Prejudice, Proxemic Space, and Social Odor: The Representation of the ‘Outsider’ Through an Evolutionary Metaverse Psychology Perspective
Abstract
1. Introduction
1.1. Experimental Hypotheses
1.2. Aim
2. Materials and Methods
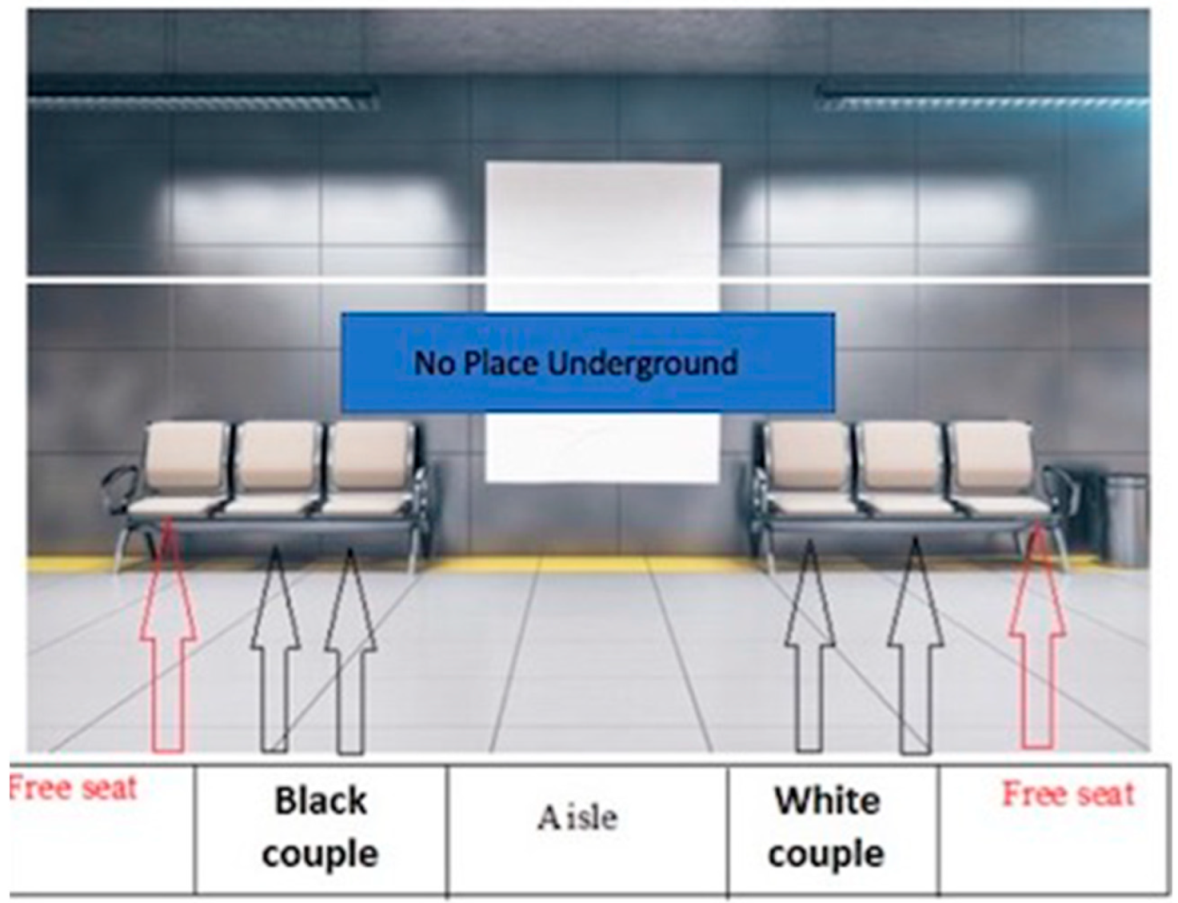
2.1. Experimental Protocol
2.2. Olfactory Stimuli
2.3. Participants
2.4. Data Collection Procedure and Study Assessment
2.5. Experimental Designs
2.5.1. Experiment 1: The Influence of Social Odors on Prejudice in the Metaverse
2.5.2. Experiment 2: Neural Correlates of Social Odor Bias in the Metaverse
2.5.3. Experiment 3: The Role of Social Odors in Implicit Association Related to Prejudice
2.6. Data Analyses
3. Discussion
3.1. Study Limitation
3.2. Relevance of the Research and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, H.S.; Bohlen, J. Implicit Bias. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- FitzGerald, C.; Hurst, S. Implicit Bias in Healthcare Professionals: A Systematic Review. BMC Med. Ethics 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Ellemers, N. Gender Stereotypes. Annu. Rev. Psychol. 2018, 69, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.J.; Chapman, M.V.; Lee, K.M.; Merino, Y.M.; Thomas, T.W.; Payne, B.K.; Eng, E.; Day, S.H.; Coyne-Beasley, T. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am. J. Public Health 2015, 105, e60–e76. [Google Scholar] [CrossRef] [PubMed]
- Zestcott, C.A.; Blair, I.V.; Stone, J. Examining the Presence, Consequences, and Reduction of Implicit Bias in Health Care: A Narrative Review. Group Process. Intergroup Relat. 2016, 19, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Saluja, B.; Bryant, Z. How Implicit Bias Contributes to Racial Disparities in Maternal Morbidity and Mortality in the United States. J. Women’s Health 2021, 30, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M. The Behavioural Immune System and the Psychology of Human Sociality. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.; Olofsson, J.K.; Lindholm, T.; Blomkvist, A.; Liuzza, M.T. Body Odor Disgust Sensitivity Is Associated with Prejudice towards a Fictive Group of Immigrants. Physiol. Behav. 2019, 201, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Liuzza, M.T.; Lindholm, T.; Hawley, C.B.; Gustafsson Sendén, M.; Ekström, I.; Olsson, M.J.; Olofsson, J.K. Body Odour Disgust Sensitivity Predicts Authoritarian Attitudes. R. Soc. Open Sci. 2018, 5, 171091. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.; Jaeger, B.; Tybur, J.M. A Behavioural Immune System Perspective on Disgust and Social Prejudice. Nat. Rev. Psychol. 2023, 2, 676–687. [Google Scholar] [CrossRef]
- Aarøe, L.; Petersen, M.B.; Arceneaux, K. The Behavioral Immune System Shapes Political Intuitions: Why and How Individual Differences in Disgust Sensitivity Underlie Opposition to Immigration. Am. Political Sci. Rev. 2017, 111, 277–294. [Google Scholar] [CrossRef]
- Stevenson, H. BELMAS Report: Reflections upon a Year of BELMAS Council Membership. Manag. Educ. 2009, 23, 3–4. [Google Scholar] [CrossRef]
- Olsson, M.J.; Lundström, J.N.; Kimball, B.A.; Gordon, A.R.; Karshikoff, B.; Hosseini, N.; Sorjonen, K.; Olgart Höglund, C.; Solares, C.; Soop, A.; et al. The Scent of Disease: Human Body Odor Contains an Early Chemosensory Cue of Sickness. Psychol. Sci. 2014, 25, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Semin, G.R.; Groot, J.H.B. de The Chemical Bases of Human Sociality. Trends Cogn. Sci. 2013, 17, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Lübke, K.T.; Pause, B.M. Always Follow Your Nose: The Functional Significance of Social Chemosignals in Human Reproduction and Survival. Horm. Behav. 2015, 68, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Semin, G.R.; Scandurra, A.; Baragli, P.; Lanatà, A.; D’Aniello, B. Inter- and Intra-Species Communication of Emotion: Chemosignals as the Neglected Medium. Animals 2019, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Coppin, G.; Pool, E.; Delplanque, S.; Oud, B.; Margot, C.; Sander, D.; Van Bavel, J.J. Swiss Identity Smells like Chocolate: Social Identity Shapes Olfactory Judgments. Sci. Rep. 2016, 6, 34979. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.J.; Coppin, G.; Van Bavel, J.J. Perceiving the World through Group-Colored Glasses: A Perceptual Model of Intergroup Relations. Psychol. Inq. 2016, 27, 255–274. [Google Scholar] [CrossRef]
- Lundström, J.N.; Olsson, M.J. Functional Neuronal Processing of Human Body Odors. Vitam. Horm. 2010, 83, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Oren, C.; Shamay-Tsoory, S.G. Women’s Fertility Cues Affect Cooperative Behavior: Evidence for the Role of the Human Putative Chemosignal Estratetraenol. Psychoneuroendocrinology 2019, 101, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.B.; Pierzchajlo, S.R.; Mitchell, D.G.V. Neural Correlates of Social and Non-Social Personal Space Intrusions: Role of Defensive and Peripersonal Space Systems in Interpersonal Distance Regulation. Soc. Neurosci. 2020, 15, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Patel, H.; D’Cruz, M.; Cobb, S. What Makes a Space Invader? Passenger Perceptions of Personal Space Invasion in Aircraft Travel. Ergonomics 2017, 60, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- McCall, C.; Blascovich, J.; Young, A.; Persky, S. Proxemic Behaviors as Predictors of Aggression towards Black (but Not White) Males in an Immersive Virtual Environment. Soc. Influ. 2009, 4, 138–154. [Google Scholar] [CrossRef]
- Lisi, M.P.; Fusaro, M.; Tieri, G.; Aglioti, S.M. Humans Adjust Virtual Comfort-Distance towards an Artificial Agent Depending on Their Sexual Orientation and Implicit Prejudice against Gay Men. Comput. Hum. Behav. 2021, 125, 106948. [Google Scholar] [CrossRef]
- Patané, I.; Lelgouarch, A.; Banakou, D.; Verdelet, G.; Desoche, C.; Koun, E.; Salemme, R.; Slater, M.; Farnè, A. Exploring the Effect of Cooperation in Reducing Implicit Racial Bias and Its Relationship with Dispositional Empathy and Political Attitudes. Front. Psychol. 2020, 11, 510787. [Google Scholar] [CrossRef] [PubMed]
- Fini, C.; Verbeke, P.; Sieber, S.; Moors, A.; Brass, M.; Genschow, O. The Influence of Threat on Perceived Spatial Distance to Out-Group Members. Psychol. Res. 2020, 84, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Lucifora, C.; Gangemi, A.; D’Italia, G.; Culicetto, L.; Ferraioli, F.; Grasso, G.M.; Vicario, C.M. PanicRoom: A Virtual Reality-Based Pavlovian Fear Conditioning Paradigm. Front. Psychol. 2024, 15, 1432141. [Google Scholar] [CrossRef] [PubMed]
- Lucifora, C.; Schembri, M.; Poggi, F.; Grasso, G.M.; Gangemi, A. Virtual Reality Supports Perspective Taking in Cultural Heritage Interpretation. Comput. Hum. Behav. 2023, 148, 107911. [Google Scholar] [CrossRef]
- Lucifora, C.; Grasso, G.M.; Nitsche, M.A.; D’Italia, G.; Sortino, M.; Salehinejad, M.A.; Falzone, A.; Avenanti, A.; Vicario, C.M. Enhanced Fear Acquisition in Individuals with Evening Chronotype. A Virtual Reality Fear Conditioning/Extinction Study. J. Affect. Disord. 2022, 311, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Vicario, C.M.; Culicetto, L.; Lucifora, C.; Ferraioli, F.; Massimino, S.; Martino, G.; Tomaiuolo, F.; Falzone, A.M. The Power of Belief? Evidence of Reduced Fear Extinction Learning in Catholic God Believers. Front. Public Health 2025, 12, 1509388. [Google Scholar] [CrossRef] [PubMed]
- Karakoc, C.; Lucifora, C.; Massimino, S.; Nucera, S.; Vicario, C.M. Extending Peri-Personal Space in Immersive Virtual Reality: A Systematic Review. Virtual Worlds 2025, 4, 5. [Google Scholar] [CrossRef]
- Vicario, C.M.; Martino, G.; Vicario, C.M.; Martino, G. Psychology and Technology: How Virtual Reality Can Boost Psychotherapy and Neurorehabilitation. AIMS Neurosci. 2022, 9, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Vicario, C.M.; Salehinejad, M.A.; Lucifora, C.; Martino, G.; Falzone, A.M.; Grasso, G.; Nitsche, M.A. Combining Virtual Reality Exposure Therapy with Non-Invasive Brain Stimulation for the Treatment of Post-Traumatic Stress Disorder and Related Syndromes: A Perspective. In Translational Methods for PTSD Research; Pinna, G., Ed.; Springer: New York, NY, USA, 2023; pp. 231–245. ISBN 978-1-07-163218-5. [Google Scholar]
- Ferraioli, F.; Culicetto, L.; Cecchetti, L.; Falzone, A.; Tomaiuolo, F.; Quartarone, A.; Vicario, C.M. Virtual Reality Exposure Therapy for Treating Fear of Contamination Disorders: A Systematic Review of Healthy and Clinical Populations. Brain Sci. 2024, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The Great Pheromone Myth; JHU Press: Baltimore, MD, USA, 2010. [Google Scholar]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Reproducible Research into Human Chemical Communication by Cues and Pheromones: Learning from Psychology’s Renaissance. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190262. [Google Scholar] [CrossRef] [PubMed]
- Berglund, H.; Lindström, P.; Savic, I. Brain Response to Putative Pheromones in Lesbian Women. Proc. Natl. Acad. Sci. USA 2006, 103, 8269–8274. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.J.; Brooksbank, B.W.L. Human Exposure to Putative Pheromones and Changes in Aspects of Social Behaviour. J. Steroid Biochem. Mol. Biol. 1991, 39, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.N.; Gonçalves, M.; Esteves, F.; Olsson, M.J. Psychological Effects of Subthreshold Exposure to the Putative Human Pheromone 4,16-Androstadien-3-One. Horm. Behav. 2003, 44, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Keshmiri, S.; Mazzatenta, A.; Grasso, A.; Romano, D.; Bona, F.; Shiomi, M.; Sumioka, H.; Ishiguro, H. Perception of Social Odor and Gender-Related Differences Investigated Through the Use of Transfer Entropy and Embodied Medium. Front. Syst. Neurosci. 2021, 15, 650528. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, L.; Di Dio, C.; Manzi, F.; Massaro, D.; Schito, A.; Mazzatenta, A.; Cangelosi, A.; Invitto, S.; Marchetti, A. Sensing Robots and Social Odor: How Relational Engagement and Trust Change. IEEE Trans. Affect. Comput. 2025; early access. [Google Scholar] [CrossRef]
- Invitto, S.; Keshmiri, S. Chemosensory Neuro-Olfactometry, Pheromones Perceptions, and EEG Signal Processing Methods. In Neuromethods; Springer: New York, NY, USA, 2024; Volume 206, pp. 109–114. [Google Scholar]
- Preti, G.; Wysocki, C.J.; Barnhart, K.T.; Sondheimer, S.J.; Leyden, J.J. Male Axillary Extracts Contain Pheromones That Affect Pulsatile Secretion of Luteinizing Hormone and Mood in Women Recipients. Biol. Reprod. 2003, 68, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K. Effects of 5alpha-Androst-16-En-3alpha-Ol on the Pulsatile Secretion of Luteinizing Hormone in Human Females. Chem. Senses 2000, 25, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Laktionova, T.; Kvasha, I.; Voznessenskaya, V. Male Body Odor Affects Emotional State, LH, and Cortisol Secretion in Women of Different Age Groups. Brain Sci. 2024, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Neguț, A.; Matu, S.-A.; Sava, F.A.; David, D. Virtual Reality Measures in Neuropsychological Assessment: A Meta-Analytic Review. Clin. Neuropsychol. 2016, 30, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Piraino, G.; Ciccarese, V.; Carmillo, L.; Caggiula, M.; Trianni, G.; Nicolardi, G.; Di Nuovo, S.; Balconi, M. Potential Role of OERP as Early Marker of Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Boscolo-Rizzo, P.; Fantin, F.; Bonifati, D.M.; de Filippis, C.; Emanuelli, E.; Frezza, D.; Giopato, F.; Caggiula, M.; Schito, A.; et al. Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway? Bioengineering 2023, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moallem, I.; Paller, K.A.; Gottfried, J.A. Subliminal Smells Can Guide Social Preferences. Psychol. Sci. 2007, 18, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Boulkroune, N.; Wang, L.; March, A.; Walker, N.; Jacob, T.J.C. Repetitive Olfactory Exposure to the Biologically Significant Steroid Androstadienone Causes a Hedonic Shift and Gender Dimorphic Changes in Olfactory-Evoked Potentials. Neuropsychopharmacology 2007, 32, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M.; Krauel, K.; Schrader, C.; Sojka, B.; Westphal, E.; Müller-Ruchholtz, W.; Ferstl, R. The Human Brain Is a Detector of Chemosensorily Transmitted HLA-Class I-Similarity in Same- and Opposite-Sex Relations. Proc. R. Soc. B Biol. Sci. 2005, 273, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.N.; Olsson, M.J.; Schaal, B.; Hummel, T. A Putative Social Chemosignal Elicits Faster Cortical Responses than Perceptually Similar Odorants. Neuroimage 2006, 30, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Allport, G.W. The Nature of Prejudice; Addison-Wesley: Oxford, UK, 1954; p. xviii, 537. [Google Scholar]
- Herek, G.M.; McLemore, K.A. Sexual Prejudice. Annu. Rev. Psychol. 2013, 64, 309–333. [Google Scholar] [CrossRef]
- Batson, C.D.; Schoenrade, P.; Ventis, W.L. Religion and the Individual: A Social-Psychological Perspective; Oxford University Press: New York, NY, USA, 1993; p. viii, 427. ISBN 978-0-19-506208-3. [Google Scholar]
- Tsang, J.-A.; Rowatt, W.C. The Relationship between Religious Orientation, Right-Wing Authoritarianism, and Implicit Sexual Prejudice. Int. J. Psychol. Relig. 2007, 17, 99–120. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Nosek, B.A.; Banaji, M.R. Understanding and Using the Implicit Association Test: I. An Improved Scoring Algorithm. J. Personal. Soc. Psychol. 2003, 85, 197–216. [Google Scholar] [CrossRef]
- Allport, G.W.; Ross, J.M. Personal Religious Orientation and Prejudice. J. Personal. Soc. Psychol. 1967, 5, 432–443. [Google Scholar] [CrossRef]
- Cuevas, J.A.; Dawson, B.L. An Integrated Review of Recent Research on the Relationships Between Religious Belief, Political Ideology, Authoritarianism, and Prejudice. Psychol. Rep. 2021, 124, 977–1014. [Google Scholar] [CrossRef] [PubMed]
- Payne, B.K. Prejudice and Perception: The Role of Automatic and Controlled Processes in Misperceiving a Weapon. J. Pers. Soc. Psychol. 2001, 81, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Hugenberg, K.; Bodenhausen, G.V. Facing Prejudice: Implicit Prejudice and the Perception of Facial Threat. Psychol. Sci. 2003, 14, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, I.; Zellag, M.; Hernout, J.; Dumais, A.; Potvin, S.; Lavoie, M.E. Alpha and Theta Oscillations during the Cognitive Reappraisal of Aversive Pictures: A Spatio-Temporal qEEG Investigation. Int. J. Psychophysiol. 2023, 192, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Stein, L.; Bentin, S. Motor and Attentional Mechanisms Involved in Social Interaction--Evidence from Mu and Alpha EEG Suppression. Neuroimage 2011, 58, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.M.; Hampton, R.S.; Spinrad, T.L.; Varnum, M.; Blais, C.; Eisenberg, N.; Gal-Szabo, D.E.; Berger, R.H.; Xu, J.; Xiao, S.X. Children’s Mu Suppression Is Sensitive to Witnessing Others’ Social Victimization. Soc. Neurosci. 2020, 15, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Siqi-Liu, A.; Harris, A.M.; Atkinson, A.P.; Reed, C.L. Dissociable Processing of Emotional and Neutral Body Movements Revealed by μ-Alpha and Beta Rhythms. Soc. Cogn. Affect. Neurosci. 2018, 13, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Gutsell, J.N.; Simon, J.C.; Jiang, Y. Perspective Taking Reduces Group Biases in Sensorimotor Resonance. Cortex 2020, 131, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Schickel, R.J.; Jauk, E.; Fink, A.; Neubauer, A.C. Alpha Power Increases in Right Parietal Cortex Reflects Focused Internal Attention. Neuropsychologia 2014, 56, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Bergner, S.; Könen, T.; Fink, A.; Neubauer, A.C. EEG Alpha Synchronization Is Related to Top-down Processing in Convergent and Divergent Thinking. Neuropsychologia 2011, 49, 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Mignozzi, A.; Piraino, G.; Capone, S.; Montagna, G.; Siciliano, P.; Mazzatenta, A.; Rocco, G.; De Feudis, I.; Brunetti, A.; et al. Smell and Meaning: An OERP Study. In Multidisciplinary Approaches to Neural Computing; Esposito, A., Faundez-Zanuy, M., Morabito, F.C., Pasero, E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 289–300. ISBN 978-3-642-20924-6. [Google Scholar]
- Invitto, S.; Mazzatenta, A. Olfactory Event-Related Potentials and Exhaled Organic Volatile Compounds: The Slow Link Between Olfactory Perception and Breath Metabolic Response. A Pilot Study on Phenylethyl Alcohol and Vaseline Oil. Brain Sci. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.J.; Carbine, K.A. Sample Size Calculations in Human Electrophysiology (EEG and ERP) Studies: A Systematic Review and Recommendations for Increased Rigor. Int. J. Psychophysiol. 2017, 111, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Martoni, R.M.; Rancoita, P.M.V.; Di Serio, C.; Brombin, C. Validating the Italian Version of the Disgust and Propensity Scale-Revised. Front. Psychol. 2017, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Liuzza, M.T.; Zakrzewska, M.Z.; Olofsson, J.K. Italian Validation of the Body Odor Disgust Scale. Front. Behav. Neurosci. 2024, 18, 1389905. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, A.; D’Alessio, M. Il Percorso Di Validazione Della Religious Attitude Scale (RAS). G. Ital. Psicol. 2010, 37, 465–488. [Google Scholar] [CrossRef]
- Bò, E.D.; Gentili, C.; Spoto, A.; Bruno, G.; Castellani, A.; Tripodi, C.; Fischmeister, F.P.S.; Cecchetto, C. The Social Odor Scale: Development and Initial Validation of a New Scale for the Assessment of Social Odor Awareness. PLoS ONE 2021, 16, e0260587. [Google Scholar] [CrossRef]
- Grigor, J.; Van Toller, S.; Behan, J.; Richardson, A. The Effect of Odour Priming on Long Latency Visual Evoked Potentials of Matching and Mismatching Objects. Chem. Senses 1999, 24, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Hummel, T. Olfactory (Chemosensory) Event-Related Potentials. Toxicol. Ind. Health 1994, 10, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M. Processing of Body Odor Signals by the Human Brain. Chemosens. Percept. 2012, 5, 55–63. [Google Scholar] [CrossRef]
- van Overveld, M.; de Jong, P.J.; Peters, M.L.; van Hout, W.J.P.J.; Bouman, T.K. An Internet-Based Study on the Relation between Disgust Sensitivity and Emetophobia. J. Anxiety Disord. 2008, 22, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Loos, H.M.; Schaal, B.; Pause, B.M.; Smeets, M.A.M.; Ferdenzi, C.; Roberts, S.C.; de Groot, J.; Lübke, K.T.; Croy, I.; Freiherr, J.; et al. Past, Present, and Future of Human Chemical Communication Research. Perspect. Psychol. Sci. 2025, 20, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; You, Y.; Farias, A.R.; Simon, J.; Semin, G.R.; Smeets, M.A.; Li, W. Human Chemosignals of Disgust Facilitate Food Judgment. Sci. Rep. 2018, 8, 17006. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, L.; Hummel, T.; Croy, I. The Design Matters: How to Detect Neural Correlates of Baby Body Odors. Front. Neurol. 2019, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M.; Storch, D.; Lübke, K.T. Chemosensory Communication of Aggression: Women’s Fine-Tuned Neural Processing of Male Aggression Signals. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190270. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.G.; McGhee, D.E.; Schwartz, J.L. Measuring Individual Differences in Implicit Cognition: The Implicit Association Test. J. Personal. Soc. Psychol. 1998, 74, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Nosek, B.A.; Greenwald, A.G.; Banaji, M.R. The Implicit Association Test at Age 7: A Methodological and Conceptual Review. In Social Psychology and the Unconscious: The Automaticity of Higher Mental Processes; Psychology Press: Sussex, UK, 2007; pp. 265–292. [Google Scholar]
- Montefinese, M.; Ambrosini, E.; Fairfield, B.; Mammarella, N. The Adaptation of the Affective Norms for English Words (ANEW) for Italian. Behav. Res. Methods 2014, 46, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.G.; Brendl, M.; Cai, H.; Cvencek, D.; Dovidio, J.F.; Friese, M.; Hahn, A.; Hehman, E.; Hofmann, W.; Hughes, S.; et al. Best Research Practices for Using the Implicit Association Test. Behav. Res. Methods 2022, 54, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Legrain, V.; Hummel, T.; Rombaux, P.; Mouraux, A. Time-Frequency Analysis of Chemosensory Event-Related Potentials to Characterize the Cortical Representation of Odors in Humans. PLoS ONE 2012, 7, e33221. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.D.; Lindsey, S.; Schooler, T.Y. A Model of Dual Attitudes. Psychol. Rev. 2000, 107, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Liuzza, M.T.; Lindholm, T.; Hawley, C.; Sendén, M.G.; Ekström, I.; Olsson, M.J.; Larsson, M.; Olofsson, J.K. The Body Odor Disgust Scale (BODS): Development and Validation of a Novel Olfactory Disgust Assessment. Chem. Senses 2017, 42, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liuzza, M.T. Olfaction and Disgust: Sensory and Affective Processes to Avoid Disease; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-84485-1. [Google Scholar]
- Darwin, C. The Expression of the Emotions in Man and Animals; John Murray: London, UK, 1872; p. vi, 374. [Google Scholar]
- Susskind, J.M.; Lee, D.H.; Cusi, A.; Feiman, R.; Grabski, W.; Anderson, A.K. Expressing Fear Enhances Sensory Acquisition. Nat. Neurosci. 2008, 11, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Rozin, P.; Haidt, J.; McCauley, C. Disgust: The Body and Soul Emotion in the 21st Century. In Disgust and Its Disorders: Theory, Assessment, and Treatment Implications; American Psychological Association: Washington, DC, USA, 2009; pp. 9–29. ISBN 978-1-4338-0397-0. [Google Scholar]
- Miller, W.R.; Thoresen, C.E. Spirituality, Religion, and Health: An Emerging Research Field. Am. Psychol. 2003, 58, 24–35. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.A. Spirituality: Description, Measurement, and Relation to the Five Factor Model of Personality. J. Personal. 2000, 68, 153–197. [Google Scholar] [CrossRef] [PubMed]
- Terrizzi, J.A., Jr.; Shook, N.J.; Ventis, W.L. Disgust: A Predictor of Social Conservatism and Prejudicial Attitudes toward Homosexuals. Personal. Individ. Differ. 2010, 49, 587–592. [Google Scholar] [CrossRef]
- Terrizzi, J.A.; Shook, N.J.; McDaniel, M.A. The Behavioral Immune System and Social Conservatism: A Meta-Analysis. Evol. Hum. Behav. 2013, 34, 99–108. [Google Scholar] [CrossRef]
- Tybur, J.M.; Merriman, L.A.; Caldwell Hooper, A.E.; McDonald, M.M.; Navarrete, C.D. Extending the Behavioral Immune System to Political Psychology: Are Political Conservativism and Disgust Sensitivity Really Related. Evol. Psychol. Int. J. Evol. Approaches Psychol. Behav. 2010, 8, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, B.O. Disgust, Scrupulosity and Conservative Attitudes about Sex: Evidence for a Mediational Model of Homophobia. J. Res. Personal. 2008, 42, 1364–1369. [Google Scholar] [CrossRef]
- Olatunji, B.O.; Tolin, D.F.; Huppert, J.D.; Lohr, J.M. The Relation between Fearfulness, Disgust Sensitivity and Religious Obsessions in a Non-Clinical Sample. Personal. Individ. Differ. 2005, 38, 891–902. [Google Scholar] [CrossRef]
- Tybur, J.M.; Lieberman, D.; Griskevicius, V. Microbes, Mating, and Morality: Individual Differences in Three Functional Domains of Disgust. J. Personal. Soc. Psychol. 2009, 97, 103–122. [Google Scholar] [CrossRef] [PubMed]

| Positive/Pleasant | Negative/Unpleasant |
|---|---|
| FELICE (happy) | DEPRESSO (depressed) |
| AMATO (loved) | SLEALE (disloyal) |
| LIBERO (free) | SGRADEVOLE (nasty) |
| ACCOGLIENTE (cozy) | STRESSATO (distressed) |
| ONESTO (honest) | PERDENTE (loser) |
| FORTUNATO (lucky) | EGOISTA (selfish) |
| SOCIEVOLE (social) | SCONFITTO (defeated) |
| BELLO (beautiful) | OSTILE (hostile) |
| RISPETTOSO (respectful) | RESPINTO (rejected) |
| SIMPATICO (nice) | BRUTTO (ugly) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invitto, S.; Ferraioli, F.; Schito, A.; Costanzo, G.; Lucifora, C.; Pompili, A.; Vicario, C.M.; Curcio, G. Prejudice, Proxemic Space, and Social Odor: The Representation of the ‘Outsider’ Through an Evolutionary Metaverse Psychology Perspective. Brain Sci. 2025, 15, 779. https://doi.org/10.3390/brainsci15080779
Invitto S, Ferraioli F, Schito A, Costanzo G, Lucifora C, Pompili A, Vicario CM, Curcio G. Prejudice, Proxemic Space, and Social Odor: The Representation of the ‘Outsider’ Through an Evolutionary Metaverse Psychology Perspective. Brain Sciences. 2025; 15(8):779. https://doi.org/10.3390/brainsci15080779
Chicago/Turabian StyleInvitto, Sara, Francesca Ferraioli, Andrea Schito, Giulia Costanzo, Chiara Lucifora, Assunta Pompili, Carmelo Mario Vicario, and Giuseppe Curcio. 2025. "Prejudice, Proxemic Space, and Social Odor: The Representation of the ‘Outsider’ Through an Evolutionary Metaverse Psychology Perspective" Brain Sciences 15, no. 8: 779. https://doi.org/10.3390/brainsci15080779
APA StyleInvitto, S., Ferraioli, F., Schito, A., Costanzo, G., Lucifora, C., Pompili, A., Vicario, C. M., & Curcio, G. (2025). Prejudice, Proxemic Space, and Social Odor: The Representation of the ‘Outsider’ Through an Evolutionary Metaverse Psychology Perspective. Brain Sciences, 15(8), 779. https://doi.org/10.3390/brainsci15080779









