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the Yale Epilepsy Surgery Program. Ann. Neurol. 2016, 80, 35–45. [Google Scholar] [CrossRef]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 2018, 10, 451. [Google Scholar] [CrossRef]
- Eid, T.; Thomas, M.J.; Spencer, D.D.; Runden-Pran, E.; Lai, J.C.K.; Malthankar, G.V.; Kim, J.H.; Danbolt, N.C.; Ottersen, O.P.; De Lanerolle, N.C. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, A. The Pilocarpine Model of Temporal Lobe Epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Bagdy, G.; Kecskemeti, V.; Riba, P.; Jakus, R. Serotonin and epilepsy. J. Neurochem. 2007, 100, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kaneko, S.; Hirano, T.; Ishida, M.; Kondo, T.; Otani, K.; Fukushima, Y. Effects of zonisamide on extracellular levels of monoamine and its metabolite, and on Ca2+ dependent dopamine release. Epilepsy Res. 1992, 13, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Fowler, L.J.; Whitton, P.S. Effects of Antiepileptic Drugs on Neurotransmitter Levels. Epilepsy Res. 2005, 63, 141–149. [Google Scholar] [CrossRef]
- Dailey, J.W.; Naritoku, D.K.; Jobe, P.C. Effects of Fluoxetine on Convulsions and Brain Serotonin in Epilepsy-Prone Rats. J. Pharmacol. Exp. Ther. 1992, 260, 533–540. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Anselmo-Franci, J.A.; Perosa, S.R.; de Castro-Neto, E.F.; Bellissimo, M.I.; de Oliveira, B.H.; Cavalheiro, E.A.; da Graça Naffah-Mazzacoratti, M.; Da Cunha, C. Amino acid and monoamine alterations in the cerebral cortex and hippocampus of mice submitted to ricinine-induced seizures. Pharmacol. Biochem. Behav. 2002, 72, 779–786. [Google Scholar] [CrossRef]
- Faingold, C.L.; Tupal, S.; Randall, M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011, 22, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J.; Seeley, W.W.; Kilgard, M.; Schreiner, C.E.; Tecott, L.H. Sound-Induced Seizures in Serotonin 5-HT2C Receptor Mutant Mice. Nat. Genet. 1997, 16, 387–390. [Google Scholar] [CrossRef]
- Kinney, H.C.; Richerson, G.B.; Dymecki, S.M.; Darnall, R.A.; Nattie, E.E. The Brainstem and Serotonin in the Sudden Infant Death Syndrome. Annu. Rev. Pathol. 2009, 4, 517–550. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.S.; Trachtenberg, F.L.; Thompson, E.G.; Belliveau, R.A.; Beggs, A.H.; Darnall, R.; Chadwick, A.E.; Krous, H.F.; Kinney, H.C. Multiple Serotonergic Brainstem Abnormalities in Sudden Infant Death Syndrome. JAMA 2006, 296, 2124–2132. [Google Scholar] [CrossRef]
- Duncan, J.R.; Paterson, D.S.; Hoffman, J.M.; Mokler, D.J.; Borenstein, N.S.; Belliveau, R.A.; Krous, H.F.; Haas, E.A.; Stanley, C.; Nattie, E.E.; et al. Brainstem Serotonergic Deficiency in Sudden Infant Death Syndrome. JAMA 2010, 303, 430–437. [Google Scholar] [CrossRef]
- Haynes, R.L.; Trachtenberg, F.; Darnall, R.; Haas, E.A.; Goldstein, R.D.; Mena, O.J.; Krous, H.F.; Kinney, H.C. Altered 5-HT2A/C receptor binding in the medulla oblongata in the sudden infant death syndrome (SIDS): Part I. Tissue-based evidence for serotonin receptor signaling abnormalities in cardiorespiratory- and arousal-related circuits. J. Neuropathol. Exp. Neurol. 2023, 82, 467–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mueller, S.G.; Nei, M.; Bateman, L.M.; Knowlton, R.; Laxer, K.D.; Friedman, D.; Devinsky, O.; Goldman, A.M. Brainstem Network Disruption: A Pathway to Sudden Unexplained Death in Epilepsy? Hum. Brain Mapp. 2018, 39, 4820–4830. [Google Scholar] [CrossRef]
- Keller, S.S.; Wilke, M.; Wieshmann, U.C.; Mackay, C.E.; Denby, C.E.; Webb, J.; Roberts, N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: Effects of side of seizure onset and epilepsy duration. J. Neurol. Neurosurg. Psychiatry 2002, 73, 648–655. [Google Scholar] [CrossRef]
- Ciumas, C.; Savic, I. Structural Changes in Primary Generalized Tonic-Clonic Seizures. Neurology 2006, 67, 683–686. [Google Scholar] [CrossRef]
- Li, J.; Ming, Q.; Lin, W. The Insula Lobe and Sudden Unexpected Death in Epilepsy: A Hypothesis. Epileptic Disord. 2017, 19, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lacuey, N.; Zonjy, B.; Theerannaew, W.; Loparo, K.A.; Tatsuoka, C.; Sahadevan, J.; Lhatoo, S.D. Left-Insular Damage, Autonomic Instability, and Sudden Unexpected Death in Epilepsy. Epilepsy Behav. 2016, 55, 170–173. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Q.; Yu, X.; Xia, W.; Luo, C.; Huang, X.; Tang, H.; Gong, Q.; Zhou, D. A Resting-State Functional Connectivity Study in Patients at High Risk for Sudden Unexpected Death in Epilepsy. Epilepsy Behav. 2014, 41, 33–38. [Google Scholar] [CrossRef]
- Harper, R.M.; Kumar, R.; Macey, P.M.; Harper, R.K.; Ogren, J.A. Impaired Neural Structure and Function Contributing to Autonomic Symptoms in Congenital Central Hypoventilation Syndrome. Front. Neurosci. 2015, 9, 415. [Google Scholar] [CrossRef]
- Bozorgi, A.; Chung, S.; Kaffashi, F.; Loparo, K.A.; Sahoo, S.; Zhang, G.Q.; Kaiboriboon, K.; Lhatoo, S.D. Significant Postictal Hypotension: Expanding the Spectrum of Seizure-Induced Autonomic Dysregulation. Epilepsia 2013, 54, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Kimmerly, D.S.; O’Leary, D.D.; Menon, R.S.; Gati, J.S.; Shoemaker, J.K. Cortical Regions Associated with Autonomic Cardiovascular Regulation during Lower Body Negative Pressure in Humans. J. Physiol. 2005, 569, 331–345. [Google Scholar] [CrossRef]
- Harper, R.M.; Gozal, D.; Bandler, R.; Spriggs, D.; Lee, J.; Alger, J. Regional Brain Activation in Humans during Respiratory and Blood Pressure Challenges. Clin. Exp. Pharmacol. Physiol. 1998, 25, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Liebenthal, J.A.; Wu, S.; Rose, S.; Ebersole, J.S.; Tao, J.X. Association of Prone Position with Sudden Unexpected Death in Epilepsy. Neurology 2015, 84, 703–709. [Google Scholar] [CrossRef]
- Richerson, G.B. Serotonin: The Anti-SuddenDeathAmine?: Serotonin & SUDEP. Epilepsy Curr. 2013, 13, 241–244. [Google Scholar] [PubMed]
- Benarroch, E.E. Adenosine and Its Receptors: Multiple Modulatory Functions and Potential Therapeutic Targets for Neurologic Disease. Neurology 2008, 70, 231–236. [Google Scholar] [CrossRef]
- Zuchora, B.; Wielosz, M.; Urbańska, E.M. Adenosine A1 Receptors and the Anticonvulsant Potential of Drugs Effective in the Model of 3-Nitropropionic Acid-Induced Seizures in Mice. Eur. Neuropsychopharmacol. 2005, 15, 85–93. [Google Scholar] [CrossRef]
- Weltha, L.; Reemmer, J.; Boison, D. The Role of Adenosine in Epilepsy. Brain Res. Bull. 2019, 151, 46–54. [Google Scholar] [CrossRef]
- Shen, H.; Li, T.; Boison, D. A Novel Mouse Model for Sudden Unexpected Death in Epilepsy (SUDEP): Role of Impaired Adenosine Clearance. Epilepsia 2010, 51, 465–468. [Google Scholar] [CrossRef]
- Friedman, D.; Kannan, K.; Faustin, A.; Shroff, S.; Thomas, C.; Heguy, A.; Serrano, J.; Snuderl, M.; Devinsky, O. Cardiac Arrhythmia and Neuroexcitability Gene Variants in Resected Brain Tissue from Patients with Sudden Unexpected Death in Epilepsy (SUDEP). npj Genom. Med. 2018, 3, 9. [Google Scholar] [CrossRef]
- Goldman, A.M.; Behr, E.R.; Semsarian, C.; Bagnall, R.D.; Sisodiya, S.; Cooper, P.N. Sudden Unexpected Death in Epilepsy Genetics: Molecular Diagnostics and Prevention. Epilepsia 2016, 57 (Suppl. S1), 17–25. [Google Scholar] [CrossRef]
- Gano, L.B.; Grabenstatter, H.L. Modulation of Abnormal Sodium Channel Currents in Heart and Brain: Hope for SUDEP Prevention and Seizure Reduction. Epilepsy Curr. 2017, 17, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Wandschneider, B.; Koepp, M.; Scott, C.; Micallef, C.; Balestrini, S.; Sisodiya, S.M.; Thom, M.; Harper, R.M.; Sander, J.W.; Vos, S.B. Structural Imaging Biomarkers of Sudden Unexpected Death in Epilepsy. Brain 2015, 138, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Isokawa, M.; Babb, T.L.; Crandall, P.H. Functional Connections in the Human Temporal Lobe: I. Analysis of Limbic System Pathways Using Neuronal Responses Evoked by Electrical Stimulation. Exp. Brain Res. 1990, 82, 279–292. [Google Scholar] [CrossRef]
- Rugg-Gunn, F.J.; Holdright, D. Epilepsy and the Heart. Br. J. Cardiol. 2010, 17, 223–229. [Google Scholar]
- Devinsky, O. Effects of Seizures on Autonomic and Cardiovascular Function. Epilepsy Curr. 2004, 4, 43–46. [Google Scholar] [CrossRef]
- Koos, B.J.; Chau, A.; Matsuura, M.; Punla, O.; Kruger, L. Thalamic Locus Mediates Hypoxic Inhibition of Breathing in Fetal Sheep. J. Neurophysiol. 1998, 79, 2383–2393. [Google Scholar] [CrossRef]
- Saper, C.B. Convergence of Autonomic and Limbic Connections in the Insular Cortex of the Rat. J. Comp. Neurol. 1982, 210, 163–173. [Google Scholar] [CrossRef]
- Engel, J., Jr. Introduction to Temporal Lobe Epilepsy. Epilepsy Res. 1996, 26, 141–150. [Google Scholar] [CrossRef]
- Bertram, E.H. Temporal Lobe Epilepsy: Where Do the Seizures Really Begin? Epilepsy Behav. 2009, 14, 32–37. [Google Scholar] [CrossRef]
- Sutula, T.; Cascino, G.; Cavazos, J.; Parada, I.; Ramirez, L. Mossy Fiber Synaptic Reorganization in the Epileptic Human Temporal Lobe. Ann. Neurol. 1989, 26, 321–330. [Google Scholar] [CrossRef]
- Wozny, C.; Gabriel, S.; Jandova, K.; Schulze, K.; Heinemann, U.; Behr, J. Entorhinal Cortex Entrains Epileptiform Activity in CA1 in Pilocarpine-Treated Rats. Neurobiol. Dis. 2005, 19, 451–460. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G.; Bedner, P. Astrocyte Dysfunction in Temporal Lobe Epilepsy: K+ Channels and Gap Junction Coupling. Glia 2012, 60, 1192–1202. [Google Scholar] [CrossRef]
- Theodore, W.H.; Bhatia, S.; Hatta, J.; Fazilat, S.; DeCarli, C.; Bookheimer, S.Y.; Gaillard, W.D. Hippocampal Atrophy, Epilepsy Duration, and Febrile Seizures in Patients with Partial Seizures. Neurology 1999, 52, 132. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.J.; So, E.L.; Meyer, F.B.; Parisi, J.E.; Jack, C.R. Progressive Hippocampal Atrophy in Chronic Intractable Temporal Lobe Epilepsy. Ann. Neurol. 1999, 45, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Flowers, K.A.; Walster, K.L. Attentional Control in Patients with Temporal Lobe Epilepsy. J. Neuropsychol. 2014, 8, 140–146. [Google Scholar] [CrossRef]
- Meador, K.J. Cognitive Outcomes and Predictive Factors in Epilepsy. Neurology 2002, 58 (Suppl. S5), S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.; Lin, J.J.; Seidenberg, M.; Hermann, B. The Neurobiology of Cognitive Disorders in Temporal Lobe Epilepsy. Nat. Rev. Neurol. 2011, 7, 154–164. [Google Scholar] [CrossRef]
- York, M.K.; Rettig, G.M.; Grossman, R.G.; Hamilton, W.J.; Armstrong, D.D.; Levin, H.S.; Mizrahi, E.M. Seizure Control and Cognitive Outcome after Temporal Lobectomy: A Comparison of Classic Ammon’s Horn Sclerosis, Atypical Mesial Temporal Sclerosis, and Tumoral Pathologies. Epilepsia 2003, 44, 387–398. [Google Scholar] [CrossRef]
- Pulliainen, V.; Kuikka, P.; Jokelainen, M. Motor and Cognitive Functions in Newly Diagnosed Adult Seizure Patients before Antiepileptic Medication: Cognitive Functions in Newly Diagnosed Seizure Patients. Acta Neurol. Scand. 2000, 101, 73–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.E.; Park, C.; Yoo, J.H.; Lee, H.W. Gray and White Matter Volumes and Cognitive Dysfunction in Drug-Naïve Newly Diagnosed Pediatric Epilepsy. BioMed Res. Int. 2015, 2015, 923861. [Google Scholar] [CrossRef]
- Soria, F.N.; Pérez-Samartín, A.; Martin, A.; Gona, K.B.; Llop, J.; Szczupak, B.; Chara, J.C.; Matute, C.; Domercq, M. Extrasynaptic Glutamate Release through Cystine/Glutamate Antiporter Contributes to Ischemic Damage. J. Clin. Investig. 2014, 124, 3645–3655. [Google Scholar] [CrossRef]
- Kim, E.-H.; Ko, T.-S. Cognitive Impairment in Childhood Onset Epilepsy: Up-to-Date Information about Its Causes. Korean J. Pediatr. 2016, 59, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Baxter, P.; Kassubek, R.; Albrecht, P.; Van Liefferinge, J.; Westhoff, M.-A.; Halatsch, M.-E.; Karpel-Massler, G.; Meakin, P.J.; Hayes, J.D.; et al. Phosphoinositide 3-Kinases Upregulate System xc− via Eukaryotic Initiation Factor 2α and Activating Transcription Factor 4—A Pathway Active in Glioblastomas and Epilepsy. Antioxid. Redox Signal. 2014, 20, 2907–2922. [Google Scholar] [CrossRef]
- Gallagher, M. The System Cystine/Glutamate Antiporter as an Antiepileptogenic Target. Epilepsy Curr. 2020, 20, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.S.; Hewett, J.A.; Hewett, S.J. Decreased Epileptogenesis in Mice Lacking the System xc− Transporter Occurs in Association with a Reduction in AMPA Receptor Subunit GluA1. Epilepsia Open 2019, 4, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, K.; Van Liefferinge, J.; Albertini, G.; Neveux, M.; Dardenne, S.; Mairet-Coello, G.; Vandenplas, C.; Deprez, T.; Chong, S.; Foerch, P.; et al. Anticonvulsant and Antiepileptogenic Effects of System Xc− Inactivation in Chronic Epilepsy Models. Epilepsia 2019, 60, 1412–1423. [Google Scholar] [CrossRef]
- Boison, D. Adenosine Kinase: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef]
- Chen, J.-F.; Sonsalla, P.K.; Pedata, F.; Melani, A.; Domenici, M.R.; Popoli, P.; Geiger, J.; Lopes, L.V.; De Mendonça, A. Adenosine A2A Receptors and Brain Injury: Broad Spectrum of Neuroprotection, Multifaceted Actions and “Fine Tuning” Modulation. Prog. Neurobiol. 2007, 83, 310–331. [Google Scholar] [CrossRef]
- Lopes, L.V.; Cunha, R.A.; Kull, B.; Fredholm, B.B.; Ribeiro, J.A. Adenosine A2A Receptor Facilitation of Hippocampal Synaptic Transmission Is Dependent on Tonic A1 Receptor Inhibition. Neuroscience 2002, 112, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Baer, S.B.; Gesese, R.; Cook, J.M.; Weltha, L.; Coffman, S.Q.; Wu, J.; Chen, J.-F.; Gao, M.; Ji, T. Adenosine-A2A Receptor Signaling Plays a Crucial Role in Sudden Unexpected Death in Epilepsy. Front. Pharmacol. 2022, 13, 910535. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Sutula, T.P. Is Epilepsy a Progressive Disorder? Prospects for New Therapeutic Approaches in Temporal-Lobe Epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Boison, D. Adenosinergic Signaling in Epilepsy. Neuropharmacology 2016, 104, 131–139. [Google Scholar] [CrossRef]
- Dragunow, M. Purinergic Mechanisms in Epilepsy. Prog. Neurobiol. 1988, 31, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Ochiishi, T.; Takita, M.; Ikemoto, M.; Nakata, H.; Suzuki, S.S. Immunohistochemical Analysis on the Role of Adenosine A1 Receptors in Epilepsy. Neuroreport 1999, 10, 3535–3541. [Google Scholar] [CrossRef]
- Young, D.; Dragunow, M. Status Epilepticus May Be Caused by Loss of Adenosine Anticonvulsant Mechanisms. Neuroscience 1994, 58, 245–261. [Google Scholar] [CrossRef]
- Von Lubitz, D.K. Adenosine in the Treatment of Stroke: Yes, Maybe, or Absolutely Not? Expert Opin. Investig. Drugs 2001, 10, 619–632. [Google Scholar] [CrossRef]
- Barros-Barbosa, A.R.; Ferreirinha, F.; Oliveira, Â.; Mendes, M.; Lobo, M.G.; Santos, A.; Rangel, R.; Pelletier, J.; Sévigny, J.; Cordeiro, J.M.; et al. Adenosine A2A Receptor and Ecto-5′-Nucleotidase/CD73 Are Upregulated in Hippocampal Astrocytes of Human Patients with Mesial Temporal Lobe Epilepsy (MTLE). Purinergic Signal. 2016, 12, 719–734. [Google Scholar] [CrossRef]
- Rebola, N.; Lujan, R.; Cunha, R.A.; Mulle, C. Adenosine A2A Receptors Are Essential for Long-Term Potentiation of NMDA-EPSCs at Hippocampal Mossy Fiber Synapses. Neuron 2008, 57, 121–134. [Google Scholar] [CrossRef]
- Marchi, M.; Raiteri, L.; Risso, F.; Vallarino, A.; Bonfanti, A.; Monopoli, A.; Ongini, E.; Raiteri, M. Effects of Adenosine A1 and A2A Receptor Activation on the Evoked Release of Glutamate from Rat Cerebrocortical Synaptosomes. Br. J. Pharmacol. 2002, 136, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Lee, H.-K.; Choi, S.-S.; Han, K.-J.; Han, E.-J.; Suh, H.-W. Roles of Adenosine Receptors in the Regulation of Kainic Acid-Induced Neurotoxic Responses in Mice. Mol. Brain Res. 2004, 125, 76–85. [Google Scholar] [CrossRef]
- Rosim, F.E.; Persike, D.S.; Nehlig, A.; Amorim, R.P.; de Oliveira, D.M.; da Silva Fernandes, M.J. Differential Neuroprotection by A1 Receptor Activation and A2A Receptor Inhibition Following Pilocarpine-Induced Status Epilepticus. Epilepsy Behav. 2011, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yekhlef, L.; Breschi, G.L.; Lagostena, L.; Russo, G.; Taverna, S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J. Neurophysiol. 2015, 113, 1616–1630. [Google Scholar] [CrossRef]
- Puhahn-Schmeiser, B.; Leicht, K.; Gessler, F.; Freiman, T.M. Aberrant hippocampal mossy fibers in temporal lobe epilepsy target excitatory and inhibitory neurons. Epilepsia 2021, 62, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kwon, O.Y.; Jung, S.; Kim, Y.S.; Kim, S.K.; Kang, H.; Park, K.J.; Choi, N.C.; Lim, B.H. Relationship between Hyperventilation-Induced Electroencephalographic Changes and PCO2 Level. J. Epilepsy Res. 2012, 2, 5–9. [Google Scholar] [CrossRef]
- Schmeiser, B.; Zentner, J.; Prinz, M.; Brandt, A.; Freiman, T.M. Extent of mossy fiber sprouting in patients with mesiotemporal lobe epilepsy correlates with neuronal cell loss and granule cell dispersion. Epilepsy Res. 2017, 129, 51–58. [Google Scholar] [CrossRef]
- Burtscher, J.; Schwarzer, C. The Opioid System in Temporal Lobe Epilepsy: Therapeutic Potential. Front. Mol. Neurosci. 2017, 10, 245. [Google Scholar] [CrossRef]
- Houser, C.R.; Miyashiro, J.E.; Swartz, B.E.; Walsh, G.O.; Rich, J.R.; Delgado-Escueta, A.V. Altered Patterns of Dynorphin Immunoreactivity Suggest Mossy Fiber Reorganization in Human Hippocampal Epilepsy. J. Neurosci. 1990, 10, 267–282. [Google Scholar] [CrossRef]
- Lynd-Balta, E.; Pilcher, W.H.; Joseph, S.A. AMPA Receptor Alterations Precede Mossy Fiber Sprouting in Young Children with Temporal Lobe Epilepsy. Neuroscience 2004, 126, 105–114. [Google Scholar] [CrossRef]
- Wang, I.-T.J.; Allen, M.; Goffin, D.; Zhu, X.; Fairless, A.H.; Brodkin, E.S.; Siegel, S.J.; Marsh, E.D.; Blendy, J.A.; Zhou, Z. Loss of CDKL5 Disrupts Kinome Profile and Event-Related Potentials Leading to Autistic-like Phenotypes in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 21516–21521. [Google Scholar] [CrossRef]
- Verrier, R.L.; Pang, T.D.; Nearing, B.D.; Schachter, S.C. The Epileptic Heart: Concept and Clinical Evidence. Epilepsy Behav. 2020, 105, 106946. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Cardiorespiratory Findings in Sudden Unexplained/Unexpected Death in Epilepsy (SUDEP). Epilepsy Res. 2004, 59, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Delogu, A.B.; Spinelli, A.; Battaglia, D.; Dravet, C.; De Nisco, A.; Saracino, A.; Romagnoli, C.; Lanza, G.A.; Crea, F. Electrical and Autonomic Cardiac Function in Patients with Dravet Syndrome. Epilepsia 2011, 52 (Suppl. S2), 55–58. [Google Scholar] [CrossRef]
- Trosclair, K.; Dhaibar, H.A.; Gautier, N.M.; Mishra, V.; Glasscock, E. Neuron-Specific Kv1.1 Deficiency Is Sufficient to Cause Epilepsy, Premature Death, and Cardiorespiratory Dysregulation. Neurobiol. Dis. 2020, 137, 104759. [Google Scholar] [CrossRef] [PubMed]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in Adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Rossi, M.; Janse van Rensburg, M.; Santangelo, G.; Ricciardi, D.; Vico, C.; Russo, M.; Fachileoli, G.; Campana, L.; Rossi, F.; De Lucia, M.; et al. Wearable Devices in Epilepsy: From Seizure Detection to SUDEP Prevention. Epilepsy Behav. 2021, 104, 106935. [Google Scholar]
- Esmaeili, B.; Kaffashi, F.; Theeranaew, W.; Dabir, A.; Lhatoo, S.D.; Loparo, K.A. Post-Ictal Modulation of Baroreflex Sensitivity in Patients with Intractable Epilepsy. Front. Neurol. 2018, 9, 793. [Google Scholar] [CrossRef]
- Glasscock, E. Kv1.1 Potassium Channel Deficiency Reveals Brain-Driven Cardiac Dysfunction as a Candidate Mechanism for Sudden Unexplained Death in Epilepsy. J. Neurosci. 2010, 30, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A Systematic Review and Meta-Analysis of Heart Rate Variability in Epilepsy and Antiepileptic Drugs. Epilepsia 2012, 53, 272–282. [Google Scholar] [CrossRef]
- Zaccara, G.; Franciotta, D.; Lattanzi, S. The Seizure-Heart Connection: Perspectives and Potential Impact of Heart Rate Variability (HRV) in Epilepsy. Epilepsia 2018, 59, 1969–1981. [Google Scholar]
- Moseley, B.D.; Nickels, K.C.; Britton, J.W.; Tyler-Kabara, E.C.; Cervenka, M.C.; Schuele, S.U.; Rueda, S.; Armstrong, C.; Smith, R.L.; Saadi, A. Sudden Unexpected Death in Epilepsy: An Analysis of 24 Years of Autopsies from a Single Center. Epilepsia 2013, 54, 119–125. [Google Scholar]
- Hirsch, L.J.; LaRoche, S.M.; Gaspard, N.; Herman, S.T.; Jennum, P.; Kapadia, M.Z.; Lee, H.; Leitinger, M.; Lin, J.J.; Milligan, T.M.; et al. Risk Factors for Sudden Unexpected Death in Epilepsy: Current Understanding and Future Directions. Epilepsy Curr. 2021, 21, 13–23. [Google Scholar]
- Aurlien, D.; Leren, T.P.; Taubøll, E.; Gjerstad, L. New Directions in the Prevention of Sudden Unexpected Death in Epilepsy. Seizure 2016, 38, 30–36. [Google Scholar]
- Nashef, L.; So, E.L.; Ryvlin, P.; Tomson, T. Unifying the Definitions of Sudden Unexpected Death in Epilepsy. Epilepsia 2012, 53, 227–233. [Google Scholar] [CrossRef]
- Nei, M.; Ho, R.T.; Abou-Khalil, B.W.; Drislane, F.W.; Liporace, J.D.; Romeo, A.; Schmitt, S.E.; Singh, G.; Spencer, D.C.; Sperling, M.R.; et al. Postictal Autonomic Dysfunction and Cardiac Arrhythmias in Epilepsy. Neurology 2023, 100, e1804–e1815. [Google Scholar]
- Cui, W.; Wang, Y.; Hu, Y.; Gao, Y.; Zhang, Y.; Li, Y.; Wang, J. Wearable Electrocardiogram Monitoring Devices for Epilepsy Patients: Advancing Real-Time Seizure Management. J. Biomed. Inform. 2021, 121, 103882. [Google Scholar]
- van Westrhenen, A.; Geertsema, E.E.; van Rootselaar, A.F.; Stolker, R.J.; Hendrikse, J. Cardiac Effects of Epilepsy and Antiepileptic Drugs: A Systematic Review. CNS Drugs 2020, 34, 233–256. [Google Scholar]
- Beniczky, S.; Ryvlin, P. Standards for Testing and Clinical Validation of Seizure Detection Devices. Epilepsia 2018, 59 (Suppl. S1), 9–13. [Google Scholar] [CrossRef]
- Myers, K.A.; Bello-Espinosa, L.; Ladbon-Bernard, D.; Farrell, K.; Gofton, T.; Mackenzie, A.; McLachlan, R.S.; Wirrell, E.C. Cardiac Monitoring in Epilepsy: Are We Utilizing It Effectively? Seizure 2018, 58, 47–54. [Google Scholar]
- Magruder, K.P.; Grant, L.; Spencer, D.C. Wearable Devices for Epilepsy: The Use of Heart Rate and Machine Learning in Monitoring Seizures. Epilepsy Res. 2019, 156, 106181. [Google Scholar]
- Rugg-Gunn, F.J. Adapting to New Technologies in Epilepsy Monitoring: The Future of Seizure Detection. J. Neurol. Neurosurg. Psychiatry 2021, 92, 301–310. [Google Scholar]
- Surges, R.; Strzelczyk, A.; Scott, C.A.; Walker, M.C. Postictal Generalized EEG Suppression and SUDEP: Mechanisms and Implications. Epilepsy Behav. 2018, 86, 123–127. [Google Scholar]
- Cogan, D.; Birjandtalab, J.; Nourani, M. Machine Learning Applications in Seizure Prediction and Epilepsy Management. IEEE Rev. Biomed. Eng. 2020, 13, 30–46. [Google Scholar]
- Milosevic, M.; Van de Vel, A.; Bonroy, B.; Ceulemans, B.; Lagae, L.; Van Huffel, S.; Vanrumste, B. Automated Detection of Tonic-Clonic Seizures Using a Wearable Accelerometer Device. Epilepsia 2021, 62, 1187–1196. [Google Scholar]
- Conradsen, I.; Beniczky, S.; Wolf, P.; Kjaer, T.W.; Sams, T.; Sørensen, H.B.D. Automatic Multi-Modal Intelligent Seizure Acquisition (MISA) System for Detection of Motor Seizures from Electromyographic Data and Motion Data. Comput. Methods Programs Biomed. 2012, 107, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Vilella, L.; Lacuey, N.; Hampson, J.P.; Rani, M.S.; Loparo, K.; Sainju, R.K.; Friedman, D.; Nei, M.; Strohl, K.; Allen, L.; et al. Incidence, Recurrence, and Risk Factors for Peri-Ictal Central Apnea and Sudden Unexpected Death in Epilepsy. Front. Neurol. 2019, 10, 166. [Google Scholar] [CrossRef]
- Devinsky, O.; Friedman, D.; Lai, Y.C.; Macher, K.; Tran, T.T.; Philbrook, B. Combined Cardiac and Respiratory Monitoring to Predict Sudden Unexpected Death in Epilepsy. Epilepsia 2018, 59, 1904–1914. [Google Scholar]
- Shorvon, S.; Tomson, T. Sudden Unexpected Death in Epilepsy. Lancet 2011, 378, 2028–2038. [Google Scholar] [CrossRef]
- Hesdorffer, D.C.; Tomson, T.; Benn, E.; Sander, J.W.; Nilsson, L.; Langan, Y. Combined Analysis of Risk Factors for SUDEP. Epilepsia 2020, 61, e27–e32. [Google Scholar] [CrossRef]
- Surges, R.; Sander, J.W. Sudden unexpected death in epilepsy: Mechanisms, prevalence, and prevention. Curr. Opin. Neurol. 2012, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lhatoo, S.D.; Faulkner, H.J.; Dembny, K.; Trippick, K.; Johnson, C.; Bird, J.M. An Electroclinical Case-Control Study of Sudden Unexpected Death in Epilepsy. Ann. Neurol. 2010, 68, 787–796. [Google Scholar] [CrossRef]
- Purnell, B.S.; Thijs, R.D.; Buchanan, G.F. Deadly Rhythms: Neurological Modulation of Heart Rate Precedes Sudden Unexpected Death in Epilepsy. Front. Neurol. 2021, 12, 654576. [Google Scholar]
- Ali, A.; Ali, I.; Keil, A.; Diaz, G.; Kabbani, H.; Detyniecki, K. Peri-Ictal Breathing Patterns and SUDEP Risk. Epilepsy Behav. 2017, 77, 96–100. [Google Scholar]
- Lucey, B.P.; Nelson, S.E.; Lopez, J.E.; Howlett, J.A.; Green, A.L.; Koehler, P.J. Cardiorespiratory Changes Associated with Seizures in Adults. J. Clin. Neurophysiol. 2016, 33, 282–289. [Google Scholar]
- Nobili, L.; Proserpio, P.; Cicolin, A.; Sforza, E.; Braghiroli, A.; Nobili, F. Cardiorespiratory and Autonomic Alterations in Epilepsy: Implications for SUDEP Prevention. Clin. Neurophysiol. Pract. 2020, 5, 129–138. [Google Scholar]
- Tupal, S.; Faingold, C.L. Serotonin and Sudden Death: Differential Effects of Serotonin Receptor Subtypes in the Brainstem. Neurosci. Lett. 2018, 703, 147–153. [Google Scholar]
- Glasscock, E. The Role of Respiratory and Autonomic Dysfunction in Sudden Unexpected Death in Epilepsy (SUDEP). J. Physiol. 2018, 596, 2247–2260. [Google Scholar]
- Kinney, H.C.; Poduri, A.H.; Cryan, J.B.; Haynes, R.L.; Teot, L.; Sleeper, L.A.; Holm, I.A.; Berry, G.T.; Prabhu, S.P.; Warfield, S.K.; et al. Hippocampal Formation Maldevelopment and Sudden Unexpected Death Across the Pediatric Age Spectrum. J. Neuropathol. Exp. Neurol. 2016, 75, 981–997. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Smith, C.A.; Blain, G.M.; Xie, A. Role of Chemoreception in Sudden Unexpected Death in Epilepsy (SUDEP). J. Physiol. 2020, 598, 2335–2347. [Google Scholar]
- Cooper, M.S.; McIntosh, A.; Crompton, D.E.; McMahon, J.M.; Schneider, A.; Farrell, K.; Ganesan, V.; Gill, D.; Kivity, S.; Lerman-Sagie, T.; et al. Mortality in Dravet Syndrome. Epilepsy Res. 2021, 169, 106525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, M.Y.; Seh, B.A.; Zahra, S.; Legradi, A. The Crucial Interplay Between the Lungs, Brain, and Heart to Understand Epilepsy-Linked SUDEP: A Literature Review. Brain Sci. 2025, 15, 809. https://doi.org/10.3390/brainsci15080809
Mir MY, Seh BA, Zahra S, Legradi A. The Crucial Interplay Between the Lungs, Brain, and Heart to Understand Epilepsy-Linked SUDEP: A Literature Review. Brain Sciences. 2025; 15(8):809. https://doi.org/10.3390/brainsci15080809
Chicago/Turabian StyleMir, Mohd Yaqub, Bilal A. Seh, Shabab Zahra, and Adam Legradi. 2025. "The Crucial Interplay Between the Lungs, Brain, and Heart to Understand Epilepsy-Linked SUDEP: A Literature Review" Brain Sciences 15, no. 8: 809. https://doi.org/10.3390/brainsci15080809
APA StyleMir, M. Y., Seh, B. A., Zahra, S., & Legradi, A. (2025). The Crucial Interplay Between the Lungs, Brain, and Heart to Understand Epilepsy-Linked SUDEP: A Literature Review. Brain Sciences, 15(8), 809. https://doi.org/10.3390/brainsci15080809





